Abstract
Background
Decreased blood perfusion at an intestinal anastomosis may contribute to postoperative anastomotic leak (AL) resulting in substantial morbidity and mortality. Near-infrared (NIR) laparoscopy in conjunction with indocyanine green (ICG) allows for visualization of the microcirculation before formation of the anastomosis, thereby allowing the surgeon to choose the point of transection at an optimally perfused area.
Methods
This is a retrospective case-control analysis examining the effectiveness of NIR + ICG in reducing the rate of AL after low anterior resection (LAR) for rectal cancer. Records of patients undergoing robot-assisted LAR for rectal cancer with and without ICG were analyzed for the years 2011 and 2012.
Results
Among the 40 patients who underwent robotic LAR, NIR + ICG was used in 16 cases (41 %). Male patients accounted for the majority of cases in both groups (74 %). The median level of the anastomosis was 3.5 cm in the NIR + ICG group and 5.5 cm in the control group. There was no difference in the use of diverting ileostomy. In 3 patients (19 %), the use of NIR + ICG resulted in revision of the proximal bowel (colonic) transection point before formation of the anastomosis. The distal transection point was never revised. The rate of AL in the NIR + ICG group was 6 % versus 18 % in control group.
Conclusions
ICG fluorescence may play a role in anastomotic tissue perfusion assessment and affect the AL rate. Larger prospective studies are needed to further validate this novel technology.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
In the past two decades, long-term outcomes for rectal cancer have improved as a result of advances in surgical techniques and the use of neoadjuvant and adjuvant therapies. Abdominoperineal resection, which was once considered the gold standard for rectal cancer treatment, has been largely replaced with sphincter-sparing surgery [1]. Better understanding of the biology of rectal cancer and mastering total mesorectal excision (TME) as a surgical technique for mid to low rectal cancers have improved oncological outcomes [1–5]. Novel technological advances and improved surgical skills have allowed surgeons to perform low and ultralow anastomosis instead of a permanent colostomy [1, 6]. Despite these surgical advances, postoperative mortality rates are still reported at about 1–8 % [2, 7]. Symptomatic anastomotic leak after low anterior resection (LAR) has been reported to occur in 1–39 % of patients with an associated risk of mortality of 2–24 % [1, 2, 6, 8–10].
Anastomotic dehiscence is one of the most dreaded complications after LAR, ultimately leading to increased length of stay, higher cost, and higher local recurrence and mortality rates [11, 12]. Independent risk factors for leak include male gender, level of anastomosis (<5 cm), preoperative radiation, and the presence of intraoperative adverse events [2, 6, 12–14]. This complication can lead to frequent reoperation and multiple drainage procedures in 88–95 % of cases [6, 11, 15–17]. Although the cause of anastomotic leak is multifactorial, it can be argued that perfusion abnormalities and technical factors play a substantial role in the development of anastomotic failures [11, 18–21].
Intestinal perfusion at the time of anastomotic formation may be evaluated with indocyanine green (ICG) and a near-infrared (NIR) laparoscopic systems capable of visualizing inducible fluorescence. NIR laparoscopy in conjunction with ICG allows for visualization of the microcirculation before formation of anastomosis, which allows the surgeon to choose the point of division at an ideally perfused area, possibly optimizing tissue perfusion at the anastomosis. This study aims to evaluate the effect of ICG fluorescence on bowel transection point selection and the anastomotic leak rates after LAR.
