Abstract
Insect sex pheromones play a crucial role in the mate finding and calling behavior of Lepidoptera pests. Currently, little is known about the chemical ecology of Diaphania angustalis Snellen (Lepidoptera: Crambidae), a severe and important defoliator attacking the medicinal plant, Alstonia scholaris. In the present study, the pheromone components of D. angustalis females were investigated using electrophysiological and behavioral methods. Distilled hexane extracts of female pheromone glands were analyzed through electroantennogram (EAG) and gas chromatography-electroantennogram detector (GC-EAD), and the active compounds were identified through gas chromatography-mass spectrometry (GC-MS). Production peak of female sex pheromone occurred on the third day of age at 5 h into the scotophase with the EAG test, and the hexane extracts were attractive to males in the wind tunnel test. GC-EAD analysis of virgin males to gland extracts that were subsequently evaluated showed two active compounds, (E,E)-10,12-hexadecadienal (E10E12-16:Ald) and (E,E)-10,12-hexadecadien-1-ol (E10E12-16:OH), based on comparison of retention time and mass spectrum, with suitable synthetic compounds. Under laboratory conditions, the blend of E10E12-16:Ald and E10E12-16:OH in a ratio of 9:1 elicited a stronger EAG response than other treatments or a single component. In the field, more male moths were captured by traps baited with the mixture of E10E2-16:Ald and E10E2-16:OH in a ratio of 9:1, whereas a mixture of 8:1 and 10:1 also caught males. Accordingly, E10E2-16:Ald and E10E2-16:OH were regarded as the major sex pheromone components in D. angustalis females.
Similar content being viewed by others

Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Alstonia scholaris, distributed in southern China, is a very important medicinal and economic plant. The leaf extracts of A. scholaris, as a traditional Chinese medicine, have been used to treat chronic respiratory diseases and cold symptoms in hospitals (Ministry of Public Health, People’s Republic of China 1997; Shang et al. 2010). It is also a potential source of herbicidal constituents for weeds management (Javaid et al. 2010; Wang et al. 2014).
Diaphania angustalis Snellen (Lepidoptera: Crambidae) is the most important and severe defoliator of A. scholaris in southern China (Huang et al. 2007; Shu and Li 2014). The larvae ate mesophyll to cause the fallen leaves, even balding of branches. It seriously impacted the growth of A. scholaris, which caused severe economic losses. The essential biology and population dynamics of D. angustalis have been particularly described: the females deposit their eggs (oval 0.3–0.5 mm × 0.1–0.2 mm) on the leaves, and the larvae have six instars (Lei and Xie 2009). The insect has 6 generations per year in Puer, Yunnan Province, China (Li et al. 2006), and 7 generations in Maoming, Guangdong Province, China (Chen 2009).
Current insecticide control strategy could easily lead to environmental pollution, and these insecticides are not only broad spectrum and resistance problem but also kill the beneficial insect pest as well as the targeted pests. Consequently, it is noteworthy that chemical control of D. angustalis is not feasible due to the medicinal plant (A. scholaris). Currently, diel activity patterns and sexual communication in moths are thought to be mediated by sex pheromone, which plays a very important role in chemical communication of female and male adults (Sass 1983; Kawazu and Tatsuki 2002; Mazor and Dunkelblum 2005; Sadek et al. 2012; Groot 2014). Nowadays, sex pheromone and lure application technology, as an ideal tool, have been widely used for monitoring and controlling insect pests, for its high efficiency, nonpolluting, nontoxicity, and specificity to target species (Csonka and Tóth 2005; Strong et al. 2008; Witzgall et al. 2010; Weeks et al. 2011; Ma et al. 2014, 2015, 2016; Ioriatti and Lucchi 2016; Hanks and Millar 2016). Current researches are mainly focused on ecological aspects of D. angustalis. Therefore, the paper is aimed to study the production and identification of D. angustalis sex pheromone, and their attractiveness is further evaluated with field bioassay. Afterwards, the sex pheromone could provide a basis and useful tool in monitoring and controlling this pest, either mating disruption or mass trapping in environment-friendly integrated pest management programs.
