Abstract
The sex pheromones of two species of hawk moth, Deilephila elpenor lewisii (Butler) and Theretra oldenlandiae oldenlandiae (Fabricius), were analyzed using gas chromatography–electroantennographic detection (GC–EAD) and GC–mass spectrometry (GC–MS). Two and three EAD-active components were found in D. elpenor lewisii and T. oldenlandiae oldenlandiae, respectively. GC–MS analyses using authentic compounds and extracts derivatized by dimethyl disulfide and 4-methyl-1,2,4-triazoline-3,5-dione identified the two components in D. elpenor lewisii as (E)-11-hexadecenal (E11–16:Ald) and (10E,12E)-10,12-hexadecadienal (E10,E12–16:Ald), and the three in T. oldenlandiae oldenlandiae as E11–16:Ald, E10,E12–16:Ald, and (10E,12Z)-10,12-hexadecadienal (E10,Z12–16:Ald). In field-trap tests, no males of either species were attracted to any single components. Male moths of D. elpenor lewisii were specifically attracted to a binary blend of E11–16:Ald and E10,E12–16:Ald at a ratio of 85:15, whereas males of T. oldenlandiae oldenlandiae were attracted to a ternary blend of E11–16:Ald, E10,Z12–16:Ald and E10,E12–16:Ald at a ratio of 30:40:30. We therefore conclude that the sex pheromone of D. elpenor lewisii is a mixture of E11–16:Ald and E10,E12–16:Ald and that of T. oldenlandiae oldenlandiae is E11–16:Ald, E10,Z12–16:Ald and E10,E12–16:Ald.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sphingidae, commonly known as hawk moths, is the largest family of Bombycoidea comprising 1,400 species around the world. Species diversity can be facilitated by a species-specific mating system, which is generally mediated by sex pheromones (Linn and Roelofs 1995). However, sex pheromones or sex attractants of hawk moths have been identified in only a few species. Starratt et al. (1979) identified (10E,12Z)-10,12-hexadecadienal (E10,Z12–16:Ald) (bombykal) from Manduca sexta (Linnaeus), and Tumlinson et al. (1994) found (10E,12E,14Z)-10,12,14-hexadecatrienal (E10,E12,Z14–16:Ald) to be a new minor component essential for male attraction in this species. Although no field-trap tests were done, hexadecenals and hexadecadienals are also reported as sex pheromone candidates in other hawk moth species (Bestmann et al. 1992; Wakamura et al. 1996). Field screenings using synthetic chemicals demonstrated that five species of Sphingidae males are attracted to bombykal family compounds, and these compounds were discovered in pheromone gland extracts of Sphinx drupiferarum (J. E. Smith), Hyles galii (Rottemburg), and Amphion floridensis (B.P. Clark) by gas chromatographic (GC) analyses, although the complete chemical profiles were not described (Reed et al. 1987; Landolt et al. 1989).
As many species of hawk moths such as M. sexta and Agrius convolvuli (Linnaeus) are important pest insects in field crops and natural vegetation, information on their sex pheromones is useful for developing pheromone lures for pest management and understanding mechanisms of reproductive isolation and evolution of pheromone systems in hawk moths. Deilephila elpenor lewisii (Butler), a subspecies of the elephant hawk moth and the impatience hawk moth Theretra oldenlandiae oldenlandiae (Fabricius), are common species and widely distributed in Japan, that damage orchard and vegetable crops including grapes, taro, and rose balsam (Japanese Society of Applied Entomology and Zoology 2006). In the study presented here, we identified candidates of sex pheromone components extracted from virgin females of the two species by using GC coupled with an electroantennographic detector (EAD) and a mass spectrometer (MS). Furthermore, activities of the identified chemicals were evaluated using field-trap experiments to develop effective pheromone lures for males of D. elpenor lewisii and T. oldenlandiae oldenlandiae.
