Abstract
Homologs of bombykal, (10E,12Z)-10,12-hexadecadienal, have been reported to be sex pheromones or sexual attractants of several species of sphingid moths. In this study, we identified novel bombykal analogs as sex pheromone components from a Japanese sphingid moth, Dolbina tancrei. Staudinger (Sphingidae: Lepidoptera). Sex pheromone gland extracts from calling female moths were subjected to gas chromatography/electroantennograhic detection (GC/EAD), gas chromatography/mass spectrometry (GC/MS), and gas chromatography (GC) analyses. GC/EAD analyses showed two active components in the crude pheromone extracts. GC/MS analysis determined these two components to be pentadecadienals. GC/MS of their MTAD derivatives showed conjugated double bonds at the 9- and 11-positions, indicating 9,11-pentadecadienals. The isomeric configurations of these candidates were determined by comparison of their Kováts retention indices with those of synthetic compounds. Field bioassays with the four isomers of 9,11-pentadecadienal and their mixtures confirmed that the two sex pheromone components of D. tancrei are (9E,11Z)-9,11-pentadecadienal and (9Z,11Z)-9,11-pentadecadienal, with the highest male catches observed for a 90:10 blend. This is the first report of 9,11-pentadecadienals as sex pheromone components in lepidopteran species.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The family Sphingidae is one of the largest in the Bombycoidea and comprises ca. 1,400 species worldwide (Kawahara et al. 2009). However, sex pheromones or sexual attractants of the Sphingidae remain largely unexplored as compared to the Tortricidae, Noctuidae, Pyralidae, Gelechiidae, and Sessiidae, which include many important agricultural pests (El-Sayed 2011). Hexadecadienyl compounds such as (10E,12Z)-10,12-hexadecadienal (bombykal, E10,Z12-16:Ald) have been reported as sex pheromones or sexual attractants for a few sphingid species (Bestmann et al. 1992; Landolt et al. 1989; Reed et al. 1987; Starratt et al. 1979; Tumlinson et al. 1994), and (11E,13Z)-11,13-hexadecadienal (E11,Z13-16:Ald) attracted Agrius convolvuli (Wakamura et al. 1996). Most of these studies involved attraction of male moths in field screening tests, with no detailed analyses of pheromone gland extracts. More detailed chemical analyses, complete structural identifications, and field bioassays are essential for understanding pheromone diversity and function in the Sphingidae.
The sphingid moth Dolbina tancrei commonly occurs in Japan and is a sporadic pest of Oleaceae ornamental plants including sweet osmanthus, sweet scented olive, and privet (Anonymous 2006). However, the composition of the female-produced sex pheromone of this species is unknown. In the present study, we identified components of the female sex pheromone of D. tancrei and demonstrated that the synthetic compounds attract male moths in the field.
Methods and Materials
Insects
Larvae of Dolbina tancrei were collected from privet, Ligustrum obtusifolium (Lamiales, Oleaceae), on the campus of Tottori University and reared on the same plants at 25 ± 2 °C under natural photoperiodic conditions. Pupae were transported to the University of Tsukuba, where they were kept at 25 ± 1 °C and 60 % in RH under a 15 L:9D photoperiod until emergence. Newly emerged adults were sexed and isolated in different cages under the same conditions.
Extraction of Sex Pheromone
Sex pheromone gland extracts were obtained from 2-d-old virgin females in the first 3 to 6 hr of the light period, when calling behavior was observed. After anesthetization with carbon dioxide, female abdominal tips including the sex pheromone gland were excised with micro-scissors and extracted with approximately 50 μl of redistilled hexane per gland for 15 min under ambient temperature. The extracts were pooled into several stocks and stored at −20 °C before use.