Materials and methods
Insects
D. angustalis larvae were collected from A. scholaris tree, Conghua (N 23.57°, E 113.55°), Guangzhou. Larvae were reared until pupation in the laboratory in plastic containers (60 × 30 × 30 cm). Rearing conditions were 25–28 °C, 14 L:10 D photoperiod, and 75–80% RH. Pupae were separated by sex within 24 h of pupation (Cai et al. 2014) and placed separately into plastic cages (60 × 60 × 40 cm). Adult moths were supplied with 10% sugar-honey solution for nourishment.
Pheromone gland extracts
Abdominal tips of virgin females, including 3 days old at different times into scotophase and 1 to 5 days old at 5 h into scotophase, were extracted with distilled hexane (10 μl hexane per abdominal tip) at ambient temperature for 50 min and stored in a clean glass vial (2 ml, Agilent Technologies, USA) at − 20 °C until used.
EAG response of males to pheromone gland extracts
Electroantennogram (EAG) recordings were made with a Syntech EAG (Germany), consisting of a probe/micromanipulator (MP-15), a data acquisition interface box (IDAC-2), and a stimulus air controller (CS-55). An excised male antenna was mounted onto an antenna holder for the EAG probe (PRG-2) with electrically conductive gel (Spectra 360, Parker Lab. Inc., Orange, NJ, USA). EAG responses (mV) to different stimuli were measured consecutively from the antenna. The process was repeated three times with the same antenna, for each of the five antennae. Stimuli were applied to a strip of filter paper (0.5 × 3.0 cm), inserted into a 15-cm-long Pasteur pipette. The tip of the pipette was introduced into the main airflow tube (8 mm diameter), in which a moistened air stream (100 ml/min) was flowing continuously toward the antenna. Stimuli were applied to the antenna by puffing the humidified air for 0.3 s from the Pasteur pipette. The male antenna was stimulated by pheromone gland extracts from calling females at different times into scotophase and of different ages. The EAG values obtained from each standard were averaged. Two experiments were conducted to detect the presence of D. angustalis female sex pheromone.
Experiment 1: hour into the scotophase
Male EAG response to solvent extracts of 3-day-old virgin females at different times into scotophase (1, 2, 3, 4, 5, 6, 7, and 8 h) was measured. Thirty female moth abdomens were extracted per sample, and the concentration was 1 FE/10 μl (female equivalent).
Experiment 2: female age
Virgin females of different ages (1, 2, 3, 4, and 5 days old) at 5 h into scotophase were extracted in solvents, and the extracts were tested by measuring EAG activity. Thirty female moth abdomens were extracted per sample, and the concentration was 1 FE/10 μl.
Wind tunnel test
The responses of males to pheromone gland extracts were evaluated using the flow wind tunnel (200 cm long × 40 cm wide × 40 cm high) (Fig. 1). The airflow was purified through an activated carbon filter and produced by a controlled suction pump. The airflow speed was set at approximately 800–1000 ml/min through an anemometer, and outlet air was exhausted to the outside. The flight chamber was separated from the windward chamber by a fine wire mesh with an additional wire mesh at the other end. Experimental environment conditions during the wind tunnel tests were 25–28 °C and 75–80% RH. A dimmed red light was used for visual observation during the wind tunnel test. The rubber-baited pheromone gland extracts of 3-day-old calling female moths were hung at the tunnel entrance, and blank rubber was only injected with distilled hexane. For each experiment, 30 male moths were tested and placed at the end of the tunnel; three replicates were done for each odor. After the end of each test, the whole apparatus was washed with hexane. Each insect and each odor sample were used only once. During the experiment, a series of behavioral steps was recorded: (a) activation (wing fluttering), (b) take off (leaving the location of habitat), (c) oriented upwind flight (flying upwind toward the stimuli), (d) getting close to lure and landing (getting close to the stimuli and landing near the lure), and (e) copulation (trying to mate).
