Abstract
Tylophora indica (Burm. f.) Merrill, an ethno-pharmacologically important perennial climber of Asclepiadaceae, is commonly known as Antamul or Indian ipecac. It is essentially accredited for its medicinal properties owing to its wide range of alkaloids in the form of bioactive secondary metabolites, such as tylophorine, tylophorinine, and tylophorinidine. Accelerated mass propagation of Tylophora is challenging because of its reduced seed germination frequency that consequently headed the pursuit for efficient protocols on in vitro propagation for the large-scale regeneration, conservation as well as sustainable supply of quality propagules. Ample tissue culture-mediated biotechnological investigations have been carried out on this medicinal plant till date and several micropropagation protocols have been standardized as well. The present review compares between several typical methods as well as factors, involving on direct and indirect organogenesis of Tylophora along with various up-to-date and modified techniques such as somatic embryogenesis, protoplast culture, synthetic seed production, genetic transformation, and in vitro interventions for the secondary metabolite production that have been reported in last two decades. This compilation will allow assessing the achievements and trends of Tylophora research so far, as well as will advance the research more rapidly, since many aspects, basic and applied, have yet to be explored.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tylophora [Tylophora indica (Burm. f.) Merrill syn. Tylophora asthmatica (L.F.) Wight & Arn.], an ethno-pharmacologically essential perennial vine, is generally popular as Antamul or Indian ipecac (Faisal et al. 2007). It is an important member of Asclepiadaceae (milkweed family), which has now been fallen into Apocynaceae (Endress and Bruyns 2000). Tylophora, highly recognized predominantly for its therapeutic potential, is a source of secondary metabolites including a wide range of alkaloids. Tylophora roots harbor several bioactive compounds that exhibit a number of antifeedant, bacteriostatic, cathartic, diaphoretic, emetic, expectorant, stimulant, and stomachic properties (Varrier et al. 1994). Moreover, Tylophora root comprises an impending anti-tumor alkaloid tylophorinidine (Mulchandani et al. 1971). Owing to the enormous potential in pharmaceutical industry, this plant population has been over exploited, which is eventually compelling this plant species curving its way towards the list of the threatened species. As a result, it is obligatory to devise methods for the en masse multiplication and improvement of the species for commercial production. On this account, various tissue culture-based biotechnological tools that have contributed significantly for the germplasm maintenance, improvement, and multiplication in past two decades have been discussed comprehensively in this review.
Geographical distribution
Tylophora normally dwells dense patches in planes, hills, and forests. It prefers well-drained soil rich in humus (Rani et al. 2012). It necessitates the support of a host plant to survive on a sunny location, but grows well in plains. It is native to India and resides up to an altitude of 1260 m in the sub-Himalayan tract extending from the west of Uttar Pradesh to Far East of Meghalaya states. It is dispersed throughout the southern and eastern part of India including the states of West Bengal, Odisha, Andhra Pradesh, Tamil Nadu, Karnataka, and Kerala (Joshi 2000). Apart from the plains of India, it is also found in Malay island, Africa, Australia, Ceylon, and Borneo (Fig. 1).
Botanical description
Tylophora is a perennial climber vine and branches profusely (Fig. 2a) and grows up to a height of 1.5 m or more with green shiny 3–10 cm-long and 1.5–7 cm-wide leaves that are obviate-oblong to elliptic-oblong in shape and leathery cordate at base (Kirtikar and Basu 1991). Roots are widespread with longitudinally fissured corky bark. Inflorescence is umbellate cymes and contains small, 5–6 mm flowers, which are greenish-yellow outside and purplish inside, and lobes are oblong and acute. Calyx is divided almost to its base with dense hair and lanceolate segments. Fruit is 10 cm-long and 1–2 cm-wide, striated, and divaricated follicle. Seeds are elongated to 2–2.5 cm long and ovate (Gupta 2003). Flowering and subsequent fruiting generally occur during the months of October to December. The plant flowers abundantly, but fruit setting has hardly been noticed under North Indian climatic state of affairs.
a Actively growing Tylophora indica plantlet in field (bar, 5 cm), b multiple shoot initiation and proliferation from shoot tip explants on MS medium fortified with 8.88 µM BA (bar, 1 cm), c formation of friable calli from leaf explant on MS medium fortified with 13.59 µM 2,4-D (bar, 2 mm), d formation of compact and organogenic calli from leaf explant on MS medium fortified with 4.53 µM 2,4-D plus 2 µM TDZ (bar, 2 mm), e regeneration, multiplication, and elongation of multiple shoots from organogenic calli on MS medium plus 4.5 µM TDZ (bar, 1.5 cm), f cell suspension culture derived from friable calli (bar, 100 µm), g formation of embryoid-like structure from embryogenic calli on MS medium plus 1.5 µM TDZ and 9.06 µM 2,4-D (bar, 1 mm), h synthetics seeds, developed from in vitro regenerated nodal segments using 3% (w/v) sodium alginate and 75 mM calcium chloride, and its regeneration on half-strength liquid MS medium (inset) (bars, 5 mm), and i complete plantlet with shoot and root regenerated in vitro (inset) (bar, 1 cm), primary acclimatization of plantlet (bar, 2 cm) (Source: unpublished data and photos of S. Gantait)
Phytochemistry and therapeutic uses
Tylophora, comprising numerous bioactive compounds such as flavonoids, saponins, alkaloids, and tannins (Rao et al. 1971, Benjamin and Mulchandani 1973), is currently going to be threatened due to its excessive demand for the plant regarding pharmaceutical purposes. Different significant key alkaloids, namely, tylophorine, tylophorinine, tylophorinidine, and tylophorindine, are extracted from the roots and leaves (Kaur and Singh 2012). Tylophorine is chiefly used for curing anti-inflammatory-related problems such as rheumatism, hay fever, bronchial asthma, and bronchitis. Tylophora is universally called ‘asthma herb’ because of several uses as an anti-asthmatic plant (Gupta et al. 2010). Kaur and Singh (2012) demonstrated in a pharmacological experiment that two alkaloids, namely, phenanthroindolizidine (ficuseptine-A) and tylophorine, compelled the suppression of nitric oxide production in RAW264.7 cells by imitating acute inflammation. Moreover, upon ethanol treatment, methanolic leaf extracts displayed superior response by inhibiting various biochemical, physical, and functional modifications on account of ethanol-induced hepatotoxicity. Even for treating cancer, tylophorine has proven to be much effective, since it arrests the cell growth at G1 phase and S phase of cell cycle. Based on different experiments performed on cells of lung carcinoma, it was detected that in HepG2 cells, cAMP’s action as the secondary messenger was hindered and thus incorporated in various innovative drug development methodologies. Two alkaloids belonging to phenanthroindolizidine groups, namely, tylophorinidine and pergularine, have also been shown to possess anti-cancer activity. Alkaloids from leaf extracts of Tylophora also have antibacterial as well as anti-spasmodic characteristics and have been proven from its response against numerous bacterial strains (reviewed by Gupta et al. 2010). The extracted alkaloids, when testified for anti-allergic, immunomodulatory, and diuretic activity, responded positively (Kaur and Singh 2012). Besides alkaloids, various other non-alkaloid compounds have also been obtained either from the leaves or from the roots of the Tylophora plant that includes acetyl-alcohol, β-sitosetrol, pigments, quercetin, sigmasterol, tetratriacontanol, tyloindane, tannins, etc. Mohan et al. (2014) conducted a phyto-pharmacological assessment and deduced that the saponins were considerably effective in curing syphilis and other venereal ailments. This was in agreement with earlier reports (Okwu and Okwu 2004) that proposed that the saponins enclose antibiotic properties. It acts as a mild detergent and also used for staining, since it facilitates the imaging of intracellular proteins. The most essential plant pigment is flavonoid that gives yellow coloration to flower petals. Flavonoids such as kaempferol, obtained from Tylophora, are also distinguished for their use against adjuvant-induced arthritis. In addition, lysosomal enzyme inhibiting activity was also displayed by the flavone fraction extracted from leaves (reviewed by Gupta et al. 2010).
Conventional growing condition, propagation, and its demerits
Tylophora is conventionally propagated by means of seeds. However, the seeds show considerably low rate of germination, even fruiting is rare. Seeds commence germination in around 10 days and complete in 3 weeks. The 3-month-old plantlets can be transplanted in the field, but only if rainy season is available, and distance between the plants should also be kept constant. Vegetative propagules such as stem cuttings can also be employed for clonal propagation. Moist climate is optimum for its growth and it needs annual rainfall of 1000–1500 mm. The plant grows well in shady regions and humus-rich soil; nevertheless, loamy-to-clay soil enriched with farmyard manure, and ambient temperatures are desirable (Rani et al. 2012).
Seed-derived plantlets normally possess unnecessary genetic variation that is detrimental to commercial propagation. Besides, the survival of the plant is threatened on account of the destruction caused due to frequent harvesting of roots as a potential source of drugs. Thus, large-scale demand necessitates rapid multiplication of Tylophora that remains difficult due to its poor vegetative propagation, which eventually led the quest for effective micropropagation protocol for the regeneration in bulk to sustain the supply as and when required.
