Abstract
Transgenic plants of Tylophora indica obtained via somatic embryogenesis through genetic transformation by Agrobacterium rhizogenes have been maintained in vitro for over 6 years and the comparison of long term cultures and field grown normal and transformed plants on various morphological, biochemical and molecular characterisation described. Ri-transformed plants were found to be morphologically stable after 6 years in axenic culture as well as after transfer to the field. Genetic stability for rolA, rolB, rolC and rolD genes were obtained in Ri-transformed plants prior to and after transfer to field. The chromosome number, 2n = 22 was maintained in all the transgenic plants. The Ri-transformed plants retained and expressed all the four rol genes after transfer to the field and were able to synthesise and accumulate tylophorine. Thus, the Ri-transformed plants were found to be stable for more than 6 years in vitro and after 1year of field transfer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tylophora indica (Burm.f.) Merrill (Asclepiadaceae) is a perennial climber native to South and Southeast Asia. This indigenous medicinal plant has been traditionally used for the treatment of a number of diseases including bronchial asthma, jaundice, dermatitis, etc. [8, 24]. Tylophorine, the major alkaloid and tylophorinidine, the minor alkaloid have been proved to have several therapeutic properties including- anti-inflammatory, anti-tumor, immunosuppressive, anti-amoebic, anti-candidal, antileukemic, etc. [14, 16, 18].
Agrobacterium rhizogenes infects higher plants and causes hairy root disease by transferring its T-DNA from the root-inducing (Ri) plasmid to the host genome. The Ri plasmids of agropine strains of A. rhizogenes carries two separate T-DNA regions- the TL-DNA and the TR-DNA [29, 48]. During infection process, these two T-DNA regions are known to be transferred independently to the host plant genome [46]. TR-DNA shows homology with the T-DNA of Ti plasmid. The TR-DNA contains auxin synthesising genes (aux1 and aux2), mannopine synthesising genes (mas1 and mas2) and agropine synthesising gene (ags). In the TL-DNA, four genes are important for root induction and hairy root morphology known as root loci. The four rol genes- rolA, rolB, rolC and rolD corresponds to the ORFs 10, 11, 12 and 15 respectively [29, 40, 48]. The hairy roots formed at the infection sites can be excised to establish axenic root cultures and can be indefinitely grown in phytohormone free medium. Regeneration of Ri-transformed plants from the hairy root cultures is reported in number of plant species, which can be either spontaneous or induced by phytohormones, as reviewed earlier [11, 12, 30]. We have [5, 6] reported earlier the genetic transformation of T. indica with A. rhizogenes agropine strain A4, and spontaneous regeneration of Ri-transformed plants in the species for the first time.
Analysis of Ri-transformed plants in long term culture is reported in a very few species [31]. But there are some reports of instability in the Ri-transformed plants like spontaneous deletion of T-DNAs in Ri-transformed potato plants [22] and somaclonal variation among the Ri-transformed plants in Hyoscyamus muticus [39].
In the present study we evaluated the morphological, molecular, cytological and biochemical stability of Ri-transformed plants of T. indica maintained in vitro for more than 6 years in axenic culture and characterised them after transfer to field.
Materials and methods
Plant materials
Axenic Ri-transformed plants of Tylophora indica regenerated from A4-transformed roots of T. indica [6] were used for the present study. The plants were maintained for more than 6 years in axenic culture by regular subculture on Murashige and Skoog’s [28] basal medium (MS) supplemented with 3 % (w/v) sucrose and 0.75 % (w/v) agar at 24 ± 1 °C at approximately 50–60 % relative to humidity in the presence of light (with a 16/8 h light/dark photoperiod provided by cool-white fluorescent tubes at an intensity of 48 μmol m−2 s−1). The plants were sub cultured at an interval of every 4 weeks by culturing nodal and shoot tip explants on solid MS medium in presence of light. Non-transformed plants of T. indica [4] were maintained and used for present study in similar way.
Morphological characterisation of Ri-transformed plants in vitro
Morphological characterisation of the non-transformed and Ri-transformed plants were performed based on morphological descriptors such as the length of shoots, number of nodes / plant, length of internodes, presence / absence of wrinkled leaves, leaf length, presence / absence of aerial roots, number of axillary shoots / plant, number of primary roots / plant, lateral density of roots (number of laterals/cm), among others [6, 21, 25, 38].