Gas chromatography-electroantennogram detector
Coupled gas chromatography-electroantennogram detector (GC-EAD) analysis of pheromone gland extracts was done with an Agilent 7820A GC equipped with a flame ionization detector (FID). A 30-m × 250-μm i.d. × 0.25-μm film thickness DB-5 MS-fused silica capillary column (Agilent Technologies, USA) and a Y splitter (5181-3397, Agilent Technologies, USA) were used for the analyses. At GC column effluent, carrier gas (hydrogen and nitrogen) was split 1:1 for the simultaneous detection between the FID and EAD apparatus. A 1-μl sample was injected (splitless, inlet temperature 260 °C). The GC oven temperature was programmed at 80 °C for 2 min, increased to 200 °C at 5 °C/min, and then to 270 °C at 10 °C/min (held for 10 min). Antennal depolarization was detected with a high-resistance EAD probe, a Signal Interface Box, Type IDAC-02, and an Intelligent Data Acquisition Controller (CS-55) (Syntech, Germany). A fresh and excised male antenna from 2- to 3-day-old moth was mounted onto an antenna holder for the EAG probe (PRG-2) with an electrode gel (Spectra 360, Parker Lab. Inc., Orange, NJ, USA) using a micromanipulator (Syntech, Germany). Charcoal-filtered and humidified air (1 l/min) passed through a glass tube connected to a GC transfer line, which tracked the GC oven temperature. Up to 10 antennae, placed into the outlet of glass tube, were tested with calling female pheromone gland extracts for GC-EAD analyses. A compound was considered to be electroantennographic active components when it elicited antennal responses at least 10 times.
Gas chromatograph-mass spectrometry
Hexane extract analyses were carried out on an Agilent 7890A GC interfaced with an Agilent 5975C triple quadrupole MS-selective detector operated at 70 eV electron ionization. The samples (pheromone gland extracts) were run on DB-5 MS capillary column (30 m × 0.25 mm internal diameter, 0.25 μm film thickness, Agilent Technologies, USA) with the following chromatographic methods: the oven was maintained at 80 °C for 2 min then increased to 200 °C at 5 °C/min, then to 270 °C at 10 °C/min, and finally, held at the temperature for 10 min. Helium was used as the carrier gas at a constant flow rate of 1 ml/min, and GC inlet temperature was held at 260 °C. One-microliter gland extracts were manually injected in the splitless mode. Pheromone active compounds were identified based on retention time and mass spectra with those of synthetic compounds.
Chemicals
(E,E)-10,12-Hexadecadienal (E10E2-16:Ald) and (E,E)-10,12-hexadecadien-1-ol (E10E2-16:OH) were purchased from Ning-lu Technology Co., Ltd. (Changzhou, Jiangsu Province, China). They were utilized as analytical standards and behavioral experiments and stored at − 20 °C until used.
Field test
Field trapping experiments were conducted in Conghua, Guangdong Province, from October to November 2014, with triangle trap and blank dispensers (small rubber septa, 8 mm diameter, Pherobio Technology Co. Ltd., China). The rubber was baited with hexane and a single component or blend (200 μg) in different ratios. Synthetic compounds were dissolved in distilled hexane and pipetted into the groove of the blank dispensers. The rubber baited with distilled hexane was used as control. All lures were stored at − 20 °C until used in the field. The 42 traps were divided into two rows, 21 traps in each row of 20 m apart and at 10-m intervals within each trap line. The bottom of the traps was covered with adhesive paper to capture the male moth. Each trap was placed randomly near the A. scholaris at the height of 1.5 m above the ground. The number of captured males was counted every 7 days and the lure was replaced after 7 days. After each check, the traps were successively rotated to eliminate any positional effects.