In vitro regeneration
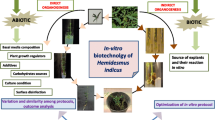
Direct and indirect (via an intermediary callus phase) regenerations are the two efficient methods for in vitro regeneration. Amid these two modes, indirect organogenesis is less followed due to the probability of occurrence of somaclonal variation. Therefore, a direct method of complete plantlet formation is mostly opted for clonal propagation in general (Gantait et al. 2016). Until date, many investigations have been carried out on this medicinal plant and a number of protocols have been standardized (Fig. 3a). Yet, due to limited time and resources, it has become mandatory to relate between several techniques reported and categorize them according to their efficiency, eventually to select the apposite protocol for in vitro regeneration as per requirement. Therefore, the key objective of this review is to compare between established micropropagation methods of Tylophora, such as direct and indirect organogenesis with some new and modified techniques such as synthetic seed production, Agrobacterium-mediated genetic transformation, protoplast culture, etc. This review also explores the usage of several types of physical factors, media compositions, and applications of different key plant growth regulators (PGRs) while exploiting diverse explant sources of Tylophora for its efficient regeneration in either direct or indirect modes (Fig. 3b–d).
Overview on successful reports of in vitro culture of Tylophora indica. a Yearwise relative frequency (number) of in vitro regeneration studies on Tylophora indica as well as a comparison on frequency of reports on direct organogenesis, indirect organogenesis, and somatic embryogenesis-oriented reports, b selected reports showing influence of BA alone on direct shoot regeneration frequency, c selected reports showing influence of IAA alone on root induction frequency, and d selected reports showing influence of 2,4-D alone on callus induction frequency
Source of explants and their reaction in vitro
A wide range of explant sources used for the initial in vitro culture establishment of Tylophora results in substantially variegated growth response. An array of explants was employed for in vitro growth of Tylophora. Shoot, leaf, nodal segments, green and white root segments as well as petiole were used as explants. Amid all the explants used for organogenesis or embryogenesis, it was notably detected and reported that leaf was the most widely used explant source (Jayanthi and Mandal 2001; Singh et al. 2009a, 2010; Anand et al. 2012; Koilpillai 2012; Jahan et al. 2013; Chaturvedi and Chowdhary 2013; Sadguna et al. 2013; Rathod et al. 2014; Sharma et al. 2014) that effectively exhibited adventitious multiple shoots (Kaur et al. 2011a; Nayeem et al. 2014), calli, or somatic embryo formation. Reddy et al. (2010) reported the use of leaves that confirmed even induction of in vitro rooting. Haque and Ghosh (2013) reported the regeneration of shoot primordia through direct organogenesis and formation of nodular meristemoids via indirect organogenesis both from old and young leaves. Furthermore, Anjum et al. (2014) reported the regeneration of organ from leaf segments. Besides using leaves as explants, majority of the other researchers have proposed the use of shoot tips and nodal segments for both direct and indirect methods of regeneration (Faisal et al. 2007; Singh et al. 2009a; Rathore et al. 2010; Rani and Rana 2010; Kaushik et al. 2010; Sellathurai et al. 2013; Rajavel and Stephan 2014a, b). In another report, employing tender stem explant Kaur et al. (2011b) attained the proliferation of green calli and finally completed plantlets through indirect organogenesis. Hence, from the above reported events, it is well understood that leaf, shoot, and nodal segments proved to be versatile as an initial plant material. On the contrary, only a few researchers reported effective plant micropropagation through the use of root segments also. Chaudhuri et al. (2004) used the green and white root segments and effectively attained the regeneration of nodular meristemoids that were later put forth for the induction of somatic embryos. In a considerable fact, it was noticed that during majority of the instances for the production of somatic embryos, leaf explants as well as leaf-derived embryogenic cultures were employed. Conversely, the ultimate outcome of medicinal plant propagation lies in its ability to remain phenotypically persistent so as to sustain the quality and quantity of the preferred secondary metabolite. Hence, selection of explants should be in such a way that it retains the phenotypical consistency from cycle to cycle regeneration. It was interesting to observe and quite contrary to the usual evidences that the plantlets regenerated from root segment-derived calli (Koilpillai 2012) did not display any phenotypic disparity.
Surface disinfection
It is the foremost requirements to surface sterilize all the explant sources so as to eradicate all the possible latent microbial contamination. The type of sterilant and the duration of explants’ exposure to the sterilant are the two crucial factors of disinfection. The explants collected from fields were washed properly for 10–30 min under running tap water followed by treatment with 0.1% (w/v) Bavistin® (fungicide) and 5% Teepol™ (v/v) (mild detergent) solution for 5 min, and again thoroughly washed with sterile distilled water (Faisal et al. 2005; Anand et al. 2012; Kaushik et al. 2010; Rathod et al. 2014). Haque and Ghosh (2013) accounted the use of TWEEN® 20 for 3 min for sterilizing young leaves of Tylophora. Some authors further investigated the usage of 70–90% (v/v) alcohol for 50 s−2 min and found superior results (Singh et al. 2009a, 2010; Mahesh et al. 2011; Rathore et al. 2010). The reports of Manjula et al. (2000) and Faisal et al. (2007) also stated the use of 5% Labolene for 5 min, while the efficacy of 0.01% (w/v) tetracycline for 15–20 min instead of Teepol™ solution was assessed by Rathore et al. (2010). Subsequent step of disinfection was carried out under laminar airflow with 0.1% (w/v) HgCl2 solution for 3 min, and then, the explants were rinsed the explants with autoclaved distilled water for 5 min followed by inoculation in the culture medium.
Basal media
Basal medium in plant tissue culture contains necessary nutrients in the form of inorganic salts, vitamins, and other organic supplements to necessitate the explant for its in vitro growth and development. Majority of the researchers employed Murashige and Skoog (MS) (1962) media for in vitro micropropagation of Tylophora (Manjula et al. 2000; Jayanthi and Mandal 2001; Faisal and Anis 2003, 2010; Chaudhuri et al. 2004; Faisal et al. 2005; Thomas and Philip 2005; Chandrasekhar et al. 2006; Faisal et al. 2007; Singh et al. 2009a, b; Kaushik et al. 2010; Rani and Rana 2010; Rathore et al. 2010; Reddy et al. 2010; Sahai et al. 2010a; b; Devendra et al. 2011; Kaur et al. 2011a, b, c; Mahesh et al. 2011; Anand et al. 2012; Koilpillai 2012; Chaturvedi and Chowdhary 2013; Haque and Ghosh 2013; Jahan et al. 2013; Sadguna et al. 2013; Sellathurai et al. 2013; Anjum et al. 2014; Nayeem et al. 2014; Rathod et al. 2014; Sharma et al. 2014). Reddy et al. (2010) investigated the efficacy of four diverse basal media, namely, MS, Linsmaier and Skoog (LS) (Linsmaier and Skoog 1965), B5 (Gamborg et al. 1968), and L6 (Kumar et al. 1988), and validated that MS media favored the highest rate of shoot bud induction in comparison with the three other media compositions. Moreover, Koilpillai (2012) substantiated the use of B5 media for the development of friable callus from Tylophora leaves used as an explant source. The completion of in vitro regeneration depends on the rooting efficiency of plantlets, and during this analysis, it was reconfirmed that the half-strength MS medium supported superior frequency of rooting rather than full-strength MS. A notable research reported by Rajavel and Stephan (2014a) who compared widely used MS basal media against a Low-Cost (LC) media. In this medium, amongst many significant differences, agar is replaced by tapioca as a solidifying agent and noticed that more number of shoot buds were comparatively induced in the LC media.
Carbohydrates sources
Carbohydrates serve the main source of energy and also act as the osmotic regulator in a culture medium. They regulate both the development and the morphogenesis of plant tissues by modifying the gene expression. In case of Tylophora, most of the reports validate the use of 3% (w/v) sucrose in MS medium primarily for the reason that it assisted easy translocation and absorption of the energy sources that promoted dynamic plant growth (Manjula et al. 2000; Jayanthi and Mandal 2001; Chaudhuri et al. 2004; Chandrasekhar et al. 2006; Faisal et al. 2007; Singh et al. 2009a, b, 2010; Rani and Rana 2010; Devendra et al. 2011; Mahesh et al. 2011; Koilpillai 2012; Chaturvedi and Chowdhary 2013; Haque and Ghosh 2013; Nayeem et al. 2014). Contrastingly, there are few reports that employed only 0.7–2% sucrose (Kaur et al. 2011a, b, c; Anand et al. 2012; Koilpillai 2012). Rajavel and Stephan (2014b) made an extensive study on diverse carbohydrate sources in two different concentrations (2 and 3%). It was observed that 3% sucrose was the most efficient in shoot proliferation followed by white-refined sugar, unrefined brown sugar, and the bottommost results are indicated by jaggery and sugarcane juice. Consequently, white-refined sugar or table sugar can be commended for its minimal cost and efficient alternate for sucrose.
