Abstract
Aims
Longer root hair length (RHL) increases root surface area, thereby contributing to increased phosphorous uptake. This study aimed to identify useful germplasms and quantitative trait loci (QTL) for RHL improvement in wheat.
Methods
The genetic variation in RHL was examined using hydroponic culture in 117 hexaploid wheat strains. Rhizosheath size and phosphorous uptake ability were also examined using high P-fixing soils with extremely low phosphorous availability (Andisols). QTL analysis was performed using backcross inbred lines from a cross between a Spanish spelt strain and a bread wheat variety.
Results
A wide range in genetic variation was found, especially in spelt wheat and landraces of bread wheat, whereas modern bread wheat varieties tended to show shorter RHL. Interestingly, remarkably longer RHL were observed in Spanish spelt strains than in strains from other countries. RHL exhibited a significant positive correlation with rhizosheath size and phosphorous uptake in soils. A QTL on chromosome 2A (QRhl.obu-2A) conferring longer RHL by the allele of a Spanish spelt strain was identified. It was co-located with TaRSL4-2AS, a key regulator of root hair elongation.
Conclusions
Spanish spelt wheat strains could be unique germplasms that provide a useful allele for increasing RHL to enhance phosphorous uptake ability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phosphorus (P) is an essential nutrient for growth and development in plants and its deficiency causes a reduction in crop productivity in agriculture. Large amounts of P fertilizer have been applied to maximize the yield, especially in high-input agricultural systems in developed countries. The use of P fertilizer has been increasing with global population growth and rising food demand (Heuer et al. 2017); however, limits in the availability of P fertilizer will possibly occur as phosphate rock is a non-renewable natural resource and is expected to become exhausted in the future. Recently, the price of P fertilizer became highly volatile owing to its increasing demand and an unequal distribution of phosphate rock which could influence food security (Khabarov and Obersteiner 2017; Mew 2016). In the field, most of the applied P (generally about 80%) is not absorbed by crops and tends to be immobilized by interactions with the soil (Lambers et al. 2006). Thus, breeding for enhanced P uptake ability is an urgent goal for sustainable food production around the world.
Plants have various mechanisms to acquire P from the soil, such as the development and plasticity of root systems and associations with microorganisms, such as arbuscular mycorrhizal fungi (Heuer et al. 2017; Lambers et al. 2006). Because P is poorly mobile in the soil, a large root system with abundant fine roots and long root hairs could enhance P uptake ability or uptake efficiency. This occurs due to a high root surface area and increased opportunities for root-soil contact. Root hairs are developed from root epidermis cells as tubular outgrowths (Dolan 2017). Root hair length (RHL) is associated with the P uptake ability of various crop species, including rice, common bean, soybean, barley, and wheat (Gahoonia et al. 1997; Miguel et al. 2015; Nestler and Wissuwa 2016; Vandamme et al. 2013). Wheat is a very important staple cereal, thus the improvement of its P uptake ability is necessary for sustainable global food production. A wide range of natural genetic variations in root hair traits are reported in Arabidopsis and barley (George et al. 2014; Stetter et al. 2015); however, information about genetic diversity for RHL within species is still poor, including in wheat. Furthermore, there are few studies in wheat regarding the genetic control of natural variations in RHL or the size of the rhizosheath, which includes the soils that adhere around the root and has frequently been used as an indicator of RHL (Delhaize et al. 2015; Horn et al. 2016; Liu et al. 2017). A survey of useful germplasms and the identification of quantitative trait loci (QTL) or genes is necessary to improve RHL.
In modern wheat breeding programs, much effort has been made to expand genetic variation by introducing genes from landraces and wild relatives because genetic homogenization has been enhanced by breeding of high-yielding elite varieties, especially after the “Green Revolution” (Reif et al. 2005; Tester and Langridge 2010). Wheat comprises several species and subspecies that have been used as food cereals for thousands of years, but at present, only bread wheat (Triticum. aestivum subsp. aestivum), which is a hexaploid wheat with a free threshing trait, is widely cultivated around the world. Spelt wheat (T. aestivum subsp. spelta) is a subspecies of bread wheat with hulled or non-free threshing traits (Longin and Wurschum 2016; Matsuoka 2011). Although spelt wheat was a major cereal until the last century, especially in European countries, it was replaced by modern bread wheat varieties because of its lower yield and inefficiency of harvesting owing to the non-free threshing trait (Miedaner and Longin, 2016). Spelt wheat diverged from bread wheat in its genome, as revealed by the evaluation of DNA sequence polymorphisms such as single nucleotide polymorphisms (SNP) (Kippes et al. 2015; Takenaka et al. 2019). The potential of spelt wheat as a genetic resource for the breeding of traits such as yield and yield components, disease resistance, and abiotic stress tolerance, including root traits has been reported (Sakai et al. 2018; Xie et al. 2017; Xie et al. 2015); however, variation in RHL has not been comprehensively examined until now.
In the present study, we evaluated genetic variation in RHL in spelt and bread wheat strains, including modern varieties and landraces, to identify the useful germplasms for the improvement of RHL. In addition, we examined rhizosheath size and P uptake ability in high P-fixing soils with extremely low P availability. Furthermore, we performed a QTL analysis using backcrossed inbred lines derived from a cross between a Spanish spelt strain with long RHL and an elite bread wheat variety with short RHL. We report here that spelt wheat, especially the strains in Spain, are unique germplasms that provide a useful allele for increasing RHL in bread wheat breeding programs.
Materials and methods
Plant materials
The genetic variation in RHL was examined in 117 spring wheat strains (Table S1). We used forty-one spelt strains (including two macha wheat strains) and 60 strains of bread wheat landraces, originating from diverse regions. Sixteen modern varieties, defined as varieties developed by cross breeding, were also used. Spelt strains were provided by the National BioResource Project-Wheat, with support, in part, from the National BioResource Project of the Ministry of Education, Culture, Sports, Science & Technology (MEXT), Japan. Landrace strains were derived from the genetic resources preserved in the Laboratory of Plant Genetics and Breeding in Okayama University, Okayama, Japan. All strains were grown for seed propagation in a vinyl greenhouse at Obihiro University, Obihiro, Japan, and used for the following experiments.
A population of backcross inbred lines (BILs) were developed from the cross between ‘Harukirari’ and ‘KU-1025’. ‘Harukirari’ is a spring wheat variety, bred in 2007 at the Central Agricultural Research Station in Hokkaido, Japan. ‘KU-1025’ is a spelt strain collected from the Asturias region in Spain. The F1 plants were developed from the cross between ‘Harukirari’ and ‘KU-1025’ and B1F1 plants were developed from the ‘Harukirari’ (female parent) × F1 plants (male parent) cross. One hundred and twenty BILs of B1F8 or later generations were developed by the single-seed descent method from B1F1 plants and used for map construction and QTL analysis.