Statistical analysis
Data analyses were done using one-way analysis of variance (ANOVA: Tukey’s honestly significant difference test) for mean comparison. The proportion of calling female moths and mating behavior was evaluated as independent factors. Both EAG responses to pheromone gland extracts (mV) and antennal responses were normalized. The mean response was compared to that of hexane, and the data from comparison of different steps of wind tunnel test were analyzed with paired t test (n = 2). All analyses were performed using SPSS 17.0 (Inc., Chicago, IL, USA). In all tests, the probability level of significance was set at α = 0.05.
Results
EAG response of males to pheromone gland extracts
Male EAG responses to solvent collection of 3-day-old virgin females revealed that the presence of pheromone started from 4 h into the scotophase (Fig. 2). The pheromone production peaked at 5 h into the scotophase and then decreased gradually. Extracts from females at 5 h elicited significantly higher male EAG responses than those from females at 6 h (P < 0.05), and those from females at 6 h elicited significantly higher male EAG responses than those at the other hours tested (P < 0.05). We conclude that female Diaphania glauculalis released the greatest amount of pheromone during the 6-h period of the scotophase.
EAG response of male moths to pheromone crude extract from 3-day-old virgin females at different times during scotophase. Each treatment, consisting of 30 females, was replicated five times (n = 5, or 5 male antennae). Means followed by the different lowercase letters in every bar are significantly different (P < 0.05)
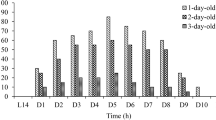
The response of male antennae to pheromone gland extracts was observed staring from the second day after emergence (Fig. 3). It is not known whether the females do not produce any pheromone at all on their first day after emergence. Sex pheromone production increased significantly each day and peaked at 3 days (P < 0.05) and then decreased gradually. Extracts of 3-day-old females elicited significantly higher male EAG responses than did those of 1-, 2-, 4-, and 5-day-old females. Extracts from 5-day-old female moths still produced significant EAG responses. As females grew older, pheromone production increased and then decreased.
EAG response of male moths to pheromone crude extract from 1- to 5-day-old virgin females, 5 h into scotophase. Each treatment, consisting of 30 females, was replicated five times (n = 5, or 5 male antennae). Means followed by the different lowercase letters in every bar are significantly different (P < 0.05)
Response of male adults in the wind tunnel
In the wind tunnel test, there were significantly more males that performed directional flights near the extract and landing on lures when pheromone gland extract was present compared to the control (Fig. 4). However, the percentage of males taking off (t = 3.381, df = 4, P < 0.05) and having upwind flights (t = 5.579, df = 4, P < 0.05) was significantly different between the treatment and control. Very few males of getting close to the lure and copulation to the control were observed (Fig. 4). But the pheromone gland extract could elicit the changes in the responses (getting close to the lure and copulation) of D. angustalis males at a low proportion, and males arrived, left, and flew back to the lure several times, and finally, males attempted to finish mating behavior.
Chemical analysis of sex pheromone gland extracts
GC-EAD analysis of pheromone gland extracts from D. angustalis females on a DB-5 MS column showed two components that could elicit active response to male moths (Fig. 5). Additional pheromone gland extracts were analyzed with gas chromatography-mass spectrometry (GC-MS) to provide chemical structure and retention time, which revealed the presence of aldehydes and alcohols with C 16. Peak I showed a mass spectrum characterized by a small molecular ion at m/z 236, and peak II with a clear molecular ion at m/z 238. Also, fragment ion at m/z 67 (C5H7 +, base peak), 81, 95 or 96, and 109 (C8H13 +) showed the characteristic ions of conjugated aliphatic dienes (Fig. 6) (Adati and Tatsuki 1999). In addition, the relatively high intensity of M+ ion and two significant diagnostic ions at m/z 96 (C7H12 +) and 109 (C8H13 +) indicated two conjugated double bonds at the 10- and 12-positions (ω4 and ω6) in a straight carbon chain (Ando et al. 1988). The identity of the compounds confirmed the retention times and mass spectra by the natural standards with the references previously identified. Therefore, the two compounds that evoked consistent depolarization were identified as (E,E)-10,12-hexadecadienal (E10E2-16:Ald), RT 25.160 min, and (E,E)-10,12-hexadecadien-1-ol (E10E2-16:OH), RT 26.633 min.
