Abstract
Synthetic hexaploid wheat (SHW) possesses numerous genes for resistance to stress, including phosphorus (P) deficiency. Root diameter (RDM) plays an important role in P-deficiency tolerance, but information related to SHW is still limited. Thus, the objective of this study was to investigate the genetic architecture of RDM in SHW under P-deficient conditions. To this end, we measured the RDM of 138 F9 recombinant inbred lines derived from an F2 population of a synthetic hexaploid wheat line (SHW-L1) and a common wheat line (Chuanmai32) under two P conditions, P sufficiency (PS) and P deficiency (PD), and mapped quantitative trait loci (QTL) for RDM using an enriched high-density genetic map, containing 120,370 single nucleotide polymorphisms, 733 diversity arrays technology markers, and 119 simple sequence repeats. We identified seven RDM QTL for P-deficiency tolerance that individually explained 11–14.7% of the phenotypic variation. Five putative candidate genes involved in root composition, energy supply, and defense response were predicted. Overall, our results provided essential information for cloning genes related to P-deficiency tolerance in common wheat that might help in breeding P-deficiency-tolerant wheat cultivars.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The uptake of soil phosphorus (P) affects crop growth and yield. Plants absorb P as inorganic phosphate; however, up to 80% of applied P fertilizer is fixed into organic forms (Holford 1997), and thus the concentration of inorganic phosphate in the soil solution is usually low (Raghothama 1999) and its supply to the root surface by diffusion is slow (Fitter and Hay 2012). Consequently, P deficiency is one of the major abiotic stresses worldwide (Sharpley 1985; Hayes et al. 2000; Mudge et al. 2003; Yadav et al. 2014), and the development of wheat cultivars with P-deficiency tolerance is considered essential in wheat production.
Synthetic hexaploid wheat (SHW) obtained from the distant hybridization of Triticum turgidum L. and Aegilops tauschii is a source of novel genetic variability associated with the D genome of A. tauschii (Mares and Mrva 2008). SHW lines show a significantly better performance in disease resistance, abiotic stress tolerance, suitable quality, anti-sprouting ability (Lage et al. 2003; Trethowan and Mujeeb-Kazi 2008; Yang et al. 2016), and P-deficiency tolerance compared with the tetraploid and common wheat lines (Wang et al. 2015).
Previous studies indicated that root diameter (RDM) is an important trait for evaluating root development, because it defines the volume of soil that comes in contact with the roots (Atkinson 1990). It has been reported that the primary RDM in Arabidopsis thaliana, Zea mays, and Quercus robur is strongly correlated with the root length and weakly correlated with the elongation rate (Cahn et al. 1989; Pages 1995). RDM was believed to directly influence phosphorus uptake in low phosphorus soils. A previous study showed that barley RDM highly contributes to P uptake under low P conditions (Chen et al. 2015). However, most reports on root morphology quantitative trait loci (QTL) have focused on root length and weight (Coudert et al. 2010; Den Herder et al. 2010; Cao et al. 2014). Compared with the identified RDM QTL for P-deficiency tolerance in A. tauschii, Z. mays, and Phaseolus vulgaris (Beebe et al. 2006; Chen et al. 2009; Liu et al. 2015), our knowledge on the genetic base of RDM under P-deficient conditions is still limited. The objective of this study was to identify RDM QTL and putative candidate genes for P-deficiency tolerance in SHW, in order to obtain useful information for cloning RDM genes and breeding wheat cultivars with P-deficiency tolerance.
Materials and methods
Plant material
A total of 138 F9 recombinant inbred lines (RILs) derived by single-seed descent from the F2 population of SHW-L1/Chuanmai 32 was used in this study. SHW-L1 is a SWH derived from a cross between T. turgidum ssp. turgidum AS2255 (AABB) and A. tauschii ssp. tauschii AS60 (DD). Chuanmai 32 is a commercial cultivar of hexaploid wheat grown in the southwest winter wheat areas of China (Yu et al. 2014).
