Abstract
A new method to obtain cellulose sulfates from available and inexpensive raw material—aspen wood was developed. This method integrates catalytic peroxide delignification and sulfamic acid sulfation stages. Solvents such as acetic acid and water were used for isolation of pure cellulose by wood peroxide delignification with TiO2 catalyst. Low-aggressive and less-toxic sulfating agent—sulfamic acid–urea mixture was used to obtain cellulose sulfates.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Biologically active cellulose sulfates are used in perfumery and cosmetics as sorbent materials, and they have good prospects for application in medicine as antimicrobial, antiviral and anticoagulant agents (Zhang et al. 2015; Lin et al. 2012; Shibata 2011).
The most common raw material for obtaining cellulose is wood. At present, the manufacture of cellulose from wood is an environmentally hazardous process because dangerous chemicals are involved in the wood delignification and pulp bleaching (Sixta 2006).
Catalytic delignification of wood in hydrogen peroxide–acetic acid–water medium at 120–130 °C allows producing pure cellulose by a one-step process without the use of ecologically dangerous delignification and bleaching agents (Kuznetsov et al. 2008). However, the different side reactions of low molecular mass products condensation with the formation of so-called “pseudo-lignin” (Hu et al. 2012) take place in the processes of wood peroxide delignification at elevated temperatures. Intensive mixing of a reaction medium and fine grinding of wood were employed to prevent the condensation reactions in aspen wood peroxide delignification with sulphuric acid catalyst (Kuznetsov et al. 2013). Pure cellulose with a low content of residual lignin (less 1%) was obtained from aspen wood sawdust at a temperature of 100 °C and atmospheric pressure. The obtained cellulose is suitable for the production of different ethers, including cellulose sulfates.
The sulfating agents such as H2SO4 (Chen et al. 2013), SO3 (Qin et al. 2014), ClSO3H (chlorosulfonic acid) with solvents Py, DMF, DMSO (Zhu et al. 2014), 1,4-dioxane (Kuznetsov et al. 2015), ionic liquids (Wang et al. 2009) are used to obtain the cellulose sulfates. The most effective sulfating agents are SO3 complexes with organic bases, such as tertiary amines, Py, DMF, etc. However, SO3, H2SO4, and ClSO3H are rather aggressive and toxic reagents.
The sulfamic acid, which is produced in industry by the reaction of urea with H2SO4 is a less dangerous sulfating agent. The sulfation of cellulose by sulfamic acid in an N2O4–DMF system was reported (Wagenknecht et al. 1993). But the degree of substitution of cellulose sulfates obtained by this method was less than 0.4 under the conditions employed.
The sulfation of cellulose with NH2SO3H in DMF was described by Zhang et al. (2011). In comparison with chlorosulfonic acid, the sulfamic acid reacts with cellulose only at elevated temperature (70–80 °С). Obtained cellulose sulfates had the degree of substitution 1.78–1.89 and they were soluble in water. The main disadvantage of this method is a quite long reaction time (up to 24 h). In a paper by Huang and Zhang (2012), the reaction of cotton cellulose with NH2SO3H was carried out in a laboratory oven at high temperature which promoted the partial destruction of sulfated products.
The new method for obtaining cellulose sulfates from aspen wood, which integrates the stages of pure cellulose isolation by wood peroxide delignification with TiO2 catalyst and further sulfation of cellulose by sulfamic acid–urea mixture, is described in the present paper. In contrast to previously studied method of aspen wood oxidative delignification with H2SO4 catalyst (Kuznetsov et al. 2013), the non-toxic and non-corrosive TiO2 catalyst is used for pure cellulose isolation from aspen wood under mild conditions (100 °C, 0.1 MPa). The less-aggressive and less-toxic sulfating agent—sulfamic acid–urea mixture is employed for cellulose sulfation instead of dangerous traditional sulfating agents (e.g. chlorosulfonic acid–pyridine mixture).