EI mass spectra of Diaphania angustalis pheromone active components, also showing their structure, formula, and molecular weight (MW): a mass spectra of comp I (upper, natural E10E12-16:Ald) and synthetic E10E12-16:Ald (lower); b mass spectra of comp II (upper, natural E10E12-16:OH) and synthetic E10E12-16:OH (lower)
Subsequently, EAG response of male antenna to E10E2-16:Ald and E10E2-16:OH in different ratios, diluted to 0.1% per μl with hexane, was tested. The synthetic mixtures of the two pheromone components in a ratio of 9:1 could elicit the strongest EAG response, followed by a ratio of 8:1 and 10:1. Also, other ratios did not elicit significant EAG responses in D. angustalis males (Fig. 7).
Field test
To evaluate the biological activity of D. angustalis pheromone candidates, field trapping experiments were conducted in Conghua, Guangzhou, China. The blend of E10E2-16:Ald and E10E2-16:OH, baited with a 9:1 ratio found in the pheromone gland hexane extracts, was attractive to D. angustalis males than other treatments, but the male moths were still captured baited with 8:1 and 10:1 of E10E2-16:Ald and E10E2-16:OH. The other treatments could not attract males (Fig. 8). In the experiment, no other male moth species were captured, and the data was consistent with the EAG test of laboratory condition.
Discussion
The sex pheromone of D. angustalis calling females as a blend of E10E2-16:Ald and E10E2-16:OH was identified and detected with the EAG, GC-MS, and GC-EAD. Field tests also indicated that E10E2-16:Ald and E10E2-16:OH could effectively attract more males in a ratio of 9:1; however, a small number of male moths was caught in our experiment. This might be due to the small numbers of adult emergence at that time or as a result of other weather conditions (rainfall, temperature, etc). Currently, there is no doubt that E10E2-16:Ald and E10E2-16:OH were the sex pheromone components of D. angustalis, and the amount of E10E2-16:Ald is more than that of E10E2-16:OH. However, the main sex pheromone components could have significant field trapping in some pests. For instance, E4Z10-14:OAc and Z10-14:OAc were identified as the sex pheromones of Phyllonorycter ringoniella, and 1 mg E4Z10-14:OAc per lure achieved the highest moth catch (Du et al. 2013). Similarly, Z7Z10-16:Ald, Z7-16:Ald, Z9-16:Ald, and Z9Z12-18:Ald were detected from the Chilecomadia valdiviana calling females, and traps baited with Z7Z10-16:Ald captured remarkably more moths (Herrera et al. 2016). Their minor components might play a part in interspecies chemical communication to those of certain polyenes in Operophtera fagata (Szöcs et al. 2004). But in our research, D. angustalis males were not attracted to the single component, E10E2-16:Ald; accordingly, in addition to E10E2-16:OH, other minor components might be not detected in the D. angustalis calling females. Furthermore, further research is needed to detect the active components and then optimize trapping technology of the pheromone components.
