Abstract
The synthesis of guar gum sulfates by a complex of sulfur trioxide with 1,4-dioxane was studied. The influence of temperature, process duration, and the volume of chlorosulfonic acid on the degree of substitution of guar gum sulfates was studied. The sulfation process has been optimized using the Box-Behnken design. It was shown that the optimal conditions for sulfation of guar gum with a complex of sulfur trioxide-1.4-dioxane: temperature 60 °C, duration 2.9 h, and a volume of chlorosulfonic acid of 3.1 ml. Sulfate groups embedding into the structure of guar gum was confirmed by elemental analysis and FTIR. The initial and sulfated guar gum were also characterized by methods: X-ray diffraction, scanning electron microscopy, and gel permeation chromatography. Using X-ray diffraction, it was shown that amorphization of guar gum occurs during sulfation. Using scanning electron microscopy, it was shown that the morphology of guar gum changes in the process of sulfation. Using gel permeation chromatography, it was shown in the process of guar gum sulfation by a complex of sulfur trioxide with 1,4-dioxane, the molecular weight decreases from 600 to 176 kDa. The geometric parameters of all complexes were carried out by using the DFT/B3PW91 method with a 6-31 + G (d,p) basis set. These structures are optimized to predict the important properties of a theme. MEP with contour map has been performed to obtain the electronic properties. Frontier molecular orbital HOMO-LUMO orbital diagram has been obtained for different energy levels and their band gap energies have been computed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant biomass, consisting of lignin, cellulose, and hemicelluloses, is a renewable resource and an important source of valuable chemicals [1]. Plant polysaccharides are widely used as biomedical preparations with antioxidant, prebiotic, antimutagenic, immunomodulatory, mitogenic, hepatoprotective, and lipid-lowering properties [2]. Due to the combination of the unique properties of polysaccharides (water solubility, low toxicity, biodegradability, dispersing properties, and the ability to retain moisture and bind fat), they are widely used in veterinary medicine, medicine, and in the manufacture of food and cosmetic products [3].
Guar gum is galactomannan—plant polysaccharide consisting of β-(1–4)-d-mannose and α-(1–6)-d-galactose [4, 5]. The main sources of galactomannans from gums are Ceratonia siliqua (locust bean), Cyamopsis tetragonoloba (guar gum), Caesalpinia spinosa (tara gum), and Trigonellafoenum-graecum L. (fenugreek gum). Among them, only locust bean and guar gums are of considerable industrial and commercial importance [5].
Galactomannans are non-toxic compounds actively used in the production of food, textile, pharmaceuticals, and medicine as food additives, stabilizers, flocculants, thickeners, and gelling agents [6,7,8,9]. The pharmacological studies showed that galactomannans and their derivatives exhibit the anticoagulant [10], hepatoprotective, and analgesic properties [11, 12].
Guar gum (GG) is a food grade carbohydrate polymer that is used as a thickener and as a reagent for absorption and hydrogen bonding with mineral and polysaccharide surfaces [13, 14]. Guar gum consists of a direct chain of mannose units connected to galactopyranose units. GG is used in explosives, food products, cosmetics, and pharmaceuticals, as well as in the mining, paper, and textile industries, mainly as a binder for water [13, 15].
Derivatives of guar gum are widely used in the oil industry [16] corrosion inhibitors [17, 18], viscosity modifiers [16, 19, 20], fracturing fluids [16, 20], and for the preparation of water-in-oil emulsions [20, 21].
One of the promising directions for the modification of guar gum is the preparation of derivatives containing a sulfate group. Embedding of a sulfate group into biopolymer macromolecule increases its biodegradability and dissolution in water [22]. Sulfated derivatives of guar gum may find application in pharmaceuticals as antiviral drugs, antioxidants, and anticoagulants [23,24,25].
Currently, complexes of sulfur trioxide with various bases, which are used not only to obtain a sulfating mixture but also as a reaction medium, are widely used as sulfating reagents for hydroxyl-containing organic compounds [26].
The synthesis of guar gum sulfates is mainly based on methods in which complexes of sulfur trioxide with toxic amines (in particular pyridine) are used as a sulfating mixture [23,24,25].