Evaluation of RHL by hydroponic culture
The genetic variation in RHL in 117 strains was evaluated in hydroponic culture. Seeds were germinated on a wetted filter paper in a petri dish at 10 °C for 3 days and then 15 °C for one to two days. When the primary seminal root grew about 2 to 4 cm, it was measured and the root tips of the secondary seminal roots were cut off. Then, seedlings were transplanted on a plastic plate (31.0 × 23.5 cm), in which 128 small holes were made, floating on 6 L of nutrient solution in a plastic container (31.5 × 24.0 × 13 cm). Half strength Hoagland solution (Hoagland and Arnon 1950) was used as a nutrient solution, in which P concentration was adjusted to 0, 10, or 100 μM depending on the experiments. pH was adjusted to 5.5 at the beginning of the experiment and when the nutrient solution was refreshed. Plants were grown at a constant temperature (18 °C, 14 h day/10 h night) in a growth chamber (LH-410S, NK System, Osaka, Japan). The solution was aerated and refreshed once on the second day. On the fourth day, the length of the primary seminal root was measured again. Then, the primary seminal root was sampled and stored in 70% ethanol until RHL measurement. The root hair was stained using a 0.05% solution of Toluidine blue O (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) and an image of root segments (approximately 1.5 cm) was captured using digital camera (DP21, OLYMPUS, Tokyo, Japan) attached to a stereoscopic microscope (SZX7, OLYMPUS, Tokyo, Japan). Images showed the maximum RHL in the middle of each root sample where root hair was fully developed. For each root segment, three points were selected where root hairs tended to be uniform length and the length of one root hair at each point (three root hairs per sample) was measured using imaging software (cellSens version 1.11, OLYMPUS, Tokyo, Japan). The seminal root length (SRL) was calculated as the elongated root length (final SRL - initial SRL).
A full set of strains comprising 117 strains (one plant for each strain) was grown in a plastic container for an evaluation of genetic variation. Similarly, 119 BILs (excluding one strain because of low germination rate) and two parental strains (one plant for BILs and four plants for each parental strain) were grown in a plastic container for QTL analysis of RHL and SRL. In total, six replicates (six plastic containers) were performed in each experiment.
Evaluation of the rhizosheath size and P uptake ability in Andisols
The rhizosheath size and P uptake ability were examined with pot experiments. Soil was collected from the experimental field at Obihiro University of Agriculture and Veterinary Medicine, Obihiro, Hokkaido, Japan, where cultivation by plough and harrow have been performed only to test machines, but no crop has been cultivated and no fertilizer has been applied for at least 30 years. The soil is classified as Andisols (Soil Survey Staff 2014) derived from volcanic ash and rich in amorphous clay minerals, such as allophane and imogolite. The soil had an extremely high ability to fix P and make P unavailable to crops through the specific adsorption of phosphate ions on colloidal surfaces (Wada 1989). Available P content evaluated by the Truog method (Truog 1930) was 14.4 mg-P2O5/kg, which was considerably lower than growers’ field levels in the same area, ranging from 72 to 611 with an average of 230 mg-P2O5/kg (Gondwe et al. 2017). This soil was selected to demonstrate crop response to P fertilization, since soil P availability is much lower than other soils in the world.
The rhizosheath size was measured based on the methods of Delhaize et al. (2012) with modifications. Eleven strains including six spelt strains (‘KU-1025’, ‘K152’, ‘st. Rumania’, ‘K15013’, ‘K19092A’, and ‘K6535’), one landrace strain (‘Chinese Spring’), and four modern varieties (‘Harukirari’, ‘Haruyokoi’, ‘Norin 61’, and ‘Zenkoujikomugi’) were used for the experiment. Soil collected from the field described above was sieved through 2 mm mesh. 450 g of soil was moistened to 60% maximum water capacity and filled in a pot to a bulk density of approximately 1.1 g cm−3 (9 cm in diameter and approximately 400 ml in volume) without fertilization. Seeds were germinated on filter paper in a petri dish at 3 °C for one day and 15 °C for one to two days. The three germinated seeds were transplanted in a pot. Three replicates (pots) for each line (9 plants for each line) were grown at constant temperature (25 °C, 14 h day/10 h night) in a growth chamber (LH-410S, NK System, Osaka, Japan). Pots were arranged in a plastic box covered by transparent plastic sheets and the humidity was kept above 80% to retain soil moisture. Water (approximately 6 ml) was provided every day to moisten the soil surface. After three days, one seminal root per plant was sampled without disturbing the rhizosheath. After root length was measured, the rhizosheath was dried at 80 °C for three days and then weighed to minimize differences in soil moisture content and root biomass. The rhizosheath size was expressed as g m−1. For the measurement of RHL in soil, rhizosheath samples of ‘Harukirari’ and ‘KU-1025’ were stored in 50% ethanol until use. Soils adhered with root hairs were carefully removed using a soft brush and the root hair was stained using 0.05% Toluidine blue solution (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan). Root segment images were captured where root hair showed the maximum length for each sample, using the same methods as those of the hydroponic culture experiment.
The P uptake ability was compared among eight strains including three spelt strains (‘KU-1025’, ‘st. Rumania’, and ‘K6535‘), one landrace strain (‘Chinese Spring’), and four modern varieties (‘Harukirari’, ‘Haruyokoi’, ‘Norin 61′, and ‘Zenkoujikomugi’). Soils of approximately 2.5 kg moistened to 60% maximum water capacity were mixed with vermiculite (soil:vermiculite = 5:1) and filled in a pot (18 cm in diameter and 3 L in volume). Three levels of phosphate fertilizer, 127 mg P2O5/pot (low P fertilizer level), 254 mg P2O5/pot (middle P fertilizer level), and 382 mg P2O5/pot (high P fertilizer level) were applied corresponding to 50, 100, and 150 kg-P2O5/ha, respectively. Other nutrients (254 mg N/pot, 254 mg K2O/pot, and 153 mg MgO/pot, corresponding to 100 kg N/ha, 100 kg K2O/ha, and 60 kg MgO/ha, respectively) were applied at the same rate for all treatments. Seeds were germinated on filter paper in a petri dish at 3 °C for one day and 15 °C for one to two days. The three germinated seeds were transplanted in a pot on 28 April 2011. Four replicates (pots) for each line were grown in a vinyl greenhouse in Obihiro, Hokkaido, Japan. Shoots and roots from each pot were separately harvested 40 days after sowing and weighed after drying at 80 °C for two days. Total P content in shoots and roots was measured by sulphuric acid digestion with hydrogen peroxide and followed by the molybdate blue method (Murphy and Riley 1962) to determine P concentration in the acid digested samples. P uptake ability was compared among strains (varieties) by evaluating three traits (biomass, P concentration and P content in shoot, root and total plant) and their relative values to high P fertilizer level.