Based on our study, E10E2-16:Ald was the main sex pheromone component of D. angustalis female chemical communication, whereas to date, diunsaturated 16 C aldehydes have been identified as the attractant or sex pheromone in many lepidopteran species. For example, E10Z2-16:Ald was found in the gland extracts of Manduca sexta, Deilephila elpenor, and Theretra oldenlandiae oldenlandiae (Starratt et al. 1979; Bestmann et al. 1992; Uehara et al. 2012). Z10E2-16:Ald was attractive to Smerinthus jamaicensis and Hemaris diffinis, and Z10Z2-16:Ald was the only sex pheromone component of Diaphania hyalinata (Reed et al. 1987; Cabezas and Oehlschlager 1999). Additionally, E11E13-16:Ald was reported in Agrius convolvuli and Z11Z13-16:Ald is a pheromone of Amyelois transitella (Wakamura et al. 1996; Coffelt et al. 1979). Z7Z10-16:Ald was also identified as the main sex pheromone from Chilecomadia valdiviana (Herrera et al. 2016), and Acrobasis nuxvorella was attracted to the E9Z11-16:Ald (Millar et al. 1996; Passaro and Webster 2003). Hence, differentiation of geometric configurations or double bond positions of sex pheromone components would be very crucial for the chemical communication of both sexes. And these results also imply that diunsaturated 16 carbon aldehydes are widespread in the sex pheromone systems of lepidopteran species.
The compounds, E10E2-16:Ald and E10E2-16:OH, were type I pheromone structures, which have a straight chain with C10 to C18, a terminal oxygenated functional group (Ando and Yamakawa 2011). E10E2-16:Ald and E10E2-16:OH were not new compounds, but the vacancy of D. angustalis female sex pheromone was preferably remedied. Besides, they have been reported as the sex pheromone components in other lepidopteran species (El-Sayed 2016). At present, E10E2-16:Ald was identified from 23 species in Sphingidae, Schreckensteiniidae, Saturniidae, Crambidae, and Noctuidae, and E10E2-16:OH was from 12 species in Saturniidae, Crambidae, Noctuidae, and Bombycidae, but the two pheromone components were only found from 6 species (4 Crambidae, 1 Noctuidae, 1 Saturniidae) at the same time (Fig. 9). This indicates not only the species specificity of the insect pheromone but also the multiplicity of the pheromone system (Tamaki 1977).
E10E2-16:Ald and E10E2-16:OH have already been utilized as sex pheromones or attractants in insect pests. Yet, the identification of the female-produced sex pheromone of D. angustalis would represent a crucial step toward developing environment-friendly methods based on pheromone for monitoring of low-density populations and control of this pest insect in China.
References
Adati T, Tatsuki S (1999) Identification of the female sex pheromone of the legume pod borer, Maruca vitrata and antagonistic effects of geometrical isomers. J Chem Ecol 25:105–115
Ando T, Ogura Y, Uchiyama M (1988) Mass spectra of lepidopterous sex pheromones with a conjugated diene system. Agric Biol Chem 52:1415–1423
Ando T, Yamakawa R (2011) Analyses of lepidopteran sex pheromones by mass spectrometry. Trends Anal Chem 30:990–1002
Bestmann HJ, Erler J, Garbe W, Kern F, Martischonok V, Schäfer D, Vostrowsky O, Wasserthal LT (1992) Pheromone components of the female elephant hawk-moth, Deilephila elpenor, and the silverstriped hawk-moth, Hippotion celerio. Experientia 48:610–613
Cabezas JA, Oehlschlager AC (1999) Stereospecific synthesis of (E,Z)- and (Z,Z)-hexadeca-10,12-dienal. Sex pheromone components of Diaphania hyalinata. Synthesis 1:107–111
Cai WD, Cai WQ, Liu ZT, Wen XJ (2014) Sex identification of the pupae of Diaphania angustalis Snellen. For Dis Pest 33(5):17–20 (In Chinese)