Influence of physical factors
Together with proper basal medium and initial plant material (explant), the environmental condition of the incubation room plays a critical role in the growth and development of in vitro plant cell, tissue, and organ culture. Light, temperature, and relative humidity are the three key physical factors that must be considered during any plant tissue culture experiments. Plants need light mainly for two purposes, one is photosynthesis and the other one is photomorphogenesis. The advancement of plant growth is proportional to the duration (photoperiod) and intensity of light to which the plant material is exposed. In case of Tylophora, the majority of the reports state that the cultures were incubated under 40.5 μmol/m2/s photosynthetic photon flux density (PPFD) or in some cases 50–60 μmol/m2/s (Faisal et al. 2005, 2007; Faisal and Anis 2010; Chandrasekhar et al. 2006; Devendra et al. 2011; Mahesh et al. 2011; Koilpillai 2012; Haque and Ghosh 2013; Nayeem et al. 2014) for a photoperiod of 16 h illumination with cool fluorescent lights. Not only has the duration of light exposure on the plant material, but also the presence and absence of light affected the plant growth. For callus induction (Chaturvedi and Chowdhary 2013) and seed germination, it is a basic requirement that the culture tubes were kept in a dark room or in a place far away from light. As temperature influences the rate of photosynthesis and respiration (rapidly increases with the rise in temperature), maintaining an ambient temperature required for suitable in vitro regeneration is another vital physical factor. The temperature of the growth room was kept at 25 ± 2 °C by the researchers, whose main aim was to induce shoot buds from the plant material (Faisal et al. 2007, 2010; Reddy et al. 2010; Kaur et al. 2011a; Anjum et al. 2014; Nayeem et al. 2014; Rajavel and Stephan 2014a, b; Soni et al. 2015b), as well as callus from explants (Kaushik et al. 2010; Sahai et al. 2010a,b; Kaur et al. 2011b, c; Anand et al. 2012; Sadguna et al. 2013; Rathod et al. 2014; Soni et al. 2015a; Jogdand et al. (2016), or even somatic embryogenesis (Manjula et al. 2000; Chandrasekhar et al. 2006; Sahai et al. 2010a). Multiple shoot regeneration was reported by Rathore et al. (2010) by allowing the nodal shoot segments to grow under a bit higher temperature 27 ± 2 °C. Besides callus induction from node, internode, and leaf segments, Singh et al. (2009a) maintained 26 ± 2 °C temperature with a light intensity of 27 μmol/m2/s which is comparatively lower than the range of light intensity used in multiple shoot regeneration. Therefore, from this, it can be inferred that for callus regeneration, lower light intensity is preferred. Higher relative humidity increases the chances of contamination in the growth room, so it is very important to uphold the rate of relative humidity, also higher humidity deteriorates the growth rate of the sapling due to the malfunctioning of the stomata in the plant cells, and the other reason is hyperhydricity (caused due to excessive hydration). Therefore, as reported by most of the researchers, the physical conditions as maintained by them for the regeneration of Tylophora the general physical condition would be 25 ± 2 °C temperature, 27–40.5 μmol/m2/s of light intensity, a photoperiod of 16 h illuminated with cool fluorescent tubes, and 50–60% relative humidity (Singh et al. 2009a, b; Reddy et al. 2010; Kaushik et al. 2010; Sadguna et al. 2013; Rathod et al. 2014; Rajavel and Stephan 2014a, b). In an interesting experiment carried out by Rathod et al. (2014), the regeneration of multiple shoots from the nodal segments was obtained by maintaining the culture room temperature at 27 ± 2 °C, relative humidity 60%, and light intensity of 30–40 μmol/m2/s for a photoperiod of 12–14 h, and subsequently, roots from those multiple shoots were induced under 26 ± 2 °C temperature and 80% relative humidity. Another significant exception perceived in a work reported by Chaturvedi and Chowdhary (2013) is that the light intensity was as low as 13.5 μmol/m2/s for induction of callus that can refer that the light intensity might be kept around 10–15 μmol/m2/s for the induction of callus.
Influence of plant growth regulators
In plant tissue culture, the term ‘direct organogenesis’ entails the de novo genesis of plant organs such as shoots (adventitious as well as multiple) and roots from different types of plant materials. The complete plantlet redevelopment of Tylophora via the process of direct organogenesis involves three basic steps: (i) shoot bud initiation (Fig. 2b); (ii) shoot elongation as well as multiplication (Fig. 2b); and (iii) rooting of the regenerated shoots (Fig. 2h). In case of regeneration via indirect organogenesis, the basic steps are: (i) callus induction (Fig. 2c); (ii) embryogenic/organogenic callus proliferation (Fig. 2d); and (iii) regeneration of callus into multiple shoots and roots (Fig. 2e). Through a different mode of regeneration, friable callus might result into cell suspension culture (Fig. 2f) and the embryogenic callus might develop somatic embryos (Fig. 2g). In the course of in vitro regeneration of Tylophora, PGRs play the key role in determining the mode of regeneration (reviewed by Teixeira da Silva and Jha 2016). The interplay between PGRs and critical environmental condition of the growth room defines the success of in vitro propagation. Auxins, cytokinins, and gibberellin A3 (GA3) are the three types of key PGRs. Auxin stimulates cell elongation by instigating the expression of certain genes that are involved with the cell elongation factors (elastin) that loosen the cell walls. Cytokinins are largely related to cytokinesis in plant shoots and roots. The parenchyma cells that are present in the explant only elongate but do not divide when cultured with cytokinins alone, but when cultured with auxin, the cells elongate as well as divide. If more cytokinin is present in the basal media, then shoot buds are initiated, and if auxin is present in much higher amount than cytokinin, then root initiation takes place from the explant. GA3 are mainly associated with cell elongation seed germination by breaking seed’s dormancy.
Multiple shoot formation
Quite often, variations during the selection of PGRs have controlled the success of in vitro direct induction of adventitious or multiple shoots of Tylophora. A very narrow range of PGRs, including 2,4-dichlorophenoxyacetic acid (2,4-D), N6-benzyladenine (BA), α-naphthalene acetic acid (NAA), GA3, have been used for this purpose, but in almost all the cases, BA alone or in seldom combination with either 2,4-D or NAA or GA3 efficiently initiated multiple shoots from explants under study. The trend of most successful concentrations of BA for multiple shoot culture of Tylophora is highlighted in Fig. 3a. Chaudhuri et al. (2004) used root segment explants, where nodular meristemoids were induced with the involvement of 26.8 µM BA, and by further dwindling the concentration to 10.72 µM, BA shoots were originated. On the basis of these successful findings, other researchers displayed their interest in this method and experimented with various explants using various concentrations of PGR but preferably BA (Haque and Ghosh 2013; Reddy et al. 2010; Rathore et al. 2010; Kaur et al. 2011a, b, c; Fig. 3b). Faisal et al. (2007) and Faisal and Anis (2010) attained the regeneration of multiple numbers of shoots from nodular segments by supplementing the MS medium with BA in addition to NAA. Rani and Rana (2010) used GA3 in combination with BA to get higher number and elongation of multiple shoots. Their report suggests the usage of cytokinin (BA) along with appropriate concentrations of GA3 not only ensured the formation of shoot buds without callus formation, but also faster bud break rate and shoot elongation due to its stimulatory effect. Hence, it is well understood that GA3 can be employed for shoot regeneration from nodal segments containing axillary buds that naturally remain dormant. On the other hand, Kaur et al. (2011b) developed an efficient protocol for the regeneration of nodular meristemoids from suitable stem explants when cultured on an MS medium containing solely 8.8 µM BA, and later, those meristemoids were cultured on the same medium through the supplementation of 2.25 µM thidiazuron (TDZ) and other additive that initiated the growth of adventitious shoot buds. According to Nayeem et al. (2014), it was further demonstrated that a diverse range of PGR concentrations used for the growth and proliferation of plantlets and the lone presence of 2,4-D displayed no effect on the plants and repressed organogenesis. However, on the same year, using shoot node as an explant Rajavel and Stephan (2014a) inveterate the usage of 2,4-D for shoot propagation along with 0.5 mg/l NAA without any undesirable callus formation. Generation of adventitious shoot buds directly from the explants was unquestionably a more advantageous process than callus-mediated organogenesis, because it assists in the generation of more true-to-type plants, which maintained genetic homogeneity and this was well ahead confirmed by Sharma et al. (2014) using cluster analysis by un-weighted pair group method with arithmetic averaging (UPGMA) method that there was significant dissimilarity in the genotypes between the in vitro saplings from callus-mediated organogenesis and the in vivo plantlets that were developed directly from the mother plant.