Plant growth and experimental treatments
The RILs, along with the parental lines, were hydroponically cultured for measuring RDM. Thirty uniformly sized seeds from each line were surface-sterilized by soaking in 10% sodium hypochlorite for 5 min, and then rinsed three times with deionized water. Seeds were then germinated on filter paper in petri dishes at 25 °C (± 1 °C) for 7 d. After removing residual endosperm materials, the uniform seedlings with coleoptile (ca. 1–2 cm in length) were transplanted into a different hydroponic system under two P conditions, P sufficiency (PS) and P deficiency (PD), in a completely randomized design with four replications. PS and PD treatments contained modified Hoagland’s nutrient solution (Hoagland and Arnon 1950) that consisted of Ca(NO3)2·4H2O (4 mmol l−1), KNO3 (6 mmol L−1), MgSO4·7H2O (2 mmol l−1), H3BO3 (46 μmol−1), Na·Fe·EDTA (100 μmol−1), MnCl2 (9.146 μmol−1), ZnSO4 (0.76 μmol−1), CuSO4 (0.32 μmol−1), and (NH4)6Mo7O24 (0.0161 μmol−1) with and without NH4H2PO4 (1 mmol l−1), respectively. The hydroponic system was formed by a cystosepiment substrate that was placed into plastic tanks (50 cm × 40 cm × 30 cm) filled with 21 l modified Hoagland’s nutrient solution. The nutrient solution was continuously aerated by pumps and renewed every 4 d. The sponge-wrapped seedlings were fixed on the cystosepiment substrate and grown at 25 °C (± 1 °C) during the day (16 h) and 22 °C (± 1 °C) during the night (8 h).
Collection and analysis of phenotypic data
At 16 d of growth, seedlings were carefully washed with clean water, and the RDM was measured using an Epson XL (11,000 ×) scanner with the WinRHizo Pro 2008a image analysis system. The experiment was repeated three times to increase the credibility of RDM measurements. The three replications were designated as R1, R2, and R3. Phenotypic data were the means of four replications in one independently repeated experiment. For estimating random effects, we used a mixed mode called the best linear predictors (BLUPs) to obtain BLUP-RDM values (Piepho et al. 2008). The BLUP model for the phenotypic value of plant Yi was calculated as follows: Yi = Xi f + ai + ei, where f is a vector of fixed effects, Xi is an incidence vector, ei is environment deviation, and ai is the phenotypic value (Goddard 1992). Analysis of variance (ANOVA) was performed using SAS 9.1.3 (SAS Institute, Cary, NC, USA) to estimate the effects of genotype on RDM. The estimated broad-sense heritability of RDM was calculated as follows: h2 = σ2 G/(σ2 G + σ2 e/r), where σ2 G is the genetic variance, σ2 e is the residual variance, and r is the number of replicates per genotype.
QTL mapping
An enriched high-density genetic map that contained 120,370 single nucleotide polymorphisms (Axiom™ Wheat 660 k Arrays), 733 diversity arrays technology markers, and 119 simple sequence repeats (121,222 markers in total) and had a total length of 17,889. For the QTL analysis, 62 cM was used (Yang 2016). The average distance between markers was 0.148 cM, which corresponds to 143 kb (wheat genome size according to the International Wheat Genome Sequencing Consortium database). QTL screening was conducted using interval mapping (IM) by MapQTL 6.0 (Kyazma, Wageningen, Netherlands). Logarithm of odds (LOD) threshold values for IM were determined based on 1,000 permutations to declare significant QTL at p < 0.05, and the QTL with LOD values <2.0 were excluded to ensure the authenticity and reliability of reported QTL. QTL that explained more than 10% of the phenotypic variation for RDM were considered major QTL.
Prediction of candidate genes
For predicting candidate/flanking genes, the nearest flanking marker sequence was aligned using BLAST against the EnsemblPlants database (http://plants.ensembl.org/hmmer/index.html) to determine the position with the highest identity and detect genes within 5,000 bp upstream and 5,000 bp downstream of this position. To predict the function of candidate genes, we conducted gene ontology (GO) annotation and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis at p < 0.05 using A. thaliana, Oryza sativa, and Z. mays as background species with KOBAS 3.0 (http://kobas.cbi.pku.edu.cn/) (Table S1).