Molecular marker analysis and linkage map construction
Total DNA from each BIL was extracted from young leaves as described by Sakai et al. (2018). For the analysis of molecular markers, amplification by polymerase chain reaction (PCR) was performed using GoTaq® DNA polymerase (Promega Corporation, Madison, WI, USA) or PrimeSTAR® GXL DNA polymerase (Takara Bio Inc., Shiga, Japan) or KOD FX and KOD FX Neo DNA polymerase (Toyobo Co., Ltd., Osaka, Japan), according to manufacturer protocols. PCR products were visualised using electrophoresis in 1 to 4% agarose gels or 10% acrylamide gels (acrylamide:N,N′-methylenebisacrylamide = 29:1). After staining with ethidium bromide, genotypes were evaluated based on the size of the PCR product.
One hundred and eighty-six simple sequence repeat (SSR) markers, originally reported in Somers et al. (2004) and GrainGenes (https://wheat.pw.usda.gov/GG3/), were used to construct the linkage map in BILs. In addition, two gene specific markers (Q and Rht-B1 genes) were added. The Q locus on chromosome 5A is a major gene for threshability and spike morphology. Bread wheat has the Q allele conferring the free-threshing trait and spike morphology of the square head type, whereas spelt wheat has the q allele conferring the non-free threshing trait and lax spike morphology (speltoid type) (Simons et al. 2006). The gene-specific markers of the Q locus developed by Asakura et al. (2009), that could distinguish the Q and q alleles, were used for map construction. ‘Harukirari’ is a semi-dwarf variety carrying the Rht-B1b (semi-dwarf) allele on chromosome 4B, whereas spelt wheat ‘KU-1025’ carries no semi-dwarf alleles. The marker of the Rht-B1 locus (Ellis et al. 2002) was used for map construction. In total, the genotypes of 120 BILs were determined using 188 markers (186 SSR markers and two gene specific markers) and the linkage map was constructed using JoinMap® 4 (van Ooijen 2006).
QTL analysis and statistical analysis
The multiple QTL model (MQM) mapping method was used for QTL analysis using MapQTL® 6 (van Ooijen 2002). First, interval mapping was performed and the putative QTLs with logarithm of the odds (LOD) greater than 2.5 were identified. Then, one marker was selected as a cofactor that showed the highest LOD peak at each putative QTL detected by interval mapping and MQM mapping was performed. If a new set of cofactors was selected based on the results of the first round of MQM mapping, then, MQM mapping was repeated. A set of cofactors was finally determined after multiple rounds of analysis. The permutation test was conducted in MapQTL® 6 to determine the genome-wide LOD threshold at the 5% level for each trait (Churchill and Doerge, 1994).
Broad-sense heritability (h2) in hexaploid wheat strains or BILs was estimated as the ratio of the genetic variance component (VG) to the total phenotypic variance (VP). VG and VP were calculated from the results of one-way analysis of variance (ANOVA) as described in Sokal and Rohlf (1995). All statistical analysis was performed using PASW® statistics 18 (SPSS Inc., Chicago, IL, USA).
Results
Genetic variation in RHL in spelt and bread wheat
The genetic variation in RHL was evaluated by hydroponic culture. First, to determine the effects of P levels on variation in RHL, three spelt strains (‘st. Rumania’, ‘K6535’, and ‘KU-1025’) and three bread wheat strains or varieties (‘Chinese Spring’, ‘Haruyokoi’, and ‘Harukirari’) were evaluated under three P levels (0 μM, 10 μM, and 100 μM). RHL in all strains tended to increase as P levels decreased (Fig. S1). Significant genetic variation was found in all conditions and broad-sense heritability at 10 μM (87.5%, p < 0.01) was slightly higher than at 0 μM (81.5%, p < 0.01) and 100 μM (80.9%, p < 0.01). P concentration in soil solution was usually lower than 10 μM (Bieleski 1973). Therefore, the genetic variation in RHL in hexaploid wheat was evaluated at P levels of 10 μM.
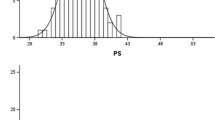
Across 117 strains, we observed a large genetic variation in RHL ranging from 200 to 600 μm (Fig. 1). The spelt wheat and landraces of bread wheat had a wider range of variation than the modern varieties, in which several strains had RHL longer than 400 μm. In contrast, modern bread wheat varieties tended to have shorter RHL of less than 400 μm. No apparent relationships between the variation in RHL and the origin of strains were found, except in Spanish strains of spelt wheat, which had specifically longer RHL in observed hexaploid strains (Fig. 1 and S2). RHL was negatively correlated with SRL (Fig. 2; r = −0.73, p < 0.001). Spanish spelt strains had shorter SRL with longer RHL. In contrast, modern bread wheat varieties had longer SRL with shorter RHL.
Genetic variation in root hair length (RHL) in 117 strains in hexaploid wheat. Based on the mean value of each line (six replicates), the number of lines is represented incrementally in each RHL category by 20 μm. Arrow indicates mean value of each group. Different letters indicate the significant difference between groups (Tukey-Kramer test, P < 0.05). h2: broad-sense heritability. *** shows significance at the 0.1% level
Relationships between RHL in hydroponic culture and the rhizosheath size and P uptake in soil condition
To verify the relationships between RHL in hydroponic culture and those in soil condition, the rhizosheath size was evaluated 11 strains including six spelt strains (‘KU-1025’, ‘K152’, ‘st. Rumania’, ‘K15013’, ‘K19092A’, and ‘K6535’), one landrace strain (‘Chinese Spring’), and four modern varieties (‘Harukirari’, ‘Haruyokoi’, ‘Norin 61’, and ‘Zenkoujikomugi’). Rhizosheath size exhibited a significant positive correlation with RHL in hydroponic culture (r = 0.80, p < 0.01, Fig. 3). A Spanish spelt strain (‘KU-1025’) with long RHL in hydroponic culture and large rhizosheath size exhibited longer RHL in soils than a modern variety (‘Harukirari’) with a short RHL in hydroponic culture and small rhizosheath size (Fig. S3). Accurate RHL measurement in the soil (Andisols) used in this experiment would be difficult because root hairs were remarkably wavy and might be damaged during removing soil adhered tightly to them (Fig. S3).
