Abstract
Hawk moths are classified into the subfamilies Sphinginae, Macroglossinae and Smerinthinae. The sex pheromones of hawk moths have been intensively investigated recently. However, these reports were mainly on Sphinginae and Macroglossinae and there are only a few reports on Smerinthinae. Here, we identified sex pheromone components from the Smerinthinae, Smerinthus tokyonis Matsumura (Lepidoptera: Sphingidae). Observation of female calling behavior showed that the behavior started immediately after the photo-phase started. Gas chromatography (GC) coupled with electroantennography detection analysis indicated that male antenna responded to three components in the pheromone gland extract. GC–MS and GC analyses demonstrated that the three components were (10Z,12E)–, (10E,12Z)–, and (10Z,12Z)–hexadecadienyl acetates in a 6:7:87 ratio. We subsequently performed behavioral assays in cages. We observed the orientation and contact behavior of males in response to different odor sources, including a solvent control, calling female, pheromone gland extract, and synthetic blend. Males did not respond to the solvent control, but did respond to the other sources. Since males responded more to the calling female than to the synthetic blend, additional cues seem to be required for complete mating behavior. Nevertheless, the pheromone components determined in this first study of a Smerinthinae species are important chemicals in mating communication.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sphingidae (hawk moths) consists of the subfamilies Sphinginae, Macroglossinae, and Smerinthinae (Pittaway 1993). There are 1,450 described species worldwide (Nieukerken et al. 2011). The phylogenetic relationships of this group have been well-studied (Kawahara et al. 2009) and enable interpretation of evolutionary processes, such as the acquisition of anti-bat ultrasonic production (Kawahara et al. 2015), evolution of anti-predator eyespots in larvae (Ponce et al. 2015), and ancestral diversification of Manduca in Central America (Kawahara et al. 2013). To understand the evolution of sex pheromone communication in hawk moths, we have investigated sex pheromones from several species and identified a blend of C16 monoene aldehydes and C15 and C16-conjugated diene aldehydes as sex pheromones (Uehara and Honda 2020). However, those samples were mainly from Macroglossinae and Sphingidae; knowledge of Smerinthinae remains limited. Here, we studied the sex pheromones of Smerinthus tokyonis Matsumura, which belongs to Smerinthinae.
The adults of many species of Smerinthus are distinguished by a blue and black eyespot on the hind-wing (Pittaway 1993). Smerinthus tokyonis (Lepidoptera: Sphingidae) has such an eyespot. This species used to be relatively rare in Japan, being limited to a mountainous area. Recently, the moth has spread to an urban area with planting of the larval food plant Enkianthus perulatus (Miq.) C.K.Schneid. as a greening tree (Kishida 2011), and is an emerging pest of the tree. To reveal components of sex pheromones in Smerinthinae, we analyzed extracts of female pheromone grands of S. tokyonis and assayed male behavioral responses to them.
Materials and methods
Insects
We caught a wild-mated female of S. tokyonis on the campus of the University of Tsukuba (N 36º6′, E 140º5′), and obtained eggs in a cage (D: 23.5 cm × W: 30 cm × H: 33.5 cm) containing a bunch of E. perulatus. The larvae were reared with E. perulatus leaves in a plastic cage (D: 16 cm × W: 24 cm × H: 5 cm) under a 15L:9D photoperiod at 25 ± 2 °C and 60% relative humidity. After pupation, the pupae were separated by sex and kept in different cages under the same conditions. The calling behavior of female moths was checked every 30 min to determine the peak calling time.
Chemicals
Four geometric isomers of 10,12–hexadecadienyl acetate were available from a stock library in our laboratory. The purity of all compounds was confirmed to exceed 99.5% by gas chromatography (GC; HP-5MS column). Standard hydrocarbons were purchased from TCI Chemical Industry (Tokyo, Japan).
Extraction
Abdominal terminal segments including pheromone glands were excised by scissors during calling behavior. The pheromone glands were transferred to 250 μL glass insert vials (Agilent Technologies, Santa Clara, CA, USA) and immersed in 150 μL of distilled hexane for 30 min. The crude extracts from every three female moths were combined, concentrated with a gentle nitrogen stream and stored in a freezer at − 20 °C until use.