Results
RDM variation in RILs
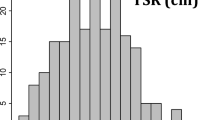
The RDM values of SHW-L1 were significantly higher than those of Chuanmai32 in PD. In PS, the mean RDM of SHW-L1 was 0.397 mm and that of Chuanmai32 was 0.360 mm. In PD, the mean RDM of SHW-L1 showed an increase of 25.693 % and that of Chuanmai32 showed an increase of 17.778%. The frequency distribution of RDM among the 138 RILs was continuous in both PS and PD (Fig. 1), indicating its polygenic inheritance. The RDM values ranged from 0.296 mm to 0.417 mm in PS, whereas in PD, the values ranged from 0.341 mm to 0.493 mm. RDM values showed significant differences among the RILs and high heritability (h2 = 0.75 and 0.86 in PS and PD respectively; Table 1).
P-deficiency-response QTL for RDM
A total of 16 QTL for RDM was detected, of which seven major RDM QTL located on chromosomes (Chr.) 1B, 1D, 2B, 3B, 3D, and 7D were detected only in PD and individually explained 11–14.7% of the phenotypic variation (Table 2; Fig. 2). These QTL for RDM were contributed by positive alleles from SWH-L1. Among them, three QTL ( QRDM.sicau-1D, QRDM.sicau-3D, and QRDM.sicau-7D) were detected in the D-genome.
Chromosomal locations of quantitative trait loci for root diameter (QRDM) and associated markers in the SHW-L1/Chuanmai 32 recombinant inbred line (RIL) population under phosphorus sufficient (PS) and phosphorus deficient (PD) conditions. The bar points to the LOD peak of QTL. Empty bar, black bar, and striped bar indicate QTLs identified only in PS, only in PD, and in both of them, respectively
Putative candidate genes associated with significant loci
Five candidate genes that may underlie QTL for RDM in PD were identified: TRIAE_CS42_1BL_TGACv1_030534_AA0093380 and TRIAE_CS42_3DL_TGACv1_251990_AA0886810 regulated the proliferation and differentiation of cells, and the latter was also involved in N-glycan biosynthesis in PD. TRIAE_CS42_2BL_TGACv1_129475_AA0385390 regulated the actin cytoskeleton organization and inhibited ligand-induced endocytosis. TRIAE_CS42_3B_TGACv1_227160_AA0821780 affected composition of Golgi apparatus. And TRIAE_CS42_7DS_TGACv1_621992_AA2030780 was involved in cell energy conversion and defense response (Table S1).
Discussion
The important role of the D genome in the P-deficiency tolerance of SHW
Previous studies have reported many QTL for P-deficiency tolerance in the D genome of wheat. Under P-deficient conditions, Chr. 2D, 3D, 4D, 5D, and 6D have been reported to harbor QTL for numerous traits related to P utilization efficiency, including biomass yield per plant, 1,000-grain weight, grain number per ear, P accumulated in the shoot per plant, and shoot dry weight (Su et al. 2006, 2009). Additionally, Chr. 4D, 5D, 6D, and 7DL harbored QTL for seedling root traits (length, number, and dry matter; Huiru et al. 2007), whereas Chr. 6D also contained QTL for shoot height (Guo et al. 2012). In the present study, three of the seven RDM QTL detected in PD were mapped in the D genome, and comparison of their genetic locations with those of previously reported RDM QTL indicated that they were novel. Liu et al. (2015) reported a highly relevant RDM-P-deficiency tolerance index QTL on Chr. 7DS in A. tauschii; however, it was not in the same loci as the QTL we detected on Chr. 7DS. Thus, the presence of these three novel RDM QTL detected in the D genome indicated that some unknown genes for P-deficiency tolerance might be introduced to SHW from A. tauschii. Our results along with those reported previously indicated that the D genome could play a key role in P-deficiency tolerance. Undoubtedly, numerous important genes were lost during the domestication of common wheat. For instance, Ma et al. (2016) suggested that during the formation of hexaploid wheat, genes that are upregulated in the root are prone to extinction. Thus, integrating genes from A. tauschii into common wheat by artificially synthesizing could be an efficient strategy for wheat improvement (Yang et al. 2016).