Callus induction
Plant cells that proliferate in an unorganized manner on a semi-solid gel substrate and turn into amorphous mass of tissue are termed ‘callus’. This occurs in vitro when the explant is treated with appropriate levels of PGRs and favorable environmental conditions. The inoculated explants may either produce friable callus that is optimum for single cell culture or else compact callus that supports somatic embryogenesis and subsequent initiation of shoot buds. A significant transformation in the approach of indirect organogenesis of Tylophora was reported by Jayanthi and Mandal (2001). They established embryogenic callus by supplementing the MS basal media with 2 mg/l 2,4-D and 0.5 mg/l 6-furfurylaminopurine (kinetin), and from that, somatic embryos were induced subsequently and turned into plantlets later on. Two synthetic auxins, i.e. 2,4-D and 2,4,5-trichlorophenoxyacetic acid (2,4,5-T), were observed to act commendably (especially 2,4-D; Fig. 3d) and proved to be the most effective PGRs to proliferate callogenesis mostly when used individually (Jayanthi and Mandal 2001; Faisal and Anis 2003; Gupta et al. 2010; Kaushik et al. 2010; Jahan et al. 2013) and alternatively in combination with BA or kinetin or TDZ (Faisal et al. 2005; Thomas and Philip 2005; Rathinavel and Sellathurai 2010; Patel and Patel 2012; Patel and Nadgauda 2014; Dhokrat et al. 2015; Kakde et al. 2016). Interestingly, Sahai et al. (2010a) induced embryogenic calli from leaf segments employing 5 µM BA along with 2.5 µM TDZ devoid of 2,4-D or 2,4,5-T. Later on, different combinations of PGRs, such as NAA, IBA, indole-3-acetic acid (IAA), kinetin, and BA (Verma et al. 2010; Kaur et al. 2011a, b, c; Mahesh et al. 2011; Anand et al. 2012; Chaturvedi and Chowdhary 2013; Sharma et al. 2014), were tested to improve the callus induction efficiency. Sellathurai et al. (2013) reported the induction of callus using only single cytokinin (BA) proves that callus formation is possible by means of cytokinin alone, yet more exploration needs to be carried out in this area. One very prominent report by Koilpillai (2012) attained friable callus by supplementing the MS media with 2,4-D and BA. Tylophora being a very useful medicinal plant secretes its active ingredient tylophorine in the form of secondary metabolite in the process of callus formation and can be extracted using cell suspension culture method (which has been discussed later in this review).
Induction of somatic embryo
‘Somatic embryogenesis’ is an in vitro technique that involves the regeneration of plantlets from embryos developed through a cluster of somatic cells. Somatic embryogenesis is generally used for large-scale production and genetic transformation. Somatic embryos can be formed from green-compact callus by manipulations in various concentrations of the PGR. Somatic embryogenesis follows two diverse pathways: first is origination of somatic embryos directly from the explant tissue and the second involves the intermediate callus stage. Higher cytokinin level induces somatic embryos formation by preventing the action of the auxins that hinders embryogenesis (Manjula et al. 2000). Manjula et al. (2000) reported the first research work on the formation of somatic embryos. In that tryout, BA alone or together with kinetin was used to induce callus, which revealed all the stages of embryogenesis viz., globular-, heart-, torpedo-, globular-shaped embryos after 60 days. Jayanthi and Mandal (2001) obtained distinct bipolar embryos from leaf-derived callus by means of 2 µM 2-isopentyladenine (2iP) along with 0.1 µM IBA from leaf-derived callus. After this, Chandrasekhar et al. (2006), Sahai et al. (2010a), and Devendra et al. (2011) established successful plantlets developed from somatic embryos employing leaf-derived callus. Chaudhuri et al. (2004) explored a new pathway of developing somatic embryos using green root segments as explant source, and this examination revealed two important factors accountable for somatic embryo formation: the age of the explant which is 24-week-old green root segments and the type of cytokinin used which was BA alone. Chandrasekhar et al. (2006) reported the efficacy of TDZ along with 2,4-D to be the most suitable for somatic embryogenesis, and subsequently, 25% of globular-shaped embryos were converted into cotyledonary stage. In another report (Thomas 2006), the use of 2 µM 2,4-D alone was competent enough to induce as high as 70% somatic embryogenesis from internode-derived callus. From all these investigations that were carried, the researchers would be able to optimize a standard protocol for Tylophora. Yet, the main concern for any medicinal plant propagation lies in maintaining the genetic homogeneity of the regenerated plantlets and should be assessed through biochemical, cytological, as well as genetic parameters.
Root formation
The success behind the effective micropropagation technique lies in the rooting percentage of the freshly produced saplings. This can be achieved using higher concentrations of auxin along with cytokinins. Many researchers applied IAA, IBA, and NAA as the PGRs for rooting (Chaudhuri et al. 2004; Faisal et al. 2007, 2010; Rani and Rana 2010; Rathore et al. 2010; Reddy et al. 2010; Haque and Ghosh 2013; Anjum et al. 2014). In vitro rooting of shoots can be induced even at low concentration of MS media devoid of any PGRs (Kaur et al. 2011a, b, c; Anand et al. 2012). However, the efficiency of rooting increases following the inclusion of auxins (such as IAA or IBA) (Fig. 2i). It was interesting to observe that IBA was used for most of the cases, where there was the necessity of in vitro rooting following callus-mediated shoot formation. On the other hand, IAA was majorly used to induce roots following direct shoot initiation (Fig. 3c). Among the successful reports on in vitro rooting of Tylophora, an interesting and efficient combination of PGRs for rooting was 2.5 mg/l IBA along with 3.0 mg/l BA and 2.5 mg/l NAA supplemented in MS medium that was reported by Nayeem et al. (2014), having 73.3% successful rooting rate. After this above experiment, it might be presumed that for an increased rooting percentage, the use of cytokinins could also be favorable along with the auxins. In spite of the fact that most of the researchers experimented with full-strength MS media, some tried to induce rooting in half-strength MS media and was successful in doing so with about 90% root regeneration percentage (Chaudhuri et al. 2004; Faisal et al. 2005, 2007; Rani and Rana 2010; Kaur et al. 2011a, b, c), even half-strength media was found to be superior over the full-strength one for rooting purposes (Faisal et al. 2005). Hence, it can be deduced that some inherent and external factors might be responsible for root regeneration in addition to external application of auxins (Wilson and van Staden 1990). In case of indirect organogenesis, the use of the same popular PGRs was observed, although in one exceptional case, the use of a cytokinin–Zeatin along with an auxin–(NAA) resulted in 100% root regeneration from the calli, induced from the internodal segments, and this can be considered as an effective rooting protocol for Tylophora (Mahesh et al. 2011). Earlier, Sahai et al. (2010a) reported an interesting fact about the formation of white- (NAA) and green-pigmented roots (IBA) by exploiting 3–4 cm of microshoots as the explant. A superior result was obtained in IBA than NAA as the root initiation was delayed in the latter one proving IBA as more efficient auxin for root initiation. Moreover, the roots displayed deformed growth in the presence of NAA as reported earlier by Chaudhuri et al. (2004).
Influence of additives
Sometimes, apart from the major PGRs, the fortification of additional chemicals as well as organic substances in basal media becomes essential to enhance the in vitro regeneration efficiency. These supplementary chemicals are considered as additives. In fact, supplementation of additives eventually improves the availability of some micronutrients in the media by acting as chelating agents, acts as buffers in any kind of pH variations, or even contributes nutrients directly for efficient regeneration of plants. Activated charcoal, adenine sulphate, casein hydrolysate (CH), coconut milk, and ascorbic acid (AA) are few among the commonly used additives during in vitro culture of Tylophora. In Tylophora, Faisal et al. (2007) used AA (50–500 mg/l) in the MS basal media beside PGRs such as BA and NAA that warranted the highest frequency of healthy shoot formation. Henceforth, the role of AA in plant tissue culture is not only as an anti-oxidant and anti-browning compound, but additionally an agent that enhances cell division and elongation. Interestingly, the regeneration efficiency of multiple shoots was suppressed when the concentration of AA was increased to twofold. This signifies the optimization of concentrations of the additives to ensure favorable results. CH, another additive, is an organic nitrogen supplement comprising a mixture of amino acids. This additive can be used in the basal media that lack sufficient nitrogen. As described by Singh et al. (2009a, 2010), the use of increased concentration of CH activated the callus regeneration frequency and rate of callus growth from nodes, internodes, and leaves of Tylophora. Amid some natural additives, coconut water, tomato extract, banana extract, carrot extract, and papaya extract could be opted for enhanced multiplication of shoots, shoot length, and fresh weight of the shoots (Swamy et al. 2014). Certain organic acids, such as malic acid, glutamine, citric acid, etc., are also used as additives in plant tissue culture media. Even though the optimization of the levels of additives solely depends on plant species and genotypes, yet, it is confirmed that complementing the basal media with little amounts of chemicals or organic supplements can be enrich in vitro growth and morphogenesis to a large extent.
