Abstract
We examined chromosomal distribution of major ribosomal DNAs (rDNAs), clustered in the nucleolar organizer regions (NORs), in 18 species of moths and butterflies using fluorescence in situ hybridization with a codling moth (Cydia pomonella) 18S rDNA probe. Most species showed one or two rDNA clusters in their haploid karyotype but exceptions with 4–11 clusters also occurred. Our results in a compilation with previous data revealed dynamic evolution of rDNA distribution in Lepidoptera except Noctuoidea, which showed a highly uniform rDNA pattern. In karyotypes with one NOR, interstitial location of rDNA prevailed, whereas two-NOR karyotypes showed mostly terminally located rDNA clusters. A possible origin of the single interstitial NOR by fusion between two NOR-chromosomes with terminal rDNA clusters lacks support in available data. In some species, spreading of rDNA to new, mostly terminal chromosome regions was found. The multiplication of rDNA clusters without alteration of chromosome numbers rules out chromosome fissions as a major mechanism of rDNA expansion. Based on rDNA dynamics in Lepidoptera and considering the role of ordered nuclear architecture in karyotype evolution, we propose ectopic recombination, i.e., homologous recombination between repetitive sequences of non-homologous chromosomes, as a primary motive force in rDNA repatterning.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Chromosomes of moths and butterflies (Lepidoptera) are small, numerous and uniform in both shape and size (Robinson 1971; Bedo 1984). They lack a distinct primary constriction (the centromere) and, as a result, sister chromatids separate by parallel disjunction during mitotic metaphase; they are thus regarded as holokinetic with kinetochores extended over a large portion of the chromosome surface (Wolf 1996). Holokinetic nature of chromosomes is supposed to facilitate chromosome fusions and fissions (Wrensch et al. 1994). However, the karyotype of Lepidoptera appears to be relatively stable as it preserves cytological similarities not only within this large and diverse order but also shares common features with the sister order Trichoptera, caddis-flies (Suomalainen 1966; Wolf et al. 1997), which diverged from a common ancestor about 200 MYA (Grimaldi and Engel 2005). Chromosome numbers range from n = 29 to 31 in the majority of moths and butterflies (Robinson 1971; Brown et al. 2007). The modal, i.e. the most frequent chromosome number of n = 31, occurring from basal to highly derived clades, has been proposed as an ancestral karyotype of Lepidoptera (Lukhtanov 2000). Moreover, Yasukochi et al. (2006) and Pringle et al. (2007) reported unusually high degree of conserved synteny of genes between Bombyx mori (Bombycoidea) and Heliconius melpomene (Papilionoidea), which suggests evolutionary stability of lepidopteran linkage groups during last 103 MY. Conserved synteny of genes including conserved gene order between chromosome 15 of B. mori and a chromosome of Manduca sexta (Sphingidae, Bombycoidea) also supports these findings (Sahara et al. 2007). Recently, this has been corroborated by Beldade et al. (2009), who observed conserved assignment of genes to linkage groups between B. mori and Bicyclus anynana (Papilionoidea).
Uniformity of lepidopteran chromosomes, the absence of morphological landmarks (e.g., the centromeres) together with the lack of convenient differential techniques (cf. Bedo 1984) disabled identification of individual chromosomes by standard cytogenetic methods and for a long time restricted their study to simple chromosome counts. Mainly because the mitotic chromosomes were unfavourable for conventional cytogenetics, much longer meiotic chromosomes in the pachytene stage were employed for the so-called pachytene mapping (Traut 1976). But even the chromomere pattern of pachytene bivalents could not be generalized for chromosome identification in Lepidoptera. Nevertheless, this approach made possible the study of sex chromosome systems (WZ/ZZ and derived variants) as the female-specific W chromosome could be distinguished by its heterochromatin in a number of species (reviewed by Traut et al. 2007). More recently, modern approaches such as genomic in situ hybridization (GISH), comparative genomic hybridization (CGH), and fluorescence in situ hybridization (FISH) with W-chromosome painting probes allowed detailed studies on the evolution and molecular structure of the W chromosome (Traut et al. 1999; Mediouni et al. 2004; Fuková et al. 2005, 2007; Yoshido et al. 2005b, 2006; Vítková et al. 2007). However, autosomes still remain unexplored except B. mori, in which all chromosomes were mapped by the so-called BAC-FISH with probes prepared from bacterial artificial chromosome (BAC) clones (Sahara et al. 2003; Yoshido et al. 2005a).
Problems with chromosome identification, similar to those in Lepidoptera, occur in other groups of invertebrates as well. The only autosomes consistently identified in different species are chromosomes bearing tandem arrays of major ribosomal RNA (rRNA) genes (i.e., genes for 18S, 5.8S, and 28S rRNA). These arrays of the so-called rDNA are located in one or several nucleolar organizer regions (NORs). Series of works showed that the number and location of rDNA (or NOR) can be a useful marker for the study of karyotype evolution (e.g., Hirai et al. 1996; Roy et al. 2005; Bombarová et al. 2007). However, data on NORs in lepidopteran genomes are scarce, often obtained as a by-product of principal research by conventional cytogenetic methods detecting only active nucleoli. In Lepidoptera, no particular study on NOR-chromosomes has been accomplished to date. In this work, we examined the number and distribution of rDNA clusters in 18 selected species of moths and butterflies by FISH with 18S rDNA probe with the aim to understand the role of rDNA in the karyotype evolution of Lepidoptera. Our study is the first systematic survey of NOR-chromosomes not only in Lepidoptera but also in species with holokinetic chromosomes in general. The latter is of a particular interest as holokinetic systems may be fundamentally different from monocentric ones in their constraints (Wrensch et al. 1994).
Materials and methods
Insects
Species examined along with their origin are given in Table 1. Specimens were either obtained from laboratory stocks or collected in natural populations. Detailed rearing conditions and diet of laboratory stocks are given in works cited in Table 1. In the field-collected species, except Orgyia recens, Nymphalis xanthomelas, and Polyommatus bellargus, fertilized females were captured, kept in plastic containers at laboratory conditions, and let to lay eggs. Hatched larvae were reared on appropriate host plants chosen according to species bionomy, at room temperature and native day/night regime. In O. recens and N. xanthomelas, larvae were collected on a hawthorn shrub and Chinese hackberry, respectively, and then reared on the same host plant at laboratory conditions. In the case of P. bellargus, adult males collected in the field were dissected.