P uptake ability was examined under three P fertilizer levels for eight strains showing diverse RHL, including three spelt (‘KU-1025’, ‘st Rumania’, and ‘K6535’), one landrace of bread wheat (‘Chinese Spring’) and four modern varieties (‘Harukirari’, ‘Haruyokoi’, ‘Norin 61’, and ‘Zenkouzikomugi’). P uptake ability was evaluated for traits including biomass, P concentration, and P content in shoot, root and total plant and their relative values to the high P fertilizer level (Table S2). The contribution of RHL to P uptake ability was assessed as the correlation coefficients of RHL with all traits examined (Table S2). The highest positive correlation was observed in total P content at the high P fertilizer level (Table S2). Although no significant correlation with total P content was found at low and middle P fertilizer levels (Fig. 4a and b), the P content of each strain and the correlation coefficient with RHL gradually increased with an increase in P fertilizer levels and a significant positive correlation (r = 0.82, p < 0.05) was observed only at the high P fertilizer level (Fig. 4c). In this condition, a Spanish spelt strain (‘KU-1025’) showed the highest P content among eight strains (Fig. 4c). At the high P fertilizer level, the correlation of RHL and P content was observed only in root (r = 0.78, p < 0.05) but not in shoot (0.45, p = 0.26) (Table S2). All other traits were not significantly correlated with RHL, except the root biomass (r = 0.77, p < 0.05) and the relative P concentration at the low P fertilizer level (r = −0.77, p < 0.05) (Table S2).
Correlation between root hair length (RHL) in hydroponic culture and total P content of plants grown under three P fertilizer levels in the pot experiment. (a), (b), and (c) indicate correlations with P content under low, middle, and high P fertilizer levels, respectively. * shows significance at the 5% level. ns indicates no significance
QTL analysis for RHL and SRL in BILs
To identify QTLs responsible for RHL, we selected a modern bread wheat variety with short RHL (‘Harukirari’) and a Spanish spelt strain with longer RHL (‘KU-1025’) (Fig. 2 and 4a) and developed BILs from the cross between them. A wide range in variation of RHL (220 to 600 μm) was observed in BILs, in which conspicuous transgressive segregation toward shorter RHL than ‘Harukirari’ was observed (Fig. 5b). SRL also showed continuous distribution in BILs (Fig. S4). A significant negative correlation (r = −0.29, p < 0.01) was observed between RHL and SRL (Fig. 6), while the correlation coefficient was lower than that observed in the natural variation of the 117 hexaploid wheat strains (r = −0.73, Fig. 2).
Segregation of root hair length (RHL) in BILs. (a) Root hairs of parental strains and two BILs showing contrasting RHL. (b) Frequency distribution of RHL in BILs. Based on the mean value of each line (six replicates), the number of lines is represented incrementally in each RHL category by 20 μm. h2: broad-sense heritability. *** shows significance at the 0.1% level
A linkage map was constructed using 188 molecular markers including the Q and Rht-B1 loci (Fig. S5) and MQM mapping for RHL was performed. Two QTLs were identified on chromosomes 2A (QRhl.obu-2A) and 6B (QRhl.obu-6B). QRhl.obu-2A had a large effect (LOD = 6.6, Phenotypic variance explained (PVE) = 20.6%) and conferred longer RHL associated with an allele from spelt strain, ‘KU-1025’ (Table 1). In contrast, QRhl.obu-6B conferred longer RHL associated with the ‘Harukirari’ allele with small effect (LOD = 2.7, PVE = 7.7%), whereas it was not significant (p = 0.90) (Table 1). To examine the interaction of QRhl.obu-2A and QRhl.obu-6B, BILs were classified into four classes of allele combinations based on the flanking marker genotypes of two QTLs (Fig. S6). Results of the two-way ANOVA for RHL showed that the main effects of both QRhl.obu-2A (p < 0.001) and QRhl.obu-6B (p < 0.05) were significant whereas the effect of QRhl.obu-6B was small. QRhl.obu-2A × QRhl.obu-6B was not significant, indicating that QRhl.obu-2A and QRhl.obu-6B affected RHL additively. Regarding SRL, three significant QTLs (QSrl.obu-4A, QSrl.obu-5A, and QSrl.obu-7A) were detected on chromosomes 4A, 5A, and 7A (Table 1), differing from those for RHL.
Possible candidate gene for QRhl.obu-2A
Han et al. (2016) reported that TaRSL4-2AS genes, an orthologue of AtRSL4 that is a key regulator controlling root hair elongation in Arabidopsis (Yi et al. 2010), was located on chromosome 2A of wheat. To compare the location of QRhl.obu-2A and TaRSL4-2AS, the TaRSL4-2AS cDNA sequence reported by Han et al. (2016) (KX619657.1) was subjected to a BLAST search against the International Wheat Genome Sequencing Consortium (IWGSC) RefSeq v1.0 reference genome (International Wheat Genome Sequencing Consortium 2018). Results indicated that the location of TaRSL4-2AS was close to the marker Xgwm448 (= WMS448), and the physical distance was 7.9 Mbp (Fig. 7b). MQM mapping for RHL in BILs found the maximum LOD values on the marker Xgwm448 (Fig. 7a). These results indicated that TaRSL4-2AS could be a possible candidate gene of QRhl.obu-2A.
Comparison between the location of QRhl.obu-2A and that of the TaRSL4-2AS gene on the chromosome 2A. (a) Results of MQM mapping for root hair length (RHL) in BILs. (b) Physical map of the TaRSL4-2AS gene and SSR markers based on the reference genome sequence (Wheat Genome Sequencing Consortium RefSeq v1.0; International Wheat Genome Sequencing Consortium 2018)
Discussion
Varieties with improved P uptake ability and P use efficiency are urgently required, because the limitation in availability of P fertilizer could be a serious risk for the stability of wheat production in the near future (Heuer et al. 2017; Lambers et al. 2006). Increased RHL is considered the most effective method to improve P uptake ability, since the carbon cost for the formation of root hair could be lower than the formation of other components of the root system (Brown et al. 2013). Identification of useful germplasms and their responsible genetic loci (or QTLs) should accelerate breeding for the P uptake ability (Wang et al. 2018).
Relevance of RHL measurement in hydroponic culture
RHL measurement directly in soil can be difficult depending on the soil type. Root hair adheres tightly to soils and the development of root hairs is greatly affected by various environmental factors, including nutritional levels, toxic ions such as Al3+, soil strength, and soil water availability (Delhaize et al. 2012; Haling et al. 2013). Alternative methods include measuring RHL in artificial conditions, in which roots are grown on agar or paper or in hydroponic culture. In this study, RHL in hydroponic culture revealed a large genetic variation in hexaploid wheat strains. However, RHL tended to be shorter than that observed by other methods (e.g. RHL of ‘Chinese Spring’ was 293.4 μm in this study but more than 2.5 mm on agar at similar young seedling stages (Horn et al. 2016). In addition, the same strains or varieties showed variable RHL in different experimental conditions of this study (e.g. ‘Harukirari’ was 259 μm (Fig. 2) and 394 μm (Fig. 6), ‘KU-1025’ was 401 μm (Fig. 2) and 601 μm (Fig. 6). These differences may be owing to uncontrolled environmental factors such as dissolved oxygen concentration; however, heritability was relatively high in each experiment (more than 40% in Figs. 1 and 5).