Assessment of clonal fidelity
Clonal fidelity assessment is imperative for large-scale in vitro propagation of any medicinally important plant. Not only for meeting the commercial demand, but also for in vitro conservation of germplasm escaping the detrimental effect of somaclonal variations, post-organogenic evaluation of genetic trueness is indispensable (Gantait et al. 2014a). The somaclonal variation is considered as the usual drawback amongst in vitro regenerated plantlets, which might be owing to gene augmentation, chromosomal aberrations, point mutation, and modification in DNA methylation through in vitro culture (Saker et al. 2000). In this context, the fidelity of clones derived from in vitro cultures is compared with their corresponding mother plants from which they are regenerated, employing PCR-based molecular markers [such as Random Amplified Polymorphic DNA (RAPD), Inter Simple Sequence Repeats (SSR), Amplified Fragment Length Polymorphisms (AFLP), and Simple Sequence Repeats (SSR)] and cytological studies in several other medicinal plants (reviewed by Gantait et al. 2014b). Amid a number of existing PCR-based molecular systems, RAPD is preferred owing to the their simplicity and cost-effectiveness and these markers which amplify diverse sections of the genome permit for improved assay of genetic constancy/variation amid somaclones (Mallón et al. 2010; reviewed by Gantait et al. 2012). On the other hand, ISSRs are DNA fragments of 100–3000 bp located between the adjacent, oppositely oriented microsatellite regions. They are dispersed throughout the genome and vary in the number of repeat units. ISSRs are proved to be significantly reproducible (Gantait et al. 2009, 2010a, b, 2011) for several plants with pharmaceutical importance. Nevertheless, both the RAPD and ISSR systems do not require any prior sequence information and use limited amounts of DNA sample that is quite easy to handle. The first report on the assessment of clonal fidelity using molecular marker-based approach was by Jayanthi and Mandal (2001). They compared 14 in vitro Tylophora regenerants and the mother plant against their mother plant using 20 arbitrary RAPD oligonucleotides. Amplified products were monomorphic among all the plants ascertaining the genetic integrity and true-to-type nature of the regenerants. Chaturvedi et al. (2012), for the first time in Tylophora, employed both RAPD and ISSR markers to achieve a comprehensive status of in vitro regenerated clones. In their study, all the bands amplified through RAPD were monomorphic, and hence, it was proved that the regenerants are true-to-type within themselves as well as to the mother plant. Contrarily, during ISSR assay, banding pattern revealed 45.71% polymorphism in the regenarants in comparison with their mother plant. Haque and Ghosh (2013) examined the clonal fidelity of micropropagated Tylophora plants employing cytological assay as well as using RAPD marker analysis. They observed that all the micropropagated plants have shown normal diploid 2n = 22 chromosomes, the same as that of the mother plant. Whereas, in the RAPD marker analysis, a total of 86 bands amplified through 20 out of 24 primers revealed that all the regenerants are genetically true without detecting any somaclonal variants. In the same year, Pathak et al. (2013) reported the detection of somaclonal variation in Tylophora when they employed ten random decamer primers that were used, and out of them, nine primers gave reproducible results. Total 58 amplified products were observed and the size of these amplified products ranges from 50 to 1200 bp. An average polymorphism was found to be 62.07%. On the contrary, Roychowdhury et al. (2015a, b) found no variation in banding pattern in RAPD profiles of Ri-transformed hairy root lines of Tylophora. This was corroborated with an earlier report, where Sharma et al. (2014) used six ISSR primers to validate the clonal fidelity and a sum of 71 reproducible bands was produced. The arrangement of banding of individual primer was alike and analogous to mother plant revealing around 93% homology through UPGMA. ISSR analysis confirmed the genetic stability of in vitro-raised plants. Nevertheless, from the above literatures, it can be concluded that instead of using single marker system, a combination of two or more marker system would be apposite towards ascertaining the clonal fidelity.
Synthetic seed production
Synthetic seed technology is an advanced and growing field of plant biotechnological research. It has become an attractive approach in the present era owing to its extensive use in en masse multiplication, short-term conservation, and transportation of germplasm of endangered as well as commercially significant species (Gantait et al. 2015a). The technology includes the employment of any meristematic tissue of the plant materials such as shoot tips, nodes, or somatic embryos, thus decreasing the dependence on the conventional propagation or micropropagation. The procedure uses synthetic gelling matrix to encapsulate plant organs (Fig. 2h) and has been recognized as a fairly competent system chiefly for plants with pharmaceutical importance (Gantait et al. 2015b). Chandrasekhar et al. (2006) published the first report on Tylophora synthetic seed development via 2% (w/v) sodium alginate encapsulation with six weeks of storage efficiency. Faisal and Anis (2007) reported the same in Tylophora in 2007 from nodal segment explant. They optimized the levels of complexing agent and gel matrix using 3% (w/v) sodium alginate and 100 mM CaCl2·2H2O to ascertain a maximum germination frequency of 91%. The substrate for conversion was MS medium supplemented with 0.5 μM BA and 0.5 μM NAA. They further were successful to store the synthetic seeds in 4 °C up to 8 weeks. Later on, Devendra et al. (2011) reported the synthetic seed production using somatic embryos directly induced from leaf explants. As per their report, 3% sodium alginate and 50 mM CaCl2·2H2O resulted in consistent bead formation with a germination frequency of 22.4% in half-MS medium that was not promising in terms of large-scale synthetic seed production. However, during the storage of synthetic seeds, 4 °C was better than 22 °C as ambient temperature. They have reported the maximum of 60 days of storage with 15% post-storage survival on a mixture of soil, peat, and perlite. Recently, Gantait et al. (2017) standardized the formation of synthetic seed employing nodal segments and its short-term storage as well as post-storage conversion of Tylophora. They found a 93.3% conversion frequency of synthetic seeds prepared with 75 mM CaCl2·2H2O plus 3% (w/v) sodium alginate. Furthermore, the earliest conversion of synthetic seeds was occurred in half-strength liquid MS medium. The synthetic seeds were then stored in three different temperature regimes [5(±1) °C, 15(±1) °C, and 25(±1) °C], and attained the highest conversion frequency (90%) at 15(±1) °C after 15 day storage. However, 70% conversion frequency was recorded after 30 days of storage at 15(±1) °C without further decline even following 45 day storage, which justifies that the lower temperature 15(±1) °C is optimum for storage and subsequent conversion of Tylophora synthetic seeds.
Agrobacterium-mediated genetic transformation
Biotechnological modification through transgenic method could be sensible for the enhancement of pharmaceutically important medicinal plants in terms of its commercial exploitation for biochemical products (Rout et al. 2000). Transfer DNA (T-DNA) from root-inducing (Ri) plasmid of Agrobacterium rhizogenes is employed for the transformation of plant genome (Gelvin 2003). The accelerated growth and genetic uniformity of Ri-mediated transformed root cultures mark its importance in plant tissue culture. Such root cultures accumulate secondary metabolites at a level that is comparably much higher than produced ex vitro (Bhojwani and Dantu 2013). Banerjee et al. (1998) accounted that the ameliorated-secondary metabolite production in hairy root cultures might be due to augmented biosynthetic competency in contrast to non-transformed plants. However, several aspects such as strains of bacteria or target sites for the transfer of T-DNA into the host tissue are responsible for the successful genetic transformation. There are scores of reports on genetic transformation in Tylophora (reviewed by Teixeira da Silva and Jha 2016). According to Chaudhuri et al. (2005), the explants (such as shoot tips or tender leaves) that are able to synthesize auxins are expected to exhibit frequent occurrence of transformation. For the first time, transgenic Tylophora was reported by Chaudhuri et al. (2005) via two wild-type A. rhizogenes agropine strains, namely, A4 (pRiA4) and LBA9402 (pRi1855), to induce in vitro leaf and stem segments for hairy root production. It was evident from the reports that the success of transformation was dependent on the strain of A. rhizogenes, type of explant, as well as site of inoculation. Amid the two utilized strains, only A. rhizogenes strain A4 (pRiA4) was competent enough with 60% rate of transformation, but also considerable variability of tylophorine accumulation among the clones was noticed. Later, the same authors (Chaudhuri et al. 2006) further reported that the plantlets produced from Ri-transformed roots exhibited increased biomass collection (350–510% in the roots and 200–320% in the whole plants) as well as tylophorine accumulation (20–60%) in the shoots, ultimately yielded 160–280% growth in tylophorine content in vitro. Roychowdhury et al. (2013) obtained Ri-mediated transgenic plants of Tylophora through somatic embryogenesis and maintained for 6 years. The comparison on the basis of morphological, biochemical, as well as molecular characterization was analyzed among the long-term transformed cultures and field grown normal and transformed plants. Morphologically, the transgenic plants were found to be stable in in vitro as well as field transferred after 6 years. In addition, genetic uniformity confirmation for rolA, rolB, rolC, and rolD genes was evidenced in Ri-transformed plants before and after relocation to the field. Most recently, Basu et al. (2017) reported co-transformation of cryptogein gene via A. rhizogenes-mediated approach successfully. They observed the stimulatory effect of crypt gene on the accumulation of phenolic compounds. The use of crypt-transformed plants as a tool for elucidation of biochemical basis of defense responses could be efficient to create a defense barrier against pathogenic infection, as suggested.