Materials and methods
This is a retrospective case-control review of rectal cancer cases treated surgically via robot-assisted low and ultralow anterior resection, as well intersphincteric resection (ISR) between 2011 and 2012. All patients provided informed consent, and institutional review board approval was obtained. Cases performed in 2011 and 2012 without the use of ICG were used as controls. The use of ICG fluorescence was at the discretion of the attending surgeon. Two patients with permanent colostomy were excluded. The control group contained 22 total patients, and the NIR + ICG group contained 16 patients. All patients underwent robot-assisted LAR by three experienced colorectal surgeons. Patient demographics were collected and included age, gender, body mass index, American Society of Anesthesiologist class, preoperative stage, preoperative chemotherapy and radiotherapy, and comorbidities including anemia, diabetes, chronic kidney disease, hyperlipidemia, hypothyroidism, cardiac disease, pulmonary disease, and history of smoking or alcohol. Intraoperative factors including operative time, estimated blood loss (EBL), intraoperative occurrences, and level of anastomosis were collected. Postoperative complications including anastomotic leak, bleeding, urinary tract infection, urinary retention, ileus, sepsis, cardiac occurrences, and wound infection were collected. Anastomotic leak was defined as any disruption of the anastomosis occurring within 60 days of surgery as visualized by contrast enema study or endoscopy. Reoperation and readmission rates were analyzed.
Surgical technique
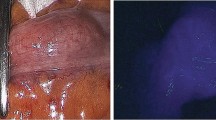
The surgical technique of robot-assisted LAR and ultralow LAR + ISR was carried out in the fashion described by Pigazzi et al. [22, 23]. The procedure was conducted by laparoscopic medial to lateral dissection with the division of the inferior mesenteric artery and vein. The four-arm da Vinci robot was then utilized for the total mesorectal dissection to the pelvic floor. This was followed by the division of the rectum based on tumor location and division of the mesentery of the descending or sigmoid colon either intra- or extracorporeally. The optimal point of transection was then marked by the surgeon under white (visible) light (Fig. 1, video 1) followed by intravenous injection of 6–8 mg of ICG. The bowel was then visualized via NIR laparoscopy, and the surgeon decided whether to revise the point of transection of either the proximal or distal bowel on the basis of the ICG perfusion assessment (Fig. 2, video 1). The bowel was then divided, and an end-to-end or colonic J pouch anastomosis was created. An air leak test was performed via flexible sigmoidoscopy, which also allowed for the assessment of the integrity of the anastomosis and inspection of the mucosa using white (visible) light.
NIR camera system
The NIR camera system is provided by multiple manufacturers including Olympus Corporation (Tokyo, Japan), Karl Storz GmbH (Tuttlingen, Germany), Stryker Corporation (Portage, MI, USA), and Novadaq Technologies (Ontario, Canada). The first three systems use modifications of current endoscopic systems, while Novadaq uses an entirely new device [24]. The da Vinci Si Surgical System (Intuitive Surgical, Sunnyvale, CA, USA) incorporates a fluorescence-capable da Vinci Si HD vision system (Firefly). The latter was used during the robot-assisted proctectomies described above. All mentioned devices can be used for standard laparoscopic visible imaging mode and can be switched to NIR fluorescence mode by means of button control on the camera head, on the stack console, or via foot pedal [24].
Indocyanine green
ICG is a sterile, water-soluble, tricarbocyanine compound that absorbs NIR light with a peak spectral absorption at 800 nm. ICG is injected intravenously, and it rapidly and extensively binds to plasma protein. It remains intravascular, with minimal leakage into the interstitium. It is cleared by the liver in 3–5 min into bile with no known metabolites. It has a maximal daily dose of 2 mg/kg. It is a nontoxic and nonionizing agent; only a single case of sore throat and hot flashes has been reported. There have been rare described cases of anaphylactic shock, hypotension, tachycardia, dyspnea, and urticaria [24–26].
Results
A total of 40 patients underwent robot-assisted LAR or ISR between February 2011 and August 2012. ICG fluorescence was used in 16 of the patients. The average age of patients in ICG + NIR was 58 versus 63 years in the control group (Table 1). Obesity (44 vs. 27 %), hyperlipidemia (13 vs. 9 %), and cardiac disease (19 vs. 9 %) were more prevalent in the ICG + NIR group compared to control group. Diabetes mellitus (18 vs. 0 %), pulmonary disease (23 vs. 6 %), and history of smoking (27 vs. 13 %) was more prevalent in the control group than in the ICG + NIR group (Table 2).