Chromosome preparations
Spread preparations of mitotic and meiotic chromosomes of all species except P. bellargus were made from both male and female gonads of 3–5th instar larvae as described in Mediouni et al. (2004). In P. bellargus spread chromosome preparations were obtained from gonads of male imago following the same procedure. The preparations were passed through an ethanol series (70, 80, and 100%; 30 s each) and stored at –20°C until further use.
Fluorescence in situ hybridization with 18S rDNA probe (rDNA-FISH)
Unlabelled, about 1,650 bp long 18S rDNA probe was generated by PCR from the colding moth, Cydia pomonella, genomic DNA. PCR was carried out using the 18S-Gal forward (5′-CGATACCGCGAATGGCTCAATA-3′) and 18S-Gal reverse (5′-ACAAAGGGCAGGGACGTAATCAAC-3′) primers as described in Fuková et al. (2005). The probes were labelled with biotin-14-dATP by nick translation using a Bionick Labeling System (Invitrogen, Life Technologies Inc., San Diego, CA, USA).
For rDNA-FISH, we used the procedure as previously reported in Fuková et al. (2005). Briefly, chromosome preparations were treated with 100 μg/mL RNase A to remove an excessive amount of rRNAs. After denaturation the chromosomes were hybridized with a probe cocktail containing 15 ng of biotinylated 18S rDNA probe and 25 μg of sonicated salmon sperm DNA per slide. Hybridization signals were detected with Cy3-conjugated streptavidin (Jackson ImmunoRes. Labs. Inc., West Grove, PA, USA), amplified with biotinylated anti-streptavidin (Vector Labs. Inc., Burlingame, CA, USA). The preparations were counterstained with 0.5 μg/mL of DAPI and mounted in antifade based on DABCO (for composition, see Mediouni et al. 2004).
Preparations were inspected in a Zeiss Axioplan 2 microscope equipped with a cooled F-View CCD camera and AnalySIS software, version 3.2 (Soft Imaging System GmbH, Münster, Germany). Black-and-white images were captured separately for each fluorescent dye and then pseudocoloured (light blue for DAPI and red for Cy3) and superimposed with Adobe Photoshop, version 7.0.
Results
Biotin-labelled probe, prepared from the 18S rRNA gene of the codling moth, Cydia pomonella (Tortricoidea: Tortricidae), was first hybridized by FISH to mitotic and meiotic chromosomes of the source species. In accordance with results of Fuková et al. (2005), rDNA-FISH revealed two clusters of rDNA, localized in both subtelomeric regions of a single chromosome pair. Subsequently, the C. pomonella 18S rDNA probe was hybridized to chromosomal preparations of other species from four large lepidopteran superfamilies, Pyraloidea, Bombycoidea, Papilionoidea, and Noctuoidea. Due to evolutionary stability of the 18S rRNA gene, reliable hybridization signals were obtained in all species examined.
Pyraloidea
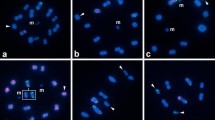
In order to verify published data on NOR-chromosomes obtained by other cytogenetic methods, several previously examined lepidopteran species were included in this study. One of these species was the Mediterranean flour moth, Ephestia kuehniella (Pyralidae). In male pachytene complements of the flour moth consisting of 30 chromosome pairs, rDNA-FISH revealed two rDNA clusters localized at the ends of two bivalents (not shown). This confirmed electron microscopy observation of Marec and Traut (1993), who designated these NOR-bearing chromosomes for their distinctive length as NORS (“small”) and NORL (“large”). Pachytene nuclei of another representative of the superfamily Pyraloidea, the European corn borer (Ostrinia nubilalis) (Crambidae), showed 31 bivalents, which is consistent with the number stated by Keil et al. (1990). Five of them showed rDNA signals it their terminal regions (Fig. 1a).
Localization of rDNA clusters in spread chromosome preparations from gonads of species belonging to superfamilies Pyraloidea (a), Bombycoidea (b, c), and Papilionoidea (d–l) as revealed by FISH with 18S rDNA probe (red signals, arrowheads). Chromosomes were counterstained with DAPI (blue). a Male pachytene complement of Ostrinia nubilalis (Crambidae). b Male pachytene complement of Manduca sexta (Sphingidae). c Female pachytene nucleus of Antheraea pernyi (Saturniidae). d Mitotic metaphase of male Pieris brassicae (Pieridae) consisting of 2n = 30 chromosomes. e Male pachytene complement of P. brassicae; note small DAPI-positive subterminal blocks of heterochromatin in some bivalents (arrows). f Pieris rapae (Pieridae) spermatogonial metaphase with 2n = 50 chromosomes. g Male pachytene complement of Colias hyale (Pieridae). h Spermatogonial metaphase of Polyommatus icarus (Lycaenidae) consisting of 2n = 46 chromosomes. i Male pachytene complement of P. icarus. j Meiotic metaphase I of Polyommatus bellargus male (Lycaenidae) with n = 45 bivalents. k Female pachytene complement of Nymphalis xanthomelas (Nymphalidae); note the proximity of two rDNA clusters, each located at the end of a different NOR-bivalent; the two NORs form a single common nucleolus. l Female pachytene complement of Inachis io (Nymphalidae) originating from a Czech population showing eleven rDNA clusters. Bar = 10 μm (a–c, e, g, i, k, and l); bar = 5 μm (d, f, h, and j)
Bombycoidea
Mitotic spermatogonia of the tobacco hornworm, Manduca sexta (Sphingidae), showed 2n = 56 chromosomes of uniform shape (cf. Sahara et al. 2007). The 18S rDNA probe hybridized to two of these chromosomes (not shown). In pachytene nuclei of spermatocytes, the probe identified a single bivalent with an rDNA cluster located in an interstitial position near one end of the bivalent (Fig. 1b). This result corroborates recent finding of Sahara et al. (2007), who observed a bivalent associated with a nucleolus in a similar position after staining of pachytene oocytes with lactic acetic orcein. The Chinese oak silkmoth, Antheraea pernyi (Saturniidae), is a very interesting species for its high chromosome number of 2n = 98 (Robinson 1971). Pachytene complements of spread oocytes indeed contained numerous, relatively short bivalents, which were difficult to count. However, there was only one bivalent bearing a single rDNA cluster (NOR) in a terminal region (Fig. 1c). The NOR-bivalent belonged to the longest bivalents in A. pernyi pachytene complements.