2 Materials and methods
2.1 Cellulose obtained from aspen wood
Air dry sawdust (fraction 2–5 mm) of aspen wood (Populus tremula L.) harvested in the forest area near Krasnoyarsk city was used as initial raw material. Experimentally defined composition of aspen wood (% on abs. dry wood) is cellulose 46.5 ± 1.4; lignin 21.7 ± 0.7; hemicelluloses 23.5 ± 0.8; mineral part 0.43 ± 0.01.
Cellulose was isolated from aspen wood using the original method of oxidative delignification by H2O2 with TiO2 catalyst under the mild conditions. Commercial TiO2 (GOST 9808-84) with an average particle size near 10 µm, phase composition: rutile 92%, anatase 8%, BET surface area 3 m2/g was used as a catalyst.
Delignification of wood sawdust was carried out in a glass reactor of 0.25 × 10−3 m3 volume equipped with mechanical stirrer, condenser and thermometer. Aspen wood sawdust (10 g) was placed into the reactor and a mixture of acetic acid, hydrogen peroxide, distilled water and TiO2 was added. The reaction mixture was heated up to the required temperature and vigorously stirred (700 rpm) for 1–4 h. After that, the reaction mixture was cooled and the obtained cellulosic product was separated under vacuum using Buchner funnel, following washing by distilled water and drying at 105 °C until constant weight.
Cellulosic product yield was estimated by gravimetric method and calculated as follows: yield = (m/m o × 100%), where m is the mass of abs. dry cellulosic product (g), m o is the mass of abs. dry wood (g).
Three replicates were used for each case and average yields of cellulose were calculated. The deviations for experimental data are given in Tables 1 and 2.
2.2 Sulfation of cellulose with sulfamic acid–urea mixture
Cellulose (1.62 g), urea (1.80 g), sulfamic acid (3.40 g) and diglyme or DMF (40 ml) were put in a 3 necked flask (100 ml) equipped with stirrer, thermometer and reflux. The mixture was boiled under reflux at vigorous stirring within 1–4 h. After that, the mixture was cooled to 15–20 °C, poured into 50 ml of 75% water–ethanol solution containing 3–4% sodium hydroxide and stirred. The resulting sodium salt of cellulose sulfate was separated by filtration, washed with 96% ethanol and dried under vacuum. The obtained product was then dissolved in 50–60 ml of distilled water, filtered and dialyzed on cellophane against distilled water. After dialysis the aqueous solution of sodium salt of cellulose sulfate (NaCS) was concentrated under vacuum until complete removal of water.
The yield of NaCS (Y) was calculated according to Eq. 1:
where mol (NaCS)—is the mol of NaCS (g) and mol (Cell)—is the mol of initial cellulose.
Number of cellulose moles was calculated as the ratio of the mass of cellulose (g) to the molar mass of anhydroglucopyranose unit [C6H7O2(OH)3] equal to 162.1.
Three replicates were used in each case and average yields of NaCS were calculated. The deviations are given for experimental data throughout the text.
2.3 Characterization methods
Elemental analysis (C, H, N, S, O) was performed with the use of Flash EATM 1112 analyzer (Thermo Quest, Italy).
The degree of substitution (DSS) of cellulose sulfates was calculated according to Eq. 2 (Gericke et al. 2009):
where W S is the sulfur content (%).
The contents of cellulose, lignin and hemicelluloses in solid products were defined by analytical methods common in wood chemistry (Sjöström and Alen 1999).
The content of cellulose was determined gravimetrically according to Kürschner and Hoffer method (Sjöström and Alen 1999). The 1 g of cellulosic product was put in an Erlenmeyer flask. The mixture of 25 ml nitric acid in ethanol (1:4, v/v) was added followed by 1-h boiling under reflux. The solid was separated from the solution using a glass filter (40 μm). The glass filter was then dried at 105 °C and weighed.
The content of holocellulose was determined by gravimetric method (Rossberg et al. 2014). The cellulosic product (0.3 g), water (45 ml), glacial acetic acid (60 ml) and sodium chloride (0.3 g) were put in a flask. The mixture was kept at 75 °C in a shaking device for 5 h. Acetic acid and sodium chloride were added after every hour. The holocellulose was separated by filtration using a glass filter (40 μm), washed, dried at 105 °C and weighed.
