Abstract
Galactomannans are plant polysaccharides with the beneficial properties, which have a wide range of industrial application. The most important galactomannan derivatives are sulfates, which exhibit the anticoagulant and other biological activities. Here, we report on the results of investigations of the effect of urea-based solvents and activators on the sulfation of galactomannan guar gum with sulfamic acid. It has been shown that 1,4-dioxane is the most effective solvent, while urea is the most effective activator of the sulfation process. The numerical optimization (the Box‒Behnken design) of sulfation of galactomannan guar gum with sulfamic acid in the presence of the most effective activator has been carried out. It has been established that the optimal conditions for obtaining the galactomannan sulfates are a sulfamic acid amount of 34 mmol per 1 g of galactomannan, a temperature of 85 °C, and a time of 2.6 h. The introduction of a sulfate group into a galactomannan molecule has been proven by Fourier transform infra-red spectroscopy. It has been found that the Fourier transform infra-red spectrum of sulfated galactomannan contains absorption bands at 1249 and 817 cm‒1, which correspond to vibrations of the sulfate group. It has been demonstrated using gel permeation chromatography that, during sulfation of guar gum galactomannan by a complex of sulfamic acid and 1,4-dioxane, the molecular weight decreases from 8.5 × 105 to 3.0 × 105 g/mol and the polydispersity value increases from 1.816 to 2.049.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Polysaccharides are complex polymers consisting of monosaccharide units linked by the glycosidic bonds with the formation of long branched or unbranched chains [1]. Plant polysaccharides are valued for their nontoxicity, biodegradability, safety, renewability, biocompatibility, and availability [2].
Plant polysaccharides are used in food, pharmaceutical, and cosmetic industries [3]. Recently, there has been an increase in industrial use of plant polysaccharides as thickeners, moisturizers, emulsifiers, suspending agents, and wound healing agent [1].
Natural gums are polysaccharides of natural origin, which exhibit the increasing viscosity of a solution at low concentrations. They can have different linear chain lengths, branching characteristics, molecular weights, and some other properties [4,5,6]. Natural gums represent hydrophilic polysaccharides obtained from plants or microbes [4].
Guar gum is a powdered endosperm of seeds of a Cyamopsis tetragonolobus legume crop [1]. It consists of a straight chain of mannose units linked to galactopyranose units. Galactomannan guar gum is a food grade carbohydrate polymer, which is used as a thickener and an absorption agent [7, 8] and in the explosives, food, cosmetics, pharmaceutical, mining, paper, and textile industries, mainly as a water-soluble binder [7, 9]. The guar gum derivatives find application in the oil industry [10] and as corrosion inhibitors [11, 12], fracturing fluids [10, 13], viscosity modifiers [10, 13, 14], etc.
Guar gum galactomannan is rich in the reactive hydroxyl groups, which opens up broad prospects for its chemical modification. Among a wide variety of the guar gum derivatives obtained by now are carboxymethyl guar gum [15, 16], hydroxymethyl guar gum [17], hydroxypropylethyl guar gum [18], O-carboxymethyl- and O-hydroxypropyl guar gum [19], acryloyloxy guar gum [20], O-carboyxymethyl-O-2 hydroxy-3- (trimethylammonia propyl) guar gum [9], and many others. Special attention has been paid to the sulfated guar gum galactomannan derivatives characterized by the biological activity.
At present, the aggressive sulfating agents, including sulfuric acid, sulfur trioxide, and chlorosulfonic acid, are widely used in sulfation of galactomannans [21,22,23,24]. However, their use in the large-scale production of galactomannan sulfates faces numerous limitations. In addition, guar gum galactomannan can be sulfated with a deep eutectic solvent representing a sulfamic acid‒urea mixture [25]. A drawback of this method is the side carbamation reactions, which can affect the biological activity of a product. It should be noted that, in all the abovementioned methods, the galactomannan polymer chains are significantly depolymerized.
The aim of this study was to investigate the effect of solvents and urea-based activators on the guar gum galactomannan sulfation process, numerically optimize the latter, and examine the products using Fourier transform infra-red (FTIR) spectroscopy and gel permeation chromatography.
2 Experimental
Galactomannan from Sigma-Aldrich guar gum was investigated. The galactose/mannose (G/M) ratio was 1.0:1.8.