Gas chromatography–electroantennography detection
Candidate sex pheromone components in the female pheromone gland extract were surveyed using a gas chromatography–electroantennography detector (GC–EAD). The GC (HP-5890 series II; Hewlett-Packard, Palo Alto, CA, USA) was equipped with an HP-5MS column (30 m × 0.32 mm diam. × 0.25 μm film thickness; Agilent Technologies). Helium was the carrier gas (constant flow of 37 cm/s). The temperatures of the injection and detection (flame ionization detector; FID) ports, and the interface with the EAD, were set at 250 °C, 250 °C, and 300 °C, respectively. The GC oven temperature was held at 130 °C for 2 min, and then increased from 130 °C to 250 °C at 5 °C/min. Effluents from the column were split in a 1:1 ratio between the FID and EAD with a Y splitter (Hewlett Packard). Humidified air at 23 °C delivered the GC effluent to the antennal preparation bridged between an electrode (PRG-2 probe; Syntech, Kirchzarten, Germany) with an electro-conductive gel (Spectra 360; Parker Laboratories, Orange, NJ, USA). The EAD responses of male moths were recorded and converted with an IDAC-2 instrument (Syntech). Aliquots of extracts (0.1 female equivalent [FE]) were injected in splitless mode and chromatographed using helium as a carrier gas (37 cm/s).
Gas chromatography mass spectrometry (GC–MS)
Sex pheromone candidates were analyzed by GC–MS using a MS-600H instrument (JEOL, Tokyo, Japan) at 70 eV coupled with a 6890 N GC (Agilent Technologies). The GC was equipped with a DB-5MS column (30 m × 0.25 mm diam. × 0.25 μm film thickness; Agilent Technologies). Helium was the carrier gas (constant flow of 1 mL/min). The temperature of the injector port and interface was 280 °C and all samples were injected in splitless mode. The GC oven temperature was held at 100 °C for 1 min, and then increased from 100 to 320 °C at a rate of 10 °C/min.
To determine the double bond position, the extract was reacted with 4–methyl–1,2,4–triazoline–3,5–dione (MTAD; Sigma-Aldrich, St. Louis, MO, USA). MTAD (1%) in dichloromethane was added to one FE extract in dichloromethane until a slight pink color persisted (Young et al. 1990). The reaction mixture was analyzed by GC–MS, as described above.
Gas chromatography
Geometric isomers were analyzed based on the GC retention indices (RIs) using two GC columns with different polarities. The analyses were conducted with the GC-17A instrument (Shimadzu, Kyoto, Japan) equipped with a non-polar HP-5MS column, and the 6890 GC (Agilent Technologies) with a polar DB-23 column (30 m × 0.25 mm diam. × 0.25 μm film thickness; Agilent Technologies). The oven temperature for the non-polar column was maintained at 130 °C for 2 min, and then raised to 250 °C at a rate of 5 °C/min and held for 10 min. The polar column was held at 100 °C for 2 min, and the temperature was then increased to 250 °C at a rate of 3 °C/min and held for 10 min. In both analyses, the temperatures of the injector port and FID were 250 °C, and all samples were injected in splitless mode. The RIs were calculated in accordance with Dool and Kratz (1963).
Cage test
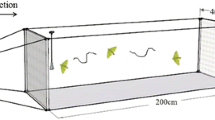
To evaluate the activity of candidate sex pheromones, we observed male behavior in a square pyramid-like cage (D: 200 cm × W: 180 cm × H: 145 cm) for 5 min in response to an odor stimulus. The tests involved 2- to 5-day-old male moths (15 individuals) and 3-day-old female moths reared under the conditions described above. The same individuals were subjected to the test several times, but only once a day to avoid effects from the previous test.
As an odor stimulus, a calling female, pheromone gland extract (10 FE), synthetic pheromone (10 FE (1 μg), ZE:EZ:ZZ = 6:7:87), and solvent control (n-hexane) were used. Odor samples were loaded on filter paper (1 cm × 1 cm) and placed in a spherical metal mesh (8 cm diam.). The calling female was also placed in the mesh. The mesh was suspended 10 cm from the ceiling of the cage.
Moth calling behavior begins immediately after the light is turned on. However, male moths show phototaxis to a ceiling light, so that orientation behavior to the pheromone source is difficult to observe in the laboratory. We placed the cage outside, without direct sunlight, and then conducted the test. Tests were conducted from 5 to 6 AM (sunrise was at about 5:45 AM) in October 2015.