ISR was performed in 13 % of patients in the ICG + NIR group and 9 % of patients in the control group (Table 3). In two patients (one per group), flexible sigmoidoscopy with visualization of the anastomosis at the conclusion of the operation revealed mucosa of questionable viability despite an airtight anastomosis. Flexible sigmoidoscopy revealed slightly dusky mucosa in one case in the ICG + NIR cohort. However, ICG fluorescence had revealed good perfusion to distal and proximal margins, and the anastomosis was thus not revised. This patient did not experience any postoperative complications. Likewise, flexible sigmoidoscopy revealed congested mucosa in one case in the control group; the anastomosis was not revised, and this patient had a postoperative leak.
There were no intraoperative or anesthetic complications. Revision of the point of transection as indicated by bowel perfusion after ICG injection occurred in three patients (19 %). In the control group, revision of the anastomosis as indicated by visual cues of dusky bowel under white (visible) light occurred in one patient (4.5 %) (Table 3). None of the patients who had revision of the transection point or of the anastomosis experienced anastomotic leak.
There was one incidence of a delayed leak in the ICG + NIR in which the patient presented on postoperative day 46 with persistent rectal pain. He underwent transanal drainage of a subcentimeter-size presacral abscess discovered during endoscopy. There was one incidence of reoperation resulting from an iatrogenic colon traction injury resulting in delayed perforation at a site other than the anastomosis causing sepsis in the ICG + NIR group. Postoperative complications are listed in Table 4.
In the control group, postoperative leaks occurred in four patients, two of whom were diverted at the index operation. The two patients who were not diverted required reoperation on postoperative days 1 and 7. Both were found to have a posterior disruption of the anastomosis. The two diverted patients presented 4 weeks after surgery and underwent drainage procedures (Table 5).
Discussion
Anastomotic leak is the most feared complication after LAR. There are multiple patient risk factors, such as gender, age, nutritional status, radiation status, and tumor location, beyond the influence of the surgeon. The two basic considerations for the success of intestinal anastomosis are mechanical integrity and tissue viability. The vascularity of the tissue surrounding the staple line is of fundamental importance to the healing of an anastomosis [11, 18–21, 27]. Therefore, surgical technique and adequate perfusion of the anastomosis are essential in the reduction of the risk of anastomotic leak [12, 28].
Our preliminary results indicated that the use of ICG fluorescence to delineate the perfusion of colorectal anastomosis may result in relatively frequent revision of the bowel transection point. This leads to altering the segment of bowel used, which may ultimately lead to a decreased rate of anastomotic leaks. Randomized controlled studies assessing LAR report an anastomotic leak rate of 2–12 % [29, 30]. In this series, despite the low median level of anastomosis (3.5 vs. 5.5 cm), the use of ICG fluorescence decreased the overall risk of leak by 12 %. Three cases required revision of the point of resection as a result of apparently poor perfusion as dictated by the ICG fluorescence technology. Previous subjective methods of assessing bowel perfusion would not have necessarily influenced the choice of the transection point. An essential observation in this study is that the hypoperfused bowel visualized with NIR mode may appear normal in standard (white) light mode. It can therefore be hypothesized that this technology can improve the naked eye’s ability to detect areas of poor blood supply.
Previous studies have concluded that there is a lack of reliable intraoperative predictive tests for anastomotic leakage by the surgeon [10, 12]. To date, surgeons have been relying on subjective data to assess the integrity and perfusion of colorectal anastomosis. The only tool at a surgeon’s disposal is experience, as well as subjective measures such as active bleeding from resection margin, palpable pulsation in the mesentery, and lack of discoloration [12]. However, this method is highly unreliable and fails to accurately predict postoperative leak [10]. Laser Doppler flowmetry and laser fluorescence angiography have been used as a reliable intraoperative predictive test [19–21, 27, 31, 32]. The NIR fluorescence technology provides a new method that will allow for an accurate evaluation of the perfusion of the proximal and distal margins of resection. ICG fluorescence technology can give objective data as a real-time image, indicating the perfusion status of the intestine with great convenience.