The aim of this work was to optimize the process of guar gum sulfation by a complex of sulfur trioxide with 1,4-dioxane and to study the obtained guar gum sulfates by elemental analysis, IR spectroscopy, X-ray diffraction, scanning electron microscopy, gel permeation chromatography, and DFT. In view of the abovementioned findings and as a continuation of our previous work, we have reported herein the synthesis of new compounds and the structural parameters of new synthesis compounds were obtained by theoretical calculations using the density functional theory (DFT) method with the common B3PW91 function using 6-31 + G (d, p) basis sets.
Experimental
The guar gum (Sigma) was used in the work.
Obtaining a sulfating complex
The complex of 1,4-dioxane and sulfur trioxide, used for sulfation of guar gum, was obtained by the interaction of 1,4-dioxane with chlorosulfonic acid. To do this, 25 ml of dioxane was placed in a three-necked flask equipped with a thermometer, a mechanical stirrer, and a dropping funnel, and with vigorous stirring at a temperature of 20 °C, 1–4 ml (15.2–60.8 mmol) of chlorosulfonic acid was added dropwise.
Sulfation of guar gum
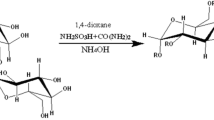
Sulfation of guar gum with a previously obtained complex of sulfur trioxide with 1,4-dioxane was carried out according to the method developed by us. To a complex of sulfur trioxide and 1,4-dioxane, 1.0 g of guar gum was added with stirring at a temperature of 20–40 °C; the reaction mixture was stirred at this temperature for 60–180 min. At the end of the sulfation process, the reaction mass was neutralized with an aqueous solution of sodium hydroxide (or ammonium hydroxide) to a pH of 8 to 9.
Dialysis of sulfated GG
The product was dialyzed against water in a plastic bag of MF-503-46 MFPI brand (USA) with a pore size of 3.5 kDa for 10 h to remove the reactants excess. The water was changed every hour (Fig. 1). In the dialysis process, high molecular weight compounds are purified from low molecular weight impurities (including inorganic compounds). The dissolved high molecular weight part remained in the dialysis bag. The low molecular weight products passed through the membrane of the dialysis bag into the external environment-distilled water.
The degree of substitution (DS) was calculated according [23] to Eq. (1).
S% is the sulfur content determination by elemental analysis.
For physico-chemical studies, a sample was taken with a DS of 0.90.
Methods of physico-chemical analysis
Elemental analysis
FlashEA-1112 elemental analyzer (ThermoQuest, Italia) was used for the sulfated guar gum elemental analysis.
Fourier transform infrared spectroscopy
Shimadzu IR Tracer-100 spectrometer (Japan) was used for the obtaining FTIR spectra of initial guar gum and sulfated guar gum within the wavelength range of 400–4000 cm−1. OPUS program (version 5.0) was used for processing the FTIR spectra. Pills in a KBr matrix (2-mg sample/1000 mg KBr) were used for analysis of solid samples.
X-ray diffraction
DRON-3 X-ray diffractometer (CuKα monochromatized radiation (λ = 0.154 nm), voltage 30 kV, current 25 mA) was used for the X-ray diffraction phase analysis. For analysis, the interval of the Bragg angles 2Θ from 5.00 to 70.00 Θ was used.
Scanning electron microscopy
Hitachi TM-1000 scanning electron microscope (Japan) (accelerating voltage of 15 kV and a magnification from × 100 to × 10,000 with a resolution of 30 nm) was used for obtaining electron micrographs. The electron micrographs were processed using the ImageJ software (version 1.8.0_112).
Gel permeation chromatography
Agilent 1260 Infinity II Multi-Detector GPC/SEC System chromatograph (with two detections: refractometer (RI) and viscometer (VS)) was used for obtaining data on the average molecular mass (Mn), average molecular weight (Mw), and polydispersity of the initial and sulfated guar gum. Two Aquagel-OH columns were used for the separation. The solution of 0.2MNaNO3 + 0.01 M NaH2PO4 in water (pH = 7) as the mobile phase was used. Calibration was performed using polyethylene glycol standards (Agilent, USA). The flow rate of the eluent was 1 ml/min, with a volume of the used sample—100 μl. Before analysis, the samples were dissolved in the mobile phase (1–5 mg/ml) and filtered through a 0.22-μm PES membrane filter (Agilent). Agilent GPC/SEC MDS software was used for data analysis.