Secondary metabolite production in vitro
Advantageous supply of secondary metabolites can be attained right from in vitro organogenesis in a more sustainable way than from in vivo or wild plant populations. So far, tissue culture has not been commercially exploited as a source of secondary metabolites in the case of Tylophora. There are several reports available on the standardization of micropropagation (Fig. 3); however, extraction and estimation of tylophorine, the chief bioactive compound of Tylophora, have been investigated in only two of the reports (Kaur et al. 2011c, 2014). Kaempferol [3,5, 7-trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one], another important anti-cancer flavonoid from Tylophora, was produced in vitro by Chaturvedi et al. (2014). They reported an enhanced production of Kaempferol (3.31%) via immobilization of plant cell culture and proposed the biosynthetic pathway of this flavonoid (as, Coumaryl coA → Naringenin → Kaempferol). For tylophorine, the estimated level from directly and indirectly in vitro grown plants was recorded 71 and 80 μg/ml, respectively (Kaur et al. 2011c). Later on, Kaur et al. (2014) showed the highest tylophorine in the leaves (80 μg/ml) of in vitro regenerated plants and subsequently in callus (24.46 μg/ml) and suspension (28.30 μg/ml) cultures. However, they have not measured the level of tylophorine in in vivo- and in vitro-raised plantlets, which were compared in detail later by Soni et al. (2015a). Tylophorine concentration at different stages of in vitro differentiation, i.e., callus, in vitro shoots, and in vivo plant, was estimated separately. It was interesting to note that the concentration of tylophorine was elevated as the differentiation progressed. The callus showed 1.89-fold increase in tylophorine, which gradually reached 2.75-fold in shoots and 5.64-fold in a complete plantlet when compared to ex vitro mother plant. Furthermore, Soni et al. (2015a) tried precursor feeding to in vitro cultures for increasing the accumulation of secondary metabolites. The data suggested that the treatment with 2 mg/l tyrosine for 48 h was the most appropriate for obtaining the highest tylophorine accumulation (27.71 μg/g DW). The attained concentration was 5.87-fold higher than in vivo plants and 2.81-fold higher than control cultures. The Ri-mediated hairy root culture also offers a competent technique for ameliorated tylophorine and other secondary metabolites with genetic uniformity. Chaudhuri et al. (2006) demonstrated that T-DNA insertion in the Tylophora genome altered the level of tylophorine content. The production of tylophorine in transgenic shoots was found to be 20–60% higher than control cultures. Even, tylophorine was investigated for its continuous production and content until 6 years of its transformation (Roychowdhury et al. 2013). Level of tylophorine was considerably higher in the Ri-transformed leaves (3.75 ± 0.12 mg/g DW) in comparison with the non-transformed ones (1.81 ± 0.15 mg/gDW). Similar result was also evident in a recent most report of Basu et al. (2017) who co-transformed crypt gene via A. rhizogenes. A significant enhancement of phenolic production (caffeic acid, 1.8- to 2.9-fold; p-coumaric acid, 1.9-fold and ferulic acid, 1.5- to 2-fold) due to crypt gene expression was reported when compared to the conventional Ri-transformed root lines. The elevated production of caffeic acid (1.19-fold) and ferulic acid (1.53-fold) was also observed in comparison with Ri-transformed plants. Collectively, the in vitro grown calli, cell suspension, and hairy roots can be further studied for exploring biosynthetic pathways of essential phytochemicals. Advanced research is required for the commercial exploitation for in vitro production of secondary metabolites.
Future directions
The in vitro interventions on Tylophora along with the described optimum parameters for the success of these events are discussed essentially in this appraisal. We have recognized the frequently employed factors during in vitro organogenesis or callogenesis or somatic embryogenesis of Tylophora. Nevertheless, there are quite a number of lacunas to be filled up in the arena of Tylophora in vitro biotechnological research. For instance, merely, a couple of reports on synthetic seed production, where ample factors to be examined, further strengthen this system for Tylophora in particular. Encapsulation of asexually regenerated propagules using calcium alginate beads could be advantageous for the interchange, storage, and micropropagation of germplasms of this threatened medicinal plant. Another significant approach for germplasm conservation of Tylophora is cryopreservation in ultra-low temperature. Unfortunately, no attempt has been made to adopt this system for long-term storage instead of the only report of short-term (4 weeks) storage through synthetic seed production. Furthermore, from our survey of literature, we can observe that genetic transformation-mediated advancement plays a key role in Tylophora biotechnology. Genetic transformation via protoplast could be an apt route for the incorporation of genes with agronomical importance (for instance, genes for disease or insect resistance and sterility). There is a lone report by Thomas (2009) on mesophyll cell-derived protoplast-based regeneration system in Tylophora that could be considered as a stepping-stone towards the application of biotechnological techniques such as somatic hybridization and direct gene transfer to this plant. However, since this area of Tylophora biotechnology has not been explored much, there is plentiful scope for auxiliary exploration dealing with the biosynthetic prospective of Agrobacterium-transformed root cultures for tylophorine or other associated secondary metabolite production alongside the possibility of using genomics approaches, biochemical pathway prediction, and its utilization for alkaloid production. This appraisal offers the utmost inclusive evaluation of the Tylophora in vitro literature to date, briefing the finest conditions as described by several researchers. The present compilation will let Tylophora research to improve promptly, since various facets, fundamental and applied, have yet to be explored.
Abbreviations
- 2,4-D:
-
2,4-Dichlorophenoxyacetic acid
- AA:
-
Ascorbic acid
- BA:
-
N 6-benzyladenine
- CH:
-
Casein hydrolysate
- GA3 :
-
Gibberellin A3
- IAA:
-
Indole-3-acetic acid
- IBA:
-
Indole-3-butyric acid
- Kinetin:
-
6-furfurylaminopurine
- MS:
-
Murashige and Skoog (1962)
- NAA:
-
α-Naphthalene acetic acid
- PGR:
-
Plant growth regulator
- TDZ:
-
Thidiazuron
References
Anand M, Kaur H, Goyal D (2012) A micropropagation system for Tylophora indica and extraction and purification of tylophorine from cultures and in vitro regenerated plants. In: International Conference on Environmental, Biomedical and Biotechnology IPCBEE vol 41, 2012
Anjum A, Narula A, Khan M, Kamaluddin A (2014) Establishment of an in vitro micropropagation protocol for a medicinal herb Tylophora indica. J Cell Tiss Res 14:4309–4314
Banerjee S, Rahman L, Uniyal GC, Ahuja PS (1998) Enhanced production of valepotriates by Agrobacterium rhizogenes induced hairy root cultures of Valeriana wallichii DC. Plant Sci 131:203–208
Basu A, Roychowdhury D, Joshi RK, Jha S (2017) Effects of cryptogein gene on growth, phenotype and secondary metabolite accumulation in co-transformed roots and plants of Tylophora indica. Acta Physiol Plant 39:3
Benjamin BD, Mulchandani NB (1973) Studies in biosynthesis of secondary constituents in tissue culture of Tylophora indica. Planta Med 23:394–397
Bhojwani SS, Dantu PK (2013) Plant tissue culture: an introductory text. Springer, Heidelberg
Chandrasekhar T, Hussain TM, Gopal GR, Rao JVS (2006) Somatic embryogenesis of Tylophora indica (Burm.f.) Merril., an important medicinal plant. Int J Appl Sci Eng 4:33–40
Chaturvedi P, Chowdhary A (2013) Enhancement of antioxidant compound in Tylophora indica (Asclepeadaceae) callus. Adv Appl Sci Res 4:325–330
Chaturvedi P, Chowdhary A, Kawalkar H (2012) Molecular characterization of Tylophora indica regenerated plants in vitro by RAPD and ISSR analysis. Int J Res Phytochem Pharmacol 2:175–179