The content of hemicelluloses was calculated as the difference between the weights of holocellulose and cellulose.
Acid-insoluble lignin (Klason lignin) was determined according to TAPPI (1998). The cellulosic product (1 g) was dissolved in 15.0 ml of 72% sulfuric acid at 20 °C while stirring for 2 h. Then 300 ml of water was added to a flask to dilute solution to 3% concentration of sulfuric acid. The solution was boiled for 4 h, maintaining constant volume by using a reflux condenser. Then, the lignin was separated by filtration, washed by hot water and dried in an oven at 105.3 °C to constant weight.
The lignin content was calculated as follows:
where A weight of lignin, g ; Wp oven-dry weight of test specimen, g.
The degree of cellulose polymerization was calculated according to ASTM 1795 (2013). Dry cellulose (0.25 g) and distilled water (25 ml) were put in 100 ml glass flask that was tightly closed by a screw cap. The flask was shaken to disperse the cellulose sample. The air from the flask was swept out with a stream of nitrogen. Then cupriethylenediamine (25 ml) was added, the cap was inserted tightly and the solution was shaken in a mechanical shaker bath at 25 °C until the cellulose completely dissolved. Then 10 ml of the cellulose solution was transferred by pipette to a viscometer (VPG-3) for measuring the viscosity of cellulose. Degree of polymerization of cellulose was calculated by the formula (Pala et al. 2007):
where DP degree of polymerization; [η] intrinsic viscosity.
All experiments were performed in triplicate and the average values were reported. Experimental errors calculated as the relative standard deviation, are shown in text and in the tables.
FTIR analysis was carried out in transmission mode using the Tensor 27 spectrometer (Bruker, Germany). Samples of products (3 mg for each) were prepared in KBr matrix. Spectra were recorded in the wavelength range 400–4000 cm−1. Spectral data were processed by the program OPUS/YR (version 5.0).
FT Raman spectra of the samples were recorded using Bruker RFS 100/S spectrometer with a liquid-nitrogen cooled Ge diode as detector over a range of 3500–100 cm−1. A cw-Nd:YAG-laser with an exciting line of 1064 nm was applied as light source for the excitation of Raman scattering. The analysis of the data was executed with OriginPro 7.0 (OriginLab Corp. MA USA).
X-ray diffraction analysis was carried out on PANalytical X’Pert Pro diffractometer using Cu-Kα source (A = 0.154 nm) in the 2θ range 5°–70° and scanning step width of 0.01°/scan.
Crystallinity index (CI) was calculated from the ratio of the height between the intensity of the crystalline peak (I 002 – I AM) and total intensity (I 002) after subtraction of the background (Park et al. 2010).
13C NMR spectra were recorded at a temperature of 25 °C with spectrometer Bruker Avance III 600 MHz. The samples were dissolved in D2O.
The electron images were obtained with scanning electron microscope TM-1000 HITACHI (Japan) with accelerating potential 15 kV. Samples were coated on carbon support.
3 Results and discussion
The developed method for obtaining cellulose sulfates from aspen-wood includes the steps of cellulose isolation by wood peroxide delignification in acetic acid–water medium in the presence of suspended TiO2 catalyst and the sulfation of obtained cellulose by sulfamic acid–urea mixture in diglyme or DMF (Fig. 1).
3.1 Peroxide delignification of aspen wood with TiO2 catalyst
In the present study, the regularities of aspen wood delignification in the medium “hydrogen peroxide–acetic acid–water” were first studied in the presence of solid TiO2 catalyst under mild conditions (temperatures 70–100 °C, atmospheric pressure).
The influence of temperature, concentrations of hydrogen peroxide and acetic acid on the dynamics of lignin removal from wood was studied at fixed liquid/wood ratio (LWR) equal to 15.
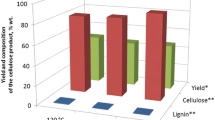
The increase in temperature and time of delignification process significantly reduces the content of residual lignin in the cellulosic product (Fig. 2). However, at the same time the yield of cellulosic product is decreased (Table 1).
