Abstract
An efficient protocol is described for the rapid in vitro multiplication of an endangered medicinal plant, Tylophora indica (Burm. f.) Merrill, via enhanced axillary bud proliferation from nodal explants collected from young shoots of a two-year-old plant. The physiological effects of growth regulators [6-benzyladenine (BA), kinetin (Kin) thidiazuron (TDZ), indole-3-acetic acid (IAA), indole-3-butyric acid (IBA) or α-naphthalene acetic acid (NAA)], ascorbic acid (AA), different strengths of Murashige and Skoog (MS) medium and various pH levels on in vitro morphogenesis were investigated. The highest number (8.6 ± 0.71) of shoots and the maximum average shoot length (5.2 ± 0.31 cm) were recorded on MS medium supplemented with 2.5 μM BA, 0.5 μM NAA and 100 mg/l AA at pH 5.8. Rooting was best achieved on half-strength MS medium augmented with 0.5 μM IBA. The plantlets regenerated in vitro with well-developed shoot and roots were successfully established in pots containing garden soil and grown in a greenhouse with a 90% survival rate. The regenerated plants did not show any immediate detectable phenotypic variation. The described method can be successfully employed for large-scale multiplication and long-term in vitro conservation of T. indica.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tylophora indica (Burm. f.) Merrill, commonly called Antamul or Indian ipecac, is an important medicinal plant belonging to the family Asclepiadaceae. It is a perennial, woody, climbing shrub and is found on plains, hilly slopes and the outskirts of the forests of eastern and southern India. The plant is used as folk remedy in certain regions of India for the treatment of bronchial asthma, inflammation, bronchitis, allergies, rheumatism and dermatitis (CSIR 2003). It also seems to be a good traditional medical remedy for psoriasis, seborrhea, anaphylaxis, leucopenia and it is an inhibitor of the Schultz–Dale reaction. The powdered leaves and roots contain the alkaloids tylophorine (responsible for strong anti-inflammatory action; Gopalakrishnan et al. 1980) and tylophorinine. The roots also contain a potential anticancer alkaloid, tylophorinidine (Mulchandani et al. 1971). Several pharmaceutical companies (Acron Chemicals, Mumbai, India; Sabinsa Corporation, Piscataway, NJ, USA) are marketing T. indica extracts as antiasthmatic herbal drugs.
The lack of proper cultivation practices and the indiscriminate way in which this plant is collected from its natural habitat pose a serious threat to its existence in the wild. Moreover, propagation, either by seed or by vegetative cuttings, is rather difficult. Stem cuttings failed to produce proper root when treated with different growth regulators (Chandrasekhar et al. 2006). Propagation through tissue culture offers a viable alternative for this species because it can also be used as a complimentary strategy for conservation and utilization of genetic resources. Further, in vitro plant regeneration through axillary bud culture is an easy and economic way of obtaining a large number of consistently uniform and true-to-type plants within a short span of time. So far a single report on the micropropagation of T. indica via axillary shoot proliferation has been reported (Sharma and Chandel 1992); this approach produced only a few shoots. There is an obvious need to develop an efficient regeneration system for effective conservation and rapid multiplication in order to meet market demands and to replenish highly impoverished populations.
The objectives of the study reported here were to (1) optimize the culture conditions applied for the initiation and proliferation of shoots from nodal explants of T. indica through enhanced axillary branching, and (2) induce rooting in microshoots and establish the plantlets in outdoor conditions.
Materials and methods
Plant material and surface sterilization
Young, healthy shoots of T. indica were collected from a two-year-old plant grown in the Botany Department, Aligarh Muslim University, Aligarh. The shoot segments were washed under running tap water for 30 min and then soaked in a 5% (v/v) detergent, labolene (Qualigens, Mumbai, India), for 5 min. After through washing, their surfaces were sterilized with 0.1% (w/v) HgCl2 for 3 min and finally rinsed 4–5 times with sterile distilled water. Shoot segments measuring 4–7 mm were cut into single node explants and inoculated vertically on sterile nutrient media.