2.1 Sulfation of guar gum galactomannan
Guar gum galactomannan was sulfated in a three-necked flask (100 ml) equipped with a thermometer, a mechanical stirrer, and a glycerol bath using a modified procedure described in [26]. Galactomannan (2.1 g) and an organic solvent (1,4-dioxane, pyridine, morpholine, piperidine, dimethyl formamide (DMF), or diglyme) (50 ml) were placed in a flask and the mixture was stirred at 50 °C. Then, 50‒150 mmol of a urea or urea-based activator (thiourea, methyl urea, ethyl urea, hydroxyethyl urea, or biuret) and 50‒150 mmol of sulfamic acid were added and stirred at 75‒85 °C for 0.5‒3.0 h, according to the sulfation conditions given in Table 4. After the termination of sulfation, the reaction mixture was cooled to room temperature, neutralized with the 25% ammonia solution until neutral, and poured into ethanol (150 ml). The obtained viscous product was triply washed with 96% ethanol (each portion was 10 ml) until the formation of a solid precipitate. The precipitate, which was a sulfated derivative of galactomannan in the form of an ammonium salt, was filtered, washed on a filter with 96% ethanol (15 ml), and dried in air.
The ammonium salt of sulfated galactomannan was purified by dialysis on cellophane against distilled water. The product was dialyzed for 10–15 h with changing water each 1‒2 h using an MF-5030–46 MFPI dialysis bag (US) with a pore size of 3.5 kDa and a width of 46 mm.
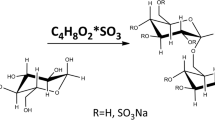
The scheme of guar gum galactomannan sulfation is shown in Fig. 1.
A sample taken for the physicochemical analysis had a degree of sulfation (DS) of 0.78; this value was obtained with sulfamic acid in the presence of urea in 1,4-dioxane at 80° C for 3 h.
2.2 Numerical optimization of the guar gum sulfation
The guar gum galactomannan sulfation process was numerically optimized using the Statgraphics Centurion XVI software, Design of Experiment (DOE) block [27, 28].
2.3 Methods of the physicochemical analysis
2.3.1 Elemental analysis
The elemental analysis of the sulfated guar gum galactomannan was carried out with a ThermoQuest FlashEA-1112 elemental analyzer (Italy).
The DS value was calculated using the following equation [29]:
where sulfur content S% is determined by an elemental analysis, 1.62 is the molar weight of the monosaccharide unit in the (C6H11O5)/100 polymer, and 1.02 is the molar weight of the (SO3Na)/100 sulfate substituent.
2.3.2 Fourier transform infrared spectroscopy study
The FTIR spectra of the initial and sulfated guar gum in the wavelength range of 400–4000 cm‒1 were obtained on a Shimadzu IR Tracer-100 spectrometer (Japan) and processed with the OPUS software (version 5.0). The solid samples for the analysis were pills in a KBr matrix (2 mg sample/1000 mg KBr).
2.3.3 Gel permeation chromatography
The data on the number-average molecular weight (Mn), weight-average molecular weight (Mw), and polydispersity of the initial and sulfated guar gum were obtained on an Agilent 1260 Infinity II Multi-Detector GPC/SEC system with two detectors: refractometer (RI) and viscometer (VS) The separation was made on two Agilent PL aquagel-OH columns. As a mobile phase, the solution of 0.1 mol/l LiNO3 in deionized water was used. Calibration was performed using Agilent polyethylene glycol standards (US). The eluent flow rate was 1 ml/min at a sample volume of 100 μl. The samples to be analyzed were dissolved in the mobile phase (1‒5 mg/ml) and filtered through a 0.22-μm Agilent PES membrane filter. The data obtained were analyzed with the Agilent GPC/SEC MDS software.
3 Results and discussion
3.1 Effect of a solvent
To elucidate the effect of solvents on sulfation of galactomannan with sulfamic acid in the presence of urea, we studied the organic solvents 1,4-dioxane, pyridine, piperidine, morphilin, N, N-dimethylformamide (DMF), and diglyme.