Numerical optimization of the sulfation guar gum
The software Statgraphics Centurion XVI, DOE block (design of experiment), was used for numerical optimization of the guar gum sulfation process, according to references [27, 28].
Computational details
To determine the molecular electronic structure using computer simulation, the theory of the density functional plays an important role. The molecular parameters of all compounds (ground state) were obtained by performing DFT calculations using a functional hybrid B3PW91 with a basic set of 6-31 + G (d, p). In the DFT methods, the Becke three-parameter exact exchange-functional functional (B3) [29] in combination with the correlation-corrected functional with the PW91 gradient is the best prediction result for molecular geometry and vibrational wavenumbers for a moderately larger molecule [30]. All calculations were obtained by adding a polarization function and a diffuse function on heavy atoms and a polarization and diffuse function on hydrogen atoms [31]. The Gaussian 09W program [32] was used with the B3PW91/6-31+G(d, p) basis set by the DFT approach where accurate structural and chemical properties can be obtained. Optimized structure with parameters of crystal, electrostatic potential energy map (MEP), and HOMO-LUMO molecular orbital analysis has been performed using Gauss View 5.0 [33]. Additionally, reactivities and behaviors of the studied products were predicted by using calculations of frontier orbitals and the chemical potential (μ), softness (S), chemical hardness (η), electron affinity (A), ionization energy (I), electronegativity (χ), and electrophilicity index (ω) [34,35,36].
Results and discussion
Synthesis of guar gum sulfate and BBD analysis
Sulfation of guar gum with complex of sulfur trioxide with 1,4-dioxane and of guar gum sulfate was carried out according to the scheme (Fig. 1).
In the study of guar gum sulfation by a complex of sulfur trioxide with 1,4-dioxane, the duration, process temperature, and amount of chlorosulfonic acid (for obtaining a complex of sulfur trioxide with 1,4-dioxane) were varied.
Two factors are included in the study as independent variables (in parentheses the levels of their variation): X1, temperature (20, 40, 60 °C); X2, the duration of the process of sulfation of guar gum (1.0, 2.0, 3.0 h), and X3, volume of chlorosulfonic acid (to obtain a sulfating complex) (1.0, 2.5, 4.0 ml). The output parameter Y1—degree of substitution (DS)—was used as the result of the guar gum sulfation reaction. The Box-Behnken design (BBD) experiment plan was used. Each experiment was carried out in two parallels. The designations of the variables are given in Table 1.
The experimental results are shown in Table 2.
The dependence of the output parameter (Y1) on variable process factors was approximated equations (2). The results of the analysis of variance are given in Table 3 and Fig. 2.
Analysis of variance showed within the limits of the accepted conditions, the factor—the temperature of the process of sulfation of guar gum—makes the greatest contribution to the total dispersion of the output parameter. This is characterized by high values of the dispersion F ratios for the main effects, also called influence efficiencies. The information contained in columns P of the table is interpreted similarly (Table 3).
The dependence of the degree of substitution in guar gum sulfates (Y1) on variable process factors is approximated by the regression equation (2):
The prognostic properties of Eq. (2) are demonstrated in Fig. 3, which compares the values of the output parameter Y1 obtained in the experiment with the values calculated by Eq. (2). A straight line corresponds to the calculated values of Y1, points—to the results of observations. The proximity of the “experimental points” to the line confirms the good prognostic properties of Eq. (2).
The quality of the approximation is also characterized by the coefficient of determination R2adj. In the considered problem, the value of R2adj = 94.6%, which indicates a good quality of the approximation. This indicates the adequacy of Eq. (2) to the observation results and allows using it as a mathematical model of the process under study.