Culture media and culture conditions
A culture medium containing MS (Murashige and Skoog 1962) salts supplemented with macro-elements, micro-elements, 3% (w/v) sucrose and 0.8% (w/v) agar was used in all experiments. The pH of the medium was adjusted to 5.8 by 1 N NaOH or 1 N HCl before being autoclaved at 121 °C for 20 min. All of the cultures were incubated under 50 μmol m−2 s−1 light provided by cool white fluorescent lamp for a photoperiod of 16 h at 25 ± 2 °C.
Shoot initiation and multiplication
For shoot induction, the nodal explants were cultured on MS medium supplemented with various plant growth regulators [6-benzyladenine (BA), kinetin (Kin) and thidiazuron (TDZ)] at different concentrations (0, 0.5, 2.5, 5.0, 7.5 and 10.0 μM), either individually or in combination with indole-3-acetic acid (IAA), indole-3-butyric acid (IBA) or α-naphthalene acetic acid (NAA) (0, 0.1, 0.5, 1.0, 1.5 and 2.0 μM). The effects of different strengths of MS medium (\(\raise0.7ex\hbox{$1$} \!\mathord{\left/ {\vphantom {1 4}}\right.\kern-\nulldelimiterspace}\!\lower0.7ex\hbox{$4$},\raise0.7ex\hbox{$1$} \!\mathord{\left/ {\vphantom {1 3}}\right.\kern-\nulldelimiterspace}\!\lower0.7ex\hbox{$3$},\raise0.7ex\hbox{$1$} \!\mathord{\left/ {\vphantom {1 2}}\right.\kern-\nulldelimiterspace}\!\lower0.7ex\hbox{$2$}\) and full strength), various pH levels (5.0, 5.4, 5.8 and 6.2) and different concentrations (50, 100, 200 and 500 mg/l) of ascorbic acid (AA) on T. indica morphogenesis were also assessed using optimal concentrations and combinations of BA (2.5 μM) and NAA (0.5 μM). All of the cultures were subcultured onto the fresh medium after every two weeks. The frequency with which explants produced shoots, the number of shoots per explant and the shoot length were recorded after six weeks of culture.
Histological observation
For histological studies, the explants were fixed in formalin:glacial acetic acid:ethanol 4:6:90 (v/v) solution. Fixed tissues were dehydrated through an ethanol/xylol series and embedded in paraffin wax (60 °C). Serial sections of 10 μM thickness were cut using a Spencer 820 microtome (American Optical Corp., Buffalo, NY, USA) and the resulting paraffin ribbons were passed through a series of deparafinizing solutions and stained in safranin and fast green solutions. The sections were examined under an optical microscope (CH20i, Olympus, Tokyo, Japan).
Rooting of regenerated shoots
Microshoots (3–4 cm) with 2–3 pairs of leaves were excised individually and transferred to half-strength MS medium supplemented with IAA, IBA or NAA (0, 0.5, 2.5 and 5.0 μM). The medium was solidified with 0.25% (w/v) Gelrite (Sigma-Aldrich, New Delhi, India). Data were recorded on the percentage of rooting, the mean number of roots per shoot and the root length after four weeks of transfer onto the rooting medium.
Acclimatization
Plantlets with well-developed shoots and roots were removed from the culture medium, washed gently under running tap water and transferred to plastic pots containing sterile garden soil, soilrite or vermiculite (Keltech Energies Ltd., Bangalore, India) under diffuse light (16:8 h photoperiod) conditions. Potted plantlets were covered with a transparent polythene membrane to ensure high humidity and watered every three days with half-strength MS salt solution for two weeks. Polythene membranes were opened after two weeks in order to acclimatize plants to field conditions. After four weeks, these plants were transferred to pots containing normal soil and maintained in a greenhouse under normal day length conditions.
Statistical analysis
All of the experiments were conducted with a minimum of 20 replicates per treatment. The experiments were repeated three times. The cultures were observed periodically and morphological changes were recorded at regular intervals. The results were analyzed statistically using SPSS version 12 (SPSS Inc., Chicago, IL, USA). The significance of differences among means was analyzed using Tukey’s test at P = 0.05.