The sulfation reaction is commonly described as the bimolecular nucleophilic substitution. As is known, the reactions proceeding by this mechanism are facilitated by the aprotic solvents. When studying the effect of the nature of an aprotic solvent on the sulfation reaction rate, it is necessary to take into account the effect of a solvent on the entire process. First, the ability of these solvents to induce dissociation of ion pairs should increase with the permittivity growth, which, in turn, can lead to an increase in the rate of decomposition of sulfamic acid into ammonia and sulfur trioxide required for the sulfation reaction. Second, the basicity of a solvent noticeably affects the sulfur content in sulfated galactan, which is related to the reactivity of the formed complex of sulfur trioxide and the solvent used. According to the data given in Table 1, the sulfated product with the maximum sulfur content was obtained in the 1,4-dioxane medium. The process of sulfation of guar gum galactomannan in the 1,4-dioxane medium using a solvent with a permittivity lower than that of other solvents is characterized by a high rate of decomposition of sulfamic acid. Sulfation of galactomannan in the solvents with a higher basicity as compared with dioxane (pyridine, morpholine, and piperidine) yielded less sulfated galactomannan. The sulfur content in the products of sulfation of galactomannan in ethers (dioxane and diglyme) with the analogous basic properties was similar (with a difference of 1.1 wt %).
According to the data given in Table 1, the highest sulfur content in sulfated galactomannan is obtained with 1,4-dioxane used as a solvent (it has the lowest permittivity among the investigated solvents). A fairly high sulfur content (9.4 wt %) in sulfated galactomannan was obtained also with diglyme used as a solvent.
3.2 Effect of an activator
The mechanism of sulfation with sulfamic acid still has been understudied. It was assumed in [30,31,32,33,34] that a donor‒acceptor complex is formed, which has a stronger sulfating ability than the initial sulfamic acid.
According to the proposed scheme (Fig. 2), under the interaction of sulfamic acid and an organic base, a donor‒acceptor complex forms and the S‒N bond weakens. This can lead to the decomposition of sulfamic acid into ammonia and sulfur trioxide, which is followed by sulfation of the polysaccharide at OH groups (Fig. 1).
Organic bases are used as activators of sulfation of polysaccharides with sulfamic acid [30, 32, 35]. However, in the cited studies, only few activators of this process were considered, which are 1,4-dioxane, N, N-dimethylformamide, urea, pyridine, morpholine, and piperidine. In this study, we examined the urea-based activators for enhancing the sulfur content in galactomannan sulfates.
According to the data given in Table 2 , without an activator, the process of sulfation of galactomannan almost does not occur. The use of an activator in the process can increase the sulfur content in galactomannan sulfate by a factor of up to 5.
With an increase in the alkyl radical length in the urea derivatives, their activating ability in the reactions of sulfation with sulfamic acid naturally decreases. The presence of a hydroxyl group in the urea derivatives almost does not affect their activating ability. The presence of an additional carbamate group in the random urea (for biuret) reduces its activating ability as compared with the original urea.
It should be noted that the use of thiourea as an activator of the galactomannan sulfation process also lowers the sulfur levels in galactomannan sulfate as compared with urea. The maximum sulfur content (10.5 wt %) is obtained with urea used as an activator.
3.3 Box‒Behnken design analysis of the galactomannan sulfation process
In this study, we investigated the effect of an amount of the sulfating complex and process temperature and time on the degree of substitution in galactomannan sulfates.
In the investigations, the three factors used as independent variables were (the values are in the parenthesis) amount X1 of the of sulfating complex per 1 g of galactomannan (1.5, 2.5, and 3.5 mmol), galactomannan sulfation temperature X2 (75, 80, and 85 °C), and process time X3 (0.5, 1.75, and 3 h). The result of sulfation was characterized by output parameter Y1, which is the degree of substitution (DS). The Box‒Behnken experiment design (BBD) was used. Each experiment was run in duplicate. The variable designations are given in Table 3.
The experimental results are given in Table 4.
An increase in the process temperature should lead to an increase in both the sulfation and depolymerization rates, but to different extents [25]. Obviously, the low molecular weight galactomannan fractions not only exhibit the high reactivity in the sulfation reaction, but also undergo the faster hydrolytic depolymerization under the action of sulfamic acid. Since the depolymerization rate increases with the process temperature [25], over time, a large amount of the low molecular weight sulfated product with a sufficiently high sulfur content is formed, which is removed during the dialysis purification (Fig. 6, Sect. 3.5, “Gel Permeation Chromatography”).