Chaturvedi P, Soundar S, Parekh K, Lokhande S, Chowdhary A (2014) Media optimization in immobilized culture to enhance the content of kaempferol in Tylophora indica (Asclepeadaceae) and curcumin in Curcuma longa (Zingiberaceae). IOSR J Pharm Biol Sci 9:86–90
Chaudhuri KN, Ghosh B, Jha S (2004) The root: a potential new source of competent cells for high-frequency regeneration in Tylophora indica. Plant Cell Rep 22:731–740
Chaudhuri KN, Ghosh B, Tepfer D, Jha S (2005) Genetic transformation of Tylophora indica with Agrobacterium rhizogenes A4: growth and tylophorine productivity in different transformed root clones. Plant Cell Rep 24:25–35
Chaudhuri KN, Ghosh B, Tepfer D, Jha S (2006) Spontaneous plant regeneration in transformed roots and calli from Tylophora indica: changes in morphological phenotype and tylophorine accumulation associated with transformation by Agrobacterium rhizogenes. Plant Cell Rep 25:1059–1066
Devendra BN, Srinivas N, Naik GR (2011) Direct somatic embryogenesis and synthetic seed production from Tylophora indica (Burm.f.) Merrill an endangered, medicinally, important plant. Int J Bot 7:216–222
Dhokrat R, Waghmare V, Pandhure N (2015) In vitro regeneration of Tylophora asthmatica (L. F.) Wight & Arn. Int J Adv Res Comput Sci Softw Eng 5:654–656
Endress ME, Bruyns PV (2000) A revised classification of the Apocynaceae s.l. Bot Rev 66:1
Faisal M, Anis M (2003) Rapid mass propagation of Tylophora indica Merrill via leaf callus culture. Plant Cell Tiss Organ Cult 75:125–129
Faisal M, Anis M (2005) An efficient in vitro method for mass propagation of Tylophora indica. Biol Plant 49:257–260
Faisal M, Anis M (2007) Regeneration of plants from alginate-encapsulated shoots of Tylophora indica (Burm. f.) Merrill, an endangered medicinal plant. J Hort Sci Biotechnol 82:351–354
Faisal M, Anis M (2010) Effect of light irradiations on photosynthetic machinery and antioxidative enzymes during ex vitro acclimatization of Tylophora indica plantlets. J Plant Interact 5:21–27
Faisal M, Singh S, Anis M (2005) In vitro regeneration and plant establishment of Tylophora indica (Burm. f.) Merrill: petiole callus culture. In Vitro Cell Dev Biol Plant 41:511–515
Faisal M, Ahmad N, Anis M (2007) An efficient micropropagation system for Tylophora indica: an endangered, medicinally important plant. Plant Biotechnol Rep 1:155–161
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–158
Gami B, Parmar M (2010) Phytochemical screening and antimicrobial activity of in vitro and in vivo developed Thylophora [sic] indica (Burm f.) Merill. Int J Drug Discov Technol 1:77–84
Gantait S, Mandal N, Bhattacharyya S, Das PK (2009) In vitro mass multiplication with genetic clonality in elephant garlic (Allium ampeloprasum L.). J Crop Weed 5:100–104
Gantait S, Mandal N, Bhattacharyya S, Das PK (2010a) Determination of genetic integrity in long-term micropropagated plantlets of Allium ampeloprasum L. using ISSR markers. Biotechnol 9:218–223
Gantait S, Mandal N, Bhattacharyya S, Das PK (2010b) A novel strategy for in vitro conservation of Aloe vera L. through long term shoot culture. Biotechnol 9:326–331
Gantait S, Mandal N, Das PK (2011) In vitro accelerated mass propagation and ex vitro evaluation of Aloe vera L. with aloin content and superoxide dismutase activity. Nat Prod Res 25:1370–1378
Gantait S, Sinniah UR, Mandal N, Das PK (2012) Direct induction of protocorm-like bodies from shoot tips, plantlet formation, and clonal fidelity analysis in Anthurium andreanum cv. CanCan. Plant Growth Regul 67:257–270
Gantait S, Debnath S, Ali N (2014a) Genomic profile of the plants with medicinal importance. 3Biotech 4:563–578
Gantait S, Sinniah UR, Das PK (2014b) Aloe vera: a review update on advancement of in vitro culture. Acta AgricScand Sec B Soil Plant Sci 64:1–12
Gantait S, Kundu S, Ali MN (2015a) Influence of encapsulating agent and matrix levels on synseed production of Bacopa monnieri (L.) Pennell. Med Plants 7:182–187
Gantait S, Kundu S, Ali MN, Sahu NC (2015b) Synthetic seed production of medicinal plants: a review on influence of explants, encapsulation agent and matrix. Acta Physiol Plant 37:98
Gantait s, Kundu S, Das PK (2016) Acacia: An exclusive survey on in vitro propagation. J Saudi Soc Agric Sci. doi: 10.1016/j.jssas.2016.03.004
Gantait S, Vijayan J, Majee A (2017) Artificial seed production of Tylophora indica for interim storing and swapping of germplasm. Horticultural Plant J 3:41–46
Gelvin SB (2003) Agrobacterium-mediated plant transformation: the biology behind the “gene-jockeying” tool. Microbiol Mol Biol Rev 67:16–37
Gupta AK (2003) Quality standards of Indian medicinal plants. ICMR 1:221–225
Gupta M, Mukhtar HM, Ahmad S (2010) Phyto-pharmacological and plant tissue culture overview of Tylophora indica (burm f.) Merill. J Pharm Sci Res 2:401–411
Haque SM, Ghosh B (2013) Field evaluation and genetic stability assessment of regenerated plants produced via direct shoot organogenesis from leaf explant of an endangered ‘Asthma Plant’ (Tylophora indica) along with their in vitro conservation. Nat Acad Sci Lett 36:551–562
Jahan N, Khatoon R, Shahzad A, Shahid M, Ahmad S (2013) Comparison of antibacterial activity of parent plant of Tylophora indica Merr. with its in vitro raised plant and leaf callus. Afr J Biotech 12:4891–4896
Jayanthi M, Mandal PK (2001) Plant regeneration through somatic embryogenesis and RAPD analysis of regenerated plants in Tylophora indica (Burm. F. Merrill.). In Vitro Cell Dev Biol-Plant 37:576–580
Jogdand V, Waghmare V, Pandhure N (2016) Micropropagation studies in medicinal plant Tylophora asthmatica (L.F.) Wight and Arn. Int J Sci Res 5:303–306
Joshi SG (2000) Medicinal plants. Oxford and IBH Publishing, New Delhi
Kakde NP, Salve MS, Pandhure N (2016) High frequency regeneration in Tylophora asthmatica (L. F.) Wight & Arn. Int J Bot Stud 1:10–11
Kalimuthu K, Jeyaraman S (2012) Morphogenetic callus and multiple shoot regeneration; and thin layer chromatography studies of Tylophora indica (Burn. f) Merill. J Med Plant Res 6:5094–5098
Kaur H, Singh K (2012) A brief phytopharmacological overview of Tylophora indica—an endangered medicinal plant. Int J Pharm Sci Res 3:4073–4076
Kaur H, Anand M, Goyal D (2011a) Optimization of potting mixture for hardening of in vitro raised plants of Tylophora indica to ensure high survival percentage. Int J Med Arom Plant 1:83–88
Kaur H, Anand M, Goyal D (2011b) Extraction of tylophorine from in vitro raised plants of Tylophora indica. J Med Plant Res 5:729–734
Kaur H, Anand M, Goyal D (2011c) Establishment of an efficient protocol for micropropagation of stem explants of Tylophora indica, an important medicinal plant. Afr J Biotechnol 10:6928–6932
Kaur H, Anand M, Goyal D (2014) HPTLC based analysis of tylophorine from cultures and in vitro regenerated plants of Tylophora indica—an endangered medicinal plant. Int J Pharma Res Sch 3:91–95
Kaushik A, Gurnani C, Sunder S, Dhingra A, Chimpa V (2010) Biochemical assessment of in vitro and in vivo culture of Tylophora indica (Burm. f.) Merr. Kathmandu Univ J Sci Eng Technol 6:1–5
Kirtikar KR, Basu BD (1991) Indian medicinal plants, 2nd edn. Periodic expert book agency, New Delhi, pp 1–5
Koilpillai YJ (2012) Leaf as a potential new source of competent cells for high frequency regeneration through indirect organogenesis in Tylophora indica (Burm. f.) Merrill (Asclepiadaceae). J Biomed Pharma Res 1:46–50
Kumar AS, Gamborg OL, Nabors MW (1988) Plant regeneration from cell suspension cultures of Vigna aconiifolia. Plant Cell Rep 7:138–141
Linsmaier EM, Skoog F (1965) Organic growth factor requirements for tobacco tissue cultures. Physiol Plant 18:100–127
Mahesh R, Muthuchelian K, Maridass M, Raju G (2011) Clonal propagation of Tylophora indica—a medicinal plant. Int J Appl Biores 1:1–4
Mallón R, Rodríguez-Oubińa J, González ML (2010) In vitro propagation of the endangered plant Centaurea ultreiae: assessment of genetic stability by cytological studies, flow cytometry and RAPD analysis. Plant Cell Tiss Organ Cult 101:31–39
Manjula S, Job A, Nair GM (2000) Somatic embryogenesis from leaf derived callus of Tylophora indica (Burm. f.) Merill. Ind J Exp Biol 38:1069–1072
Mohan C, Devi BR, Manjula P, Kiran Kumar B, Naresh B, Pratibha Devi B (2014) Phytochemical investigations and micropropagation of Tylophora indica (burm. f.) Merill from nodal explants. J Indian Bot Soc 93:25–32