For this study, shoot tips (approximately 2 cm long) of axenic Ri-transformed plants were cultured on 20 ml of solid MS for 12 weeks in culture tubes (15 × 2.5 cm) under a 16/8 h (light/dark) photoperiod at 24 ± 1 °C. One shoot tip was cultured per culture tube. At the end of 12 weeks, the plants were harvested, washed, blotted dry, fresh weight (FW) determined and morphological data taken. Then plants were oven-dried (55 °C) to obtain the dry weight (DW). Shoot tips of non-transformed plants of T. indica were used as control. For each experiment 30 explants were used and repeated thrice.
Transfer of Ri-transformed plants to field
The Ri-transformed plants maintained in vitro for over 6 years were also transferred to the field for morphological and molecular study. The shoot tips of the transgenic plants, approximately 2 cm long, were cultured on solid MS for 8 weeks in presence of light in a growth chamber (n = 50). After 8 weeks, the plants were taken out of the culture tubes, washed thoroughly with water and labelled as individual plants. Morphological characterisation of the plants was done based on the morphological descriptors mentioned for in vitro plants. The plants were then transferred to small pots with soilrite. The potted plants were watered, covered with plastic bags and kept on a tray with approximately 2.5 cm. of water. They were kept under a 16/8 h (light/dark) photoperiod at room temperature for 1 month. After 1 month, the plastic covers were removed and the pots were shifted to a polyhouse. Three days after being shifted to the polyhouse, the plants were taken out of soilrite and transferred to larger pots (15 cm. diameter) with soil. Morphological analysis was performed at every step and thereafter every month for 1 year. Non-transformed plants were similarly grown and used as control.
Molecular characterisation of Ri-transformed plants
Molecular characterisation of the Ri-transformed plants was performed based on the presence and/or absence of different T-DNA genes, i.e., the rolA, rolB, rolC and rolD genes of TL-DNA and the TR-DNA, by PCR analysis. Genomic DNA was extracted according to the procedure published by Dellaporta et al. [13] from the leaves and roots of in vitro cultures of Ri-transformed plants and from the leaves of plants after 1 year of transfer to field. Plasmid DNA (pLJ1 for TL-DNA and pLJ85 for TR-DNA), isolated following the standard alkali lysis method [33] was used as a positive control. DNA of the non-transformed plants was used as negative control. Isolated DNA was analysed by PCR using rolA [3], rolB [47], rolC [6, 38], rolD [9] and TR-DNA [2] specific primers (Table 1). To eliminate the chances of false positive PCR products due to bacterial contamination, Vir D1-specific primers were used which is present outside the T-DNA of Ri plasmid and not transferred to the host plant genome [1]. Each experiment was repeated three times.
Genomic DNA was quantified spectrophotometrically. DNA was amplified in a final volume of 25 μl containing approximately 100 ng of template DNA, 0.25 μM of each primer (forward and reverse), 100 μM of each dNTP, 2.5 μl of 10X Taq DNA Polymerase Buffer A (GeNei™) containing 1.5 mM (final concentration) of MgCl2 and 1.5 U Taq DNA polymerase (GeNei™). The PCR amplification was performed in a programmed thermal cycler (Gene Amp® PCR system 2400; Perkin Elmer, Foster City, Calif). The samples were subjected to an initial denaturation step at 94 °C for 5 min, followed by 35 cycles at 94 °C (denaturation), 30 s at the specified annealing temperature for each gene fragment (Table 1) and 1 min extension at 72 °C. This was followed by a final extension at 72 °C for 7 min. The amplified products were resolved on an agarose gel (1.2 % w/v), visualised by ethidium bromide staining under UV light and documented using the BioRad Gel Doc™ EZ Imager.
Gene expression analysis by RT-PCR
The reverse transcriptase polymerase chain reaction (RT-PCR) was performed to analyse the expression of the transgenes at the transcription level. Total RNA was extracted with the TRIzol® Reagent (Invitrogen, USA) [7] from the leaves of the Ri-transformed plants both from in vitro culture as well as from plants transferred to the field after 1 year. The isolated RNA was quantified spectrophotometrically. cDNA was synthesized for all the genes (rolA, rolB, rolC and rolD genes of TL-DNA) using gene specific primers in 20 μl reaction volume containing 2 μg total RNA, 20 pmol primers, 5X buffer, 10 mM dNTP mix, 200 U RevertAid™ M-MuLV RT and 20 U ribonuclease inhibitor. Prior to adding the RT enzyme, the reaction mix was heated at 65 °C for 5 min and after this RT was added and the reaction mixture was incubated at 42 °C for 50 min for cDNA synthesis. The reaction was stopped by heating at 75 °C for 5 min. Finally, 2 μl cDNA samples were used for PCR analysis following the same procedure described above.