Pheromone activity was evaluated in terms of the number of orientation flights and contact with the pheromone source. The behavior was observed in the mesh cage at the beginning of the photo-phase, when the females called most frequently. The numbers of orientation flights and source contacts were analyzed using generalized linear model (GLM) with a negative binomial distribution and a log link function. Significant explanatory variables were compared by Tukey’s post hoc test. The analyses were performed using R version 4.0.3 (R Core Team 2020) and the MASS (Venables and Ripley 2002) and multcomp (Hothorn et al. 2008) packages.
Results
Calling behavior
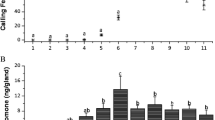
Female S. tokyonis showed typical moth calling behavior; they extended the abdominal tip, exposing the intersegmental membrane where the sex pheromone gland located. A few females started calling behavior 1 h before the photo-phase (Fig. 1). When the light was turned on, 80% of the females started calling, and they stopped gradually until 4 h after lights on.
Chemical analysis
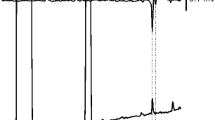
GC–EAD analysis showed that the male antenna responded to three components in female pheromone gland extracts (Fig. 2). The total amounts of these components were approximately 100 ng/female. We further analyzed these components using GC/MS (Supplementary Fig. 1). All three components had the same molecular ion at m/z 280 (M+, 25.4%), and the same base peak at 67 (C5H7+). The ions were separated by 14 mass units (m/z 67, 81, 95, and 109) and the relative intensities were inversely proportional to the mass number, suggesting an unsaturated aliphatic chain compound. Fragment ions at M–60 (12.4%) and m/z 61 (7.1%) indicate an acetic ester. The data suggested the molecular formula C18H32O2, consistent with hexadecadienyl acetate.
To determine the positions of double bonds, the components were reacted with MTAD regent and the adducts were analyzed by GC–MS. The mass spectra of the MTAD adducts had ions at m/z 393 (M+, 13.9%), m/z 208 ([M–C11H21O2]+, 100%), and m/z 350 ([M–C3H7]+, 31.2%), indicating that the components had two conjugated double bonds at the 10- and 12-positions of hexadecadienyl acetate (10,12–16:OAc).
To determine the geometric isomers of these components, the RIs of candidate components and synthetic 10,12–hexadecadienyl acetates were compared. The RIs of components A, B, and C matched those of the (Z,E)–, (E,Z)–, and (Z,Z)–isomers of 10,12–16:OAc, respectively (Table 1). The ratio deduced from the GC peak area was 6:7:87.
Cage test
In the mesh cage, male moths performed a ritual that involved approaching the pheromone source. This ritual had two components: an orientation flight, and touching the pheromone source while bending the abdomen.
In response to a calling female, male moths performed orientation and touching 2.80 ± 0.29 (mean ± SD) and 2.63 ± 0.29 times, respectively. In response to the pheromone gland extract (10 FE), male moths performed orientation and touching 1.40 ± 0.22 and 0.63 ± 0.18 times, respectively. In response to the synthetic pheromone blend (10 FE), male moths performed orientation and touching 1.15 ± 0.20 and 0.45 ± 0.12 times, respectively. No males responded to the control treatment (Table 2).
The numbers of orientation flights in response to the extract and synthetic pheromone treatments were significantly higher than in the control condition, but lower than for calling females (GLM df = 3, p < 0.001; Tukey’s post hoc test p < 0.01). The numbers of source contacts in response to the extract and synthetic pheromone treatments were also significantly higher than in the control condition, but lower than for calling females (GLM df = 3, p < 0.001; Tukey’s post hoc test p < 0.01).
Discussion
GC-EAD showed that the male antenna of S. tokyonis responds to three components in the pheromone gland extract of females. GC–MS and GC analyses showed that these components were (10Z,12E)–, (10E,12Z)–, and (10Z,12Z)–10,12–hexadecadienyl acetates in a 6:7:87 ratio. A mixture of the three components evoked orientation flights and source contact in the cage test, but not in a manner comparable to the response to calling females. These results suggested that the three components are involved in the S. tokyonis sex pheromone.