According to the data given in Table 4, the highest DS value (0.78) for galactomannan sulfates is obtained using 35 mmol of the sulfating complex at a process temperature of 80 °C and a process time of 3 h.
The results of the analysis of variance (ANOVA) are given in Table 5.
The BBD of the experiment appeared useful for the development of an accurate experimental model among the significant factors [36]. The experimental data on the sulfur content in galactomannan sulfates were analyzed by the ANOVA (see Table 5). The significant factors were defined as p < 0.05. For all the independent variables in the region of the factor space, we have p < 0.0003 (Table 6).
The ANOVA study showed that, under the specified experimental conditions, the greatest contribution to the total variance of the output parameter is made by the two factors: the amount of the sulfating complex and the time of the guar gum sulfation process. This is indicated by the high values of dispersion coefficient F for the main effects, which are also called the efficiencies. The data given in the column containing the P values in Table 5 is interpreted similarly. It should be noted that there is also the effect of the process temperature, although weaker than that of the two other factors. This is indicated by a P value of 0.0003 (with an acceptable range of 0.05). The effect of the source of variance on the output parameter is considered statistically significant if the significance level is lower than a specified critical value.
The dependence of the DS Y1 in galactomannan sulfates on the variable process factors is approximated by the regression equation:
The predictive properties of Eq. (2) are shown in Fig. 3, which compares the experimental values of output parameter Y1 with the values calculated using Eq. (2). The straight corresponds to the calculated Y1 values and symbols, to the results of the observation. The proximity of the experimental points to the straight confirms the good predictive properties of Eq. (2).
A graphic representation of Eq. (2) in the form of a response surface is shown in Fig. 4.
The quality of the approximation is also characterized by coefficient of determination R2adj. In the problem under consideration, we have R2adj = 98.6%, which points out the high approximation quality. This evidences for the consistency of Eq. (2) to the observation results and shows the adequacy of the mathematical model to the process under study.
The calculated optimal conditions for sulfation of galactomannan with sulfamic acid in dioxane in the presence of urea are a sulfamic acid amount of 34 mmol per 1 g of galactomannan, a temperature of 85 °C, and a time of 2.6 h.
Table 6 gives a comparison of the experimental and calculated DS values for galactomannan sulfates. It can be seen that the relative error ranges from 0.0 to 10.8%, depending on the experimental conditions. The mean relative error is no more than 5.5%.
3.4 FTIR spectroscopy
The initial and sulfated galactomannan were studied by FTIR spectroscopy (Fig. 3).
The introduction of the sulfate group into the galactomannan structure was confirmed by the FTIR spectroscopy data (Fig. 5). The FTIR spectra of galactomannan sulfate (in contrast to those of initial galactomannan) contain absorption bands at 1249 and 817 cm‒1, which correspond to the vibrations of sulfate groups [25, 31].
In the range of 2900‒3600 cm‒1, in galactomannan sulfate (Fig. 5), in contrast to the initial galactomannan, the absorption bands of amino groups, hydroxyl groups, and CH2 groups overlap.
In addition, note that sulfation of galactomannan in 1,4-dioxane with sulfamic acid in the presence of urea leads to the side carbamation reactions, which were observed by Akman et al. [31], Kazachenko et al. [25], and Sirviö et al. [38]. Thus, the absorption band of the C = O group (1715 cm‒1) appears in the spectra of galactomannan sulfate.
3.5 Gel permeation chromatography
In our previous studies of guar gum sulfation [24, 25], we used GM (Cyamopsis tetraganoloba, LLC “Mast-sl”) as the initial sample, which was characterized by a bimodal weight distribution of particles and had a molecular weight of ~ 6.0 × 105 g/mol.
According to the galactomannan molecular weight distribution (Table 7), the initial sample (Sigma-Aldrich) is a low molecular weight-type galactomannan with an Mw value of 8.3 × 105 g/mol, which has the monomodal mass distribution of particles with a high molecular weight fraction with an MM value of > 1.0 × 106 g/mol and a more homogeneous structure (a polydispersity of 1.816) than in our previous studies [24, 25].