Results
Effect of cytokinins
The morphogenetic responses of nodal segment explants to various cytokinins (BA, Kin and TDZ) are summarized in Table 1. Placing explants in a medium without growth regulators (control) induced 1–2 shoots (Fig. 1A). However, the multiplication rate and shoot number were higher in cultures supplemented with plant growth regulators. The percentage of response varied with the type of growth regulator used and its concentration. All concentrations of BA (0.5, 2.5, 5.0 and 10 μM), Kin (0.5, 2.5, 5.0 and 10 μM) or TDZ (0.5, 2.5, 5.0 and 10 μM) alone facilitated shoot bud differentiation. Swelling of the dormant axillary bud took place within ten days, and then differentiation into multiple shoots occurred after four weeks (Fig. 1B). The histological sections revealed direct differentiation of shoot buds from the explant with a prominent apical dome and leaf primordia (Fig. 2A,B). Among the various cytokinins tested, BA was found to be more efficient than others with respect to initiation and subsequent proliferation of shoots (Table 1). Of the various levels of BA tested, 2.5 μM proved to be most effective, as in this medium an average of 3.7 ± 0.26 shoots were developed per explant in 80% of cultures. Upon lowering the concentration of each cytokinin, a reduction in the number of shoots per culture was recorded. Similarly, at a higher concentration (10 μM) the number as well as the percent response was drastically reduced (Table 1). A callus occasionally formed at the base of the explant, retarding axillary bud formation and the subsequent growth of shoots. Therefore, precautions were taken to remove such callus growth while subculturing.
A Axillary bud proliferation on MS basal medium after four weeks of culture. B Regeneration of shoots from nodal explants on MS + Kin (2.5 μM). C Multiple shoot regeneration on MS + BA (2.5 μM) + NAA (0.5 μM) + AA (100 mg/l) after six weeks of culture. D Rooted shoot on ½MS + IBA (0.5 μM). E Acclimatized plants of T. indica after one month
Effect of auxin and cytokinin
The efficiency of the optimal concentration of BA with various auxins (IAA, IBA or NAA) was also evaluated for multiple shoot induction (Table 2). BA with NAA was found to be the most effective combination for shoot regeneration and multiplication. Nodal explants cultured on MS medium supplemented with 2.5 μM BA and 0.5 μM NAA exhibited 90% shoot regeneration. Upon increasing the concentration of NAA up to 2.0 μM, a gradual decrease in regeneration frequency and the number of shoots per explant was recorded. The elevated concentration of NAA (2.0 μM) resulted in little callusing at the cut end thus reducing the percent shoot regeneration and the number of shoots per explant. Among the various combinations of BA and IAA used, the highest shoot regeneration frequency (80%) and number of shoots per explant (5.0 ± 0.30) along with the maximum shoot length (4.2 ± 0.15 cm) were recorded on MS medium supplemented with BA (2.5 μM) + IAA (0.5 μM) after six weeks of inoculation (Table 2). Among the BA–IBA combinations, the maximum frequency (70%) of shoot bud formation and the greatest number of shoots (4.3 ± 0.41) per explant were obtained on MS medium containing 2.5 μM BA with 1.0 μM IBA.
Effect of ascorbic acid
In another experiment, nodal explants were cultured on growth regulators (2.5 μM BA + 0.5 μM NAA) containing medium supplemented with various concentrations of ascorbic acid (50–500 mg/l) (Table 3). Results obtained after six weeks of incubation revealed that 100 mg/l ascorbic acid in the presence of BA and NAA gave the maximum response with regard to overall shoot growth in terms of number of shoots and shoot length (Fig. 1C). Increasing the concentration of ascorbic acid to 500 mg/l suppressed the regeneration ability, and the shoots formed were vitrified.