Comparison of newly and previously detected QTL for P-deficiency tolerance
Since no other RDM QTL for P deficiency have been previously reported, we compared our results with QTL for other root traits that control P deficiency tolerance in wheat. Under low P conditions, QTL for root potassium (K) content per plant, shoot K content per plant, total K content per plant, root P utilization efficiency (Guo et al. 2012), and grain number per ear (Su et al. 2009) have been identified on Chr. 1BL; five QTL for tiller number per plant, ear number per plant, grain yield, and biomass yield have been identified on Chr. 2BL (Su et al. 2009); and nine QTL for P accumulation in the shoot per plant, biomass yield per plant, ear number per plant, grain yield, and shoot dry weight have been identified on Chr. 3B (Su et al. 2009). Moreover, Guo et al. (2012) reported the existence of two QTL clusters for P deficiency on Chr. 1BL and 3B. In our study, four of the seven RDM QTL for P deficiency were also identified on Chr. 1B, 2B, and 3B, indicating that these chromosomes might contain important genes for P absorption in wheat.
The biological mechanism of RDM variation response to P-deficiency
A previous study showed that different plant species can have significantly different RDM variation and different response to P deficiency (Hill et al. 2006). In PS, a relatively smaller root diameter implies that a larger soil volume per surface area unit delivers nutrients to the root, increasing the uptake rate (Fitter 1991), whereas in PD, seemingly relatively thinner roots might be more effective in absorbing soil P. However, since thin roots tend to turnover more rapidly than coarse roots, the carbon cost of producing thin roots may be higher as these are replaced more frequently (Sattelmacher et al. 1994; Gahoonia and Nielsen 2004). Consequently, it remains unclear whether the root diameter is increased or decreased to cope with P deficiency. In the present study, SHW was adapted to P deficiency by increasing the root diameter, probably because coarse roots tend to turnover more slowly and reduce energy consumption. The survival ability of roots increases with the increasing root diameter (Qiu et al. 2013). The increase in root diameter also indicated that the extent of xylem and phloem increased, which promoted the nutrient uptake (Zhao et al. 2005). The predicted candidate genes in the present study were closely related to RDM increase. TRIAE_CS42_1BL_TGACv1_030534_AA0093380 was annotated as the A. thaliana gene MYB36, which is a critical positive regulator of differentiation and a negative regulator of cell proliferation (Liberman et al. 2015). MYB36 controls the expression of the machinery required to locally polymerize lignin in a fine band in the cell wall for the formation of the Casparian strip (Kamiya et al. 2015), and outside the endodermis during the lateral root primordia development regulates the proliferation/differentiation transition in the root meristem (Fernández-Marcos et al. 2016). TRIAE_CS42_3DL_TGACv1_251990_AA0886810 was annotated as the A. thaliana gene GCS1, which encodes an alpha-glucosidase I enzyme that catalyzes the first step in N-linked glycan processing during the epidermal development in Arabidopsis. (Gillmor et al. 2002; Saint-Jore-Dupas et al. 2006; Furumizu and Komeda 2008; Boulaflous et al. 2009). TRIAE_CS42_3B_TGACv1_227160_AA0821780 that was identified by GO annotation can affect the composition of Golgi apparatus. TRIAE_CS42_2BL_TGACv1_129475_AA0385390 and TRIAE_CS42_7DS_TGACv1_621992_AA2030780 are associated with plant defense to environmental stress. The former was annotated as the A. thaliana gene EHD2, which has an inhibitory effect on endocytosis involved in the induction of plant defense responses (Bar et al. 2008), whereas the latter was identified by GO annotation to be involved in the cell energy supply and defense response (Chang et al. 2009). Previous studies have found three genes associated with phosphate transporter (PT): TaPht2; 1, TaPht1; 4, and TaPht2. (Guo et al. 2013, 2014; Liu et al. 2013). All the five candidate genes identified in this study were different from the above three genes. These novel putative functional genes, which play an important role in cell configurations, energy supply, and nutrient absorption, provide a basis for dissecting the genetic mechanism of P-deficiency tolerance in wheat.