After the sulfation process, we can observe the molecular weight redistribution in the sample. In contrast to the initial galactomannan, its sulfated derivative showed a rapid decrease in Mw (the range from 8.3 × 105 to 3.02 × 105 g/mol). Furthermore, the molecular weight distribution for sulfated galactomannan was much broader than that for the initial sample. The molecular weight distribution curves for galactomannan and its sulfated derivative are presented in Fig. 6.
The high molecular weight fraction in the sample noticeably decreases and can be attributed to the partial depolymerization of galactomannan and the ongoing competing hydrolysis reactions. The molecular weight redistribution leads to an increase in polydispersity from 1.816 to 2.049.
In our previous studies on sulfated guar gum [24, 25], the Mw value for the sulfated derivatives decreased after sulfation, which was caused by the multi-interaction of the factors, including the sulfating complex composition, temperature, and time. In summary, we can conclude that sulfation and degradation occurred simultaneously in the sulfation process in the form of a complex interaction in the acidic environment.
Thus, sulfation of galactomannan with sulfamic acid in 1,4-dioxane in the presence of urea yields sulfated galactomannan with a molecular weight of Mw = 3.02 × 105 g/mol and a polydispersity value of 2.049. This is indicative of the less aggressive sulfation conditions and therefore weaker degradation of galactomannan as a result of hydrolysis during sulfation (Table 7, Fig. 7) as compared with the other methods [24, 25].
4 Conclusions
In this study, we proposed a new method for sulfation of guar gum galactomannan with sulfamic acid in 1,4-dioxane in the presence of urea-based activators. It was found that, with an increase in the length of the N substituent of urea, its activating ability in the reactions of sulfation with sulfamic acid weakens. The optimal parameters for the sulfation of galactomannan with sulfamic acid in 1,4-dioxane in the presence of urea were established by the Box‒Behnken design method. The introduction of a sulfate group was confirmed by the elemental analysis and FTIR spectroscopy. The FTIR spectra of sulfated galactomannan, in contrast to the initial galactomannan, contain absorption bands of the sulfate group. Sulfation of galactomannan with sulfamic acid in 1,4-dioxane in the presence of urea leads to a decrease in the molecular weight by a factor of 2.7 as a result of depolymerization.
References
Thombare N, Jha U, Mishra S, Siddiqui MZ (2016) Guar gum as a promising starting material for diverse applications: a review. Int J Biol Macromol 88:361–372. https://doi.org/10.1016/j.ijbiomac.2016.04.001
Reddy K, Mohan GK, Satla S, Gaikwad S (2011) Natural polysaccharides: versatile excipients for controlled drug delivery systems. Asian J Pharm Sci 6(6):275–286
Liu J, Willför S, Xu C (2015) A review of bioactive plant polysaccharides: biological activities, functionalization, and biomedical applications. Bioact Carbohydr Diet Fibre 5(1):31–61. https://doi.org/10.1016/j.bcdf.2014.12.001
Ahmad S, Ahmad M, Manzoor K, Purwar R, Ikram S (2019) A review on latest innovations in natural gums-based hydrogels: preparations & applications. Int J Biol Macromol. https://doi.org/10.1016/j.ijbiomac.2019.06.113
Nussinovitch A (2010) Plant gum exudates of the world: sources, distribution, properties, and applications CRC Press/Taylor & Francis, Boca Raton
Williams PA (2011) Renewable resources for functional polymers and biomaterials. Polysaccharides, proteins and polyesters. RSC Polymer Chemistry Series. Royal Society of Chemistry, Cambridge.