Gas Chromatography/Electroantennograhic Detection (GC/EAD)
GC/EAD analyses were performed on an HP5890 Series II GC (Hewlett Packard, California, USA) equipped with an HP-5MS column (30 m × 0.32 mm i.d. with 0.25 μm film thickness; Agilent Technologies, California, USA). The oven temperature was held at 100 °C for 2 min and then increased to 250 °C at 5 °C/min. The temperatures of the detector and injector were 250 °C and that of the outlet for the EAD was kept at 300 °C. The GC had splitless injection and used helium as carrier gas. GC effluent from the column was split 1:1 between the FID and EAD. The effluent was delivered by humidified air (23 °C) to the antennal preparation connected to an amplifier via Ag–AgCl electrodes immersed in 0.1 M KCl.
Gas Chromatography/Mass Spectrometry (GC/MS)
GC/MS analysis was performed with a JEOL MS-600H (JEOL Ltd., Tokyo, Japan) at 70 eV coupled to an Agilent 6890N GC (Agilent Technologies). The GC instrument was equipped with a DB-5MS capillary column (25 m × 0.25 mm i.d with a 0.25 μm film thickness; Agilent Technologies). Interface and injector temperatures were 280 °C, and the oven temperature was held at 100 °C for 1 min and then increased by 10 °C/min to 320 °C and held for 7 min.
Gas Chromatography (GC)
GC analyses were performed with a GC-17A (Shimadzu Co., Ltd., Kyoto, Japan) equipped with an HP-5MS nonpolar column (30 m × 0.32 mm i.d. with 0.25 μm film thickness; Agilent Technologies) and Agilent 6890 GC (Agilent Technologies) with a DB-23 polar column (30 m × 0.25 mm i.d. with a 0.25 μm film thickness; Agilent Technologies). The oven temperature for the nonpolar column was maintained at 100 °C for 2 min, raised to 250 °C at a rate of 5 °C/min, and held for 10 min. The polar column was held at 100 °C for 2 min, increased to 250 °C at rate of 3 °C/min, and held for 10 min. In both instruments, temperatures of the injector port and detector (FID) were 250 °C, and all samples were injected in splitless mode. Retention times were converted to Retention Indices (RI) relative to the retention times of n-alkanes.
MTAD Derivatization
To determine double-bond positions, pheromone candidates with conjugated dienes in the extracts were reacted with 4-methyl-1,2,4-triazoline-3,5-dione (MTAD, Sigma-Aldrich Co., Ltd., Missouri, USA). MTAD (1 %) in dichloromethane was added to a one female equivalent (FE) extract in dichloromethane until a slight pink color persisted (Young et al. 1990). The reaction mixture was analyzed by GC/MS as described above.
Synthesis of 9,11-Pentadecadienals
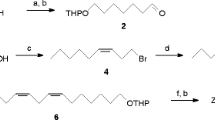
The four geometrical isomers were synthesized starting from 1,9-nonanediol (1) or 1,8-octanediol (2) as shown in Fig. 1. The configuration of each isomer was confirmed from NMR data (Ando et al. 1985). 1H and 13C NMR spectra were recorded with a Delta 2 Fourier transform spectrometer (JEOL Ltd., Tokyo, Japan) at 399.8 and 100.5 MHz, respectively, in CDCl3 solutions containing TMS as an internal standard.