Conclusions
In this study, we identified seven RDM QTL for tolerance to P deficiency that explained 11–14.7% of the phenotypic variation, as well as five putative candidate genes by GO annotation and KEGG pathway enrichment analysis. Overall, our data provided new insights into the genetic basis of RDM under different P conditions, important information for cloning genes related to P-deficiency tolerance, and a foundation for developing stress-tolerant wheat cultivars.
References
Atkinson D (1990) Influence of root system morphology and development on the need for fertilizers and the efficiency of use. In: Baligar VC, Duncan RR (eds.) Crops as enhancers of nutrient use. Academic Press, San Diego, pp. 411–450
Bar M, Aharon M, Benjamin S, Rotblat B, Horowitz M, Avni A (2008) AtEHDs, novel Arabidopsis EH-domain-containing proteins involved in endocytosis. Plant J 55:1025–1038
Beebe SE, Rojas-Pierce M, Yan X, Blair MW, Pedraza F, Munoz F, Tohme J, Lynch JP (2006) Quantitative trait loci for root architecture traits correlated with phosphorus acquisition in common bean. Crop Sci 46:413–423
Boulaflous A, Saintjoredupas C, Herranzgordo MC, Pagnysalehabadi S, Plasson C, Garidou F, Kiefermeyer MC, Ritzenthaler C, Faye L, Gomord V (2009) Cytosolic N-terminal arginine-based signals together with a luminal signal target a type II membrane protein to the plant ER. BMC Plant Biol 9:1–22
Cahn M, Zobel R, Bouldin D (1989) Relationship between root elongation rate and diameter and duration of growth of lateral roots of maize. Plant Soil 119:271–279
Cao P, Ren Y, Zhang K, Teng W, Zhao X, Dong Z, Liu X, Qin H, Li Z, Wang D (2014) Further genetic analysis of a major quantitative trait locus controlling root length and related traits in common wheat. Mol Breed 33:975–985
Chang IF, Curran A, Woolsey R, Quilici D, Cushman JC, Mittler R, Harmon A, Harper JF (2009) Proteomic profiling of tandem affinity purified 14-3-3 protein complexes in Arabidopsis thaliana. Proteomics 9:2967–2985
Chen HY, Yu HY, Chen GD, Li YX (2015) Root morphological characteristics of barley genotype with high phosphorus efficiency under phosphorus stress. Chin J Appl Ecol 26(10):3020–3026 (in Chinese with English abstract)
Chen J, Xu L, Cai Y, Xu J (2009) Identification of QTLs for phosphorus utilization efficiency in maize (Zea mays L.) across P levels. Euphytica 167:245–252
Coudert Y, Périn C, Courtois B, Khong NG, Gantet P (2010) Genetic control of root development in rice, the model cereal. Trends Plant Sci 15:219–226
Den Herder G, Van Isterdael G, Beeckman T, De Smet I (2010) The roots of a new green revolution. Trends Plant Sci 15:600–607
Fernández-Marcos M, Desvoyes B, Manzano C, Liberman LM, Benfey PN, Del Pozo JC, Gutierrez C (2016) Control of Arabidopsis lateral root primordium boundaries by MYB36. New Phytol 213:105
Fitter AH (1991) Characteristics and functions of root systems. Plant roots: the hidden half 2:1–29
Fitter AH, Hay RK (2012) Environmental physiology of plants. Academic Press, San Diego
Furumizu C, Komeda Y (2008) A novel mutation in KNOPF uncovers the role of α-glucosidase I during post-embryonic development in Arabidopsis thaliana. FEBS Lett 582:2237–2241
Gahoonia TS, Nielsen NE (2004) Root traits as tools for creating phosphorus efficient crop varieties. Plant Soil 260(1–2):47–57
Gillmor CS, Poindexter P, Lorieau J, Palcic MM, Somerville C (2002) Alpha-glucosidase I is required for cellulose biosynthesis and morphogenesis in Arabidopsis. J Cell Biol 156:1003
Goddard ME (1992) A mixed model for analyses of data on multiple genetic markers. Theor Appl Genet 83(6–7):878–886