Mulchandani NB, Iyer SS, Badheka LP (1971) Incorporation of phenylanine 2-14C into tylophorine. Phytochemistry 10:1047–1050
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol Plant 15:473–495
Nayeem A, Panchakshararadhya RM, Basappa VA (2014) In vitro plant regeneration using adventitious roots as explants in Tylophora indica. Asian J Plant Sci Res 4:15–18
Okwu DE, Okwu ME (2004) Chemical composition of Spondias mombin Linn plant parts. J Sustain Agric Environ 6:140–147
Patel P, Nadgauda R (2014) Development of simple, cost effective protocol for micropropagation of Tylophora indica (Burm f.) Merill., an important medicinal plant. Eur J Med Plants 4:1356–1366
Patel A, Patel IC (2012) Micropropagation of medicinally important climber: tylophora indica Merrill. future demand. Life Sci Leafl 2012:311–316
Pathak A, Dwivedi M, Laddha NC, Begum R, Joshi A (2013) Detection of somaclonal variants using RAPD marker in Bacopa monnieri and Tylophora indica. J Agric Technol 9:1253–1260
Rajavel L, Stephan R (2014a) Effect of low cost media to propagate the endangered medicinal plant Tylophora indica (Burm. f.) Merrill. Species 11:27–33
Rajavel L, Stephan R (2014b) Low cost in vitro propagation of Tylophora indica (Burm f.) Merrill. using different carbon sources. J Acad Ind Res 3:221–224
Rani S, Rana JS (2010) In vitro propagation of Tylophora indica- influence of explanting season, growth regulator synergy, culture passage and planting substrate. J Am Sci 6:385–392
Rani AS, Patnaik S, Sulakshanaand G, Saidulu B (2012) Review of Tylophora indica-an antiasthmatic plant. FS J Res Basic Appl Sci 1(3):20–21
Rao KV, Wilson RA, Cummings B (1971) Alkaloids of Tylophora. 3 New Alkaloids of Tylophora indica (Burm) Merrill and Tylophora dalzelii Hook. J Pharma Sci 60:1725–1726
Rashmi MP, Vinaya M, Vedamurthy AB, Nayeem A (2012) Effectiveness of auxins in inducing in vitro adventitious root formation in Tylophora indica. J Cell Tiss Res 12:3357–3360
Rathinavel S, Sellathurai T (2010) In vitro regeneration and phytochemical screening of Tylophora indica, an endangered medicinal herb. J Exp Sci 1:4–6
Rathod D, Patel A, Shrimali G, Rami E, Patel C, Panigrahi J, Patel I (2014) Biochemical changes during in vitro organogenesis of Tylophora indica (Burm. F.) Merrill. Ind J Appl Res 4:25–28
Rathore MS, Gehlot HS, Shekhawat NS (2010) Biotechnological approaches for propagation and prospecting of important medicinal plants from Indian Thar Desert. Int J Plant Prod 4:67–72
Reddy BK, Anjaneyulu E, Ramgopal M, Vaidyanath K, Hemalatha S, Balaji M (2010) An efficient protocol for shoot bud induction and regeneration of Tylophora indica, an endangered medicinal plant. Curr Trend Biotechnol Pharma 4:922–929
Rout GR, Samantaray S, Das P (2000) In vitro manipulation and propagation of medicinal plants. Biotechnol Adv 18:91–120
Roychowdhury D, Ghosh B, Chaubey B, Jha S (2013) Genetic and morphological stability of six-year-old transgenic Tylophora indica plants. Nucleus 56:81–89
Roychowdhury D, Basu A, Jha S (2015a) Morphological and molecular variation in Ri-transformed root lines are stable in long term cultures of Tylophora indica. Plant Growth Reg 75:443–453
Roychowdhury D, Chaubey B, Jha S (2015b) The fate of integrated Ri T-DNA rol genes during regeneration via somatic embryogenesis in Tylophora indica. J Bot 2015:1–16
Sadguna V, Swamy TN, Raju S, Ghani M, Suresh V, Mustafa M (2013) High frequency regeneration of plantlets from leaf derived callus cultures of Tylophora indica Burmf. an important medicinal plant. Int J Sci Eng Res 4:2704–2707
Sahai A, Shahzad A, Anis M (2010a) High frequency plant production via shoot organogenesis and somatic embryogenesis from callus in Tylophora indica, an endangered plant species. Turk J Bot 34:11–20
Sahai A, Shahzad A, Sharma S (2010b) Histology of organogenesis and somatic embryogenesis in excised root cultures of an endangered species Tylophora indica (Asclepiadaceae). Austr J Bot 58:198–205
Saker MM, Bekheer SA, Taha HS, Fahmy AS, Moursy HA (2000) Detection of somaclonal variations in tissue cultured derived date palm plants using iso-enzyme analysis and RAPD fingerprints. Biol Plant 43:347–351
Sellathurai T, Rathinavel S, Natarajan KK (2013) Screening of antimicrobial potential of in vitro calli and adult leaf extracts of Tylophora indica (Burm. f.) Merill. Afr J Biotechnol 12:958–962
Sharma MM, Verma RN, Singh A, Batra A (2014) Assessment of clonal fidelity of Tylophora indica (Burm. f.) Merrill “in vitro” plantlets by ISSR molecular markers. SpringerPlus 3:400
Singh SR, Singh R, Dhawan AK (2009a) Biochemical changes related to shoot differentiation in callus cultures of Tylophora indica Wight and Arn. J Ind Bot Sco 88:49–53
Singh SR, Singh R, Dhawan AK (2009b) Callus induction and micropropagation from different explants of Tylophora lndica Wight and Am. (Antamul). Environ Ecol 27:945–948
Singh SR, Singh R, Dhawan AK, Kumar S (2010) Changes in protein profiles during shoot differentiation in callus cultures from Tylophora indica Weight & Arn. (Antamul). Prog Agric 10(Spl Issue):57–62
Soni K, Sahni S, Abdin MZ, Narula A (2015a) Conservation and enhanced tylophorine through in vitro propagation and precursor feeding in Tylophora indica—an endangered medicinal plant. Int J Pharma Bio Sci 6:9–18
Soni V, Bhusan M, Swarnkar PL (2015b) Biotechnological approaches for conservation of Tylophora indica: an economically important endangered medicinal plant. Economol J 5:2–5
Swamy MK, Mohanty SK, Anuradha M (2014) The effect of plant growth regulators and natural supplements on in vitro propagation of Pogostemon cablin Benth. J Crop Sci Biotechnol 17:71
Teixeira da Silva JA, Jha S (2016) Micropropagation and genetic transformation of Tylophora indica (Burm. f.) Merr.: a review. Plant Cell Rep 35:2207–2225
Thomas TD (2006) Effect of sugars, gibberellic acid and abscisic acid on somatic embryogenesis in Tylophora indica (Burm. f.) Merrill. Chin J Biotechnol 22:465–471
Thomas TD (2009) Isolation, callus formation and plantlet regeneration from mesophyll protoplasts of Tylophora indica (burm. f.) merrill: an important medicinal plant. In Vitro Cell Dev Biol Plant 45:591–598
Thomas TD, Philip B (2005) Thidiazuron-induced high-frequency shoot organogenesis from leaf-derived callus of a medicinal climber, Tylophora indica (Burm. F.) Merrill. In Vitro Cell Dev Biol Plant 41:124–128
Varrier PK, Nambiar VPK, Ramankutty C (1994) Tylophora indica Indian medicinal plants—a compendium of 500 species, vol 5. Orient Longman, New Delhi, pp 66–68
Verma RN, Jamal SM, Sharma MM, Rao DV, Batra A (2010) Regulation of organogenesis using leaf, internode and petiole explants in Tylophora indica (Burm. f.) Merr. Int J Pharma Sci Rev Res 5:35–40
Wilson PJ, van Staden J (1990) Rhizocaline, rooting co-factors and concepts of promoters and inhibitors of adventitious rooting—a review. Ann Bot 66:470–490
Yadav N (2016) Conservation of some endangered and economically important medicinal plants of India—a review. J Integr Sci Technol 4:59–62
Acknowledgements
The authors acknowledge the laboratory facilities from the Faculty Centre for Integrated Rural Development and Management, School of Agriculture and Rural Development, Ramakrishna Mission Vivekananda University, and library assistance from Bidhan Chandra Krishi Viswavidyalaya. We further are thankful to the anonymous reviewers and the editor of this article for their critical comments and suggestions on the manuscript.
Author information
Authors and Affiliations
Contributions
Conception of the review—S. Gantait; survey of the literature—S. Gantait and S. Kundu; and drafting of manuscript—S. Gantait and S. Kundu. Both the authors scrutinized, corrected, and approved the final version of the manuscript prior to its submission.
Corresponding author
Ethics declarations
Conflict of interest
We, the authors of this article declare that there is no conflict of interest and we do not have any financial gain from it.
Rights and permissions
About this article
Cite this article
Gantait, S., Kundu, S. Neoteric trends in tissue culture-mediated biotechnology of Indian ipecac [Tylophora indica (Burm. f.) Merrill]. 3 Biotech 7, 231 (2017). https://doi.org/10.1007/s13205-017-0865-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-017-0865-8