Mudgil D, Barak S, Khatkar BS (2011) Guar gum: processing, properties and food applications—a review. J Food Sci Technol 51(3):409–418. https://doi.org/10.1007/s13197-011-0522-x
Yamamoto Y (2001) Hypolipidemic effects of a guar gum-xanthan gum mixture in rats fed high sucrose diet. J Jpn Soc Nutr Food Sci 54:139–145
Zhang L-M, Zhou J-F, Hui PS (2005) A comparative study on viscosity behavior of water-soluble chemically modified guar gum derivatives with different functional lateral groups. J Sci Food Agric 85(15):2638–2644. https://doi.org/10.1002/jsfa.2308
Hasan AMA, Abdel-Raouf ME (2018) Applications of guar gum and its derivatives in petroleum industry: a review. Egypt J Pet. https://doi.org/10.1016/j.ejpe.2018.03.005
Jano A, Lame GA, Kokalari TE (2012) Use of extracted green inhibitors as a friendly choice in corrosion protection of low alloy carbon steel Kemija u industriji. J Chem Chem Eng 61(11–12):497–503. https://doi.org/10.15255/KUI.2012.013
About S, Zouarhi M, Chebabe D, Damej M, Berisha A, Hajjaji N (2020) Galactomannan as a new bio-sourced corrosion inhibitor for iron in acidic media. Heliyon 6(3):e03574. https://doi.org/10.1016/j.heliyon.2020.e03574
Adewole JK, Muritala KB (2019) Some applications of natural polymeric materials in oilfield operations: a review. J Petroleum Exploration Prod Technol 9(3):2297–2307. https://doi.org/10.1007/s13202-019-0626-9
Mothé CG, Correia DZ, de França FP, Riga AT (2006) Thermal and rheological study of polysaccharides for enhanced oil recovery. J Therm Anal Calorim 85:31–36. https://doi.org/10.1007/s10973-005-7339-7
Dodi G, Hritcu D, Popa M (2011) Carboxymethylation of guar gum: synthesis and characterization. Cellul Chem Technol 45:171
Gong H, Liu M, Chen J, Han F, Gao C, Zhang B (2012) Synthesis and characterization of carboxymethyl guar gum and rheological properties of its solutions. Carbohyd Polym 88(3):1015–1022. https://doi.org/10.1016/j.carbpol.2012.01.057
Shaikh T, Kumar SS (2011) Pharmaceutical and pharmacological profile of guar gum an overview. Int J Pharm Pharm Sci 3:38–40
Lapasin R, De Lorenzi L, Pricl S, Torriano G (1995) Flow properties of hydroxypropyl guar gum and its long-chain hydrophobic derivatives. Carbohyd Polym 28(3):195–202. https://doi.org/10.1016/0144-8617(95)00134-4
Shi H-Y, Zhang L-M (2007) New grafted polysaccharides based on O-carboxymethyl-O-hydroxypropyl guar gum and N-isopropylacrylamide: synthesis and phase transition behavior in aqueous media. Carbohyd Polym 67(3):337–342. https://doi.org/10.1016/j.carbpol.2006.06.005
Shenoy MA, D’Melo DJ (2010) Synthesis and characterization of acryloyloxy guar gum. J Appl Polym Sci 117:148–154. https://doi.org/10.1002/app.31872
Mestechkina NM, Egorov AV, Shcherbukhin VD (2006) Synthesis of galactomannan sulfates. Appl Biochem Microbiol 42:326. https://doi.org/10.1134/S0003683806030185
Zhang Z, Wang H, Chen T, Zhang H, Liang J, Kong W, Yao J, Zhang J, Wang J (2019) Synthesis and structure characterization of sulfated galactomannan from fenugreek gum. Int J Biol Macromol 125:1184–1191. https://doi.org/10.1016/j.ijbiomac.2018.09.113
Wang X, Wang J, Zhang J, Zhao B, Yao J, Wang Y (2009) Structure-antioxidant relationships of sulfated galactomannan from guar gum. Int J Biol Macromol 46(1):59–66. https://doi.org/10.1016/j.ijbiomac.2009.10.004
Kazachenko AS, Akman F, Sagaama A, Issaoui N, Malyar YN, Vasilieva NY, Borovkova VS (2021) Theoretical and experimental study of guar gum sulfation. J Mol Model 27(1):5. https://doi.org/10.1007/s00894-020-04645-5
Kazachenko AS, Malyar YUN, Vasilyeva NYU, Bondarenko GN, Korolkova IV, Antonov AV, Karacharov AA, Fetisova OYU, Skvortsova GP (2020) Green synthesis and characterization of galactomannan sulfates obtained using sulfamic acid. Biomass Conv Bioref. https://doi.org/10.1007/s13399-020-00855-2
Vasilyeva NYu, Levdansky AV, Kuznetsov BN, Skvortsova GP, Kazachenko AS, Djakovitch L, Pinel C (2015) Sulfation of arabinogalactan by sulfamic acid in dioxane. Russ J Bioorg Chem 41:725–731. https://doi.org/10.1134/S1068162015070158
Kazachenko AS, Vasilyeva NYU, Sudakova IG, Levdansky VA, Lutoshkin MA, Kuznetsov BN (2020) Numerical optimization of the abies ethanol lignin sulfation process with sulfamic acid in 1 4 dioxane medium in the presence of urea. J Sib Fed Univ Chem 13(2):232–246. https://doi.org/10.17516/1998-2836-0178
Pen RZ (2014) Planning an experiment at Statgraphics Centurion. Krasnoyarsk: SibSTU, 2014. 293.