Guo C, Guo L, Li X, Gu J, Zhao M, Duan W, Ma C, Lu W, Xiao K (2014) TaPT2, a high-affinity phosphate transporter gene in wheat (Triticum aestivum L.), is crucial in plant Pi uptake under phosphorus deprivation. Acta Physiol Plant 36:1373–1384
Guo C, Zhao X, Liu X, Zhang L, Gu J, Li X, Lu W, Xiao K (2013) Function of wheat phosphate transporter gene TaPHT2; 1 in Pi translocation and plant growth regulation under replete and limited Pi supply conditions. Planta 237:1163–1178
Guo Y, Kong FM, Xu YF, Zhao Y, Liang X, Wang YY, An DG, Li SS (2012) QTL mapping for seedling traits in wheat grown under varying concentrations of N, P and K nutrients. Theor Appl Genet 124:851–865
Hayes J, Richardson A, Simpson R (2000) Components of organic phosphorus in soil extracts that are hydrolyzed by phytase and acid phosphatase. Biol Fertil Soils 32:279–286
Hill JO, Simpson RJ, Moore AD, Chapman DF (2006) Morphology and response of roots of pasture species to phosphorus and nitrogen nutrition. Plant Soil 286(1):7–19
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. Circular (California Agricultural Experiment Station 347). College of Agriculture, University of California, Berkeley CA
Holford ICR (1997) Soil phosphorus: its measurement, and its uptake by plants. Soil Research 35(2):227–240
Huiru LZNZP, Shengbao LZNXX, Qixin LGS (2007) Molecular mapping of QTLs for root response to phosphorus deficiency at seedling stage in wheat (Triticum aestivum L.) Prog Nat Sci 17:1177–1184
Kamiya T, Borghi M, Wang P, Danku JM, Kalmbach L, Hosmani PS, Naseer S, Fujiwara T, Geldner N, Salt DE (2015) The MYB36 transcription factor orchestrates Casparian strip formation. Proc Natl Acad Sci U S A 112:10533–10538
Lage J, Skovmand B, Andersen SB (2003) Expression and suppression of resistance to greenbug (Homoptera: Aphididae) in synthetic hexaploid wheats derived from Triticum dicoccum × Aegilops tauschii crosses. J Econ Entomol 96:202–206
Liberman LM, Sparks EE, Moreno-Risueno MA, Petricka JJ, Benfey PN (2015) MYB36 regulates the transition from proliferation to differentiation in the Arabidopsis root. Proc Natl Acad Sci U S A 112:12099–12104
Liu X, Zhao X, Zhang L, Lu W, Li X, Xiao K (2013) TaPht1; 4, a high-affinity phosphate transporter gene in wheat (Triticum aestivum), plays an important role in plant phosphate acquisition under phosphorus deprivation. Funct Plant Biol 40:329–341
Liu Y, Wang L, Deng M, Li Z, Lu Y, Wang J, Wei Y, Zheng Y (2015) Genome-wide association study of phosphorus-deficiency-tolerance traits in Aegilops tauschii. Theor Appl Genet 128:2203–2212
Ma J, Zheng Z, Stiller J, Lan XJ, Liu Y, Deng M, Wei Y (2016) Identification and characterization of genes on a single subgenome in the hexaploid wheat (Triticum aestivum L.) genotype ‘Chinese Spring’. Genome 60(3):208–215
Mares D, Mrva K (2008) Genetic variation for quality traits in synthetic wheat germplasm. Crop and Pasture Science 59:406–412
Mudge SR, Smith FW, Richardson AE (2003) Root-specific and phosphate-regulated expression of phytase under the control of a phosphate transporter promoter enables Arabidopsis to grow on phytate as a sole P source. Plant Sci 165:871–878
Pages L (1995) Growth patterns of the lateral roots of young oak (Quercus robur) tree seedlings. Relationship with apical diameter. New Phytol 130:503–509
Piepho H, Möhring J, Melchinger A, Büchse A (2008) BLUP for phenotypic selection in plant breeding and variety testing. Euphytica 161:209–228
Qiu XQ, Cao Y, Huang L et al (2013) Temporal and spatial distribution of root morphology of winter wheat. Agric Sci China 46(11):2211–2219 (in Chinese with English abstract)
Raghothama KG (1999) Phosphate acquisition. Annu Rev Plant Biol 50(1):665–693