The cellulosic product obtained from aspen wood at temperature 100 °C and time of delignification 4 h has the lowest content of residual lignin (0.7%). The fixed temperature and time were used to study the impact of H2O2 and CH3COOH concentrations on the yield and composition of cellulosic product (Table 2).
It was found, that the concentrations of H2O2 and acetic acid impact significantly on the content of residual lignin in cellulosic product. The increase of H2O2 concentration from 3 to 5% reduces the content of residual lignin in cellulosic product from 6.2 up to 0.7%.
According to XRD data (Fig. 3), the unit cell of cellulosic product obtained from aspen wood is identical to the monoclinic unit cell of cellulose I (Nishiyama et al. 2002). The crystallinity index of aspen cellulose (0.70) is a little less than that of the commercial microcrystalline cellulose (MCC) Vivapur 101 (0.75) (Park et al. 2010).
The similarity of FTIR spectra of cellulosic product from aspen wood and of industrial MCC Vivapur 101 (Fig. 4) indicates a similar composition of both the samples. According to literature data (Fengel 1992) the broad absorption band in the region 3400–3500 cm−1 is due to vibration of hydrogen-bonding –OH groups and the absorption peak at 2900 cm−1 belongs to CH2 groups. The absorption peaks at 1164 and 896 cm−1 belong to vibrations of C–O–C and C–H bonds accordingly.
The absence of peaks located in the range 1509–1609 cm−1, which correspond to vibrations of aromatic ring confirms the complete removal of lignin from cellulosic product. The absorption peak which corresponds to either the acetyl or uronic ester groups of hemicelluloses normally appears in the region 1700–1740 cm−1. The presence of absorption peak at 1735 cm−1 in spectrum of cellulose obtained from aspen wood indicates the presence of hemicelluloses in this sample.
According to SEM data, the samples of cellulose from aspen wood and industrial MCC consist of microfibrils with different length (Fig. 5). Some microfibrils are collected in bundles.
As a result of the accomplished study the green method of aspen-wood peroxide delignification under the mild conditions in the medium of “H2O2-CH3COOH-H2O-TiO2 catalyst” was developed.
The regularities of aspen-wood peroxide delignification in acetic acid–water medium at 70–100 °C in the presence of 1% TiO2 catalyst are the same as for catalyst 2% H2SO4 (Kuznetsov et al. 2013). For both catalysts the increase in concentrations of H2O2 and CH3COOH and time of the process reduces the content of residual lignin in cellulosic product and decreases the yield of product. The peroxide delignification processes are described satisfactorily by the first order equations for both of TiO2 and H2SO4 catalysts. However, in contrast to H2SO4, the TiO2 catalyst is non-toxic and corrosively non-active material. The more technologically convenient TiO2 catalyst at first was used to obtain the pure microcrystalline cellulose from aspen wood at mild conditions.
The cellulosic product containing 93.5 ± 2.1% of cellulose, 5.5 ± 0.1% of hemicelluloses 0.7 ± 0.1% of lignin was isolated from aspen wood with the yield 50.2 ± 1.2% at the next conditions of delignification: temperature 100 °C, concentrations of H2O2 5%, CH3COOH 25%., TiO2 1%, LWR 15, time 4 h (Table 1). This product has the degree of polymerization 450 ± 10 and crystallinity index 0.70, just like the commercial MCC.
The obtained cellulosic product was used for synthesis of cellulose sulfates.
3.2 Sulfation of cellulose from aspen wood by sulfamic acid–urea mixture
Chlorosulfonic acid (ClSO3H) is the common reagent for sulfation of cellulose (Zhu et al. 2014; Wang et al. 2007). To prevent the destruction reactions, the sulfation of cellulose by chlorosulfonic acid is usually carried out below 30 °C. This makes possible to obtain cellulose sulfates with a high degree of substitution (up to 1.70).