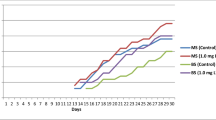
Effect of medium strength and pH
The effect of the strength of the MS medium on shoot proliferation was also examined with \(\raise0.7ex\hbox{$1$} \!\mathord{\left/ {\vphantom {1 4}}\right.\kern-\nulldelimiterspace}\!\lower0.7ex\hbox{$4$}{\text{MS}},\raise0.7ex\hbox{$1$} \!\mathord{\left/ {\vphantom {1 3}}\right.\kern-\nulldelimiterspace}\!\lower0.7ex\hbox{$3$}{\text{MS}},\raise0.7ex\hbox{$1$} \!\mathord{\left/ {\vphantom {1 2}}\right.\kern-\nulldelimiterspace}\!\lower0.7ex\hbox{$2$}{\text{MS}}\) and full-strength MS (Fig. 3). The shoot proliferation was found to be highest for full-strength MS medium and poorest on \(\raise0.7ex\hbox{$1$} \!\mathord{\left/ {\vphantom {1 4}}\right.\kern-\nulldelimiterspace}\!\lower0.7ex\hbox{$4$}{\text{MS}}{\text{.}}\) The effect of different medium pH levels (5.0, 5.4, 5.8 and 6.2) was also tested for MS medium supplemented with 2.5 μM BA + 0.5 μM NAA + 100 mg/l AA (Fig. 4). The optimum pH for shoot proliferation and elongation was found to be 5.8, and multiplication was inhibited in more acidic media. Removal of individual shoots and division of “shoot clumps” stimulated further proliferation of buds. The shoots produced in this way were either subcultured for further multiplication or transferred onto a rooting medium.
Effect of MS medium strength on shoot regeneration from nodal explants of T. indica supplemented with BA (2.5 μM) + NAA (0.5 μM) + AA (100 mg/l). Data represent the mean ± SE. Means followed by the same letter within response variables are not significantly different (P = 0.05) using Tukey’s tests. Evaluations were made after six weeks of culture
Effect of medium pH on shoot regeneration from nodal explants of T. indica supplemented with BA (2.5 μM) + NAA (0.5 μM) + AA (100 mg/l). Data represent the mean ± SE. Means followed by the same letter within response variables are not significantly different (P = 0.05) using Tukey’s tests. Evaluations were made after six weeks of culture
Rooting of regenerated shoots
The shoots regenerated in vitro were transferred to half-strength MS medium. Root formation from the basal cut portion of the shoots was observed one week after transfer to the rooting medium. The presence of an auxin (IAA, IBA or NAA) at a low concentration in half-strength MS medium was found to be more effective for rooting (Fig. 1D), and the best rooting was achieved in this medium fortified with 0.5 μM IBA; fairly good shoot numbers (4.50 ± 0.45) and root lengths per shoot (4.01 ± 0.31) were obtained (Table 4). No rooting was observed from the base of any microcuttings prior to the first week of culture. Rooting frequency increased gradually over time and reached a maximum after four weeks of culture.
Acclimatization
Plantlets with 4–5 fully expanded leaves and well-developed roots were successfully hardened off inside the growth room in a selected planting substrate (garden soil, soilrite or vermiculite) for four weeks and eventually established in natural soil (Fig. 1E). Of the three different types of planting substrates examined, the percentage survival of the plantlets was highest (90%) in vermiculite (Table 5). About 100% of the regenerated plants survived following transfer from vermiculite to natural soil and no detectable variation with respect to morphology or growth characteristics was observed.