Optimal conditions for sulfation of guar gum with a complex of sulfur trioxide-1.4-dioxane are temperature 60 °C, duration 2.9 h, and a volume of chlorosulfonic acid of 3.1 ml.
FTIR spectroscopy
The initial and sulfated guar gum was analyzed by FTIR spectroscopy (Fig. 4).
FTIR spectra of sulfated guar gum show there is an intensity band at 1256 cm−1 (υas (O=S=O)) (Fig. 4). Absorption bands in the regions of 802–817 cm−1 and 855–867 cm−1 indicate the presence of sulfated groups in sulfated guar gum. FTIR spectra of sulfated guar gum show decreased the intensity of the absorption band of OH groups in the regions of 3419–3427 cm−1 and 1370–1380 cm−1, which indicates a decrease in the number of OH groups in the sulfated product, due to their replacement with SO3 groups.
The FTIR spectrum of the ammonium salt of sulfated guar gum differs from the spectrum of sodium salt (Fig. 4). FTIR spectrum shows there is a high-intensity band at 1260 cm−1, corresponding υas (O=S=O). The absorption bands in the region of 3427–2930 cm−1, (O–H and C–H bonds) broaden due to the superposition of the absorption bands of vibrations of N–H bonds in the ammonium cation. In the FTIR spectrum of the ammonium salt, in contrast to the sodium salt, there is an intense absorption band at 1447 cm−1, corresponding to vibrations of the N–H bonds of the ammonium cation.
X-ray diffractions
The initial and sulfated guar gum was analyzed by X-ray diffraction (Fig. 5).
Guar gum samples have an amorphous structure [37]. Comparison of X-ray diffraction patterns of the samples of guar gum and sodium salt of guar gum sulfate showed (Fig. 5) that further slight amorphization of the material structure occurs upon sulfation. On the X-ray diffraction pattern of the sodium salt of guar gum sulfate, smoothing of peaks was observed in the range of angles from 12 to 30° 2Ɵ (Fig. 5).
Scanning electron microscopy
According to scanning electron microscopy, the morphology of the samples changes during the sulfation of guar gum. Before the sulfation process, the guar gum sample consists of particles with an average size of 200 to 800 μm (Fig. 6 (1)). The introduction of a sulfate group into the guar gum molecule leads to a change in the average particle size from 80 to 1200 μm (Figure 6 (2)).
Gel permeation chromatography
According to the molecular mass distribution of guar gum (Table 4), the initial sample is guar gum with a molecular mass of ~ 600 kDa, which is consistent with the results presented in [38]. At the same time, GG has a bimodal mass distribution of particles: a high molecular weight fraction with an MM of ~ 1500 kDa and a low molecular weight fraction with an MM of ~ 600 kDa.
After the sulfation process, a redistribution of molecular masses in the sample is observed. The peak of the low molecular weight fraction with an MM of ~ 600 kDa disappears, and a new peak appears, corresponding to the product of the reaction with an MM of ~ 115 kDa. The fraction of the high molecular weight fraction in the sample also decreases markedly (Fig. 7), which is probably due to the partial destruction of GG and the ongoing competing hydrolysis reactions.
Galactomannan from guar gum is consisting of α-(1–6)-d-galactose and β-(1–4)-d-mannose [4, 5]. The conformation of the 1 → 4-linked backbone of β-d-mannan (as in cellulose), which makes it insoluble in water. However, the lateral galactose groups sterically disrupt interchain association thus increase the degree of solubility in water of galactomannans. As a result, the solubility of galactomannans increases with the degree of substitution of galactose: fenugreek and guar gum dissolve in cold water [5]. Thus, it can be assumed that upon sulfation of guar gum with a complex of sulfur trioxide and 1,4-dioxane, competing hydrolysis reactions take place along α-(1–6) bonds and cleavage of galactose units. This explains the production of both soluble guar gum sulfate and a small amount of insoluble residue.
The peak with RT ~ 10.5 min (corresponding to the maximum mass) belongs to the unreacted guar gum (Fig. 8). The main peak Mw 176 kDa relates to the target product. Moreover, several peaks can be observed that can correspond to products of joint destruction and sulfation.