Materials and methods
Insects
A female adult of D. elpenor lewisii was caught from Kokufu-town, Tottori-city (35.5°N, 134.3°E) in July 2010 and offspring larvae were reared with Impatiens textori (Miquel) at 25 ± 2 °C and natural photo regime until pupation. Larvae of T. oldenlandiae oldenlandiae were obtained from Tsukuba-city (36.1°N, 140.1°E) in August 2010 and fed on Cayratia japonica (Thunberg) at 25 ± 1 °C and 60–70 % relative humidity (RH) under a reversed photo regime of L15:D9 until they emerged. The pupae of both species were sexed based on morphological differences in the genital area and allowed to emerge under the same conditions described above.
Chemicals and extracts
(E)-11-Hexadecenal (E11–16:Ald) and (Z)-11-hexadecenal were supplied by ShinEtsu Chemical Co., Ltd. (Tokyo, Japan). Four geometric isomers of 10,12-hexadecadienals were supplied from a stock library of our laboratory. The isomeric purity of all compounds was confirmed to be ≥97 % by GC (column: HP-5MS). Pheromone extraction in D. elpenor lewisii was conducted with 11 calling females for 3 h after light-off and in T. oldenlandiae oldenlandiae with eight calling females for 7 h after light-off. Female abdominal tips, including the pheromone glands of 3- 4-day-old virgin females were cut with ophthalmology scissors and extracted to a small tapered glass vial containing redistilled n-hexane for 20 min. After passing through a glass filter, the crude extracts were concentrated with a gentle nitrogen stream and stored at −20 °C until use. Pooled extracts were subjected to chemical analysis and bioassay.
Chemical analysis
Female pheromone gland extracts were analyzed with GC coupled to an GC–EAD. HP-5890 series II GS fitted with HP-5MS capillary column (30 m × 0.32 mm ID, film thickness 0.25 μm; Agilent Technologies, USA) and helium as a carrier gas (37 cm/s). EAD responses of male moths were recorded with Syntech IDAC-2 (Syntech, The Netherlands). GC oven temperature was programmed at 130 °C for 2 min, then at a rate of 5 °C/min to 250 °C and held at this temperature for 10 min. Effluents from the column were split at a ratio of 1:1 between a flame ionization detector (FID) and EAD. Humidified air at 21 °C delivered the GC effluent to the antennal preparation bridged between Ag–AgCl electrodes with electric conductible gel.
GC–MS analysis was conducted using a JEOL MS-600H MS (JEOL Ltd., Japan) coupled with HP-6890N GC (Agilent) with a DB-5MS capillary column (25 m × 0.25 mm ID, film thickness 0.25 μm, Agilent) in electro ionization mode (70 eV). Helium was used as the carrier gas at a liner velocity of 42 cm/s. GC oven temperature was maintained at 100 °C for 1 min, programmed at a rate of 10 °C/min to 320 °C for 7 min. Temperature of the injector, interface, and ion source was 280, 280, and 190 °C, respectively. The structures of EAD-active compounds in the extracts were deduced by analysis of the mass spectra of native and dimethyl disulfide (DMDS)- or 4-methyl-1,2,4-triazoline-3,5-dione (MTAD)-derivatized extracts (Buser et al. 1983; Young et al. 1990).
GC analyses were performed on Shimadzu GC-17A (Shimadzu Co., Ltd., Japan) and Agilent 6890 (Agilent) GS fitted with a nonpolar HP-5MS column and a polar DB-23 column (30 m × 0.25 mm ID, film thickness 0.15 μm; Agilent), respectively. Oven temperatures were controlled as follows: HP-5MS: 130 °C for 2 min, programmed at a rate of 5 °C/min to 250 °C for 10 min; DB-23: 100 °C for 2 min, programmed at a rate of 3 °C/min to 250 °C for 10 min. In both columns, injector and detector temperatures were 250 °C and the carrier gas was helium (32 cm/s). Retention indices (RI) of EAD-active components in the extracts and authentic chemicals were determined by comparisons with retention times of standard hydrocarbons (C14–C28). Ratios of EAD-active components in the extracts were obtained from the GC peak area of each compound.