Extraction and analysis of tylophorine by HPTLC
Leaves of Ri-transformed plants and non-transformed 1 year old plants growing in the polyhouse were oven-dried at 55 °C to constant weight and powdered. 500 mg dried tissue of each sample was defatted with 20 ml n-Hexane (AR grade; Spectrochem) for 48 h in 100 ml conical flasks. The residue was extracted with 20 ml methanol (HPLC grade; Spectrochem) for 72 h. The extract was concentrated under vacuum, and dissolved in 5 ml HPLC grade methanol [19, 20].
Chromatography was performed on 20 × 10 cm pre-coated silica gel 60 F254 TLC plates (Merck) of 0.25 mm layer thickness. A CAMAG HPTLC system (Muttenz, Switzerland) comprising a Linomat-5 automated sample applicator equipped with a 100 μl syringe, CAMAG TLC scanner with winCATS software (version: 1.4.6), a UV cabinet and a twin-trough glass tank was used for the analysis. Standard solution of tylophorine (Enzo Life Sciences) was prepared (0.5 mg/ml) to construct a calibrated graph by plotting peak areas versus amount of tylophorine injected over a range of 20–200 ng. Known amounts of samples and standards were applied to the plates as bands of 6 mm width, 8 mm from the bottom of the plate, by the use of the CAMAG Linomat-5 automated TLC applicator, with nitrogen flow. Plates were developed with toluene: ethyl acetate: diethylamine (7:2:1, v/v/v) as mobile phase, in a tank pre-saturated with mobile phase vapour for 30 min. The development distance was 8.5 cm. After development, the plate was removed, dried, and spots were visualised under UV light. Quantitative evaluation of the plates were performed in reflectance/absorbance mode at λ = 254 nm and 290 nm with a slit dimension of 5 × 0.45 mm, scan speed of 20 mm/s and data resolution at 100 μm/step. Post chromatographic derivatization was done with Dragendorff’s reagent and scanned at 560 nm. The samples were extracted and analyzed in triplicate. The tylophorine content was expressed as mg gDW−1.
Cytological analysis of Ri-transformed plants
Chromosome number was studied in the Ri-transformed plants along with the non-transformed plants in vitro. Fresh root tips were obtained from the in vitro plants grown on solid MS. The root tips were pre-treated in saturated solution of paradichlorobenzene at 18 °C for 4 h and then fixed in 3:1 absolute ethanol: propionic acid and stored at −18 °C. The root tips were then transferred to 45 % propionic acid for 15 min and stained in mixture of 2 % propiono-orcein: 1(N) HCl (9:1) overnight before squashing in 45 % propionic acid on glass slides to obtain plates with countable chromosomes. Root tips were obtained from 30 randomly selected the Ri-transformed plants. Data on chromosome number were based on at least 30 scattered metaphases/5 root tips/plant.
Statistical analysis
All of the experiments were randomised and were repeated at least twice. Data were examined by a one-way analysis of variance (ANOVA) to detect significant differences (p ≤ 0.05) in the mean [41]. A post hoc mean separation was performed by the Tukey’s multiple comparison test at the same 5 % probability level using SPSS software (version 16.0). Variability in the data was expressed as the mean ± standard deviation (SD).
Results
Morphological characterisation of Ri-transformed plants in vitro
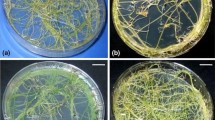
The Ri-transformed plants, maintained in vitro for more than 6 years showed stable morphological characteristics, retaining the various altered characteristics that are typically observed in a Ri-transformed plant (Fig. 1). The transgenic plants showed significant (p ≤ 0.05) reduction in length of shoot, length of leaves and internode size compared to non-transformed controls (Table 2). The leaves of the transformed plants were narrow, wrinkled and smaller than non transformed plants. There was significant (p ≤ 0.05) increase in the number of nodes, number of leaves and number of axillary shoots in the Ri-transformed plants as compared to the non-transformed ones. The Ri-transformed plants in long term culture showed an extensive root system with highly branched and plagiotropic roots which often appeared on or above the medium surface. The lateral density of the roots increased significantly (p ≤ 0.05) by 2.4 times than the non-transformed plants.