Synthetic schemes for four isomers of 9,11-pentadecadienal; (9E,11Z) isomer (Scheme A), (9Z,11Z) isomer (Scheme B), and (9Z,11E) and (9E,11E) isomers (Scheme C). Reagents: a, 3,4-dihydropyran/p-TsOH/CH2Cl2; b, (COCl)2/DMSO/Et3N/CH2Cl2; c, MeCO2C = P(PPh3)/benzene; d, LiAl(OEt)2H2/ether; e, PCC/CH2Cl2; f, PrCH = PPh3/THF; g, (CN)2C = C(CN)2/THF; h, p-TsOH/EtOH; i, HBr; j, dimethoxymethane (DMM)/p-TsOH/LiBr; k, (1) TMSacetylene/BuLi/THF-HMPA, (2) K2CO3/MeOH; l, NBS/acetone; m, PrC ≡ CH/CuCl/NH2OH۰HCl/BuNH2; n, (1) BH(C6H11)2/THF, (2) AcOH, (3) NaOH/H2O2 (30 %); o, dry HCl/MeOH; p, PPh3/Δ; q, (1) BuLi/THF and (2) (E)-2-hexenal; r, column chromatography with SiO2–AgNO3
(9E,11Z) Isomer (Scheme A): This isomer was obtained by a synthetic route similar to that designed for (4E,6Z)-4,6-hexadecadienal from 1,4-butanediol (Nishida et al. 2003). After one hydroxyl group of 1,9-nonanediol 1 was protected as a tetrahydropyranyl (THP) ether, the other was exposed to Swern oxidation to produce aldehyde (2), which was converted into (E)-2-alkenal (3) in three steps: a coupling reaction with methyl (triphenylphosphoranylidene)acetate, reduction with LiAl(OEt)2H2, and oxidation with pyridinium chlorochromate (PCC). In dry tetrahydrofuran (THF), 3 was coupled with the ylid derived from butyltriphenylphosphonium bromide, using butyllithium (BuLi) as a base, to obtain a mixture of THP ethers of two dienyl alcohols (4), (9E,11Z)-9,11-pentadecadien-1-ol and the (9E,11E) isomer, in a ratio of approximately 3:2 (Millar et al. 1996; Nishida et al. 2003). After elimination of the (9E,11E) isomer by treatment with tetracyanoethylene, the THP group was removed by heating with catalytic p-toluenesulfonic acid (p-TsOH) in ethanol. The resulting alcohol was oxidized with PCC to yield E9,Z11-15:Ald. 1H NMR δ: 0.92 (3H, t, J = 7.5 Hz, CH 3CH2), ~1.3 (10H, m), 1.63 (2H, m, CH 2CH2 CHO), 2.11 (4H, m, CH 2CH = CHCH = CHCH 2), 2.42 (2H, dt, J = 7.5, 2 Hz, CH 2CHO), 5.31 [1H, dt, J = 11, 7.5 Hz, CH = CHCH = C(12)H], 5.65 [1H, dt, J = 15, 7 Hz, C(9)H = CHCH = CH], 5.96 [1H, dd, J = 11, 11 Hz, CH = CHC(11)H = CH], 6.30 [1H, dd, J = 15, 11 Hz, CH = C(10)HCH = CH], 9.76 (1H, t, J = 2 Hz, CHO); 13C NMR δ: 13.8, 22.1.0, 22.9, 29.0, 29.1, 29.2, 29.3, 29.8, 32.8, 43.9, 125.8, 128.7, 130.0, 134.5, 203.0.
(9Z,11Z) Isomer (Scheme B): According to the synthesis of the methoxymethyl (MOM) ether of 7-bromoheptan-1-ol (Do et al. 2009), the MOM ether of 8-bromooctan-1-ol (6) was obtained by bromination of one hydroxyl group of 1,8-octanediol (5) and MOM protection of the other. Coupling of 6 with lithium trimethylsilylacetylide in a mixed solvent of THF and HMPA and desilylation of the product with potassium carbonate in methanol produced the MOM ether of 9-decyn-1-ol (7) (Do et al. 2011). The terminal alkyne was brominated with N-bromosuccinimide (NBS) and the resulting 10-bromo derivative was coupled with 1-pentyne by a Cadiot–Chodkiewicz reaction (Nash et al. 1965) to yield the MOM ether of 9,11-pentadecadiyn-1-ol (8). From 8, Z9,Z11-15:Ald was prepared by partial reduction of the two triple bonds utilizing dicyclohexylborane (Do et al. 2011), deprotection of the MOM group, and PCC oxidation. 1H NMR δ: 0.91 (3H, t, J = 7.5 Hz, CH 3CH2), ~1.3 (10H, m), 1.62 (2H, m, CH 2CH2CHO), 2.16 (4H, m, CH 2CH = CHCH = CHCH 2), 2.42 (2H, dt, CH 2C = O), 5.44 (2H, m, CH = CHCH = CH), 6.25 (2H, m, CH = CHCH = CH), 9.76 (1H, t, J = 1.7 Hz, CHO). 13C NMR δ: 13.8, 22.1, 22.8, 27.4, 29.0, 29.1, 29.2, 29.5, 43.9, 123.72, 123.74, 131.9, 132.0, 203.0.