Root hair plays an important role in the formation of the rhizosheath, which comprises the soil that adheres around the root. Rhizosheath size has been frequently used as an indicator for RHL when examining the variation in and genetics of RHL (Delhaize et al. 2012; Delhaize et al. 2015). Positive correlations between RHL and rhizosheath size are reported in various plant species, including wheat (Delhaize et al. 2012; Delhaize et al. 2015); however, they were poorly correlated in barley (George et al. 2014). The present results indicate that RHL in hydroponic culture is positively correlated with rhizosheath size in soils. This evidence suggests that RHL measured in hydroponic culture could be reflective of that in soils (Andisols). Thus, RHL measurement in hydroponic culture is relevant to the survey of genetic diversity and QTL analysis in this study.
Spanish spelt strains are unique germplasms for improvement of RHL
Spelt wheat has long been suggested to have higher adaptabilities under unfavourable environments, such as infertile soil condition and cold climate (Campbell 1997; Miedaner and Longin, 2016); however, the scientific evidence is still scarce. Our results indicate that spelt strains tend to have longer RHL in hexaploid germplasms. Interestingly, the group of strains originating in Spain tended to exhibit extremely long RHL and most of them were collected from the Asturias region, one of the last regions where spelt cultivation had been continued until the end of the last century (Caballero et al. 2006). Spanish spelt strains were suggested to have a different phylogenetic origin from spelt strains from other areas (Elía et al. 2004; Guzman et al. 2012), which is consistent with our results of genetic variation in RHL. Spelt was frequently grown with a traditional cultivation system in areas where modern bread wheat varieties were hardly grown (Caballero et al. 2006; Campbell 1997). Thus, a different history of natural and/or artificial selection from bread wheat may have allowed the accumulation of unique genes in spelt with adaptations to agroecosystems, such as in the Asturias region.
P uptake ability is related to various developmental, physiological and molecular mechanisms and the genetic variation of P uptake ability should result in differences in traits, such as biomass production, P concentration, and P content (Bilal et al. 2018; de Souza Campos et al. 2019). Our results demonstrate that RHL in hydroponic culture is positively correlated with total P content in plants in the pot experiment, indicating that long RHL could allow plants to uptake P efficiently, as previously suggested (Gahoonia et al. 1997; Miguel et al. 2015; Nestler and Wissuwa 2016; Vandamme et al. 2013). However, a lower correlation coefficient was found at lower P fertilizer levels, which was inconsistent with the result that RHL could contribute to enhanced P uptake, especially under low available P conditions in wheat (Gahoonia et al. 1997; Liu et al. 2017). The soil used in this experiment was a high P-fixing soil (Andisols) with extremely low available P content (14.4 mg-P2O5/kg). No fertilizer was applied to this soil for at least 30 years as described in the Materials and Methods section. The high P fertilizer level used in this experiment was 150 kg-P2O5/ha. However, according to the fertilization guideline distributed by our local government (Department of Agriculture, Hokkaido 2015), the application rate of P fertilizer for spring wheat grown on Andisols should be 225 kg-P2O5/ha or more, when the available P content of soil is less than 50 mg-P2O5/kg. This implies that the P availability in our pot experiments was still relatively low even for the high P fertilizer level described above. Therefore, the available P content might be too low to make contact with root hairs at lower fertilizer levels and root traits other than RHL, such as the size of root system (Wang et al. 2016) and uptake ability from sparingly soluble P (Pearse et al. 2007), might be important to increase P uptake. At the high P fertilizer level, the Spanish spelt strain ‘KU-1025’ had the highest P uptake among the eight strains examined. Interestingly, RHL at the high P fertilizer level was significantly correlated with root biomass and root P content, but not shoot biomass and shoot P content. This suggests that strains with a high P uptake ability, such as ‘KU-1025’, have increased P uptake accompanied with the promoted allocation of biomass and P to roots, as shown in the P efficient genotype reported in de Souza Campos et al. (2019). These finding indicate that Spanish spelt strains could be useful germplasms for the enhancement of P uptake ability accompanied by improved RHL.
Useful allele identified from Spanish spelt wheat at QRhl.obu-2A for improvement of RHL
Genetic control of natural variation in RHL and rhizosheath size has been examined in Arabidopsis (Stetter et al. 2015), maize (Zhu et al. 2005), common bean (Yan et al. 2004). and barley (George et al. 2014) and were recently reported in wheat using QTL analysis (Delhaize et al. 2015; Horn et al. 2016) and mapping with aneuploid lines (Liu et al. 2017). However, identification of useful genes or QTLs for crop breeding is still insufficient because of genetic complexities of root hair related traits and the difficulties of its measurement as mentioned earlier. The present study identified a QTL on the chromosome 2A (QRhl.obu-2A) with a large effect (LOD = 6.6, PVE = 20.6%). Although Horn et al. (2016) reported a QTL for RHL at a similar location on chromosome 2A, the effect of QTL was smaller (LOD score was 1.9) than QRhl.obu-2A. Identification of useful alleles with large effects should make it possible to conduct marker-assisted selection, which would accelerate the breeding of P uptake ability in wheat. Horn et al. (2016) also reported a QTL for RHL on chromosome 6A, which might be the homologous region of QRhl.obu-6B detected on chromosome 6B in this study. ‘Harukirari’, despite having the overall shorter RHL, has a positive allele at QRhl.obu-6B which could be used to further improve RHL along with the major locus at QRhl.obu-2A provided by ‘KU-1025’.