Non-transformed and Ri-transformed plants of T. Indica after culturing shoot tips on MS medium for 8 weeks under 16/8 h light/dark cycles at 24 ± 1 °C a non-transformed plant, b, c, d, e, f Ri-transformed plants. Note smaller size of leaf and internode, increased number of nodes and leaves, wrinkled leaves, and highly branched roots in Ri-transformed plants
The FW and DW accumulation of the Ri-transformed plants also significantly (p ≤ 0.05) varied from the non-transformed plants after 12 weeks of culture. The FW and DW were approximately 2.94 times and 2.32 times higher in Ri transformed rooted plants than the non-transformed rooted plants respectively. The roots of the Ri-transformed plants also showed higher FW (4.1 times) and DW (3.85 times) accumulation compared to the non-transformed ones. The root system of the transformed plants showed nearly two times biomass accumulation compared to the shoot. The evaluation of morphological features of the Ri-transformed plants of T. indica suggests morphological stability [6] in the Ri-transformed plants even after 6 years of maintenance in vitro.
Morphological stability of Ri-transformed plants after transfer to field
Fifty Ri-transformed plants were transferred to the soil and maintained in polyhouse conditions for 1 year. These plants exhibited most of the transgenic morphological characteristics even after 12 months in polyhouse conditions after transfer to the field (Table 3). The internode length of the Ri-transformed plants was smaller than that of the non-transformed controls and increased number of leaves and nodes were present in the Ri-transformed plants (Fig. 2). One hundred percent of the transgenic plants produced axillary shoots (range of axillary shoots/ plant = 1–5/plant) compared to 16 % in the non-transformed plants. The axillary buds of the Ri-transformed plants were more prominent than those of the non-transformed plants (Fig. 3a, b). Aerial roots were present in very few plants after 6 months of transfer to the field (Fig. 3c). Most of the Ri-transformed plants exhibited small expanded leaves. To the best of our knowledge, this is the first report of successful transfer and maintenance of Ri-transformed plant of T. indica in the field.
Morphology of non-transformed (a, d) and Ri-transformed (b, c, e) plants after 6 months (a, b, c) and 1 year (d, e) of transfer to field under polyhouse conditions. Note smaller internode and leaf size, increased number of nodes and leaves, increase in axillary shoot (arrow) in Ri-transformed plants
a Nodes of non-transformed plant without prominent axillary bud (arrow) (bar = 0.3 cm); b nodes of Ri-transformed plant with prominent axillary buds (arrow) after 1 month of transfer to soil (bar = 0.25 cm); c aerial roots (arrow) on Ri-transformed plant after 6 months of transfer to field (bar = 1 cm)
Molecular characterisation of Ri-transformed plants in vitro
Molecular characterisation of the Ri-transformed plants in vitro, was done by PCR analysis of different T-DNA genes. PCR analysis with the genomic DNA from leaves and roots of Ri-transformed plants demonstrated the presence of all four rol genes- rolA, rolB, rolC and rolD genes of TL-DNA and absence of TR-DNA (Fig. 4). The Ri-transformed plants regenerated from transformed roots were also reported to be TL-DNA positive and TR-DNA negative earlier by us [5, 6]. Hence, none of the T-DNA genes were lost during 6 years of maintenance in axenic culture in Ri-transformed plants studied. All the PCR products were of expected size, and were identical to those of positive control for all the genes studied. No PCR amplification was observed in negative control (i.e. genomic DNA of non-transformed plant). Absence of VirD1 specific amplified product confirmed the absence of bacterial contamination in the Ri-transformed plants in vitro.
Agarose [1.2 % (w/v)] gel electrophoresis of PCR products with rolA-, rolB-, rolC-, and rolD-specific primers. Lane 1: molecular markers (100-bp plus DNA ladder); Lane 2: positive control (pLJ1, containing TL-DNA); Lane 3: negative control (genomic DNA from non-transformed plant); Lanes 4–8: genomic DNA of Ri-transformed plants maintained for 6 years in vitro; Lane 9–13: genomic DNA of Ri-transformed plants after 1 year of transfer to field
The results from the PCR analysis were also confirmed by the simultaneous RT-PCR results (Fig. 5). Expression of all the rol genes (rolA, rolB, rolC and rolD genes of TL-DNA) at transcriptional level was observed in the plants after 6 years of maintenance in axenic culture. All the PCR products were of expected size and corresponded to positive controls (pLJ1 plasmid). The RT-PCR analysis revealed that different rol genes were not only present but were also expressed at transcriptional level in the Ri-transformed plants in long term culture.