Papilionoidea
Members of families Pieridae, Lycaenidae, and Nymphalidae were examined. In mitotic complements of the large white, Pieris brassicae (Pieridae), 2n = 30 rod-shaped chromosomes were observed as previously reported by Bigger (1975). Two obviously homologous chromosomes of a similar size carried each terminal rDNA signals (Fig. 1d). In pachytene nuclei of spermatocytes, the 18S rDNA probe hybridized to a terminal region of one NOR-bivalent (Fig. 1e). Subterminal segments of some pachytene bivalents were highlighted with DAPI, indicating the presence of subtelomeric heterochromatin (see arrows in Fig. 1e). In a closely related species, the small white Pieris rapae (Pieridae), spermatogonial nuclei had 2n = 50 chromosomes (cf. Robinson 1971). Also in this species rDNA-FISH identified two mitotic chromosomes carrying rDNA clusters. The rDNA signals appeared terminally located in these small, densely packed chromosomes (Fig. 1f). Unfortunately, we failed to get preparations of pachytene spematocytes in this species and therefore, could not determine precisely the rDNA location at a high resolution of much longer pachytene bivalents. In spermatocytes of the pale clouded yellow, Colias hyale (Pieridae), four bivalents bearing rDNA clusters were regularly observed among 31 chromosome pairs of its pachytene complements. All these clusters were localized in interstitial chromosomal regions (Fig. 1g).
In spermatogonial metaphases of the common blue, Polyommatus icarus (Lycaenidae), 2n = 46 chromosomes were observed. Hybridization signals of the rDNA probe were found in two largest chromosomes, most likely corresponding to chromosome pair no. 1 in the study of Bigger (1975; Fig. 1h). A single rDNA cluster was found in pachytene nuclei of spermatocytes. It was located in an interstitial region near one end of the NOR-bivalent (Fig. 1i). In the Adonis blue, Polyommatus bellargus (Lycaenidae), only metaphase I chromosomes were found on preparations of testes from adult males. The metaphase I complement consisted of n = 45 bivalents. Two of the bivalents showed each two clusters of hybridization signals, each cluster corresponding to one homologous chromosome. The rDNA signals of the smaller bivalent were much fainter than those of the larger bivalent, most likely indicating a small number of rDNA repeats (Fig. 1j). Positions of rDNA clusters on the bivalents could not be determined.
Two bivalents bearing strong terminal hybridization signals were observed in female pachytene nuclei of the yellow-legged tortoiseshell, Nymphalis xanthomelas (Nymphalidae), with a haploid chromosome number of n = 31 (see Robinson 1971). The rDNA signals were located at the ends of these NOR-bivalents and formed small spheres, probably reflecting distribution of rRNA genes in nucleoli. The two NOR-bivalents were often oriented in head-to-head manner by their rDNA (NOR) ends, obviously due to association of their nucleoli in a common nucleolus (Fig. 1k). The peacock butterfly, Inachis io (Nymphalidae), has also a haploid chromosome number of n = 31 (see Robinson 1971). In the Japanese subspecies I. io geisha, Yoshido et al. (2006) observed at least five NOR-bivalents in pachytene oocyte complements after genomic in situ hybridization (GISH) with female genomic DNA probe and DAPI counterstaining. We examined rDNA distribution in I. io specimens originating from a Czech population. In pachytene oocytes, rDNA-FISH identified up to 11 rDNA sites localized at the ends of seven bivalents; three bivalents had rDNA signals at one end and four bivalents at both ends (Fig. 1l). Some of the hybridization signals were relatively weak, most probably due to a small number of rDNA repeats. In addition, the number of the weak rDNA signals varied in some nuclei, probably because of a lower resolution of rDNA-FISH for the rDNA sites with low numbers of repeats.
Noctuoidea
In pachytene oocytes of Lymantria dispar, rDNA-FISH identified a single rDNA cluster located interstitially in a bivalent (not shown). This finding is in a good accord with a single NOR-bivalent observed in this species by Krider and Shields (1997). The karyotype of scarce vapourer, Orgyia recens, consists of n = 30 chromosomes (Robinson 1971). Also in this species, we found a single rDNA cluster located interstitially in the NOR-bivalent of pachytene oocytes (Fig. 2a). Metaphase I spermatocyte nuclei of the white-marked tussock moth, Orgyia leucostigma, consisted of n = 28 bivalents, which is in keeping with the earlier published data (see Robinson 1971). The 18S rDNA probe hybridized to a single bivalent of its metaphase I complement (Fig. 2b). Accordingly, one NOR-bivalent was found in pachytene nuclei of spermatocytes with an interstitial rDNA cluster (Fig. 2c). Futhermore, rDNA-FISH was carried out on chromosomal preparations of O. antiqua males. In metaphase I spermatocyte nuclei, one out of 14 bivalents showed clear rDNA signals (not shown). The presence of a single cluster of rRNA genes was confirmed in nuclei of pachytene spermatocytes, which also showed an NOR-bivalent with an interstitial rDNA site (not shown). These results are consistent with observations of a single autosome NOR-bivalent in orcein-stained pachytene oocytes of O. antiqua (Traut and Clarke 1997; Traut and Marec 1997). We also examined rDNA distribution in the garden tiger, Arctia caja, a representative of the family Arctiidae, which is closely related to the family Lymatriidae (Mitchell et al. 2006). Male mitotic metaphase complements consisted of 2n = 62 chromosomes, two of which bore hybridization signals of the rDNA probe (Fig. 2d). Accordingly, a single chromosome pair (i.e., NOR-bivalent) with an interstitial rDNA cluster was observed in pachytene nuclei of both sexes. Moreover, the WZ sex-chromosome bivalent was easily discernible in oocyte pachytene nuclei according to a conspicuous block of W-heterochromatin deeply stained with DAPI (Fig. 2e). Similar to species of Lymantriidae and Artiidae, also the cabbage moth, Mamestra brassicae (Noctuidae), with n = 31 (Robinson 1971) had only one NOR-bivalent in spermatocyte pachytene nuclei, with an rDNA site located in an interstitial region (Fig. 2f).