Molecular mass distribution may indicate different reactivity of individual sections of the guar gum chains (Fig. 8). Peaks with a release time of more than 17 min and a mass of less than 1 kDa are low molecular weight products of the destruction of guar gum.
Structural analyses
To determine the structural stability of the molecule under investigation, molecular optimization study was made by using a DFT calculation. The optimized molecular structure of pure gum (a), sulfated gum 1 (b), sulfated gum3 (c), and sulfated gum 5 (d) are represented in Fig. 9.
The SCF energy of these molecules are found to be − 1297.5124, − 2082.9407, − 3653.7564, and − 5224.6513 hartree, they belong to the C1 point group. The structural parameters of optimized geometries are computed by B3PW91 with a 6-31 + G (d, p) basis set. The bond lengths and angles of pure gum and sulfated gum 1 are listed in Table 5, whereas the calculated parameters of the two other compounds are tabulated in Table 6.
The comparison of the theoretical values of pure gum-sulfated gum 1 as well sulfated gum 3-sulfated gum 5 shows a slight difference owing to sulfation reaction. The bond length values are in the range 0.9636–1.5403 Å (pure gum) and 0.9693–2.3345 Å (sulfated gum 1); the longer bonds are C16–C20 and O47–Na49 and the shorter ones are O23–H45 and O19–H40, as it is shown in Table 5. Similarly for Table 2, the bond lengths are between 0.9645 and 2.3535 Å (sulfated gum 3), among 0.9660 and 3.5778 Å (sulfated gum 5). The longest and shortest bond lengths in the sulfated gum 3-sulfated gum 5 cases are O25–Na55/O25–Na61 and O18–H50/O17–H56. Concerning bond angles which ranging from 103.3438 to 118.0201 and from 93.3444 to 119.8288° for pure gum and sulfated gum 1, whereas for both other structures are in the interval 87.9633–121.68994° and 50.4703–135.5069°. As seen from Table 6, the largest angles are C2–O1–C4 (pure gum), O47–S45–O48 (sulfated gum 1), C21–O19–S32 (sulfated gum 3), and O41–Na63–H85 (sulfated gum 5). The O1–C2–H24, S45–O47–Na49, S24–O25–Na55, and O29–Na62–H46 bond angles are considered as the smallest ones for pure gum, sulfated gum, sulfated gum 3, and sulfated gum 5, respectively.
Frontier molecular orbitals
Frontier molecular orbitals, HOMO and LUMO, and the corresponding energies are highly significant in charge-transfer interaction analysis. The highest occupied molecular orbital (HOMO) possesses the ability to donor an electron, while the lowest unoccupied molecular orbital (LUMO) is considered like an electron acceptor owing to his ability to win an electron. In addition, a low frontier orbital gap magnitude indicates high chemical reactivity consequently low kinetic stability of the working compound [34]. The HOMO and LUMO orbitals of the studied compounds and their energy plots are illustrated in Fig. 10.
As clearly seen from this figure, there are two colors: the red color marks the positive phase and the green one associate with the negative region. The gap energy of the guar gum sulfates calculated at B3PW91/6-31 + G (d, p) level of theory is equal to 6.21 eV (pure gum), 5.95 eV (sulfated gum 1), 5.21 eV (sulfated gum 3), and 6.13 eV (sulfated gum 5). These computed energy values show that sulfated gum 3 molecule has the greatest electronic charge transfer since it mark the lowest energy band gap ∆E. In order to predict the chemical reactivity of the different species, several parameters are calculated throughout HOMO and LUMO energies and tabulated in Table 7.
The ionization energy (I) and electron affinity (A) are calculated via the following equations; I = − EHOMO and A = − ELUMO. The electronegativity (χ) and chemical hardness (μ) are computed as follows; χ = (I + A)/2 and η = − (I + A)/2, while the chemical potential, maximum charge transfer index, global softness, and electrophilicity index are defined as μ = − χ, ΔNmax = − μ/η, ς = 1/η, and ω = μ2/2η, respectively. The maximum charge transfer index is directly related to the gap energy, the lower HOMO-LUMO energy separation, the greater ΔNmax. Building on these two factors, the sulfated gum 3 molecule is considered the most reactive candidate compound since it has the weaker energy band gap (5.2180 eV) and the highest transfer index (1.6798). Compared to the other compounds, sulfated gum 3 has the largest electronic charge transfer flow between HOMO-LUMO orbitals, conversely for the most stable pure gum system with 3.3064 eV (chemical hardness)/0.3024 eV−1 (softness).