The 3-D optical technology of the robot enhances the operative experience compared to conventional laparoscopic surgery [24]. The evolution of optical data incorporating feed from electromagnetic spectra beyond that of the visible (white) light can in turn potentiate the appreciation of tissue architecture and blood flow. This technology, combined with the intravascular injection of ICG, allows for the acquisition of high-quality images of both the circulatory and lymphatic vasculature. As demonstrated by our findings, these techniques do not increase operative time and can easily be incorporated into clinical practice.
To our knowledge, only one previous study has been published regarding the use of fluorescence angiography in colorectal anastomosis. Kudszus et al. [12] used laser fluorescence angiography with ICG and a laser-mounted scope to visualize the tissue perfusion. This study demonstrated a 60 % reduction in reoperation for anastomotic leak in a retrospective study consisting of 638 patients. The authors concluded that intraoperative visualization of tissue perfusion reduces the rate of severe complications in colorectal surgery. The ICG fluorescence technology has been studied and shown to be beneficial in assessing perfusion and vascular architecture in cardiothoracic, hepatobiliary, foregut, and plastic surgery [32–36].
This study should be viewed with certain limitations. As a retrospective study, inherent biases exist. The decision to use ICG + NIR and diversion was left to the discretion of the attending surgeon, and knowledge of the surgical decision-making process is not available for all cases. The small sample size is also a limitation that decreases the power of this study.
Nevertheless, this study suggests that the ability to visualize perfusion to the anastomosis may reduce the rate of anastomotic leak after LAR. Although one cannot eliminate patient factors that will influence the rate of anastomotic leak, perfusion and technical integrity of the anastomosis depend on the surgeon’s judgment. The elimination of poor perfusion to an anastomosis may decrease the rate of anastomotic leak and thereby improve outcomes of rectal cancer surgery. However, larger randomized prospective studies need to be conducted to further validate this study’s findings.
References
Law WL, Chu KW (2004) Anterior resection for rectal cancer with mesorectal excision: a prospective evaluation of 622 patients. Ann Surg 240(2):260–268
Matthiessen P, Hallböök O, Rutegård J, Simert G, Sjödahl R (2007) Defunctioning stoma reduces symptomatic anastomotic leakage after low anterior resection of the rectum for cancer: a randomized multicenter trial. Ann Surg 246(2):207–214
Quirke P, Dixon MF (1988) The prediction of local recurrence in rectal adenocarcinoma by histopathological examination. Int J Colorectal Dis 3(2):127–131
Quirke P, Durdey P, Dixon MF, Williams NS (1986) Local recurrence of rectal adenocarcinoma due to inadequate surgical resection. Histopathological study of lateral tumour spread and surgical excision. Lancet 2(8514):996–999
Heald RJ, Husband EM, Ryall RD (1982) The mesorectum in rectal cancer surgery: the clue to pelvic recurrence? Br J Surg 69(10):613–616
Montedori A, Cirocchi R, Farinella E, Sciannameo F, Abraha I (2010) Covering ileo- or colostomy in anterior resection for rectal carcinoma. Cochrane Database Syst Rev (5):CD006878