(9Z,11E) and (9E,11E) Isomers (Scheme C): These isomers were obtained by a synthetic route similar to that designed for a mixture of (9Z,11E)- and (9E,11E)-9,11-tetradecadienal (Do et al. 2011). Specifically, in a synthetic procedure similar to that from the 1,8-octanediol 5 to 6, 1,9-nonanediol 1 was converted to the MOM ether of 9-bromononan-1-ol (10). The bromide 10 was heated with PPh3, and the product phosphonium salt was treated with BuLi to make an ylid, which was coupled with (E)-2-hexenal to obtain a mixture of THP ethers of two alcohols (11), (9Z,11E)-9,11-pentadecadien-1-ol and the (9E,11E) isomer, in a ratio of approximately 7:3 (Do et al. 2011). After deprotection, the two geometrical isomers were separated by column chromatography using silica gel impregnated with AgNO3, and the alcohols were oxidized with PCC to yield the two aldehydes. Z9,E11-15:Ald; 1H NMR δ: 1.91 (3H, t, J = 7.5 Hz, CH 3CH2), ~1.3 (10H, m), 1.63 (2H, m, CH 2CH2CHO), 2.12 (4H, m, CH 2CH = CHCH = CHCH 2), 2.42 (2H, dt, CH 2C = O), 5.29 [1H, dt, J = 11, 7.5 Hz, C(9)H = CHCH = CH], 5.66 [1H, dt, J = 15, 7 Hz, CH = CHCH = C(12)H], 5.95 [1H, dd, J = 11, 11 Hz, CH = C(10)HCH = CH], 6.29 [1H, dd, J = 15, 11 Hz, CH = CHC(11)H = CH], 9.76 (1H, t, J = 1.7 Hz, CHO); 13C NMR δ: 13.8, 22.0, 22.6, 27.6, 29.0, 29.1, 29.2, 29.6, 35.0, 43.9, 125.8, 128.7, 129.9, 134.6, 203.0. E9,E11-15:Ald; 1H NMR δ: 0.90 (3H, t, J = 7.5 Hz, CH 3CH2), ~1.3 (10H, m), 1.62 (2H, m, CH 2CH2CHO), 2.04 (4H, m, CH 2CH = CHCH = CHCH 2), 2.42 (2H, dt, CH 2C = O), 5.58 (2H, m, CH = CHCH = CH), 6.00 (2H, m, CH = CHCH = CH), 9.76 (1H, t, J = 1.7 Hz, CHO); 13C NMR δ: 13.7, 22.1, 22.6, 29.0, 29.1, 29.2, 29.3, 32.5, 34.7, 43.9, 130.4, 130.5, 132.26, 132.29, 203.0.
The purities of these four geometrical isomers of 9,11-15:Ald were 95.0–99.9 % by GC analysis.
Field Bioassays
Bioassays were conducted in a field with natural vegetation of one host plant, Osmanthus fragrans var. aurantiacus, on the campus of the University of Tsukuba from June to August 2011–2013. Sticky delta traps (Sumika-Takeda Agrochemical Co., Ltd., Tokyo, Japan) were baited with a gray rubber septum (West Company, Singapore) impregnated with 0.5 mg of the synthetic compounds or their blends. The traps were placed at ca. 10 m distances and 2 m from the ground, and they were checked and changed every 3 d. The positions of traps were rotated to avoid positional effects. Data were analyzed with ANOVA after transformation to √(x + 0.5). Treatments with no catches were excluded from the analysis. Differences among means were tested for significance with Tukey–Kramer’s honestly significant difference (HSD) test (P < 0.05).