Localization of rDNA clusters in spread chromosome preparations from gonads of species belonging to the superfamily Noctuoidea as revealed by FISH with 18S rDNA probe (red signals, arrowheads). Chromosomes were counterstained with DAPI (blue). a Female pachytene complement of Orgyia recens (Lymantriidae). b Metaphase I complement of Orgyia leucostigma (Lymantriidae) male consisting of 2n = 28 bivalents. c Male pachytene nucleus of O. leucostigma. d Spermatogonial mitotic metaphase of Arctia caja (Arctidae). e Female pachytene complement of Arctia caja; note a nucleolus (N) associated with an NOR-bivalent carrying a cluster of strong interstitial rDNA signals; also note the WZ bivalent identified by DAPI-highlighted segment of the W chromosome. f Male pachytene nucleus of Mamestra brassicae (Noctuidae). Bar = 10 μm (a, c, e, and f); bar = 5 μm (b, d)
Discussion
Number and location of rDNA clusters in Lepidoptera
In the present study, we examined chromosomal distribution of rDNA (and thus also potential NORs) in 18 species of the lepidopteran clade Ditrysia using FISH with a codling moth (C. pomonella) 18S rDNA probe. Our results in a compilation with previous data on rDNA/NORs in 32 lepidopteran species from five large superfamilies are summarized in Fig. 3 along with haploid chromosome numbers.
Summary of available data on the number and position of NORs in lepidopteran species along with their phylogenetic relationships and haploid chromosome numbers. Idiograms of NOR-bearing chromosomes show number and location (terminal or interstitial without closer specification) of rDNA clusters (red) in haploid complement. Phylogenetic relationships are based on Kristensen (1999), Wahlberg et al. (2005), Mahendran et al. (2006), Mitchell et al. (2006), and Regier et al. (2008). Colours of phylogenetic branches indicate the following superfamilies: grey, Gelechioidea; orange, Tortricoidea; violet, Pyraloidea; blue, Bombycoidea; yellow, Papilionoidea; green, Noctuoidea. *this study, 1Bedo (1984); 2Bartlett and Del Fosse (1991); 3Fuková et al. (2005); 4Marec and Traut (1993); 5Mediouni et al. (2004); 6Sahara et al. (2007); 7Traut (1976); 8Yoshido et al. (2005b); 9Kundu et al. (1991); 10Van’t Hof et al. (2008); 11Yoshido et al. (2006); 12Monti et al. (1998); 13Traut and Marec (1997); 14Krider and Shields (1997); 15Traut and Clarke (1997); 16Traut and Clarke (1996); †F. Marec, unpublished; ††H.B. Manjunatha and F. Marec, unpublished; ‡chromosome numbers differed in subspecies (2n = 25 in S. c. sp. indet.; 2n = 26 in S. c. walkeri; 2n = 27 in S. c. ricini)
The basal superfamilies of Ditrysia, Gelechioidea and Tortricoidea, showed three different patterns of rDNA distribution in four species with similar chromosome numbers, n = 28–30 (Fig. 3). No common rDNA pattern was found in three species of Pyraloidea with n = 30–31, where E. kuehniella and Ectomyelois ceratoniae have two NORs but one NOR in the latter species is interstitially located. Five rDNA clusters in another pyraloid, O. nubilalis, is most probably a derived state (Fig. 3).
One NOR in an interstitial position seems to be a typical feature of karyotypes in the superfamily Bombycoidea as it occurs in representatives of three families, M. sexta (Sphingidae), B. mori (Bombycidae), and Samia cynthia (Saturniidae; Fig. 3). Three saturnids of the genus Antheraea have either one NOR, probably terminal as in A. pernyi, or two terminal NORs as in A. yamamai (Fig. 3). Available data including chromosome numbers do not allow us to suggest whether the ancestor of A. pernyi and A. roylei lost one NOR or A. yamamai acquired one NOR.
All Noctuoidea examined so far have only one NOR-chromosome with rDNA cluster located interstitially (Fig. 3) except Spodoptera latifascia and S. descoinsi (Monti et al. 1998). The uniform pattern of NOR distribution in species with the ancestral chromosome number of n = 31 and in those with reduced chromosome numbers such as in Orgyia sp. suggests that one interstitial NOR is an ancestral character of Noctuoidea. In S. latifascia and S. descoinsi, the interstitial rDNA cluster could move to terminal locus by inversion, which is corroborated by a single interstitial NOR observed in S. litura (H. B. Manjunatha and F. Marec, unpublished data). The important role of inversions in rearrangements of NOR-bearing chromosomes was demonstrated, for example, in thorny-headed worms of the genus Pomphorhynchus (Acanthocephala) (Bombarová et al. 2007) and Myrmecia ants (Hymenoptera) (Hirai et al. 1996).
The Papilionoidea, in contrast to Noctuoidea, are diverse in the distribution of rDNA site both within and between families (Fig. 3). In Lycaenidae, the occurrence of one interstitial NOR in P. icarus versus two NORs in P. bellargus is probably related to the doubled chromosome number in the latter species. In Nymphalidae, two terminal rDNA sites as in N. xanthomelas multiplied to 11 terminal clusters in I. io. Recently, two NORs were also reported in the butterfly B. anynana, one autosomal NOR in a terminal position and the other associated with the WZ bivalent but its location was not determined (Van’t Hof et al. 2008). In Pieridae, the karyotype of C. hyale with n = 31 but four interstitial rDNA loci demonstrates unique and definitely derived pattern of rDNA distribution, which contrasts with the single terminal NOR in the closely related species of the genus Pieris with reduced chromosome numbers, P. brassicae (n = 15) and P. rapae (n = 25).