Discussion
Nodal segments containing axillary buds have quiescent or active meristems depending upon the physiological stage of the plant. These buds have the potential to develop into complete plantlets. The conventional method used for the vegetative propagation of stem cuttings relies on the axillary bud taking over the function of the main shoot in the absence of a terminal bud. In nature, these buds remain dormant for a specific period depending on the growth pattern of the plant. However, using tissue culture, the rate of shoot multiplication can be greatly enhanced by performing axillary bud culture in a nutrient medium containing suitable cytokinin or cytokinin and auxin combinations. Due to the continuous availability of cytokinin, shoots formed by the bud already present in the explant (nodal segment) develop into axillary buds, which then grow directly into shoots. Multiple shoot formation following the in vitro culture of nodal segments has proved to be an effective method of mass multiplication. The effect of BA on multiple shoot bud differentiation has been demonstrated in a number of cases using a variety of explants (Stefaan et al. 1994; Mao et al. 1995; Pattnaik and Chand 1996; Patil 1998; Jeong et al. 2001; Hiregoudar et al. 2003, 2006; Loc et al. 2005). In the present case, BA also proved to be more effective than other cytokinins. Among the different levels of BA tested, 2.5 μM produced the maximum number of shoots from nodal explants. Kin and TDZ were less effective than BA. A low concentration of auxin along with a high concentration of cytokinin was most promising for the induction and multiplication of shoots in T. indica, and MS medium supplemented with 2.5 μM BA in combination with 0.5 μM NAA proved most effective for direct shoot regeneration. The synergistic effect of BA in combination with an auxin has been demonstrated in many medicinal plants from the Asclepiadaceae family, such as Gymnema sylvestre (Reddy et al. 1998), Holostemma annulare (Sudha et al. 1998), Hemidesmus indicus (Sreekumar et al. 2000), Holostemma ada-kodien (Martin 2002), Leptadenia reticulata (Arya et al. 2003), and Ceropegia candelabrum (Beena et al. 2003). In accordance with these reports, the present study also exemplifies the positive modification of shoot induction efficacy obtained by employing a low concentration of auxin in combination with a cytokinin. Addition of ascorbic acid (100 mg/l) to the medium containing 2.5 μM BA + 0.5 μM NAA significantly enhanced shoot number and shoot length. Similarly, a stimulative effect of ascorbic acid was also reported by Sharma and Chandel (1992), and Ahmad et al. (2006).
To optimize the rooting response of plantlets raised in vitro, different auxins (IAA, IBA and NAA) were tested at various concentrations. In general, IBA was observed to induce a strong rooting response; this has been used to promote rooting in a wide range of plant species and is readily available around the world. The rooting of this plant was significantly affected by the concentration of IBA. The best rooting response was obtained in a medium containing 0.5 μM IBA. The success of IBA in promoting efficient root induction has been reported for Swaisona formosa (Jusaitis 1997), Hemidesmus indicus (Sreekumar et al. 2000), Cunila galoides (Fracaro and Echeverrigaray 2001), Holostemma ada-kodien (Martin 2002), Ceropegia candelabrum (Beena et al. 2003), and Mucuna pruriens (Faisal et al. 2006).
The period of transition during the process of hardening after transfer from the in vitro to the ex vitro environment is considered to be the most important step in tissue culture. Due to the heterotrophic mode of nutrition, a lack of adaptation or exposure to the outside environment, during lab to land transfer micropropagated plants are first placed in the hardening chamber. In general, during the period of hardening care is taken over the physical (temperature, light intensities, relative humidity, air current, atmospheric CO2) and other factors (mineral nutrition, pH and texture of soil) employed. One important factor during acclimatization is the type of potting material used. Of the three different types of planting substrates used (garden soil, soilrite and vermiculite), the highest survival rates for the micropropagated plants were achieved in vermiculite.
In conclusion, we have established a direct in vitro culture system for an important medicinal plant, T. indica, which should enable the large-scale nursery production of this valuable medicinal plant for the landscape and for pharmaceutical industries.