Molecular electrostatic potential analysis
Molecular electrostatic potential (MEP) is widely used in the quantum molecular description. It provided a visual representation of charge distribution and enabled to define nucleophilic and electrophilic molecular sites; for this reason, it was employed in numerous studies [39,40,41,42,43,44]. MEP surface of 1,4-dioxane and SO3 along with compounds under investigation is mapped with the help of the B3PW91/6-31 + G (d, p) method, as it is shown in Fig. 11.
In this schematical visualization, each electrostatic potential value is plotted by a color. The increasing order potential is as follows: red < orange < yellow < green < blue. The color code of the investigated molecule surfaces ranging from − 5.14 · 10−2 (profoundest red) to 5.14 · 10−2 (profoundest blue). The nucleophilic region is represented by red color, indicating strong repulsion (potential < 0), whereas the electrophilic area is planned with blue demonstrating strong attraction (potential > 0). The green is associated with the neutral region with zero potential value. As shown in Fig. 11a, the two oxygen atoms of the 1,4-dioxane molecule possess a negative potential (red color). Generally, the positive σ-hole potential is present in covalent groups as well as in halogen and hydrogen groups. In our case, despite the weak blue color intensity of hydrogen atoms localized at the edge of the surface, we can say that the hydrogen atoms have σ-hole electrostatic potential. Then, the positive sign of electrostatic potential is associated with σ-hole and π-holes which are used in intermolecular interactions explication [45,46,47]. Based on this fact, the SO3 compound has a positive electrostatic potential value (Fig. 11b), so it a molecule with π-holes. As clearly seen in Fig. 11c–f, the hydrogen atoms connected to hydroxyl groups located at the interface of the maps have the weaker electronic density and are plotted by blue color, while the oxygen atoms of the same group possess the greatest electronic density and it is shown as red. The MEP surface of pure gum presents four electrophilic sites with a potential value equal to 0.1087 (H28), 0.1046 (H45), 0.1023 (H29), and 0.0436 a.u (H40). Relating to nucleophilic attacks, the electrostatic potential ranging from − 0.0443 to −0.0771 a.u. Passing from pure gum to sulfated gum (1, 3, 5), the maps present some modifications. For sulfated molecules, the nucleophilic sites (red color) are concentrated not only on the oxygen atoms of the hydroxyl group but also on the oxygen of the sulfate groupment. On the other side, the blue color (electrophilic side) is localized mainly over sodium atoms. From the figure, we notice that the insertion of sulfated atoms in the pure gum increases the number of nucleophilic and electrophilic attacks which rise the chemical reactivity of the system.
Conclusions
For the first time, the synthesis of guar gum sulfates by a complex of sulfur trioxide with 1,4-dioxane was studied. The high degree of substitution (0.91) was achieved at a temperature of 60 °C, duration of 2.9 h, and a volume of chlorosulfonic acid of 3.1 ml.
The introduction of sulfate groups into the structure of guar gum was confirmed by elemental analysis, FTIR. The FTIR spectra of sulfated guar gum contain bands at 1260 cm−1 and 802–817 cm−1, which indicate the presence of sulfate groups.
The initial and sulfated guar gum was also investigated by the methods: X-ray diffraction and gel permeation chromatography. Using X-ray diffraction showed that sulfation leads to partial amorphization of guar gum.
Using gel permeation chromatography, it was shown that in the process of guar gum sulfation by a complex of sulfur trioxide with 1,4-dioxane, the molecular weight decreases from 600 to 176 kDa.