Mechanisms of rDNA changes in Lepidoptera
Ability of rDNA clusters to change their number and position was first described in the genus Allium by Schubert (1984). Later Schubert and Wobus (1985) proved that NORs jump between some preferential chromosome sites. Since then, evidence for “the mobile NOR hypothesis” has been growing (Zhang and Sang 1999; Shishido et al. 2000; Roy et al. 2005; Datson and Murray 2006; Cabrero and Camacho 2008). Mobility of rDNA seems to be rather common and thus, it is reasonable to assume the existence of a common mechanism responsible for changes in the number and position of rDNA clusters. However, no such consensual mechanism has been found so far.
In Lepidoptera, the absence of data from ‘primitive’ non-dytrisian families and insufficient data from basal lineages of Ditrysia do not allow for a plausible hypothesis on NOR distribution in the ancestral karyotype. Our study showed that most examined species with n = 28 and lower have one interstitial NOR (Fig. 3). Providing that the ancestral karyotype of n = 31 (Lukhtanov 2000) had two NOR-chromosomes, each bearing one NOR in a terminal region, the most parsimonious way to explain evolutionary dynamics of rDNA clusters in species with reduced chromosome numbers is by fusion of two chromosomes through their terminal NORs. Such fusion, resulting in a chromosome with an interstitial rDNA site, would resemble Robertsonian translocations between the NOR-bearing acrocentric chromosomes in man (Stahl et al. 1983; Choo et al. 1988) and would be facilitated by holokinetic nature of lepidopteran chromosomes (see discussion in Rego and Marec 2003), which overcomes challenge of forming unstable dicentric chromosomes (Stahl et al. 1983). The origin by fusion seems to be applicable in Bombycoidea, where three species with n = 28 have one interstitial NOR and A. yamamai with n = 31 has two terminal NORs (Fig. 3). Further, the curious NOR-autosome in the codling moth, bearing two terminal NORs, was supposed to arise by fusion of two ancestral NOR-chromosomes by their non-NOR ends (Fuková et al. 2005). In another tortricid, Zeiraphera diniana, which has the same number of chromosomes (n = 28), the only NOR-autosome with an interstitial NOR (Emelianov et al. 2004; F. Marec, unpublished data on NOR) could also originate by fusion but through NOR-ends. However, the fusion concept lacks a support in our data set as we failed to find a group of closely related species that would substantiate NOR-fusion.
Instead of fusions of NOR-bearing chromosomes, this study revealed an opposite trend in the karyotype evolution of Lepidoptera, i.e. a multiplication of rDNA clusters. Hirai et al. (1996) reported a positive correlation between a total number of chromosomes and number of NORs in ants of the genus Myrmecia. A similar multiplication of rDNA clusters was observed in the plant Hypochoeris radicata (Hall and Parker 1995). In both cases, rDNA was localized in pericentric heterochromatin, and the multiplication of rDNA clusters was thus a direct consequence of fission of the metacentric NOR-bearing chromosome, which formed two acrocentrics, each bearing a portion of the former rDNA cluster. Hall and Parker (1995) hypothesized that the NOR itself could cause instability of the centromeric region resulting in chromosomal fission. However, in Lepidoptera the mechanism of rDNA multiplication via chromosome fissions can be applied only in the case of the blue butterfly P. bellargus. The modal chromosome number in blue butterflies is reduced to n = 23–24 (Robinson 1971), the state represented by P. icarus which has a single interstitial NOR. Most probably, the chromosome number in P. bellargus increased to n = 45 by a sequence of chromosome fissions (Kandul et al. 2007). During this process, the NOR-chromosome was likely to split into two fragments resulting in two NOR-chromosomes, the number observed in this study.
The observed increase in the number of rDNA clusters is not associated with increasing chromosome numbers in O. nubilalis (Pyraloidea), C. hyale and I. io (both Papilionoidea), all having the ancestral number of n = 31. In these species, rDNA probably dispersed into new chromosomal regions. Such rDNA dispersion is often associated with chromosomal rearrangements such as inversions and translocations, which can result in transfer of an rDNA cluster or its part in a new chromosomal locus. Subtelomeric location of NORs was proposed to enhance rDNA mobility by reducing deleterious effects of these rearrangements. Therefore, more NORs and a higher variability in the number of NORs between closely related species are expected in species with terminally located rDNA clusters (Hanson et al. 1996). Observations in several plant genera such as Allium (Schubert and Wobus 1985), Paeonia (Zhang and Sang 1999), and Aloe (Adams et al. 2000), and in Phaseolus vulgaris (Pedrosa-Harand et al. 2006) are consistent with these predictions. Data available in Lepidoptera (Fig. 3) suggest that species with terminal NORs have a higher number of NORs as well and point thus to a similar mechanism of rDNA dispersion. However, Dubcovsky and Dvořák (1995) and Shishido et al. (2000) found that NOR loci have changed position in the genome without affecting linkage groups in two Poaceae genera, Triticum and Oryza, respectively. Also recent findings of Cabrero and Camacho (2008) contradict the above-mentioned premises of Hanson et al. (1996). According to these authors, rDNA mobility can be explained by transposition involving mobile elements rather than chromosomal rearrangements (see also Raskina et al. 2004; Datson and Murray 2006).
Generally, chromosomal rearrangements can be ascribed to ectopic recombination, i.e., homologous recombination between repetitive sequences dispersed throughout genome (Mieczkowski et al. 2006), which is an efficient mechanism for double strand break (DSB) repairs. Ectopic recombination has two possible resolutions, (1) a crossover configuration resulting in chromosomal rearrangements and (2) a non-crossover gene conversion, predicted to occur with an equal frequency according to the conservative DSB repair model. However, deleterious chromosome rearrangements are under selection pressure and therefore, it is reasonable to assume the existence of mechanisms favouring non-crossover resolution (Shalev and Levy 1997), such as the synthesis-dependent strand-annealing model usually generating only gene conversion (Aylon and Kupiec 2004). Connection between rDNA genes and gene conversion is proved by their concerted evolution, i.e. homogenization of ribosomal gene family (reviewed in Nei and Rooney 2005). Considering these arguments, we suggest that rDNA dynamics observed in Lepidoptera can be mainly ascribed to ectopic homologous recombination which results either in rearrangements of NOR-chromosomes (by inversion, fusion or translocation) or transfer of few ribosomal transcriptional units to a new location by gene conversion followed by their amplification via unequal crossing-over (cf. Dubcovsky and Dvořák 1995; Cabrero and Camacho 2008). The small size of rDNA clusters in I. io is consistent with this hypothesis. Recognition of ectopic recombination as a primary motive force in rDNA dynamics would be of significant consequences. It would mean that changes in number and position of rDNA clusters could be promoted by the presence of ubiquitous repetitive sequences, such as satellite DNA or mobile elements in the NOR and its vicinity (Choo et al. 1988; Maggini et al. 1991; Mieczkowski et al. 2006; Song and Boissinot 2007).