Unsaturated aliphatic acetates are common chemicals in the sex pheromone of moths. The sex pheromones of hawk moths are mostly mono- or di-unsaturated aldehydes with C16 or C15 (Bestmann et al. 1992; Landolt et al. 1989; Reed et al. 1987; Tumlinson et al. 1994; Uehara et al. 2012, 2013, 2015, 2016; Wakamura et al. 1996). Reed et al. (1987) reported that two species of Smerinthinae were attracted to C16-conjugated diene acetate. However, these components have never been identified in the female pheromone glands of any hawk moth species. This study demonstrated that a hawk moth uses acetates as pheromone components.
We identified three C16-conjugated diene acetates as candidate sex pheromones in S. tokyonis. The attractiveness of the synthetic blend of these components was comparable to that of the pheromone gland extract. However, it was inferior to calling females in terms of eliciting a response from males. These results suggested that the three compounds do not comprise the complete blend of odorants responsible for sexual attraction. The lack of minor pheromone components and other cues involved in attraction or inhibition by antagonistic components in the extract could have affected the results.
In Bombycidae, the number of sex pheromone components is related to the structure of the macroglomerular complex (MGC), which acts as the sex pheromone processing center (Namiki et al. 2014). Briefly, the use of a compound as a sex pheromone involves enlargement of a corresponding MGC glomerulus. Nirazawa et al. (2017) showed that this is the case in Sphingidae Agrius convolvuli. Neuroanatomically, the MGC glomerulus of S. tokyonis was enlarged (Namiki et al. submitted). This implies that either the C16-conjugated diene acetates act as pheromones, or that others are not emitted or are inhibitory.
The calling behavior of S. tokyonis peaked immediately after the light was turned on, which corresponded to dawn in nature. The possible contribution of visual cues to sexual attraction in this species was mentioned above. Some diurnal moths require visual cues for sexual attraction (Judd and Eby 2014; KonDo et al. 2012), although two diurnal hawk moths in which we identified sex pheromones were predominantly attracted to the sex pheromones rather than visual cues (Uehara et al. 2015, 2016). S. tokyonis is a nocturnal moth, but it mates in the early morning. In our cage test, more male moths flew to calling females than to the synthetic chemical blend. However, the females were covered with metal mesh and never seen. Therefore, olfactory cues are of primary importance in this species.
We intensively studied the sex pheromones of hawk moths and found that they were based on a few components, such as C16- and C15-conjugated diene aldehydes and acetates (Uehara and Honda 2020). Much effort has been expended to identify pheromone components, such as by examining the male antennal response and chemicals involved. However, no activity in field bioassays was seen in some species, including S. tokyonis. Additional chemical analysis might improve our understanding of the sex pheromone communication system in hawk moths.
References
Bestmann HJ, Erler J, Garbe W, Kern F, Martischonok V, Schäfer D, Vostrowsky O, Wasserthal LT (1992) Pheromone components of the female elephant hawk-moth, Deilephila elpenor, and the silver-striped hawk-moth, Hippotion celerio. Experientia 48:610–613
Dool HD, Kratz PD (1963) A generalization of the retention index system including linear temperature programmed gas–liquid partition chromatography. J Chromatogr 11:463–471
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biom J 50:346–363
Judd GJ, Eby C (2014) Spectral discrimination by Synanthedon myopaeformis (Lepidoptera: Sesiidae) when orienting to traps baited with sex pheromone or feeding attractants. Can Entomol 146:8–25
Kawahara AY, Barber JR (2015) Tempo and mode of anti-bat ultrasound production and sonar jamming in the diverse hawkmoth radiation. Proc Nat Acad Sci 112:6407–6412
Kawahara AY, Mignault AA, Regier JC, Kitching IJ, Mitter C (2009) Phylogeny and biogeography of hawkmoths (Lepidoptera: Sphingidae): evidence from five nuclear genes. PLoS ONE 4:e5719
Kawahara AY et al (2013) Evolution of Manduca sexta hornworms and relatives: biogeographical analysis reveals an ancestral diversification in Central America. Mol Phylogenet Evol 68:381–386
Kishida Y (2011) Sphingidae. In: Kishida Y (ed) The standard of moths in Japan I. Gakken Education Publishing, Tokyo, p 331 (in Japanese)
KonDo Y, Naka H, Tsuchida K (2012) Pheromones and body coloration affect mate recognition in the Japanese nine-spotted moth Amata fortunei (Lepidoptera: Arctiidae). J Ethol 30:301–308
Landolt PJ, Tumlinson JH, Brennan MM (1989) Attraction of Amphion floridensis (Lepidoptera: Sphingidae) to bombykal, (E, Z)-10,12-hexadecadienal. Fla Entomol 72:324–327
Namiki S, Daimon T, Iwatsuki C, Shimada T, Kanzaki R (2014) Antennal lobe organization and pheromone usage in bombycid moths. Biol Lett 10:20140096
Nieukerken EJ et al (2011) Order Lepidoptera Linnaeus, 1758. In: Zhang, Z.-Q. (Ed.) Animal biodiversity: an outline of higher-level classification and survey of taxonomic richness. Zootaxa 3148:212–221
Nirazawa T, Fujii T, Seki Y, Namiki S, Kazawa T, Kanzaki R, Ishikawa Y (2017) Morphology and physiology of antennal lobe projection neurons in the hawkmoth Agrius convolvuli. J Insect Physiol 98:214–222
Pittaway AR (1993) The hawkmoths of the western palaearctic. Harley books, Colchester
Ponce FV, Breinholt JW, Hossie T, Barber JR, Janzen DH, Hallwachs W, Kawahara AY (2015) A molecular phylogeny of Eumorpha (Lepidoptera: Sphingidae) and the evolution of anti-predator larval eyespots. Sys Entomol 40:401–408
R Core Team (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/.