Molecular mechanisms for root hair development have been intensively examined focusing on the model plant Arabidopsis, which illustrate the gene and hormonal networks during root hair development (Cui et al. 2018). Root hair cell development processes are regulated by three groups of genes involved in cell fate determination, root hair tip initiation, and hair-cell-tip growth (Cui et al. 2018). Transcription factor AtRSL4 acts as a key regulator of root hair elongation, in which AtRSL4 binds directly to root hair specific genes and regulates their expression (Cui et al. 2018; Han et al. 2016). A recent analysis revealed that TaRSL4, an orthologue of AtRSL4, played a significant role in increasing RHL during allopolyploidization in wheat evolution (Han et al. 2016). The upregulation of TaRSL4, especially TaRSL4-2AS on the chromosome 2A, is related to increased RHL in hexaploid wheat compared with diploid wheat (Han et al. 2016). However, no significant correlation was found between the expression of TaRSL4 and RHL within hexaploid wheat accessions despite the positive correlation in diploid and tetraploid wheats (Han et al. 2016). The present study indicates QRhl.obu-2A co-locates with the TaRSL4-2AS gene, suggesting that the TaRSL4-2AS gene was one of the possible candidate genes for QRhl.obu-2A and might be responsible for diversification within hexaploid wheat after allopolyploidization. Further analysis for fine mapping of QRhl.obu-2A should be performed to verify this hypothesis.
Unconscious selection for RHL in modern agriculture
In landraces and modern varieties of bread wheat, no apparent relationships between variation in RHL and origin of strains were observed. Remarkably, modern bread wheat varieties tended to have shorter RHL in contrast to landrace strains exhibiting a wide range of variation, suggesting the possibility that RHL has been negatively selected in modern breeding. After the “Green Revolution”, high yielding varieties have been developed for intensive agricultural systems, especially in developed countries, with high inputs of chemical fertilizer, by introducing the semi-dwarf genes, Rht-B1b and Rht-D1b. In spite of no artificial selection in root traits, the selection for yield and yield related traits under high input soil condition might cause unconscious selection for reducing root biomass (Voss-Fels et al. 2017; Waines and Ehdaie 2007). Delhaize et al. (2015) indicated that the semi-dwarf allele at the Rht-D1 locus decreased rhizosheath size but no effects were detected on the Rht-B1 locus. This was consistent with our result in which a semi-dwarf allele on the Rht-B1 locus carried by the modern variety ‘Harukirari’ had no effect on RHL.
Unconscious negative selection for long RHL implies that longer RHL might have some cost for increasing yield. Recently, the cost-benefit of developing long root hair has been evaluated using transgenic plants of Brachypodium with the enhanced expression of endogenous BdRSL2 and BdRSL3 (Zhang et al. 2018). This indicates that the longer RHL was accompanied by a pleiotropic effect to decrease plant biomass. In this study, RHL had a strong negative correlation with SRL in natural variations of hexaploid wheat; however, our QTL analysis showed that RHL and SRL had independent genetic control, at least in the early seedling stage. In contrast, increased shoot biomass was observed at the early seedling stage under nutrient-poor conditions by the overexpression of TaRSL4 (Han et al. 2016). Furthermore, Horn et al. (2016) reported that a QTL for RHL on chromosome 6A was co-located with a QTL for thousand-grain weight in the same mapping population. The development of a near isogenic line of QRhl.obu-2A is now in progress for testing whether QRhl.obu-2A is useful for breeding to improve P uptake ability without negative pleiotropic effects on other agronomic traits.
Abbreviations
- P:
-
phosphorus
- RHL:
-
root hair length
- QTL:
-
quantitative trait loci
- SNP:
-
single nucleotide polymorphism
- BIL:
-
backcross inbred line
- SRL:
-
seminal root length
- PCR:
-
polymerase chain reaction
- SSR:
-
simple sequence repeat
- MQM:
-
multiple QTL model
- LOD:
-
logarithm of the odds
- PVE:
-
phenotypic variance explained
- ANOVA:
-
analysis of variance
References
Asakura N, Mori N, Nakamura C, Ohtsuka I (2009) Genotyping of the Q locus in wheat by a simple PCR-RFLP method. Genes Genet Syst 84:233–237
Bieleski RL (1973) Phosphate pools, phosphate transport, and phosphate availability. Annu Rev Plant Physiol 24:225–252. https://doi.org/10.1146/annurev.pp.24.060173.001301
Bilal HM, Aziz T, Maqsood MA, Farooq M, Yan G (2018) Categorization of wheat genotypes for phosphorus efficiency. PLoS One 13:e0205471. https://doi.org/10.1371/journal.pone.0205471
Brown LK, George TS, Dupuy LX, White PJ (2013) A conceptual model of root hair ideotypes for future agricultural environments: what combination of traits should be targeted to cope with limited P availability? Ann Bot 112:317–330. https://doi.org/10.1093/aob/mcs231
Caballero L, Martín LM, Alvarez JB (2006) Agrobiodiversity of hulled wheats in Asturias (north of Spain). Genet Resour Crop Ev 54:267–277. https://doi.org/10.1007/s10722-005-4049-8
Campbell KG (1997) Spelt: agronomy, genetics, and breeding. Plant Breed Rev 15:187–213. https://doi.org/10.1002/9780470650097.ch6
Cui S, Suzaki T, Tominaga-Wada R, Yoshida S (2018) Regulation and functional diversification of root hairs. Semin Cell Dev Biol 83:115–122. https://doi.org/10.1016/j.semcdb.2017.10.003
Delhaize E, James RA, Ryan PR (2012) Aluminium tolerance of root hairs underlies genotypic differences in rhizosheath size of wheat (Triticum aestivum) grown on acid soil. New Phytol 195:609–619. https://doi.org/10.1111/j.1469-8137.2012.04183.x
Delhaize E, Rathjen TM, Cavanagh CR (2015) The genetics of rhizosheath size in a multiparent mapping population of wheat. J Exp Bot 66:4527–4536. https://doi.org/10.1093/jxb/erv223
Department of Agriculture, Hokkaido (2015) Hokkaido Fertiliser recommendations 2015. Department of Agriculture, Hokkaido
de Souza Campos PM, Cornejo P, Rial C, Borie F, Varela RM, Seguel A, Lopez-Raez JA (2019) Phosphate acquisition efficiency in wheat is related to root:shoot ratio, strigolactone levels, and PHO2 regulation. J Exp Bot 70:5631–5642. https://doi.org/10.1093/jxb/erz349