Results
Identification of Pheromone Components
GC/EAD analyses of crude pheromone gland extracts showed two prominent responses, corresponding to peaks A and B on the FID chromatogram (Fig. 2). In GC/MS analyses, the spectra of the active components A and B showed likely M+ ions at m/z 222 (22 %) and fragment ions at m/z 123, 109, 95, 81, 67 (C5H7 +, base peak), and 55 (Fig. 3a, b). The fragmentation pattern indicated unsaturated straight-chain components, with possible molecular formulae of C15H26O, consistent with a 15-carbon, diunsaturated aldehyde. In addition, the relatively intense M+ ions and two conspicuous diagnostic ions at m/z 96 (C7H12 +, 35 %) and m/z 109 (C8H13 +, 25 %) suggested two conjugated double bonds at the 9- and 11- (ϖ4 and ϖ6) positions in a straight carbon chain (Ando et al. 1988; Ando and Yamakawa 2011).
The positions of the double bonds in A were confirmed by derivatization with MTAD, which reacts specifically with conjugated dienes. The mass spectrum of the MTAD reaction product exhibited ions at m/z 335 (M+), m/z 208 (base peak, 100 %, [M–C8H15O]+), and m/z 292 ([M–C3H7]+) (Fig. 3c), indicating that the major component A had two conjugated double bonds at either the 9- and 11-positions or the 3- and 5-positions of a pentadecadienal. Although the MTAD derivative of component B could not be detected because of its small amount, the mass spectrum of component B was very similar to that of component A, suggesting component B to be a geometric isomer of component A.
Components A and B had RIs similar to those of the four isomers of 9,11-pentadecadienal on GC/MS and GC, on both polar and nonpolar columns. The 3,5-pentadecadienals would have been expected to elute much earlier (Ando et al. 2004). As shown in Table 1, the RIs of components A and B were similar to those of (9E,11Z)-9, 11-pentadecadienal (E9,Z11-15:Ald) and (9Z,11Z)-9,11-pentadecadienal (Z9,Z11-15:Ald), respectively, on both DB-5MS and HP-5MS columns. On a polar DB-23 column, the RI of component A matched that of E9,Z11-15:Ald, but component B eluted between the (9Z,11Z) and (9E,11E) isomers. However, the good RI matches of B with the (9Z,11Z)-isomer on the nonpolar columns, and the corresponding relatively poor RI matches of B with the (9E,11E)-isomer on the nonpolar columns suggested that B was indeed the (9Z,11Z)-isomer.
The ratio of components A and B in the crude pheromone extract was determined to be 94.3:5.7 ± 1.32 (N = 4), based on peak areas in the HP-5MS GC analysis.
Field Bioassays
No male D. tancrei moths were caught in traps baited with the (9Z,11E)-, (9Z,11Z)-, and (9E,11E)-pentadecadienal isomers alone, whereas traps baited with lures containing the (9E,11Z) isomer attracted male moths (Fig. 4). The addition of the 2.5 or 5 % of the (9Z,11Z) isomer to the (9E,11Z) isomer had no significant effect on trap catch, whereas addition of 10 % of the (9Z,11Z) isomer resulted in significant increase in trap captures compared to traps baited with the (9E,11Z) isomer as a single component (Fig. 4). In contrast, addition of the (9E,11E) isomer tended to decrease attraction, with the blend with 10 % of the (9E,11E) isomer being significantly less attractive than the (9E,11Z) isomer alone (Fig. 4).
Catches of male Dolbina tancrei in traps baited with synthetic 9,11-15:aldehydes and their mixtures. Bars with the same letters are not significantly different at P < 0.05 by Tukey–Kramer’s HSD test after ANOVA (N = 10, F = 12.9, P < 0.001). The number of trapped males was transformed to √(x + 0.5) prior to the test
We further evaluated the optimal proportion of the (9Z,11Z) isomer and the effect of the (9E,11E) isomer (Fig. 5). The most attractive blend was a 90:10 mixture of the (9E,11Z) and (9Z,11Z) isomers, with catches of males declining with increasing proportions of the (9Z,11Z) isomer. Addition of the (9E,11E) isomer to the (9E,11Z) and (9Z,11Z) isomers blend at 90:10 showing the highest activity led to a significant decrease in catches of males, indicating that the (9E,11E) isomer is antagonistic. In total, these results indicated that the pheromone of D. tancrei, is a 90:10 blend of E9,Z11-15:Ald and Z9,Z11-15:Ald.