Chen XH (2009) Biological characteristics and integrated control of Diaphania angustalis (Lepidoptera: Crambidae). Guangdong Agric Sci 8:124–126 (In Chinese)
Coffelt JA, Vick KW, Sonnet PE, Doolittle RE (1979) Isolation, identification, and synthesis of a female sex pheromone of the navel orangeworm, Amyelois transitella (Lepidoptera: Pyralidae). J Chem Ecol 5:955–966
Csonka É, Tóth M (2005) Chemical communication with volatile semiochemicals in Phyllotreta species (Coleoptera, Chrysomelidae): a mini review. Int J Hortic Sci 11:93–100
Du YJ, Li P, Chen ZQ, Li YR, Wang YH, Qin YX (2013) Field trapping of male Phyllonorycter ringoniella using variable ratios of pheromone components. Entomol Exp Appl 146:357–363
El-Sayed, AM (2016) The pherobase: database of pheromones and semiochemicals http://www.pherobase.com. Accessed 31 Dec 2016
Groot AT (2014) Circadian rhythms of sexual activities in moths: a review. Front Ecol Evol 2:43 https://doi.org/10.3389/fevo.2014.00043
Hanks LM, Millar JG (2016) Sex and aggregation-sex pheromones of cerambycid beetles: basic science and practical applications. J Chem Ecol 42:631–654
Herrera H, Barros-Parada W, Fernanda Flores M, Francke W, Fuentes-Contreras E, Rodriguez M, Santis F, Paulo HGZ, Bergmann J, Herrera H, Barros-Parade W (2016) Identification of a novel moth sex pheromone component from Chilecomadia valdiviana. J Chem Ecol 42:908–918
Huang YB, Zheng XF, Zheng DX (2007) The development and control of Diaphania angustalis. Yuedong For Sci Tech 2:5–6 (In Chinese)
Ioriatti C, Lucchi A (2016) Semiochemical strategies for tortricid moth control in apple orchards and vineyards in Italy. J Chem Ecol 42:571–583
Javaid A, Shafique S, Bajwa R, Shafique S (2010) Parthenium management through aqueous extracts of Alstonia scholaris. Pak J Bot 42:3651–3657
Kawazu K, Tatsuki S (2002) Diel rhythms of calling behavior and temporal change in pheromone production of the rice leaffolder moth, Cnaphalocrocis medinalis (Lepidoptera: Crambidae). Appl Entomol Zool 37:219–224
Lei YM, Xie LY (2009) Occurrence and control of Diaphania angustalis in Alstonia scholaris. Guangxi Trop Agric 1:46–48 (In Chinese)
Li ZH, Wang ZB, Luo CY, Huang WJ (2006) Preliminary study on biological characteristics of Diaphania angustalis (Lepidoptera: Crambidae). Chin Plant Pro 26(1):29–30 (In Chinese)
Ma T, Li YZ, Sun ZH, Wen XJ (2014) (Z,E)-9,12-tetradecadien-1-ol: a major sex pheromone component from Euzophera pyriella (Lepidoptera: Pyralididae) in Xinjiang, China. Fla Entomol 97:496–503
Ma T, Liu ZT, Lu J, Sun ZH, Li YZ, Wen XJ, Cui YZ (2015) A key compound: (Z)-9-tetradecen-1-ol as sex pheromone active component of Hypsipyla robusta (Lepidoptera: Pyralidae). Chemoecology 25:325–330
Ma T, Xiao Q, Yu YG, Wang C, Zhu CQ, Sun ZH, Chen XY, Wen XJ (2016) Analysis of tea geometrid (Ectropis grisescens) pheromone gland extracts using GC-EAD and GC×GC/TOFMS. J Agric Food Chem 64:3161–3166
Mazor M, Dunkelblum E (2005) Circadian rhythms of sexual behavior and pheromone titers of two closely related moth species Autographa gamma and Cornutiplusia circumflexa. J Chem Ecol 31:2153–2168
Millar JG, Knudson AE, McElfresh JS, Gries R, Gries G, Davis JH (1996) Sex attractant pheromone of the pecan nut casebearer (Lepidoptera: Pyralidae). Bioorg Med Chem 4:331–340
Ministry of Public Health, People’s Republic of China (1997) Drug specification promulgated by the Ministry of Public Health, People’s Republic of China, vol 14:49–50