Since, ClSO3H is a corrosive, high toxic reagent, the less-toxic sulfamic acid is the best alternative. In this study, the urea was used as a catalyst to improve the reactivity of NH2SO3H in the sulfation of cellulose obtained from aspen wood. The higher activity of sulfamic acid–urea mixture in sulfation of polysaccharides (Al-Horani and Desai 2010) is explained by the formation of donor–acceptor complex:

Cellulose sulfates, obtained by the use of sulfamic acid–urea mixture were isolated in the form of ammonium, sodium, and potassium salts, according to below scheme:

The reaction of cellulose sulfation by sulfamic acid–urea mixture in DMF or in diglyme proceeds in a heterogeneous medium.
Figure 6 illustrates the variation of yield and sulfur content in cellulose sulfates with time of cellulose treatment by sulfamic acid–urea mixture.
The rather high sulfur content in the cellulose sulfates obtained in diglyme (14.0 ± 0.3%) and in DMF (13.6 ± 0.3%) was observed during 1 h. During 2–2.5 h, the maximal sulphur content in cellulose sulfates reaches 15.2 ± 0.4% in diglyme (after 2.5 h) and 14.8 ± 0.3% in DMF (after 2 h). These values correspond to the degree of substitution 1.5 and 1.4, respectively. Further increase in the reaction time reduces the sulphur content in cellulose sulfates. The maximal yield of cellulose sulphate (51.6 ± 0.6%) was obtained in the presence of diglyme after sulfation during 2.5 h. This sample contains 15.4 ± 0.4% of sulphur and it has the degree of substitution 1.53.
The sulfation of cellulose in boiling diglyme at 161 °C results in less cellulose degradation than that in boiling DMF at 152 °C. Besides, the use of diglyme as a solvent increases the yield of cellulose sulfate by 2.5 times as compared to DMF.
According to XRD data the disordering of cellulose crystalline structure takes place during sulfation process.
FTIR and Raman spectra of cellulose sulfates (Figs. 7, 8) contain the absorption peaks corresponding to vibrations of C–O–S and O=S=O bonds (Wang et al. 2007; Zhang et al. 2010). This confirms the presence of sulfate groups in the structure of cellulose.
It is known (Wang et al. 2007) that the hydroxyl group at C6 primary carbon atom is predominantly sulfated in a glucopyranose unit of cellulose:

The hydroxyl group connected with carbon atom C2 is less reactive in the sulfation process. The hydroxyl group at C3 carbon atom is replaced on sulfate groups only under hard sulfation conditions, which promote the destruction of cellulose.
For cellulose sulfates, the chemical shifts in 13C NMR spectra corresponding to carbon atoms C1–C6 in glucopyranose units of cellulose are observed at 102 (C1), 74 (C2), 73 (C3), 79 (C4), 77 (C5) and 60 (C6) ppm (Wang et al. 2007). The introduction of sulfate groups into cellulose moves the chemical shifts of carbon atoms to lower magnetic field (Table 3).
In 13C NMR spectra of the obtained cellulose sulfates (Fig. 9), the signal of carbon atom C6 moves completely from 60 to 66 ppm. It means that all hydroxyl groups connected with carbon atoms C6 are substituted in cellulose sulfates on sulfate groups. Appearance of a new signal at 80–82 ppm indicates that only a part of OH–groups, connected with C2 atoms is substituted on sulfate groups in cellulose sulfates. No shift of a signal of C3 atom was observed, that indicates the absence of sulfate group at C3 atoms in cellulose sulfates.
Similar results were reported earlier for cellulose sulphates obtained with the use of other sulfating agents: H2SO4 (Chen et al. 2013), SO3 (Qin et al. 2014), chlorosulfonic acid (Kuznetsov et al. 2015; Zhu et al. 2014).
For the process of cellulose sulfation by different sulfating agents in the medium N2O–DMF it was established that the sulfating activity increased in the range: H2SO4 < < NH2SO3 ~ NOSO4H < SO2 < ClSO3H < SO3 < SO2Cl2 (Wagenknecht et al. 1993).