Catches of male Dolbina tancrei in traps baited with blends of E9,Z11-15:Ald, Z9,Z11-15:Ald, and E9,E11-15:Ald. Bars with the same letters are not significantly different at P < 0.05 by Tukey–Kramer’s HSD test after ANOVA (N = 9, F = 5.06, P = 0.002). The number of trapped males was transformed to √(x + 0.5) prior to the test
Discussion
E9,Z11-15:Ald and Z9,Z11-15:Ald or E9,E11-15:Ald were identified as potential components of the female sex pheromone in pheromone gland extracts of female D. tancrei, with E9,Z11-15:Ald being more abundant. Analyses on non-polar GC columns indicated that the minor component had the same retention indices as Z9,Z11-15:Ald, but this was less clear on the polar DB-23 column. However, field assays confirmed the synergistic activity of the (9Z,11Z) isomer, and the antagonistic effect of the (9E,11E) isomer, providing further proof that the minor pheromone component was indeed the (9Z,11Z) isomer. Field bioassays also demonstrated that the (9E,11Z) isomer is an essential component for attracting male D. tancrei, and that a specific ratio of the (9Z,11Z) isomer (10 % of the total) significantly increased trap catches, with other proportions being no different than the major component alone, or at high ratios, decreasing trap captures. Therefore, we conclude that the sex pheromone blend of D. tancrei consists of E9,Z11-15:Ald and Z9,Z11-15:Ald in the ratio of 90:10.
9,11-15:Aldehydes are novel sex pheromone components for Lepidoptera. To date, diunsaturated and monounsaturated 16-carbon aldehydes have been identified as sex pheromones or sex attractants from a few species of sphingid moths. For example, E10,Z12-16:Ald was identified from the pheromone gland extracts of Manduca sexta (Starratt et al. 1979; Tumlinson et al. 1994). Although no field trap tests were conducted, E10,Z12-16:Ald and E11-16:Ald were reported as sex pheromone candidates in Deilephila elpenor (Bestmann et al. 1992). By field screening with synthetic lures, Reed et al. (1987) demonstrated that Z10,E12-16:Ald was attractive to male Smerinthus jamaicensis and Hemaris diffinis, and E10,E12-16:Ald was attractive to Proserpinus flavofasiata. A combination of Z10,E12-16:Ac and/or Z10,E12-16:Ald also was attractive to S. cerisyl and Pachyspinx modesta (Reed et al. 1987). GC/EAD and GC/MS analyses provide evidence for the presence of these bombykal analogs in pheromone gland extracts of S. cerisyl, Sphinx drupiferarum, Hyles galii (Reed et al. 1987), and Amphion floridensis (Landolt et al. 1989). However, with the exception of M. sexta, full identifications of pheromone components of these species have not been performed. Recently, we identified two components, E11-16:Ald and E10,E12-16:Ald, from Deilephila elpenor lewisii and three components, E11-16:Ald, E10,Z12-16:Ald, and (10E, 12E)-10,12-hexadecadienal, from Theretra oldenlandiae oldenlandiae (Uehara et al. 2012). Our ongoing surveys also suggest that 14 other species of Japanese hawk moths commonly use bombykal-type compounds as their sex pheromones.