Furthermore, preferable movement of rDNA clusters in subtelomeric regions could be explained not only by deleterious effect of chromosomal rearrangements but also by the ordered spatial organization of nucleus (for reviews, see Marshall 2002; Cremer et al. 2006). If two loci are to interact physically, they must be located at the same place in the nucleus. Thus, non-random association of nonhomologous NOR-chromosomes within the same nucleolus, which was also observed in Lepidoptera (Fig. 1k), is likely to facilitate the interaction of the chromosomes through their rDNA repeats (Stahl et al. 1983; Marshall 2002). Similarly, it was shown that an efficiency of ectopic recombination is influenced by proximity of dispersed homologous sequences to telomeres (Goldman and Lichten 1996). Attachment of telomeres to the nuclear envelope is thought to create a separate compartment restricting their movement to two dimensions. Ectopic recombination between subtelomeric regions is thus facilitated in comparison with interstitial loci (Schlecht et al. 2004). Hence, a low efficiency of ectopic recombination in interstitial regions is likely to be responsible for the conserved rDNA pattern observed in Noctuoidea.
In conclusion, our study revealed dynamic evolution of rDNA distribution in most lepidopteran clades examined, particularly in Pyraloidea and Papilionoidea, which contrasts with the static rDNA pattern in Noctuoidea. Considering the above mechanisms of rDNA mobility the present data on the number and position of NORs in Lepidoptera suggest that repatterning of rDNA can be mainly ascribed to ectopic recombination. Evolutionary dynamics of rDNA clusters and chromosomes bearing them thus presumably reflects the ordered nuclear architecture and distribution of repetitive sequences. This assumption could be used as a framework to disclose the role of NOR-bearing chromosomes in the karyotype evolution.
References
Adams SP, Leitch IJ, Bennet MD, Chase MW, Leitch AR (2000) Ribosomal DNA evolution and phylogeny in Aloe (Asphodelaceae). Am J Bot 87:1578–1583
Aylon Y, Kupiec M (2004) DSB repair: the yeast paradigm. DNA Repair 3:797–815
Bartlett AC, Del Fosse FE (1991) The pachytene karyotype of the pink bollworm (Lepidoptera: Gelechiidae). Southwest Entomol 16:223–235
Bedo DG (1984) Karyotypic and chromosome banding studies of the potato tuber moth, Phthorimaea operculella (Zeller) (Lepidoptera, Gelechiidae). Can J Genet Cytol 26:141–145
Beldade P, Saenko SV, Pul N, Long AD (2009) A gene-based linkage map for Bicyclus anynana butterflies allows for a comprehensive analysis of synteny with the lepidopteran reference genome. PLoS Genet 5:e1000366
Bigger TRL (1975) Karyotypes of some Lepidoptera chromosomes and changes in their holokinetic organisation as revealed by new cytological techniques. Cytologia 40:713–726
Bombarová M, Marec F, Nguyen P, Špakulová M (2007) Divergent location of ribosomal genes in chromosomes of fish thorny-headed worms, Pomphorhynchus laevis and Pomphorhynchus tereticollis (Acanthocephala). Genetica 131:141–149
Brown KS, Freitas AVL, Wahlberg N, von Schoultz B, Saura AO, Saura A (2007) Chromosomal evolution in the South American Nymphalidae. Hereditas 144:137–148
Cabrero J, Camacho JPM (2008) Location and expression of ribosomal RNA genes in grasshoppers: abundance of silent and cryptic loci. Chromosome Res 16:595–607
Choo KH, Vissel B, Brown R, Filby RG, Earle E (1988) Homologous alpha satellite sequences on human acrocentric chromosomes with selectivity for chromosomes 13, 14 and 21: implications for recombination between nonhomologues and Robertsonian translocations. Nucleic Acids Res 16:1273–1284
Cremer T, Cremer M, Dietzel S, Muller S, Solovei I, Fakan S (2006) Chromosome territories—a functional nuclear landscape. Curr Opin Cell Biol 18:307–316
Datson PM, Murray BG (2006) Ribosomal DNA locus evolution in Nemesia: transposition rather than structural rearrangement as the key mechanism? Chromosome Res 14:845–857
Dubcovsky J, Dvořák J (1995) Ribosomal-RNA multigene loci: nomads of the Triticeae genomes. Genetics 140:1367–1377
Emelianov I, Marec F, Mallet J (2004) Genomic evidence for divergence with gene flow in host races of the larch budmoth. Proc R Soc Lond B 271:97–105
Fuková I, Nguyen P, Marec F (2005) Codling moth cytogenetics: karyotype, chromosomal location of rDNA, and molecular differentiation of sex chromosomes. Genome 48:1083–1092
Fuková I, Traut W, Vítková M, Nguyen P, Kubíčková S, Marec F (2007) Probing the W chromosome of the codling moth, Cydia pomonella, with sequences from microdissected sex chromatin. Chromosoma 116:135–145
Goldman ASH, Lichten M (1996) The efficiency of meiotic recombination between dispersed sequences in Saccharomyces cerevisiae depends upon their chromosomal location. Genetics 144:43–55
Grimaldi D, Engel MS (2005) Evolution of the Insects. Cambridge University Press, New York
Hall KJ, Parker JS (1995) Stable chromosome fission associated with rDNA mobility. Chromosome Res 3:417–422
Hanson RE, Islam-Faridi MN, Percival EA, Crane CF, Ji YF, McKnight TD, Stelly DM, Price HJ (1996) Distribution of 5S and 18S–28S rDNA loci in a tetraploid cotton (Gossypium hirsutum L.) and its putative diploid ancestors. Chromosoma 105:55–61