Smedh K, Olsson L, Johansson H, Aberg C, Andersson M (2001) Reduction of postoperative morbidity and mortality in patients with rectal cancer following the introduction of a colorectal unit. Br J Surg 88(2):273–277
Dehni N, Schlegel RD, Cunningham C, Guiguet M, Tiret E, Parc R (1998) Influence of a defunctioning stoma on leakage rates after low colorectal anastomosis and colonic J pouch-anal anastomosis. Br J Surg 85(8):1114–1117
Enker WE, Merchant N, Cohen AM, Lanouette NM, Swallow C, Guillem J, Paty P, Minsky B, Weyrauch K, Quan SH (1999) Safety and efficacy of low anterior resection for rectal cancer: 681 consecutive cases from a specialty service. Ann Surg 230(4):544–552
Karliczek A, Harlaar NJ, Zeebregts CJ, Wiggers T, Baas PC, van Dam GM (2009) Surgeons lack predictive accuracy for anastomotic leakage in gastrointestinal surgery. Int J Colorectal Dis 24(5):569–576
Kingham TP, Pachter HL (2009) Colonic anastomotic leak: risk factors, diagnosis, and treatment. J Am Coll Surg 208(2):269–278
Kudszus S, Roesel C, Schachtrupp A, Höer JJ (2010) Intraoperative laser fluorescence angiography in colorectal surgery: a noninvasive analysis to reduce the rate of anastomotic leakage. Langenbecks Arch Surg 395(8):1025–1030
McArdle CS, McMillan DC, Hole DJ (2005) Impact of anastomotic leakage on long-term survival of patients undergoing curative resection for colorectal cancer. Br J Surg 92(9):1150–1154
Choi HK, Law WL, Ho JW (2006) Leakage after resection and intraperitoneal anastomosis for colorectal malignancy: analysis of risk factors. Dis Colon Rectum 49(11):1719–1725
Bell SW, Walker KG, Rickard MJ, Sinclair G, Dent OF, Chapuis PH, Bokey EL (2003) Anastomotic leakage after curative anterior resection results in a higher prevalence of local recurrence. Br J Surg 90(10):1261–1266
Petersen S, Freitag M, Hellmich G, Ludwig K (1998) Anastomotic leakage: impact on local recurrence and survival in surgery of colorectal cancer. Int J Colorectal Dis 13(4):160–163
Walker KG, Bell SW, Rickard MJ, Mehanna D, Dent OF, Chapuis PH, Bokey E (2004) Anastomotic leakage is predictive of diminished survival after potentially curative resection for colorectal cancer. Ann Surg 240(2):255–259
Boyle NH, Manifold D, Jordan MH, Mason RC (2000) Intraoperative assessment of colonic perfusion using scanning laser Doppler flowmetry during colonic resection. J Am Coll Surg 191(5):504–510
Vignali A, Gianotti L, Braga M, Radaelli G, Malvezzi L, Di Carlo V (2000) Altered microperfusion at the rectal stump is predictive for rectal anastomotic leak. Dis Colon Rectum 43(1):76–82
Sheridan WG, Lowndes RH, Young HL (1987) Tissue oxygen tension as a predictor of colonic anastomotic healing. Dis Colon Rectum 30(11):867–871
Kologlu M, Yorganci K, Renda N, Sayek I (2000) Effect of local and remote ischemia-reperfusion injury on healing of colonic anastomoses. Surgery 128(1):99–104
Pigazzi A, Ellenhorn JD, Ballantyne GH, Paz IB (2006) Robotic-assisted laparoscopic low anterior resection with total mesorectal excision for rectal cancer. Surg Endosc 20(10):1521–1525
Pigazzi A, Luca F, Patriti A, Valvo M, Ceccarelli G, Casciola L, Biffi R, Garcia-Aguilar J, Baek JH (2010) Multicentric study on robotic tumor-specific mesorectal excision for the treatment of rectal cancer. Ann Surg Oncol 17(6):1614–1620
Cahill RA, Ris F, Mortensen NJ (2011) Near-infrared laparoscopy for real-time intra-operative arterial and lymphatic perfusion imaging. Colorectal Dis 13(Suppl 7):12–17