The regiospecificity is an important characteristic of cellulose sulfates defining their properties. It is known from literature that the hydroxyl group of cellulose at C6 carbon atom is predominantly sulphated, while OH-groups at carbon atoms C2 and C3 are less reactive in the traditional sulfation process (Zhang et al. 2011; Roy et al. 2009). The results obtained for cellulose sulfation by sulfamic acid–urea mixture confirm this conclusion. The special methods of OH-groups protection should be used to obtain regioselective cellulose sulfates (Fox et al. 2011; Zhang et al. 2010).
The developed method of cellulose sulfation by sulfamic acid–urea mixture has a number of advantages: sulfamic acid is less corrosive and less toxic as compared to the traditionally used sulfating agents. In contrast to cellulose sulfation by sulfamic acid in the absence of urea (Wagenknecht et al. 1993; Zhang et al. 2011), the developed method allows to obtain cellulose sulfates with a higher degree of substitution and to reduce the cellulose destruction and the reaction time.
4 Conclusion
A new method for obtaining cellulose sulfates from aspen wood was developed. This method integrates the stages of green peroxide catalytic delignification of wood in acetic acid–water medium and of cellulose sulfation by less-aggressive and less-toxic—sulfating agent–sulfamic acid–urea mixture.
First, the environmentally safe solid catalyst TiO2 was used to obtain in one-step pure cellulose from aspen wood in the medium H2O2–CH3COOH–H2O at a temperature of 100 °C and atmospheric pressure.
At selected conditions of wood peroxide delignification (100 °C, 5% H2O2, 25% CH3COOH, 1% TiO2, LWR 15, time 4 h), the cellulosic product, containing 93.5% cellulose, 5.5% hemicelluloses and 0.7% lignin was obtained with the yield near 50%.
The conditions of effective sulfation of cellulosic product by sulfamic acid–urea mixture were selected. In contrast to cellulose sulfation by sulfamic acid in the absence of urea, they allow to obtain the cellulose sulfates with a higher degree of substitution (up to 1.5). The yield of cellulose sulfates prepared in diglyme solvent was 2.5 times higher than that in the medium of DMF.
References
Al-Horani RA, Desai UR (2010) Chemical sulfation of small molecules—advances and challenges. Tetrahedron 66:2907–2918
ASTM D1795 (2013) Standard Test method for intrinsic viscosity of cellulose. ASTM International, West Conshohocken, PA, p 6
Chen G, Zhang B, Zhao J, Chen H (2013) Improved process for the production of cellulose sulfate using sulfuric acid/ethanol solution. Carbohyd Polym 95:332–337
Fengel D (1992) Characterization of cellulose by deconvoluting the OH valence range in FTIR spectra. Holzforschung 46:283–288
Fox SC, Li B, Xu D, Edgar KJ (2011) Regioselective esterification and etherification of cellulose: a review. Biomacromol 12:P 1956–1972
Gericke M, Liebert T, Heinze T (2009) Interaction of ionic liquids with polysaccharides, 8 - synthesis of cellulose sulfates suitable for polyelectrolyte complex formation. Macromol Biosci 9:343–353
Hu F, Jung S, Ragauskas A (2012) Pseudo-lignin formation and its impact on enzymatic hydrolysis. Bioresour Technol 117:7–12
Huang X, Zhang WD (2012) Preparation of cellulose sulfate and evaluation of its properties. J Fiber Bioeng Inf 3:32–39
Kuznetsov BN, Tarabanko VE, Kuznetsova SA (2008) New catalytic methods for obtaining cellulose and other chemical products from vegetable biomass. Kinet Catal 49:517–526
Kuznetsov BN, Sudakova IG, Garyntseva NV, Djakovitch L, Pinel C (2013) Kinetic study of aspen-wood sawdust delignification by H2O2 with sulfuric acid catalyst under the mild conditions. Reac Kinet Mech Cat 110:271–280