Field tests
Attractions of D. elpenor lewisii and T. oldenlandiae oldenlandiae to synthetic lures were examined on the campus of Tottori University (35.5°N, 134.2°E) and in Kokufu-town field (35.5°N, 134.3°E) in Tottori-city, Tottori Prefecture, during May and June 2011. For D. elpenor lewisii, 500 μg of two isomers of 11–16:Ald, two isomers of 10, 12–16:Ald and a binary mixture of E11–16:Ald and E10,E12–16:Ald (85:15) were used for lures. A ternary mixture of E11–16:Ald,E10,Z12–16:Ald,E10,E12–16:Ald (30:40:30) was used for T. oldenlandiae oldenlandiae as well as single candidate chemicals at a total of 500 μg per trap. Cross-attractions on the binary and ternary mixture were also assayed. The synthetic lures were protected from isomerization and oxidation with 5 % butylated hydroxy toluene (BHT), ladened on gray halo-butyl isoprene rubber septa (West Corp., Singapore) and placed in the center of a sticky board trap (SE-trap®, 30 cm × 27 cm bottom plate with a roof; Sankei Chemical Co., Ltd., Japan). Traps were placed 1.5 m above the ground and at least 10 m from each other. Numbers of captured males in each trap were counted every few days. After each count, the traps were rotated to eliminate any positional effects. Numbers of males captured per week (x) were transformed √(x + 0.5) prior to one-way analysis of variance (ANOVA), followed by a Tukey–Kramer’s honestly significant difference (HSD) test.
Results
Analysis of pheromone components
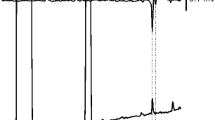
The male antennae of D. elpenor lewisii showed conspicuous responses to two components 1A and 1B in the female pheromone extracts by GC–EAD analysis (Fig. 1a), whereas EAG responses of T. oldenlandiae oldenlandiae were observed to three components 2A, 2B, and 2C (Fig. 1b). Amounts of EAD-active component are approximately 300 ng/female (1A:1B = 85:15 in a ratio) and 200 ng/female (2A:2B:2C = 30:40:30 in a ratio) on D. elpenor lewisii and T. oldenlandiae oldenlandiae, respectively.
GC–MS analysis showed components 1A and 2A produced almost identical diagnostic ion peaks, molecular ion peaks at m/z 238 (M+, 1A, 9 %; 2A, 8 %), m/z 220 ([M − 18]+, 1A, 18 %; 2A, 17 %), m/z 55 ([C4H7]+, base peak for each component), suggesting the component is hexadecenal (C16H30O). Analysis of the DMDS-derivatized extracts of both species indicated a double bond at the 11-position because of three diagnostic ion peaks: molecular ion at m/z 332 (22 %), m/z 215 ([C12H23OS]+, base peak), and m/z 117 ([C6H13S]+, 54 %), indicating an unsaturated double bond in the C11 position. Comparisons of RI of compounds 1A and 2A and authentic compounds including E11–16:Ald with the DB-23 column indicate the EAD-active components 1A and 2A are E11–16:Ald (Table 1).
Compounds 1B, 2B, and 2C gave similar diagnostic ion peaks, molecular ion peaks at m/z 236 (M+, 1B, 36 %; 2B, 29 %; 2C, 28 %), m/z 67 ([C5H7]+, base peak on each component), m/z 96 ([C7H12]+, 1B, 33 %; 2B, 32 %; 2C, 39 %) and m/z 109 ([C8H13]+, 1B, 26 %; 2B, 27 %; 2C, 26 %), suggesting a hexadecadienal (C16H28O). In addition, two characteristic ions (m/z 96 and m/z 109) and relatively high intensity of molecular ions (m/z 236) suggested a conjugated double bond at ω4 and ω6 positions, 10 and 12 positions in the hexadecadienals (Ando et al. 1988). This was confirmed by further GC–MS analysis of MTAD-derivatized female extracts of both species. MTAD-adducts corresponding to compounds 1B, 2B or 2C showed—in addition to a molecular ion peak at m/z 349 (18 %)—two other typical diagnostic ion peaks at m/z 306 (M–C3H7, 45 %) and m/z 208 (M–C9H17O, 91 %), indicating a conjugated 10,12 dienyl structure of the aldehyde. As shown in Table 1, the RI of 2B was identical to that of E10,Z12–16:Ald; the RI of 1B and 2C were identical or almost identical to that of (10E,12E)-10,12–16:Ald (E10,E12–16:Ald) on both columns used.