The optimized structures of pure and sulfated (1, 3, 5) guar gum were obtained with the help of B3PW91 with a 6-31 + G (d, p) basis set. Building on the comparison made between the four geometries, there are differences between geometrical parameters (bond length and bong angle) due to sulfation reaction. HOMO-LUMO frontier orbital analysis proves that the sulfated gum 3 is the reactive compound compared to the other ones. In addition, the MEP contour maps show the effect of sulfation on the chemical reactivity of the system. These surfaces make out the proportionality between the sulfated atoms number and the molecular reactivity.
References
Zhao X, Zhou H, Sikarwar VS, Zhao M, Park A-HA, Fennell PS, Shen L, Fan L-S (2017). Energy Environ Sci 10(9):1885–1910
Medvedeva EN, Babkin VA, Ostrouhova LA (2003). Chem Raw Plant Mater 1:27–37
Dushkin AV, Meteleva ES, Tolstikova TG, Pavlova AV, Khvostov MV (2013). Pharm Chem J 46(10):630–633
Cerqueira MA, Bourbon AI, Pinheiro AC, Martins JT, Souza BWS, Teixeira JA, Vicenta AA (2011). Trends Food Sci Technol 22:662–671
Prajapati VD, Jani GK, Moradiya NG, Randeria NP, Nagar BJ, Naikwadi NN, Variya BC (2013). Int J Biol Macromol 60:83–92
Filatova AV, Azimova LB, Turaev AS (2020). Chem Plant Raw Mater 1:33–39
Mercier T, Guldentops E, Lagrou K, Maertens J (2018). Front Microbiol 9:661
Perera N, Yang FL, Chang CM, Lu YT, Zhan SH, Tsai YT, Hsieh JF, Li LH, Hua KF, Wu SH (2017). Org Lett 19(13):3486–3489
Tolstenkov AS, Drozd NN, Lapikova EU, Makarov VA, Mestechkina NM, Bannikova GE, Ilyina AB, Varlamov VP (2007). Clinical Hematol Hemorheol in Cardiovasc Surgery 7:242–243 (in Rus)
Mestechkina NM, Anulov OV, Scherbukhin VD (1998). Appl Biochem Microbiol (Rus) 34(5):549–552
Mestechkina NM, Dovletmuradov K, Scherbukhin VD (1991). Appl Biochem Microbiol (Rus) 27(3):435–441
Krishtanova NA, Safonova MJ, Bolotova VTS (2005). Proc Voronezh St Univ Ser: Chem Biol Pharm 1:212–221 (in Russ)
Mudgil D, Barak S, Khatkar BS (2014). J Food Sci Technol 51:409–418
Yamamoto Y (2001). J Japan Soc Nutr Food Sci 54:139–145
Zhang LM, Zhou JF, Hui PS (2005). J Sci Food Agric 85:2638–2644
Hasan AMA, Abdel-Raouf ME (2018). Egypt J Pet 27:1043–1050
Jano A, Lame A, Kokalari E (2012). Kemija u industriji (J Chem Chem Eng) 61(11–12):497–503
About S, Zouarhi M, Chebabe D, Damej M, Berisha A, Hajjaji N (2020). Heliyon 6(3):e03574
Mothé CG, Correia DZ, de França FP, Riga AT (2006). J Therm Anal Calorim 85:31–36
Adewole JK, Muritala KB (2019). J Petrol Explor Prod Technol 9(3):2297–2307
Mohayeji M, Farsi M, Rahimpour MR, Shariati A (2016). J Taiwan Inst Chem Eng 60:76–82
Caputo HE, Strau JE, Grinstaff MW (2019). Chem Soc Rev 48:2338–2365
Wang X, Wang J, Zhang J, Zhao B, Yao J, Wang Y (2009). Int J Biol Macromol 46(1):59–66
Mestechkina NM, Egorov AV, Shcherbukhin VD (2006). Appl Biochem Microbiol 4:326
Wang J, Zhao B, Wang X, Yao J, Zhang J (2012). Int J Biol Macromol 50(5):1201–1206
Al-Horani RA, Desai UR (2010). Tetrahedron 66(16):2907–2918
Kazachenko AS, NYu V, Sudakova IG, Levdansky VA, Lutoshkin MA, Kuznetsov BN (2020). J Sib Fed Univ Chem 13(2):232–246
Pen RZ (2014) Planning an experiment at Statgraphics Centurion. Krasnoyarsk: SibSTU 293
Becke AD (1988). Phys Rev A 38:3098–3100
Perdew JP, Burke K, Wang Y (1996). Phys Rev B 54:16533–16539