Reed DW, Underhill EW, Giblin EM (1987) Attraction of sphingid moths (Lepidoptera: Sphingidae) to 10,12-hexadecadienyl aldehydes and acetates: evidence of pheromone components. J Chem Ecol 13:931–942
Tumlinson JH, Mitchell ER, Doolittle RE, Jackson DM (1994) Field tests of synthetic Manduca sexta sex pheromone. J Chem Ecol 20:579–591
Uehara T, Honda H (2020) Sex pheromone communication system in hawk moth. In: Ishikawa Y (ed) Insect sex pheromone research and beyond: from molecules to robots. Springer Entomology Monograph Series, pp 19–33
Uehara T, Naka H, Matsuyama S, Ando T, Honda H (2012) Identification and field evaluation of sex pheromones in two hawk moths Deilephila elpenor lewisii and Theretra oldenlandiae (Lepidoptera; Sphingidae). Appl Entomol Zool 47:227–232
Uehara T, Naka H, Matsuyama S, Vang LV, Ando T, Honda H (2013) Identification of conjugated pentadecadienals as sex pheromone components of the sphingid moth, Dolbina tancrei. J Chem Ecol 39:1441–1447
Uehara T, Naka H, Matsuyama S, Ando T, Honda H (2015) Identification of the sex pheromone of the diurnal hawk moth, Hemaris affinis. J Chem Ecol 41:9–14
Uehara T, Kitahara H, Naka H et al (2016) Single-component pheromone consisting of bombykal in a diurnal hawk moth, Neogurelca himachala sangaica. J Chem Ecol 42:517–522
Venables WN, Ripley BD (2002) Modern applied statistics with S, 4th edn. Springer, New York (ISBN 0-387-95457-0)
Wakamura S, Yasuda T, Watanabe M, Kiguchi K, Shimoda M, Ando T (1996) Sex pheromone of the sweetpotato hornworm, Agrius convolvuli (L.) (Lepidoptera: Sphingidae): identification of a major component and its activity in a wind tunnel. Appl Entomol Zool 31:171–174
Young DC, Vouros P, Holick MF (1990) Gas chromatography–mass spectrometry of conjugated dienes by derivatization with 4-methyl-1,2,4-triazoline-3,5-dione. J Chromatogr 522:295–302
Acknowledgements
This project was supported by Grants-in-Aid for JSPS Fellows and for Young Scientists (Start-up), JSPS KAKENHI Grant Number 15H06854. This research was in partial fulfillment of an MSc degree (AK) from the University of Tsukuba.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
13355_2021_743_MOESM1_ESM.pptx
Supplementary Fig. 1. Electron ionization mass spectra of EAD-active component. A main EAD-active component (a) and MTAD derivative (b) (PPTX 80 KB)
Rights and permissions
About this article
Cite this article
Kosaki, A., Uehara, T., Naka, H. et al. Identification and behavioral assays of sex pheromone components in Smerinthus tokyonis (Lepidoptera: Sphingidae). Appl Entomol Zool 56, 373–378 (2021). https://doi.org/10.1007/s13355-021-00743-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13355-021-00743-9