Abbreviations
- AA:
-
Ascorbic acid (AA)
- BA:
-
6-Benzyladenine
- IAA:
-
Indole-3-acetic acid
- IBA:
-
Indole-3-butyric acid
- Kin:
-
Kinetin
- NAA:
-
α-Naphthalene acetic acid
- MS:
-
Murashige and Skoog medium
- TDZ:
-
Thidiazuron
References
Ahmad N, Faisal M, Anis M (2006) Effect of ascorbic acid on in vitro axillary shoot multiplication of Capsicum annuum L. Physiol Mol Biol Plants 12:101–105
Arya V, Shekhawat NS, Singh RP (2003) Micropropagation of Leptadenia reticulata—a medicinal plant. In Vitro Cell Dev Biol Plant 39:180–185
Beena MR, Martin KP, Kirti PB, Hariharan M (2003) Rapid in vitro propagation of medicinally important Ceropegia candelabrum. Plant Cell Tiss Org Cult 72:285–289
Chandrasekhar T, Hussian MT, Gopal GR, Rao JVS (2006) Somatic embryogenesis of Tylophora indica (Burm. f.) Merril., an important medicinal plant. Int J App Sci Eng 4:33–40
CSIR (2003) The wealth of India: a dictionary of Indian raw materials and industrial products, vol 10. Council of Scientific and Industrial Research, New Delhi, pp 398–399
Faisal M, Siddique I, Anis M (2006) In vitro rapid regeneration of plantlets from nodal explants of Mucuna pruriens: a valuable medicinal plant. Ann App Biol 148:1–6
Fracaro F, Echeverrigaray S (2001) Micropropagation of Cunila galioides, a popular medicinal plant of south Brazil. Plant Cell Tiss Org Cult 64:1–4
Gopalakrishnan C, Shankaranarayan D, Kameswaran L (1980) Pharmacological investigations of tylophorine, the major alkaloid of Tylophora indica. Indian J Med Res 69:513–520
Hiregoudar LV, Murthy HN, Hema BP, Hahn EJ, Paek KY (2003) Multiple shoot induction and plant regeneration of Feronia limonia (L.) Swingle. Sci Hortic 98:357–364
Hiregoudar LV, Murthy HN, Bhat JG, Nayeem A, Hema BP, Hahn EJ, Paek KY (2006) Rapid clonal propagation of Vitex trifolia. Biol Plant 50:291–294
Jeong JH, Murthy HN, Paek KY (2001) High frequency adventitious shoot induction and plant regeneration from leaves of statice. Plant Cell Tiss Org Cult 65:123–128
Jusaitis M (1997) Micropropagation of adult Swaisona formosa Leguminosae: Papilionoideae: Galegeae. In Vitro Cell Dev Biol Plant 33:213–220
Loc HN, Due TD, Kwon HT, Yang SM (2005) Micropropagation of zedoary (Curcuma zedoaria Roscoe): a valuable medicinal plant. Plant Cell Tiss Org Cult 81:119–122
Mao AH, Wetten A, Fay M, Caligari PDS (1995) In vitro propagation of Clerodendrum colebrookianum Walp; a potential natural anti-hypertension medicinal plant. Plant Cell Rep 14:493–496
Martin KP (2002) Rapid propagation of Holostemma ada-kodien Schult., a rare medicinal plant, through axillary bud multiplication and indirect organogenesis. Plant Cell Rep 21:112–117
Mulchandani NB, Iyer SS, Badheka LP (1971) Structure of tylophorinidine: a new potential antitumor alkaloid from Tylophora indica. Chem Ind 19:505–506
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15:473–497
Patil VM (1998) Micropropagation studies in Ceropegia spp. In Vitro Cell Dev Biol Plant 34:240–243
Pattnaik SK, Chand PK (1996) In vitro propagation of the medicinal herb Ocimum americanum L. Syn. O. canum sims (hoary basil) and Ocimum sanctum (holy basil). Plant Cell Rep 15:846–850
Reddy PS, Gopal GR, Sita GL (1998) In vitro multiplication of Gymnema sylvestre R. Br.: an important medicinal plant. Curr Sci 75:843–845
Sharma N, Chandel KPS (1992) Effect of ascorbic acid on axillary shoot proliferation of Tylophora indica (Burm f.) Merrill. Plant Cell Tiss Org Cult 29:109–113
Sreekumar S, Seeni S, Pushpangadan P (2000) Micropropagation of Hemidesmus indicus for cultivation and production of 2-hydroxy 4-methyl benzaldehyde. Plant Cell Tiss Org Cult 62:211–218
Stefaan P, Werbrouck O, Debergh PC (1994) Applied aspects of plant regeneration. In: Dixon RA, Gonzales RA (eds) Plant cell culture: a practical approach. Oxford University Press, London, pp 127–145
Sudha GC, Krishnan PN, Pushpangadan P (1998) In vitro propagation of Holostemma annulare (Roxb.) K. Schum. A rare medicinal plant. In Vitro Cell Dev Biol Plant 33:57–63
Acknowledgments
M. Faisal is thankful to the Department of Science and Technology, Govt. of India, New Delhi for its award of a SERC Fast Track Scheme.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Faisal, M., Ahmad, N. & Anis, M. An efficient micropropagation system for Tylophora indica: an endangered, medicinally important plant. Plant Biotechnol Rep 1, 155–161 (2007). https://doi.org/10.1007/s11816-007-0025-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-007-0025-4