Hirai H, Yamamoto MT, Taylor RW, Imai HT (1996) Genomic dispersion of 28S rDNA during karyotypic evolution in the ant genus Myrmecia (Formicidae). Chromosoma 105:190–196
Kandul NP, Lukhtanov VA, Pierce NE (2007) Karyotypic diversity and speciation in Agrodiaetus butterflies. Evolution 61:546–559
Keil CB, Mason CE, Showers WB (1990) Apyrene spermatogenesis in Ostrinia nubilalis (Hübner): obligate and facultative diapausing strains. J Hered 81:72–74
Krider HM, Shields KS (1997) Meiosis in North American and Asian races of gypsy moth (Lepidoptera: Lymantriidae) and their hybrids. Ann Entomol Soc Am 90:223–229
Kristensen NP (ed) (1999) Lepidoptera, Moths and Butterflies, vol 1: Evolution, Systematics, and Biogeography. Handbook of Zoology, vol IV, Arthropoda: Insecta, part 35. Walter de Gruyter, Berlin, New York
Kundu SC, Ibotombi N, Bhagirath T (1991) Synaptonemal complex karyotype of an Indian silkworm, Antheraea roylei. Sericologia 31:545–547
Lewis LC, Lynch RE (1969) Rearing the European corn borer, Ostrinia nubilalis (Hübner), on diets containing corn leaf and wheat germ. Iowa State J Sci 44:9–14
Lukhtanov VA (2000) Sex chromatin and sex chromosome systems in nonditrysian Lepidoptera (Insecta). J Zoolog Syst Evol Res 38:73–79
Maggini F, Cremonini R, Zolfino C, Tucci GF, D′Ovidio R, Delre V, DePace C, Scarascia Mugnozza GT, Cionini PG (1991) Structure and chromosomal localization of DNA sequences related to ribosomal subrepeats in Vicia faba. Chromosoma 100:229–234
Mahendran B, Ghosh SK, Kundu SC (2006) Molecular phylogeny of silk-producing insects based on 16S ribosomal RNA and cytochrome oxidase subunit I genes. J Genet 85:31–38
Marec F, Traut W (1993) Synaptonemal complexes in female and male meiotic prophase of Ephestia kuehniella (Lepidoptera). Heredity 71:394–404
Marshall WF (2002) Order and disorder in the nucleus. Curr Biol 12:R185–R192
Mediouni J, Fuková I, Frydrychová R, Dhouibi MH, Marec F (2004) Karyotype, sex chromatin and sex chromosome differentiation in the carob moth, Ectomyelois ceratoniae (Lepidoptera: Pyralidae). Caryologia 57:184–194
Mieczkowski PA, Lemoine FJ, Petes TD (2006) Recombination between retrotransposons as a source of chromosome rearrangements in the yeast Saccharomyces cerevisiae. DNA Repair 5:1010–1020
Mitchell A, Mitter C, Regier JC (2006) Systematics and evolution of the cutworm moths (Lepidoptera: Noctuidae): evidence from two protein-coding nuclear genes. Syst Entomol 31:21–46
Monti L, Lemeunier F, Lalanne-Cassou B (1998) A chromosomal investigation of two closely related species of Spodoptera. Compt Rendus Acad Sci III Sci Vie 321:275–282
Nei M, Rooney AP (2005) Concerted and birth-and-death evolution of multigene families. Annu Rev Genet 39:121–152
Pedrosa-Harand A, de Almeida CCS, Mosiolek M, Blair M, Schweizer D, Guerra M (2006) Extensive ribosomal DNA amplification during Andean common bean (Phaseolus vulgaris L.) evolution. Theor Appl Genet 112:924–933
Pringle EG, Baxter SW, Webster CL, Papanicolaou A, Lee SF, Jiggins CD (2007) Synteny and chromosome evolution in the Lepidoptera: evidence from mapping in Heliconius melpomene. Genetics 177:417–426
Raskina O, Belyayev A, Nevo E (2004) Activity of the En/Spm-like transposons in meiosis as a base for chromosome repatterning in a small, isolated, peripheral population of Aegilops speltoides Tausch. Chromosome Res 12:153–161
Regier JC, Cook CP, Mitter C, Hussey A (2008) A phylogenetic study of the “bombycoid complex” (Lepidoptera) using five protein-coding nuclear genes, with comments on the problem of macrolepidopteran phylogeny. Syst Entomol 33:175–189
Rego A, Marec F (2003) Telomeric and interstitial telomeric sequences in holokinetic chromosomes of Lepidoptera: telomeric DNA mediates association between postpachytene bivalents in achiasmatic meiosis of females. Chromosome Res 11:681–694
Robinson R (1971) Lepidoptera Genetics. Pergamon Press, Oxford
Roy V, Monti-Dedieu L, Chaminade N, Siljak-Yakovlev S, Aulard S, Lemeunier F, Montchamp-Moreau C (2005) Evolution of the chromosomal location of rDNA genes in two Drosophila species subgroups: ananassae and melanogaster. Heredity 94:388–395
Sahara K, Yoshido A, Kawamura N, Ohnuma A, Abe H, Mita K, Oshiki T, Shimada T, Asano S, Bando H, Yasukochi Y (2003) W-derived BAC probes as a new tool for identification of the W chromosome and its aberrations in Bombyx mori. Chromosoma 112:48–55
Sahara K, Yoshido A, Marec F, Fuková I, Zhang HB, Wu CC, Goldsmith MR, Yasukochi Y (2007) Conserved synteny of genes between chromosome 15 of Bombyx mori and a chromosome of Manduca sexta shown by five-color BAC-FISH. Genome 50:1061–1065
Schlecht HB, Lichten M, Goldman ASH (2004) Compartmentalization of the yeast meiotic nucleus revealed by analysis of ectopic recombination. Genetics 168:1189–1203
Schubert I (1984) Mobile nucleolus organizing regions (NORs) in Allium (Liliaceae s. lat.)? Inferences from the specifity of silver staining. Plant Syst Evol 144:291–305
Schubert I, Wobus U (1985) In situ hybridization confirms jumping nucleolus organizing regions in Allium. Chromosoma 92:143–148
Shalev G, Levy AA (1997) The maize transposable element Ac induces recombination between the donor site and an homologous ectopic sequence. Genetics 146:1143–1151