Dolan L (2017) Root hair development in grasses and cereals (Poaceae). Curr Opin Genet Dev 45:76–81. https://doi.org/10.1016/j.gde.2017.03.009
Elía M, Moralejo M, Rodríguez-Quijano M, Molina-Cano JL (2004) Spanish spelt: a separate gene pool within the spelt germplasm. Plant Breed 123:297–299. https://doi.org/10.1111/j.1439-0523.2004.00969.x
Ellis H, Spielmeyer W, Gale R, Rebetzke J, Richards A (2002) "perfect" markers for the Rht-B1b and Rht-D1b dwarfing genes in wheat. Theor Appl Genet 105:1038–1042. https://doi.org/10.1007/s00122-002-1048-4
Gahoonia TS, Care D, Nielsen NE (1997) Root hairs and phosphorus acquisition of wheat and barley cultivars. Plant Soil 191:181–188. https://doi.org/10.1023/a:1004270201418
George TS, Brown LK, Ramsay L, White PJ, Newton AC, Bengough AG, Russell J, Thomas WT (2014) Understanding the genetic control and physiological traits associated with rhizosheath production by barley (Hordeum vulgare). New Phytol 203:195–205. https://doi.org/10.1111/nph.12786
Gondwe RL, Kinoshita R, Sano M, Suminoe T, Aiuchi D, Koaze H, Palta J, Tani M (2017) Lack of yield response in potato (Solanum tuberosum L.) to phosphate fertilizer under contrasting soil types varying in phosphate absorption coefficient and available phosphate. Soil Sci Plant Nutr 63:171–177. https://doi.org/10.1080/00380768.2017.1282300
Guzman C, Caballero L, Martin LM, Alvarez JB (2012) Waxy genes from spelt wheat: new alleles for modern wheat breeding and new phylogenetic inferences about the origin of this species. Ann Bot 110:1161–1171. https://doi.org/10.1093/aob/mcs201
Haling RE, Brown LK, Bengough AG, Young IM, Hallett PD, White PJ, George TS (2013) Root hairs improve root penetration, root-soil contact, and phosphorus acquisition in soils of different strength. J Exp Bot 64:3711–3721. https://doi.org/10.1093/jxb/ert200
Han Y, Xin M, Huang K, Xu Y, Liu Z, Hu Z, Yao Y, Peng H, Ni Z, Sun Q (2016) Altered expression of TaRSL4 gene by genome interplay shapes root hair length in allopolyploid wheat. New Phytol 209:721–732. https://doi.org/10.1111/nph.13615
Heuer S, Gaxiola R, Schilling R, Herrera-Estrella L, Lopez-Arredondo D, Wissuwa M, Delhaize E, Rouached H (2017) Improving phosphorus use efficiency: a complex trait with emerging opportunities. Plant J 90:868–885. https://doi.org/10.1111/tpj.13423
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. California agricultural Experimental Station circular 347. University of California, Berkeley
Horn R, Wingen LU, Snape JW, Dolan L (2016) Mapping of quantitative trait loci for root hair length in wheat identifies loci that co-locate with loci for yield components. J Exp Bot 67:4535–4543. https://doi.org/10.1093/jxb/erw228
International Wheat Genome Sequencing Consortium (2018) Shifting the limits in wheat research and breeding using a fully annotated reference genome. Science 361:eaar7191. https://doi.org/10.1126/science.aar7191
Khabarov N, Obersteiner M (2017) Global phosphorus fertilizer market and national policies: a case study revisiting the 2008 price peak. Front Nutr 4:22. https://doi.org/10.3389/fnut.2017.00022
Kippes N, Debernardi JM, Vasquez-Gross HA, Akpinar BA, Budak H, Kato K, Chao S, Akhunov E, Dubcovsky J (2015) Identification of the VERNALIZATION 4 gene reveals the origin of spring growth habit in ancient wheats from South Asia. Proc Natl Acad Sci U S A 112:E5401–E5410. https://doi.org/10.1073/pnas.1514883112
Lambers H, Shane MW, Cramer MD, Pearse SJ, Veneklaas EJ (2006) Root structure and functioning for efficient acquisition of phosphorus: matching morphological and physiological traits. Ann Bot 98:693–713. https://doi.org/10.1093/aob/mcl114
Liu M, Rathjen T, Weligama K, Forrest K, Hayden M, Delhaize E (2017) Analysis of aneuploid lines of bread wheat to map chromosomal locations of genes controlling root hair length. Ann Bot 119:1333–1341. https://doi.org/10.1093/aob/mcx030
Longin CFH, Wurschum T (2016) Back to the future - tapping into ancient grains for food diversity. Trends Plant Sci 21:731–737. https://doi.org/10.1016/j.tplants.2016.05.005
Matsuoka Y (2011) Evolution of polyploid Triticum wheats under cultivation: the role of domestication, natural hybridization and allopolyploid speciation in their diversification. Plant Cell Physiol 52:750–764. https://doi.org/10.1093/pcp/pcr018
Mew MC (2016) Phosphate rock costs, prices and resources interaction. Sci Total Environ 542:1008–1012. https://doi.org/10.1016/j.scitotenv.2015.08.045
Miguel MA, Postma JA, Lynch JP (2015) Phene synergism between root hair length and basal root growth angle for phosphorus acquisition. Plant Physiol 167:1430–1439. https://doi.org/10.1104/pp.15.00145
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36. https://doi.org/10.1016/S0003-2670(00)88444-5
Nestler J, Wissuwa M (2016) Superior root hair formation confers root efficiency in some, but not all, rice genotypes upon P deficiency. Front Plant Sci 7:1935. https://doi.org/10.3389/fpls.2016.01935
Pearse SJ, Veneklaas EJ, Cawthray G, Bolland MD, Lambers H (2007) Carboxylate composition of root exudates does not relate consistently to a crop species' ability to use phosphorus from aluminium, iron or calcium phosphate sources. New Phytol 173:181–190. https://doi.org/10.1111/j.1469-8137.2006.01897.x
Reif JC, Zhang P, Dreisigacker S, Warburton ML, van Ginkel M, Hoisington D, Bohn M, Melchinger AE (2005) Wheat genetic diversity trends during domestication and breeding. Theor Appl Genet 110:859–864. https://doi.org/10.1007/s00122-004-1881-8
Sakai Y, Cao L, Funata R, Shiraishi T, Yoshikawa K, Maeno K, Miura H, Onishi K (2018) QTLs for agronomic traits detected in recombinant inbred lines derived from a bread wheat x spelt cross. Breed Sci 68:587–595. https://doi.org/10.1270/jsbbs.18046
Simons KJ, Fellers JP, Trick HN, Zhang Z, Tai YS, Gill BS, Faris JD (2006) Molecular characterization of the major wheat domestication gene Q. Genetics 172:547–555. https://doi.org/10.1534/genetics.105.044727
Soil Survey Staff (2014) Key to soil taxonomy, Twelfth edn. USDA/NRCS, Washington D.C
Sokal RR, Rohlf FJ (1995) Biometry: the principles and practice of statistics in biological research, 3rd edn. WH Freeman and Company, New York