Saint-Jore-Dupas C, Nebenführ A, Boulaflous A, Follet-Gueye ML, Plasson C, Hawes C, Driouich A, Faye L, Gomord V (2006) Plant N-glycan processing enzymes employ different targeting mechanisms for their spatial arrangement along the secretory pathway. Plant Cell 18:3182–3200
Sattelmacher B, Horst WJ, Becker HC (1994) Factors that contribute to genetic variation for nutrient efficiency of crop plants. J Plant Nutr Soil Sci 157(3):215–224
Sharpley A (1985) Phosphorus cycling in unfertilized and fertilized agricultural soils. Soil Sci Soc Am J 49:905–911
Su JY, Zheng Q, Li HW, Li B, Jing RL, Tong YP, Li ZS (2009) Detection of QTLs for phosphorus use efficiency in relation to agronomic performance of wheat grown under phosphorus sufficient and limited conditions. Plant Sci 176:824–836
Su J, Xiao Y, Li M, Liu Q, Li B, Tong Y, Jia J, Li Z (2006) Mapping QTLs for phosphorus-deficiency tolerance at wheat seedling stage. Plant Soil 281:25–36
Trethowan R, Mujeeb-Kazi A (2008) Novel germplasm resources for improving environmental stress tolerance of hexaploid wheat. Crop Sci 48:1255–1265
Wang L, Liu K, Mao S, Li Z, Lu Y, Wang J, Liu Y, Wei Y, Zheng Y (2015) Large-scale screening for Aegilops tauschii tolerant genotypes to phosphorus deficiency at seedling stage. Euphytica 204:571–586
Yadav J, Verma JP, Jaiswal DK, Kumar A (2014) Evaluation of PGPR and different concentration of phosphorus level on plant growth, yield and nutrient content of rice (Oryza sativa). Ecol Eng 62:123–128
Yang J (2016) QTL mapping for pre-harvest sprouting resistance and molecular characterization of six grain germination-related genes in synthetic wheat. Triticeae Research Institute, Sichuan Agricultural University 23-25. (in Chinese with English abstract)
Yang W, Liu D, Li J, Zhang L, Wei H, Hu X, Zheng Y, He Z, Zou Y (2016) Synthetic hexaploid wheat and its utilization for wheat genetic improvement in China. J Genet Genomics 36:539–546
Yu M, Chen GY, Zhang LQ, Liu YX, Liu DC, Wang JR, Pu ZE, Zhang L, Lan XJ, Wei YM (2014) QTL mapping for important agronomic traits in synthetic hexaploid wheat derived from Aegiliops tauschii ssp. tauschii. J Integr Agric 13:1835–1844
Zhao C, Craig JC, Petzold HE, Dickerman AW, Beers EP (2005) The xylem and phloem transcriptomes from secondary tissues of the Arabidopsis root-hypocotyl. Plant Physiol 138(2):803–818
Acknowledgements
This study was supported by the National Key Research and Development Program of China (2016YFD0101004), the Outstanding Youth Foundation of the Department of Science and Technology of Sichuan Province (2016JQ0040), the Key Technology Research and Development Program of the Department of Science and Technology of Sichuan Province (2016NZ0057), the International Science & Technology Cooperation Program of the Bureau of Science and Technology of Chengdu China (No. 2015DFA306002015-GH03-00008-HZ).
Author information
Authors and Affiliations
Contributions
FW conducted data analysis and drafted the manuscript.
XY, ZW, and MD performed the phenotypic evaluation and help to data analysis.
JM helped to analyze QTL mapping.
GC performed part of population construction.
YW participated in the design of the study.
YL designed and coordinated this study and revised the manuscript.
All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Communicated by: Barbara Naganowska
Electronic supplementary material
ESM 1
(XLSX 489 kb)
Rights and permissions
About this article
Cite this article
Wu, F., Yang, X., Wang, Z. et al. Identification of major quantitative trait loci for root diameter in synthetic hexaploid wheat under phosphorus-deficient conditions. J Appl Genetics 58, 437–447 (2017). https://doi.org/10.1007/s13353-017-0406-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13353-017-0406-5