Straight-chain aliphatic alcohol derivatives with even numbers of carbon atoms are well known as sex pheromones in numerous lepidopteran species (Ando et al. 2004), but few straight-chain compounds with odd numbers of carbon atoms have been identified as sex pheromones. Acrobasis rufilimbalis males were attracted to (Z)-9-pentadecenyl acetate (Z9-15:Ac) in a field screening test (Ando et al. 1977), and the pheromone of the pyralid, Chilo auricilius, was shown to contain (Z)-10-pentadecenyl acetate by analyses of gland extracts and demonstration of attraction of male moths to synthetic lures in the field (Nesbitt et al. 1986). Tabata et al. (2009) also identified Z9-15:Ac and pentadecyl acetate as pheromone components of the female sex pheromone of A. pyrivorella. In addition to these monounsaturated 15-carbon acetates, the conjugated dienyl 15-carbon compounds, (8E,10Z)-8,10-pentadecadieyl acetate and (8E, 10Z)-8,10-pentadecadien-1-ol, attracted males of A. vaccinii (McDonough et al. 1994; Sarzynski and Liburd 2004). With A. nuxvurella, Millar et al. (1996) found slight sex attractant activity for E9,Z11-15:Ald, which is homologous to E10,Z12-16:Ald (bombykal) as a true pheromone component.
In many cases, a multi- component pheromone system or the presence of a behavioral antagonist functions as a barrier for cross-attraction of congeners or species with similar pheromone systems. Straight-chain components with an odd number of carbons such as 9,11-15:aldehydes are novel and unusual lepidopteran sex pheromones, so that they seem to offer sufficient specificity as a sex pheromone system. Nevertheless, a two-component pheromone system and a behavioral antagonist were found in D. tancrei, suggesting the possible existence of sympatric species that also use 9,11-15:aldehydes as sex pheromones. For example, in Japan a congeneric species, D. exacta, is known. Further investigations of sex pheromones in sphingid moths with emphasis on the genus Dolbina may answer this question.
References
Ando T, Yamakawa R (2011) Analyses of lepidopteran sex pheromones by mass spectrometry. Trends Anal Chem 30:990–1002
Ando T, Yoshida S, Tatsuki S, Takahashi N (1977) Sex attractants for male Lepidoptera. Agric Biol Chem 48:1485–1492
Ando T, Kurotsu Y, Kaiya M, Utiyama M (1985) Systematic syntheses and characterization of dodecadien-1-ols with conjugated double bond, lepidopterous sex pheromones. Agric Biol Chem 49:141–148
Ando T, Ogura Y, Uchiyama M (1988) Mass spectra of lepidopterous sex pheromones with a conjugated diene system. Agric Biol Chem 52:1415–1423
Ando T, Inomata S, Yamamoto M (2004) Lepidopteran sex pheromones. Top Curr Chem 239:51–96
Anonymous (2006) Major insect and other pests of economic plants in Japan. The Japanese Society of Applied Entomology and Zoology, Tokyo, 387pp. (in Japanese)
Bestmann HJ, Erler J, Garbe W, Kern F, Martischonok V, Schäfer D, Vostrowsky O, Wasserthal LT (1992) Pheromone components of the female elephant hawk-moth, Deilephila elpenor, and the silver-striped hawk-moth, Hippotion celerio. Experientia 48:610–613
Do ND, Kinjo M, Taguri T, Adachi Y, Yamakawa R, Ando T (2009) Synthesis and field evaluation of methyl-branched ketones, sex pheromone components produced by Lithosiinae female moths in the family of Arctiidae. Biosci Biotechnol Biochem 73:1618–1622
Do ND, Ohbayashi K, Naka H, Nakada K, Ando T (2011) Identification and field evaluation of se pheromone components of the pear barkminer moth, Spulerina astaurota. J Chem Ecol 37:1222–1230
El-Sayed AM (2011) The pherobase. http://www.pherobase.com/