Field-trap experiment
Pheromone activity of six combinations including E11–16:Ald, E10,Z12–16:Ald, and E10,E12–16:Ald against D. elpenor lewisii and T. oldenlandiae oldenlandiae were assessed in field-trap tests. D. elpenor lewisii males were remarkably attracted to a binary blend of E11–16:Ald and E10,E12–16:Ald at a ratio of 85:15, but not to each component alone (ANOVA, df = 5, F = 32.7, P < 0.001). T. oldenlandiae oldenlandiae males were not attracted to any single component lure, but remarkably responded to a ternary combination of E11–16:Ald, E10,Z12–16:Ald, and E10,E12–16:Ald at a ratio of 30:40:30 (ANOVA, df = 5, F = 5.2, P < 0.001). Additionally, these two species were attracted exclusively to their own pheromone blend (Table 2).
Discussion
GC–EAD-active components in female extracts of D. elpenor lewisii were E11–16:Ald and E10,E12–16:Ald. No males of D. elpenor lewisii were attracted to E11–16:Ald or E10,E12–16:Ald presented separately, but a mixture of the two elicited a substantial response, demonstrating that the binary blend is essential for male attraction. Similar results were also observed in T. oldenlandiae oldenlandiae, where three components, E11–16:Ald, E10,Z12–16:Ald, and E10,E12–16:Ald, were discovered in female extracts, and males were attracted to a ternary blend of the three but not to each single component in field tests. These results indicate that the sex pheromones of these two species consist of 10,12-hexadienals (bombykal family) as well as those of the other hawk moths previously studied. It remains to be examined whether all the three components are necessary to attract T. oldenlandiae oldenlandiae males, because activities of binary mixtures were not examined in this study. However, T. oldenlandiae oldenlandiae males have never been captured by our previous field-trap tests using binary blends of E10,Z12–16:Ald and E10,E12–16:Ald (unpublished data). Thus, at least, E11–16:Ald would be an essential component, mixed with 10,12-hexadienals, to attract T. oldenlandiae oldenlandiae and D. elpenor lewisii. Our GC–EAD surveys on Sphingidae pheromones detected E11–16:Ald as a candidate component in female pheromone gland extracts from five of 12 hawk moths examined (Uehara et al. 2011). This suggests that monoenyl aldehydes and bombykal family compounds may be key components in sphingid pheromones.
Bombykal family sex pheromone components were previously identified in M. sexta (Tumlinson et al. 1994) and D. elpenor (Bestmann et al. 1992). In addition, Reed et al. (1987) and Landolt et al. (1989) reported that single or binary combinations of 10,12-hexadecadienals attracted male moths to field traps in pheromone surveys of six other species of hawk moths. These results imply that bombykal family pheromone components are widespread in the sex pheromone systems of hawk moth species. Bombykal family compounds are also found in pheromones of other moth families, including Bombycidae (Daimon et al. 2012), Crambidae (Raina et al. 1986; Klun et al. 1986; Honda et al. 1994), Saturniidae (McElfresh and Millar 1999a, b; McElfresh et al. 2001), and Noctuidae (Cork et al. 1988).
Species specificity of insect pheromones is not only the result of component structural diversity with differences in number of carbon atoms; the number, position, and configuration of unsaturation, and functionality, but also in the multiplicity of components of pheromone systems (Tamaki 1977). In the pheromone systems of hawk moths, a single-component system was reported in A. convolvuli (11E,13E)-11, 13-hexadecadienal (E11,E13–16:Ald) (Wakamura et al. 1996) and a two-component system in D. elpenor (Bestmann et al. 1992). In this study, we also confirmed a two-component system in D. elpenor lewisii. In addition, we demonstrated for the first time a possible three-component pheromone system in Sphingidae, in T. oldenlandiae oldenlandiae. These results indicate hawk moths drive pheromone divergence by an increase/decrease of number of components as well as structural modifications.