Passaro LC, Webster FX (2003) Synthesis of (9E,11Z)-hexadeca-9,11-dienal, sex pheromone of the pecan nut casebearer, Acrobasis nuxvorella (Neunzig). Synthesis 8:1187–1190
Reed DW, Underhill EW, Giblin EM (1987) Attraction of sphingid moths (Lepidoptera: Sphingidae) to 10,12-hexadecadienyl aldehydes and acetates: evidence of pheromone components. J Chem Ecol 13:931–942
Sadek MM, Wowern GV, Löfstedt C, Rosén WQ, Anderson P (2012) Modulation of the temporal pattern of calling behavior of female Spodoptera littoralis by exposure to sex pheromone. J Insect Physiol 58:61–66
Sass H (1983) Production, release and effectiveness of two female sex pheromone components of Periplaneta americana. J Comp Physiol 152:309–317
Shang JH, Cai XH, Feng T, Zhao YL, Wang JK, Zhang LY, Yan M, Luo XD (2010) Pharmacological evaluation of Alstonia scholaris: anti-inflammatory and analgesic effects. J Ethnopharmacol 129:174–181
Shu M, Li ZH (2014) Study on occurrence and control of primary pest Diaphania angustalis in Alstonia scholaris. Modern Agric Sci Tech 3:132–133 (In Chinese)
Starratt AN, Dahm KH, Allen N, Hildebrand JG, Payne TL, Röller H (1979) Bombykal, a sex pheromone of the sphinx moth Manduca sexta. Z Naturforsch C 34:9–12
Strong WB, Millar JG, Grant GG, Moreira JA, Chong JM, Rudolph C (2008) Optimization of pheromone lure and trap design for monitoring the fir coneworm, Dioryctria abietivorella. Entomol Exp App 126:67–77
Szöcs G, Tóth M, Kárpáti Z, Zhu J, Löfstedt C, Plass E, Francke W (2004) Identification of polyene hydrocarbons from the northern winter moth Operophtera fagata, and development of a specific lure for pheromone traps. Chemoecology 14:985–100
Tamaki Y (1977) Complexity, diversity, and specificity of behavior modifying chemicals in Lepidoptera and Diptera. In: Shorey HH, Mckelvey JJ Jr (eds) Chemical control of insect behavior. Wiley, New York, pp 253–285
Uehara T, Naka H, Matsuyama S, Ando T, Honda H (2012) Identification and field evaluation of sex pheromones in two hawk moths Deilephila elpenor lewisii and Theretra oldenlandiae (Lepidoptera: Sphingidae). Appl Entomol Zool 47:227–232
Wakamura S, Yasuda T, Watanabe M, Kiguchi K, Shimoda M, Ando T (1996) Sex pheromone of the sweet potato hornworm, Agrius convolvuli (L.) (Lepidoptera: Sphingidae): identification of a major component and its activity in a wind tunnel. Appl Entomol Zool 31:171–174
Wang CM, Chen HT, Li TC, Weng JH, Han YL, Lin SX, Chou CH (2014) The role of pentacyclic triterpenoids in the allelopathic effects of Alstonia scholaris. J Chem Ecol 40:90–98
Weeks ENI, Birkett MA, Cameron MM, Pickett JA, Logan JG (2011) Semiochemicals of the common bed bug, Cimex lectularius L. (Hemiptera: Cimicidae), and their potential for use in monitoring and control. Pest Manag Sci 67:10–20
Witzgall P, Kirsch P, Cork A (2010) Sex pheromones and their impact on pest management. J Chem Ecol 36:80–100
Acknowledgements
We wholeheartedly thank the anonymous reviewers for insightful suggestions and valuable comments that improved the quality of this manuscript.
Funding information
This research was funded by the National Natural Science Foundation of China (No. 31600516) and the Young Investigator Grant of College of Forestry and Landscape Architecture (201602), South China Agricultural University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Ma, T., Liu, Z., Wang, C. et al. Production, identification, and field evaluation of sex pheromone from calling females in Diaphania angustalis (Lepidoptera: Crambidae). Environ Sci Pollut Res 24, 24485–24493 (2017). https://doi.org/10.1007/s11356-017-0119-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0119-7