Kawahara AY, Mignault AA, Regier JC, Kitching IJ, Mitter C (2009) Phylogeny and biogeography of hawkmoths (Lepidoptera: Sphingidae): evidence 25. from five nuclear genes. PLoS ONE 4:e5719
Landolt PJ, Tumlinson JH, Brennan MM (1989) Attraction of Amphion floridensis (Lepidoptera: Sphingidae) to bombykal, (E, Z)-10,12-hexadecadienal. Fla Entomol 72:324–327
McDonough LM, Averill AL, Davis HG, Smithhisler CL, Murray DA, Chapman PS, Voerman S, Dapsis LJ, Averill MM (1994) Sex pheromone of cranberry fruitworm, Acrobasis vaccinii riley (Lepidoptera: Pyralidae). J Chem Ecol 20:3269–3279
Millar JG, Knudson AE, McElfresh JS, Gries R, Gries G, Davis JH (1996) Sex attractant pheromone of the pecan nut casebearer (Lepidoptera: Pyralidae). Bioorg Med Chem 4:331–339
Nash BW, Thomas DA, Warburton WK, Williams TD (1965) The preparation of capillin and some related compounds, and of some substituted pent-4-en-2-yn-1-ones. J Chem Soc 2983–2989
Nesbitt BF, Beevor PS, Cork A, Hall DR, David H, Nandagopal V (1986) The female sex pheromone of sugarcane stalk borer, Chilo auricilius. Identification of four components and field tests. J Chem Ecol 12:1377–1388
Nishida T, Vang LV, Yamazawa H, Yoshida R, Naka H, Tsuchida K, Ando T (2003) Synthesis and characterization of hexadecadienyl compounds with a conjugated diene system, sex pheromone of the persimmon fruit moth and related compounds. Biosci Biotechnol Biochem 67:822–829
Reed DW, Underhill EW, Giblin EM (1987) Attraction of sphingid moths (Lepidoptera: Sphingidae) to 10,12-hexadecadienyl aldehydes and acetates: evidence of pheromone components. J Chem Ecol 13:931–942
Sarzynski EM, Liburd OE (2004) Effect of trap height and within-planting location on captures of cranberry fruitworm (Lepidoptera: Pyralidae) in highbush blueberries. Agric For Entomol 6:199–204
Starratt AN, Dahm KH, Allen N, Hildebrand JG, Payne TL, Röller H (1979) Bombykal, a sex pheromone of the sphinx moth Manduca sexta. Z Naturforsch C 34:9–12
Tabata J, Minamishima M, Sugie H, Fukumoto T, Mochizuki F, Yoshiyasu Y (2009) Sex pheromone components of the pear fruit moth, Acrobasis pyrivorella (Matsumura). J Chem Ecol 35:243–249
Tumlinson JH, Mitchell ER, Doolittle RE, Jackson DM (1994) Field tests of synthetic Manduca sexta sex pheromone. J Chem Ecol 20:579–591
Uehara T, Naka H, Matsuyama S, Ando T, Honda H (2012) Identification and field evaluation of sex pheromones in two hawk moths Deilephila elpenor lewisii and Theretra oldenlandiae (Lepidoptera; Sphingidae). Appl Entomol Zool 47:227–232
Wakamura S, Yasuda T, Watanabe M, Kiguchi K, Shimoda M, Ando T (1996) Sex pheromone of the sweetpotato hornworm, Agrius convolvuli (L.) (Lepidoptera: Sphingidae): identification of a major component and its activity in a wind tunnel. Appl Entomol Zool 31:171–174
Young DC, Vouros P, Holick MF (1990) Gas chromatography–mass spectrometry of conjugated dienes by derivatization with 4-methyl-1,2,4-triazoline-3,5-dione. J Chromatogr 522:295–302
Acknowledgments
We thank Dr. DeMar Taylor for his critical reading of the manuscript and Mr. Takahiro Yano for important information on D. tancrei. We are also grateful to Ms. Kanako Ohbayashi and students of the Laboratory of Applied Entomology in Tottori University for their help with insect collection and rearing.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Uehara, T., Naka, H., Matsuyama, S. et al. Identification of Conjugated Pentadecadienals as Sex Pheromone Components of the Sphingid Moth, Dolbina tancrei . J Chem Ecol 39, 1441–1447 (2013). https://doi.org/10.1007/s10886-013-0357-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-013-0357-1