Differentiation in sex pheromone systems facilitates reproductive isolation of insect subspecies. Lepidopteran subspecies may discriminate each other by different pheromone systems with minor modifications in a common basic motif of compositions and additional compounds or modified ratios (Ando et al. 1997; McElfresh and Millar 1999a; Kakizaki and Sugie 2002). D. elpenor moths are widely distributed in the Palaearctic region represented by subspecies of D. elpenor elpenor and D. elpenor lewissi (Pittaway 1993). E11–16:Ald and E10,E12–16:Ald were reported as pheromone candidates from European populations of D. elpenor, with no detailed taxonomical status (Bestmann et al. 1992). More detailed information on sex pheromone systems is needed for biogeographical understanding of reproductive isolation in D. elpenor subspecies.
We also found a new ternary pheromone system in T. oldenlandiae oldenlandiae, which is composed of E11–16:Ald, E10,E12–16:Ald and E10,Z12–16:Ald, as the first report of a three-component system from hawk moths. In addition to E11,E13–16:Ald from A. convolvuli (Wakamura et al. 1996), our ongoing pheromone survey suggests the possibility of a nonbombykal pheromone component in other hawk moth species (Uehara et al. 2011). These results provide a better understanding of sphingid sex pheromone systems that are seldom discussed. At the same time, our findings on sex pheromone components of D. elpenor lewissi and T. oldenlandiae oldenlandiae will also contribute to the development of sex pheromone lures for population monitoring as well as management of these species.
References
Ando T, Ogura Y, Uchiyama M (1988) Mass spectra of lepidopterous sex pheromones with a conjugated diene system. Agric Biol Chem 52:1415–1423
Ando T, Ohtani K, Yamamoto M, Miyamoto T, Qin X, Witjaksono K (1997) Sex pheromone of Japanese giant looper, Ascotis selenaria cretacea: identification and field tests. J Chem Ecol 23:2413–2423
Bestmann HJ, Erler J, Gerbe W, Kern F, Martischonok V, Schäfer D, Vostrowsky O, Wasserthal LT (1992) Pheromone components of the female elephant hawk-moth, Deilephila elpenor, and silver-striped hawk-moth, Hippotion celerio. Experimentia 48:610–613
Buser HR, Arn H, Guerin P, Rauscher S (1983) Determination of double bond position in mono-unsaturated acetates by mass spectrometry of dimethyl disulfide adducts. Anal Chem 55:818–822
Cork A, Chamberlain DJ, Beevor PS, Hall DR, Nesbitt BF, Campion DG, Attique MR (1988) Components of female sex pheromone of spotted bollworm, Earias vittella F. (Lepidoptera: Noctuidae): identification and field evaluation in Pakistan. J Chem Ecol 14:929–945
Daimon T, Fujii T, Yago M, Hsu Y, Nakajima Y, Fujii T, Katsuma S, Ishikawa Y, Shimada T (2012) Female sex pheromone and male behavioral responses of the bombycid moth Trilocha varians: comparison with those of the domesticated silkmoth Bombyx mori. Naturwissenschaften 99:207–215
Honda H, Himeno K, Yoshiyasu Y (1994) Chemotaxonomy of the cotton leaf-roller (Lepidoptera: Pyralidae) in Japan with special reference to differences in sex pheromones. Appl Entamol Zool 29:323–330
Japanese Society of Applied Entomology and Zoology (2006) Major insect and other pests of economic plants in Japan (revised edition). The Japanese Society of Applied Entomology and Zoology, Tokyo, p 381
Kakizaki M, Sugie H (2002) Sex pheromone of the flax bidworm, Heliothis maritime adaucta Butler (Lepidoptera: Noctuidae). Appl Entamol Zool 38:73–78