Shishido R, Sano Y, Fukui K (2000) Ribosomal DNAs: an exception to the conservation of gene order in rice genomes. Mol Gen Genet 263:586–591
Song M, Boissinot S (2007) Selection against LINE-1 retrotransposons results principally from their ability to mediate ectopic recombination. Gene 390:206–213
Stahl A, Luciani JM, Hartung M, Devictor M, Bergé-Lefranc JL, Guichaoua M (1983) Structural basis for Robertsonian translocations in man: association of ribosomal genes in the nucleolar fibrillar center in meiotic spermatocytes and oocytes. Proc Natl Acad Sci USA 80:5946–5950
Suomalainen E (1966) Achiasmatische Oogenese bei Trichopteren. Chromosoma 18:201–207
Traut W (1976) Pachytene mapping in the female silkworm, Bombyx mori L. (Lepidoptera). Chromosoma 58:275–284
Traut W, Clarke CA (1996) Cytogenetics of a moth species with a low chromosome number, Orgyia thyellina. Hereditas 125:277–283
Traut W, Clarke CA (1997) Karyotype evolution by chromosome fusion in the moth genus Orgyia. Hereditas 126:77–84
Traut W, Marec F (1997) Sex chromosome differentiation in some species of Lepidoptera (Insecta). Chromosome Res 5:283–291
Traut W, Sahara K, Otto TD, Marec F (1999) Molecular differentiation of sex chromosomes probed by comparative genomic hybridization. Chromosoma 108:173–180
Traut W, Sahara K, Marec F (2007) Sex chromosomes and sex determination in Lepidoptera. Sex Dev 1:332–346
Van’t Hof AE, Marec F, Saccheri IJ, Brakefield PM, Zwaan BJ (2008) Cytogenetic characterization and AFLP-based genetic linkage mapping for the butterfly Bicyclus anynana, covering all 28 karyotyped chromosomes. PLoS ONE 3:e3882
Vítková M, Fuková I, Kubíčková S, Marec F (2007) Molecular divergence of the W chromosomes in pyralid moths (Lepidoptera). Chromosome Res 15:917–930
Wahlberg N, Braby MF, Brower AV, de Jong R, Lee MM, Nylin S, Pierce NE, Sperling FA, Vila R, Warren AD, Zakharov E (2005) Synergistic effects of combining morphological and molecular data in resolving the phylogeny of butterflies and skippers. Proc Biol Sci 272:1577–1586
Wolf KW (1996) The structure of condensed chromosomes in mitosis and meiosis of insects. Int J Insect Morphol Embryol 25:37–62
Wolf KW, Novák K, Marec F (1997) Kinetic organization of metaphase I bivalents in spermatogenesis of Lepidoptera and Trichoptera species with small chromosome numbers. Heredity 79:135–143
Wrensch DL, Kethley JB, Norton RA (1994) Cytogenetics of holokinetic chromosomes and inverted meiosis: keys to the evolutionary success of mites, with generalization on eukaryotes. In: Houck MA (ed) Mites: Ecological and Evolutionary Analyses of Life-History Patterns. Chapman & Hall, New York, pp 282–343
Yasukochi Y, Ashakumary LA, Baba K, Yoshido A, Sahara K (2006) A second-generation integrated map of the silkworm reveals synteny and conserved gene order between lepidopteran insects. Genetics 173:1319–1328
Yoshido A, Bando H, Yasukochi Y, Sahara K (2005a) The Bombyx mori karyotype and the assignment of linkage groups. Genetics 170:675–685
Yoshido A, Marec F, Sahara K (2005b) Resolution of sex chromosome constitution by genomic in situ hybridization and fluorescence in situ hybridization with (TTAGG) n telomeric probe in some species of Lepidoptera. Chromosoma 114:193–202
Yoshido A, Yamada Y, Sahara K (2006) The W chromosome detection in several lepidopteran species by genomic in situ hybridization (GISH). J Insect Biotech Sericol 75:147–151
Zhang D, Sang T (1999) Physical mapping of ribosomal RNA genes in peonies (Paeonia, Paeoniaceae) by fluorescent in situ hybridization: implications for phylogeny and concerted evolution. Am J Bot 86:735–740
Acknowledgments
We wish to thank Marie Korchová for technical assistance and our colleagues, Oxana Habuštová for Ostrinia nubilalis, Jiří Beneš, Zdeněk Fric, Josef Jaroš, and Martin Konvička for their help with collecting and determination of some species. Our thanks are also due to Zenta Kajiura (Shinshu University, Japan) for Antheraea pernyi through NBRP, Sandra Barns and Stuart Reynolds (Bath University, UK) for Manduca sexta, Josef Chytil (Přerov, Czech Republic) for Lymantria dispar, Tomáš Hamřík (Velká Bíteš, Czech Republic) for Arctia caja, and Zdeněk Hanč (České Budějovice) for valuable advice. This work was funded by grants 206/06/1860 of the Grant Agency of the Czech Republic (GACR) and IAA600960925 of the Grant Agency of the Academy of Sciences of the Czech Republic, both Prague, and from the Entomology Institute project Z50070508. P. N. acknowledges the support from the grant SGA2006/01 of the Student Grant Agency of the Faculty of Science, University of South Bohemia, Czech Republic, and the support of his Ph.D. programme by the GACR grant 521/08/H042. K. S. and A. Y. were supported from grants 18380037 and 191114 of the Japan Society for the Promotion of Science (JSPS), respectively.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nguyen, P., Sahara, K., Yoshido, A. et al. Evolutionary dynamics of rDNA clusters on chromosomes of moths and butterflies (Lepidoptera). Genetica 138, 343–354 (2010). https://doi.org/10.1007/s10709-009-9424-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10709-009-9424-5