Cahill RA, Mortensen NJ (2010) Intraoperative augmented reality for laparoscopic colorectal surgery by intraoperative near-infrared fluorescence imaging and optical coherence tomography. Minerva Chir 65(4):451–462
Alander JT, Kaartinen I, Laakso A, Pätilä T, Spillmann T, Tuchin VV, Venermo M, Välisuo P (2012) A review of indocyanine green fluorescent imaging in surgery. Int J Biomed Imaging 2012:940585
Chung RS (1987) Blood flow in colonic anastomoses. Effect of stapling and suturing. Ann Surg 206(3):335–339
Foster ME, Brennan SS, Morgan A, Leaper DJ (1985) Colonic ischaemia and anastomotic healing. Eur Surg Res 17(3):133–139
Jayne DG, Guillou PJ, Thorpe H, Quirke P, Copeland J, Smith AM, Heath RM, Brown JM, UK MRC CLASICC Trial Group (2007) Randomized trial of laparoscopic-assisted resection of colorectal carcinoma: 3-year results of the UK MRC CLASICC Trial Group. J Clin Oncol 25(21):3061–3068
Sauer R, Fietkau R, Wittekind C, Rödel C, Martus P, Hohenberger W, Tschmelitsch J, Sabitzer H, Karstens JH, Becker H, Hess C, Raab R, German Rectal Cancer Group (2003) Adjuvant versus neoadjuvant radiochemotherapy for locally advanced rectal cancer: the German trial CAO/ARO/AIO-94. Colorectal Dis 5(5):406–415
Höer J, Töns C, Schachtrupp A, Anurov M, Titkova S, Oettinger A, Wetter O, Schumpelick V (2002) Quantitative evaluation of abdominal wall perfusion after different types of laparotomy closure using laser-fluorescence videography. Hernia 6(1):11–16
Still J, Law E, Dawson J, Bracci S, Island T, Holtz J (1999) Evaluation of the circulation of reconstructive flaps using laser-induced fluorescence of indocyanine green. Ann Plast Surg 42(3):266–274
Waseda K, Ako J, Hasegawa T, Shimada Y, Ikeno F, Ishikawa T, Demura Y, Hatada K, Yock PG, Honda Y, Fitzgerald PJ, Takahashi M (2009) Intraoperative fluorescence imaging system for on-site assessment of off-pump coronary artery bypass graft. JACC Cardiovasc Imaging 2(5):604–612
Shimada Y, Okumura T, Nagata T, Sawada S, Matsui K, Hori R, Yoshioka I, Yoshida T, Osada R, Tsukada K (2011) Usefulness of blood supply visualization by indocyanine green fluorescence for reconstruction during esophagectomy. Esophagus 8(4):259–266
Holm C, Tegeler J, Mayr M, Becker A, Pfeiffer UJ, Mühlbauer W (2002) Monitoring free flaps using laser-induced fluorescence of indocyanine green: a preliminary experience. Microsurgery 22(7):278–287
Hutteman M, van der Vorst JR, Mieog JS, Bonsing BA, Hartgrink HH, Kuppen PJ, Löwik CW, Frangioni JV, van de Velde CJ, Vahrmeijer AL (2011) Near-infrared fluorescence imaging in patients undergoing pancreaticoduodenectomy. Eur Surg Res 47(2):90–97
Disclosures
Dr. Alessio Pigazzi is a consultant for Intuitive Surgical. Dr. Steven Mills has received an Ethicon Educational grant paid to the department. Dr. Michael Stamos has had Ethicon, Olympus, NiTi/NovoGI, Adolor/GSK, and Covidien training support paid to UCI for clinical immersion courses for Lap colectomy. Dr. Joseph Carmichael, Dr. Mehraneh Jafari, Dr. Wissam Halabi, and Dr. Kang Hong Lee have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (MPG 39528 kb)
Rights and permissions
About this article
Cite this article
Jafari, M.D., Lee, K.H., Halabi, W.J. et al. The use of indocyanine green fluorescence to assess anastomotic perfusion during robotic assisted laparoscopic rectal surgery. Surg Endosc 27, 3003–3008 (2013). https://doi.org/10.1007/s00464-013-2832-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-013-2832-8