Somers DJ, Isaac P, Edwards K (2004) A high-density microsatellite consensus map for bread wheat (Triticum aestivum L.). Theor Appl Genet 109:1105–1114. https://doi.org/10.1007/s00122-004-1740-7
Stetter MG, Schmid K, Ludewig U (2015) Uncovering genes and ploidy involved in the high diversity in root hair density, length and response to local scarce phosphate in Arabidopsis thaliana. PLoS One 10:e0120604. https://doi.org/10.1371/journal.pone.0120604
Takenaka S, Nitta M, Nasuda S (2019) Population structure and association analyses of the core collection of hexaploid accessions conserved ex situ in the Japanese gene bank NBRP-wheat. Genes Genet Syst 93:237–254. https://doi.org/10.1266/ggs.18-00041
Tester M, Langridge P (2010) Breeding technologies to increase crop production in a changing world. Science 327:818–822. https://doi.org/10.1126/science.1183700
Truog E (1930) The determination of the readily available phosphorus of soils. J Am Soc Agro 22:874–882
van Ooijen JW (2002) MapQTL ® 6, software for the mapping of quantitative trait loci in experimental populations of diploid species. Kyazma B.V, Wageningen
van Ooijen JW (2006) JoinMap ® 4, software for the calculation of genetic linkage maps in experimental population. Kyazma B.V, Wageningen
Vandamme E, Renkens M, Pypers P, Smolders E, Vanlauwe B, Merckx R (2013) Root hairs explain P uptake efficiency of soybean genotypes grown in a P-deficient Ferralsol. Plant Soil 369:269–282. https://doi.org/10.1007/s11104-012-1571-2
Voss-Fels KP, Qian L, Parra-Londono S, Uptmoor R, Frisch M, Keeble-Gagnere G, Appels R, Snowdon RJ (2017) Linkage drag constrains the roots of modern wheat. Plant Cell Environ 40:717–725. https://doi.org/10.1111/pce.12888
Wada K (1989) Allophane and Imogolite. In: Dixon JB, Weed SB (eds) Minerals in soil environments. SSSA, Madison, pp 1051–1087
Waines JG, Ehdaie B (2007) Domestication and crop physiology: roots of green-revolution wheat. Ann Bot 100:991–998. https://doi.org/10.1093/aob/mcm180
Wang W, Ding G-D, White PJ, Wang X-H, Jin K-M, Xu F-S, Shi L (2018) Mapping and cloning of quantitative trait loci for phosphorus efficiency in crops: opportunities and challenges. Plant Soil 439:91–112. https://doi.org/10.1007/s11104-018-3706-6
Wang Y, Thorup-Kristensen K, Jensen LS, Magid J (2016) Vigorous root growth is a better indicator of early nutrient uptake than root hair traits in spring wheat grown under low fertility. Front Plant Sci 7:865. https://doi.org/10.3389/fpls.2016.00865
Xie Q, Fernando KMC, Mayes S, Sparkes DL (2017) Identifying seedling root architectural traits associated with yield and yield components in wheat. Ann Bot 119:1115–1129. https://doi.org/10.1093/aob/mcx001
Xie Q, Mayes S, Sparkes DL (2015) Spelt as a genetic resource for yield component improvement in bread wheat. Crop Sci 55:2753–2765. https://doi.org/10.2135/cropsci2014.12.0842
Yan X, Liao H, Beebe SE, Blair MW, Lynch JP (2004) QTL mapping of root hair and acid exudation traits and their relationship to phosphorus uptake in common bean. Plant Soil 265:17–29. https://doi.org/10.1007/s11104-005-0693-1
Yi K, Menand B, Bell E, Dolan L (2010) A basic helix-loop-helix transcription factor controls cell growth and size in root hairs. Nat Genet 42:264–267. https://doi.org/10.1038/ng.529
Zhang C, Simpson RJ, Kim CM, Warthmann N, Delhaize E, Dolan L, Byrne ME, Wu Y, Ryan PR (2018) Do longer root hairs improve phosphorus uptake? Testing the hypothesis with transgenic Brachypodium distachyon lines overexpressing endogenous RSL genes. New Phytol 217:1654–1666. https://doi.org/10.1111/nph.14980
Zhu J, Kaeppler SM, Lynch JP (2005) Mapping of QTL controlling root hair length in maize (Zea mays L.) under phosphorus deficiency. Plant Soil 270:299–310. https://doi.org/10.1007/s11104-004-1697-y
Acknowledgements
We thank Ayumi Kadota, Ayaka Kinosita, and Mayumi Tokui for their assistance. We also thank Dr. Atsushi Torada, Dr. Kiyoaki Kato and two anonymous reviewers for their valuable comments on earlier version of this manuscript. This research was supported by the research program (Agro-Eco project), at the Obihiro University, Japan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Anton Wasson.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 261 kb)
ESM 2
(PDF 22 kb)
Fig. S1
Response of RHL under three P levels (0, 10 and 100 μM) in hydroponic culture. Different letters indicate the significant difference between treatments in each strain (Tukey-Kramer test, P < 0.05). (PDF 171 kb)
Fig. S2
Relationships between variation in root hair length (RHL) and the origin of 117 strains in hexaploid wheat. Red circles: Spelt wheat, Open circles: Landraces of bread wheat, Green circles: Modern varieties of bread wheat. Number of strains evaluated was listed above the figure. * indicated macha wheat strain. (PDF 409 kb)
Fig. S3
Comparison of root hair traits between ‘Harukirari’ (A) and ‘KU-1025’ (B) grown in soils (Andisols). (PDF 185 kb)
Fig. S4
Frequency distribution of seminal root length (SRL) in BILs. Based on the mean value of each line (six replicates), the number of lines is represented incrementally in each SRL category by 0.5 cm. h2: broad-sense heritability. *** shows significance at the 0.1% level. (PDF 10 kb)
Fig. S5
A linkage map constructed using 188 molecular markers in BILs. (PDF 54 kb)
Fig. S6
Interaction between two QTLs for root hair length (RHL) on chromosomes 2A (QRhl.obu-2A) and 6B (QRhl.obu-6B). (A) Comparison of RHL among four classes of allele combinations of two QTLs. KU and Haru indicate ‘KU-1025’ and ‘Harukirari’ alleles, respectively. Alleles of QTLs were estimated by flanking markers (Xgwm448 for QRhl.obu-2A and Xgwm88 for QRhl.obu-6B). Error bars indicate standard deviation and the number of strains of each class is shown in parentheses. Different letters on bars indicate the significant differences among classes by Tukey-Kramer test (P < 0.05). (B) The result of two-way ANOVA. *** and * indicate significance at the 0.1 and 5% levels, respectively. ns indicates no significance. (PDF 134 kb)
Rights and permissions
About this article
Cite this article
Okano, N., Goto, R., Kato, T. et al. Spanish spelt is unique germplasm for improvement of root hair length in hexaploid wheat. Plant Soil 452, 171–184 (2020). https://doi.org/10.1007/s11104-020-04555-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-020-04555-8