Kuznetsov BN, Kuznetsova SA, Levdansky VA, Levdansky AV, Vasil’eva NY, Chesnokov NV, Ivanchenko NM, Djakovitch L, Pinel C (2015) Optimized methods for obtaining cellulose and cellulose sulfates from birch wood. Wood Sci Technol 49:825–843
Lin N, Huang J, Dufresne A (2012) Preparation, properties and applications of polysaccharide nanocrystals in advanced functional nanomaterials: a review. Nanoscale 4:3274–3294
Nishiyama Y, Langan P, Chanzy H (2002) Crystal structure and hydrogen-bonding system in cellulose Iβ from synchrotron X-ray and neutron fiber diffraction. J Am Chem Soc 124:9074–9082
Pala H, Mota M, Gama FM (2007) Enzymatic depolymerisation of cellulose. Carbohyd Polym 68:101–108
Park S, Baker JO, Himmel ME, Parilla PA, Jonson DK (2010) Cellulose crystallinity index: measurement techniques and their impact on interpreting cellulase performance. Biotechnol Biofuels 3:10
Qin Z, Ji L, Yin X, Zhu L, Lin Q, Qin J (2014) Synthesis and characterization of bacterial cellulose sulfates using a SO3/pyridine complex in DMAc/LiCl. Carbohyd Polym 101:947–953
Rossberg C, Steffien D, Bremer M, Koenig S, Carvalheiro F, Duarte LC, Moniz P, Hoernicke M, Bertau M, Fischer S (2014) Pulp properties resulting from different pretreatments of wheat straw and their influence on enzymatic hydrolysis rate. Biores Technol 169:206–212
Roy D, Semsarilar M, Guthrie JT, Perrier S (2009) Cellulose modification by polymer grafting: a review. Chem Soc Rev 38:2046–2064
Shibata T (2011) Cellulose and its derivatives in medical use. In: Peter A, Williams (eds) Renewable resources for functional polymers and biomaterials: polysaccharides, proteins and polyesters. RSC Publishing, Cambridge, pp 48–87
Sixta H (2006) Handbook of pulp. Wiley–VCH Verlug SmbH and Co, Weinheim
Sjöström E, Alen R (1999) Analytical methods in wood chemistry, pulping and papermaking. Springer Series in Wood Science, Springer-Verlag, Berlin
Tappi standard (1998) Acid-insoluble lignin in wood and pulp. Standard T 222 Om-98. Technical association of the pulp and paper industry, Atlanta, p 5
Wagenknecht W, Nehls I, Philipp B (1993) Studies on the regioselectivity of cellulose sulfation in an N2O4–N,N-dimethylformamide–cellulose system. Carbohyd Res 240:245–252
Wang ZM, Li L, Zheng BS, Normakhamatov N, Guo SY (2007) Preparation and anticoagulation activity of sodium cellulose sulfate. Int J Biol Macromol 41:376–382
Wang ZM, Li L, Xiao KJ, Wu JY (2009) Homogeneous sulfation of bagasse cellulose in an ionic liquid and anticoagulant activity. Bioresource Technol 100:1687–1690
Zhang K, Brendler E, Fischer S (2010) FT Raman investigation of sodium cellulose sulfate. Cellulose 17:427–435
Zhang K, Brendler E, Geissler A, Fischer S (2011) Synthesis and spectroscopic analysis of cellulose sulfates with regulable total degrees of substitution and sulfation patterns via 13C NMR and FT Raman spectroscopy. Polymer 52:26–32
Zhang Q, Lin D, Yao S (2015) Review on biomedical and bioengineering applications of cellulose sulfate. Carbohyd Polym 132:311–322
Zhu L, Qin J, Yin X, Ji L, Lin Q, Qin Z (2014) Direct sulfation of bacterial cellulose with a ClSO3H/DMF complex and structure characterization of the sulfates. Polym Advan Technol 25:168–172
Acknowledgements
The reported study was supported by Russian Science Foundation, Grant no. 16-13-10326.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kuznetsov, B., Levdansky, V., Kuznetsova, S. et al. Integration of peroxide delignification and sulfamic acid sulfation methods for obtaining cellulose sulfates from aspen wood. Eur. J. Wood Prod. 76, 999–1007 (2018). https://doi.org/10.1007/s00107-017-1262-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00107-017-1262-z