Wang X, Wang J, Zhang J, Zhao B, Yao J, Wang Y (2010) Structure–antioxidant relationships of sulfated galactomannan from guar gum. Int J Biol Macromol 46(1):59–66. https://doi.org/10.1016/j.ijbiomac.2009.10.004
Al-Horani RA, Desai UR (2010) Chemical sulfation of small molecules – advances and challenges. Tetrahedron 66(16):2907–2918. https://doi.org/10.1016/j.tet.2010.02.015
Akman F, Kazachenko AS, Vasilyeva NYu, Malyar YuN (2020) Synthesis and characterization of starch sulfates obtained by the sulfamic acid-urea complex. J Mol Struc 1208:127899. https://doi.org/10.1016/j.molstruc.2020.127899
Spillane W, Malaubier JB (2014) Sulfamic acid and its N- and O-substituted derivatives chemical reviews. 114(4):2507–2586. https://doi.org/10.1021/cr400230c
Kuznetsov BN, Vasilyeva NYU, Kazachenko AS, Skvortsova GP, Levdansky VA, Lutoshkin MA (2018) Development of the method of Abies Wood ethanol lignin sulfation using sulfamic acid. J Sib Fed Univ Chem 1(11):122–130. https://doi.org/10.17516/1998-2836-0063
Kuznetsov BN, Levdansky VA, Kuznetsova SA, Garyntseva NV, Sudakova IG, Levdansky AV (2018) Integration of peroxide delignification and sulfamic acid sulfation methods for obtaining cellulose sulfates from aspen wood. Eur J Wood Prod 76(3):999–1007. https://doi.org/10.1007/s00107-017-1262-z
Kazachenko AS, Malyar YuN, Vasilyeva NYu, Fetisova OYu, Chudina AI, Sudakova IG, Antonov AV, Borovkova VS, Kuznetsova SA (2021) Isolation and sulfation of galactoglucomannan from larch wood (Larix Sibirica). Wood Sci Technol. https://doi.org/10.1007/s00226-021-01299-1
Johny LC, Kudre TG, Suresh PV (2021) Production of egg white hydrolysate by digestion with pineapple bromelain: optimization, evaluation and antioxidant activity study. J Food Sci Technol. https://doi.org/10.1007/s13197-021-05188-0
Kazachenko AS, Tomilin FN, Pozdnyakova AA, Vasilyeva NYu, Malyar YuN, Kuznetsova SA, Avramov PV (2020) Theoretical DFT interpretation of infrared spectra of biologically active arabinogalactan sulfated derivatives. Chem Pap. https://doi.org/10.1007/s11696-020-01220-3
Sirviö JA, Ukkola J, Liimatainen H (2019) Direct sulfation of cellulose fibers using a reactive deep eutectic solvent to produce highly charged cellulose nanofibers. Cellulose 26:2303–2316. https://doi.org/10.1007/s10570-019-02257-8
Acknowledgements
This study was carried out using the equipment of the Krasnoyarsk Regional Center for Collective Use, Krasnoyarsk Science Center, Siberian Branch of the Russian Academy of Sciences.
Funding
This study was supported by the Russian Foundation for Basic Research, Government of the Krasnoyarsk Territory, and Krasnoyarsk Territorial Foundation for Support of Scientific and R&D Activities, project no. 20–43-243001. This study was carried out within the budget project #0287–2021-0017 for the Institute of Chemistry and Chemical Technology, Siberian Branch of the Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
N/A. This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kazachenko, A.S., Malyar, Y.N., Vasilyeva, N.Y. et al. Optimization of guar gum galactomannan sulfation process with sulfamic acid. Biomass Conv. Bioref. 13, 10041–10050 (2023). https://doi.org/10.1007/s13399-021-01895-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01895-y