Young D (2001) Computational chemistry: a practical guide for applying techniques to real world problems, vol 408. Wiley, New York
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, et al (2010) Gaussian, Inc., Wallingford
Dennington R, Keith T, Millam J (2010) Gauss View, Version 5. Semichem Inc., Shawnee Mission
Parr RG, Pearson RG (1983). J Am Chem Soc 105:7512–7516
Noureddine O, Gatfaoui S, Brandan SA, Marouani H, Issaoui N (2020). J Mol Struct 1202:127351
Gatfaoui S, Issaoui N, Roisnel T, Marouani H (2019). J Mol Struct 1191:183–196
Mudgil D, Barak S, Khatkar BS (2012). J Biol Macromol 50(4):1035–1039
Wang Q, Ellisa PR, Ross-Murphy SB (2003). Carbohyd Polym 53:75–83
Sagaama A, Noureddine O, Brandan SA, Jedryka AJ, Flakus HT, Ghalla H, Issaoui N (2020). Comput Biol Chem 87:107311
Issa TB, Sagaama A, Issaoui N (2020). Comput Biol Chem 86:107268
Gatfaoui S, Sagaama A, Issaoui N, Roisnel T, Marouani H (2020). Solid State Sci:106326
Noureddine O, Gatfaoui S, Brandan SA, Sagaama A, Marouani H, Issaoui N (2020). J Mol Struct 1207:127762
Jomaa I, Noureddine O, Gatfaoui S, Issaoui N, Roisnel T, Marouani H (2020). J Mol Struct 1213:128186
Noureddine O, Gatfaoui S, Brandán SA, Marouani H, Issaoui N (2020). J Mol Struct 1202:127351
Politzer P, Murray JS (2018). J Comput Chem 39:464–471
Politzer P, Murray JS (2020). ChemPhysChem 21:579–588
Politzer P, Murray JS, Clark T (2015). Top Curr Chem 358:19–42
Acknowledgments
The devices of the Krasnoyarsk Regional Center of Research Equipment of Federal Research Center “Krasnoyarsk Science Center SB RAS” were used in the work. The authors are grateful to G.N. Bondarenko for obtaining X-ray data, Korolkova I.V. for obtaining FTIR spectra and Antonov A.V. for obtaining SEM images. Besides, the authors thank Bitlis Eren University for supporting the Gaussian 09W software and Bingol University for the server.
Availability of data and material
N/A.
Funding
This work was supported by the Ministry of Higher Education and Scientific Research of Tunisia. The publication also supported by RFBR, project № 20-33-70256.
Author information
Authors and Affiliations
Contributions
Conceptualization: Aleksandr S. Kazachenko, Feride Akman, Abir Sagaama, Noureddine Issaoui; methodology: Aleksandr S. Kazachenko, Feride Akman, Noureddine Issaoui; formal analysis and investigation: Feride Akman, Abir Sagaama, Noureddine Issaoui, Yuriy N. Malyar, Valentina S. Borovkova, Aleksandr S. Kazachenko; writing—original draft preparation: Noureddine Issaoui, Feride Akman, Abir Sagaama, Aleksandr S. Kazachenko; writing—review and editing: Aleksandr S. Kazachenko, Feride Akman, Abir Sagaama, Noureddine Issaoui; supervision: Feride Akman, Abir Sagaama, Noureddine Issaoui, Natalya Yu. Vasilieva.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Consent for publication
N/A.
Ethics approval
N/A. In the course of work on this article, the authors did not conduct research on animals and humans in any form.
Consent to participate
N/A.
Code availability
N/A.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kazachenko, A.S., Akman, F., Sagaama, A. et al. Theoretical and experimental study of guar gum sulfation. J Mol Model 27, 5 (2021). https://doi.org/10.1007/s00894-020-04645-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-020-04645-5