Klun JA, Leonhardt BA, Schwarz M, Day A, Raina AK (1986) Female sex pheromone of the pickleworm, Diaphania nitidalis (Lepidoptera: Pyralidae). J Chem Ecol 12:239–249
Landolt PJ, Tumlinson JH, Brennan MM (1989) Attraction of Amphion floridensis (Lepidoptera: Sphingidae) to bombykal, (E,Z)-10,12-hexadecadienal. Fla Entamol 72:324–327
Linn CE, Roelofs WL (1995) Pheromone communication in moths and its role in the speciation process. In: Lambert DM, Spencer HG (eds) Speciation and the recognition concept. Johns Hopkins University Press, Baltimore, pp 263–300
McElfresh JS, Millar JG (1999a) Geographic variation in sex pheromone blend of Hemileuca electra from southern California. J Chem Ecol 25:2505–2525
McElfresh JS, Millar JG (1999b) Sex pheromone of the common sheep moth, Hemileuca eglanterina, from the San Gabriel Mountains on California. J Chem Ecol 25:687–709
McElfresh JS, Hammond AM, Millar JG (2001) Sex pheromone components of the buck moth Hemileuca maia. J Chem Ecol 27:1409–1422
Pittaway AR (1993) The hawkmoths of the western palaearctic. Harley books, Colchester, pp 156–157
Raina AK, Klun JA, Schwarz M, Day A, Leonhardt BA, Douglass LW (1986) Female sex pheromone of the melonworm, Diaphania hyalinata (Lepidoptera: Pyralidae), and analysis of male responses to pheromone in a flight tunnel. J Chem Ecol 12:229–237
Reed DW, Underhill EW, Giblin EM (1987) Attraction of sphingid moths (Lepidoptera: Sphindidae) to 10, 12-hecadecadienyl aldehydes and acetates: evidence of pheromone components. J Chem Ecol 13:931–942
Starratt AN, Dahm KH, Allen N, Hildebrand JG, Payne TL, Roller H (1979) Bombykal, a sex pheromone of the sphinx moth Manduca sexta. Z Naturforsch 34:9–12
Tamaki Y (1977) Complexity, diversity, and specificity of behavior-modifying chemicals in Lepidoptera and Diptera. In: Shorey HH, Mckelvey JJ Jr (eds) Chemical control of insect behavior. Wiley, NY, pp 253–285
Tumlinson JH, Mitchell ER, Doolittle RE, Jackson DM (1994) Field tests of synthetic Manduca sexta sex pheromone. J Chem Ecol 20:579–591
Uehara T, Naka H, Matsuyama S, Ando T, Honda H (2011) Sex pheromone diversity in Japanese hawkmoth. Asia-Pacific Association of Chemical Ecology, NY, p 122
Wakamura S, Yasuda T, Watanabe M, Kiguchi K, Shimoda M, Ando T (1996) Sex pheromone of the sweetpotato hornworm, Agrius convolvuli (L.) (Lepidoptera: Sphingidae): identification of a major component and its activity in a wind tunnel. Appl Entamol Zool 31:171–174
Young DC, Vouros P, Holick MF (1990) Gas chromatography–mass spectrometry of conjugated dienes by derivatization with 4-methyl-1,2,4-triazoline-3,5-dione. J Chromatogr 522:295–302
Acknowledgments
We thank ShinEtsu Chemical Co., Ltd. for supplying synthetic monoenyl aldehydes and Dr. DeMar Taylor for helpful improvement in the manuscript. We also thank Mr. Takahiro Yano for offering important information about rearing and field experiments in Deilephila elpenor. We are grateful to Mr. T. Morita, Ms. K. Ohbayashi, and students of the Laboratory of Applied Entomology in Faculty of Agriculture at Tottori University for their help with field experiments and rearing insects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Uehara, T., Naka, H., Matsuyama, S. et al. Identification and field evaluation of sex pheromones in two hawk moths Deilephila elpenor lewisii and Theretra oldenlandiae oldenlandiae (Lepidoptera: Sphingidae). Appl Entomol Zool 47, 227–232 (2012). https://doi.org/10.1007/s13355-012-0111-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13355-012-0111-0