Expression of rol genes at the transcriptional level in Ri-transformed plants observed by RT-PCR using rolA-, rolB-, rolC- and rolD- specific primers. Lane 1: molecular markers (100 bp plus DNA ladder); Lane 2: positive control (pLJ1, containing TL-DNA); Lane 3: negative control (genomic DNA of non-transformed plant); Lanes 4–5: amplified cDNAs of Ri-transformed plants maintained for 6 years in vitro; Lanes 6–7: amplified cDNAs of Ri-transformed plants after 1 year of transfer to field
Molecular characterisation of Ri-transformed plants in field
PCR and RT-PCR for rolA, rolB, rolC and rolD genes of TL-DNA and the TR-DNA was performed for the Ri-transformed plants after 6 year of transfer to field. PCR analysis of different T-DNA genes with the genomic DNA isolated from the leaves of the transgenic plants demonstrated the presence of all rol genes (rolA, rolB, rolC and rolD) and absence of TR-DNA as was found in the plants in vitro (Fig. 4). RT-PCR analysis of the total RNA isolated from the leaves of these plants also supported the fact that the rol genes were not only present but were also expressed at the transcriptional level after 1 year of transfer to the soil (Fig. 5).
Analysis of chromosome number and tylophorine content in Ri-transformed plants
Ri-transformed plants maintained in vitro for 6 years showed stable chromosome number of 2n = 22, which is the normal diploid chromosome number of this species (Fig. 6). From the chromosome study we can suggest cytological stability in the Ri-transformed plant in long term culture.
Tylophorine was detected in both the non-transformed plants as well as the Ri-transformed plants after 1 year of transfer to field. Tylophorine content was found to be significantly (p ≤ 0.05) higher in the leaves of the Ri-transformed plants (3.75 ± 0.12 mg gDW−1) compared to the non-transformed ones (1.81 ± 0.15 mg gDW−1). Thus the analysis shows continued ability of the 6 years old cultures of Ri-transformed plants to synthesise tylophorine (Fig. 7).
Tylophorine content in the leaves of non-transformed (NT) and Ri-transformed plants (Ri-T) after 1 year of transfer to field. Values represent mean ± standard deviation of three independent experiments (n = 30). Bars with the different letters are significantly different at (p ≤ 0.05) according to ANOVA
Discussion
Regeneration of whole viable plants from hairy root cultures, established from transformation with A. rhizogenes, has been reported in a number of plant species [30]. Such transgenic plants frequently show a very characteristic phenotype which differ from their normal counterparts, such as, wrinkled leaf, shortened internodes, decreased apical dominance, altered flower morphology, increase in number of branches, reduced pollen and seed production, and abundant production of highly branched plagiotropic roots. In addition to the above-mentioned changes, biennial species frequently become annuals on transformation and regeneration with A. rhizogenes [23, 43, 45].
Detailed analysis of Ri-transformed plants in long term culture is reported in a very few plant species. Development of transgenic medicinal plants is an important area of research and Tylophora indica is widely used medicinal plant. Simultaneously long term studies are essential to establish credibility of such newly developed plants. The current paper describes the comparison of long term cultures and field grown normal and transformed plants on various morphological, biochemical and molecular characterisation. The main achievement is evaluation of long term cultures and plants.
In the present study, the Ri-transformed plants of T. indica were found to be morphologically stable in long term cultures maintained in vitro for more than 6 years, retaining the transgenic characters typical for the Ri-transformed plants [12, 15]. The altered characters of Ri-transformed plants are due to the combined expression of rolA, rolB and rolC genes. The rolA gene is responsible for the decrease in the length of internode and leaf wrinkling. The rolB gene is associated with the increase in adventitious roots in the stem, in addition to reduction in length of stamens and protruding stigmas. The rolC gene causes internode shortening, reduced apical dominance and increase of axillary branches [29, 45]. The PCR and RT-PCR analysis of Ri-transformed T. indica plants maintained in vitro showed stable presence of all the four rol genes as well as their expression at the transcriptional level in the long term culture. Genetic stability of six-year-old transgenic kiwi plants has been reported [31]. However, instability in long term cultures of Ri-transformed plants is also reported. Spontaneous deletion of TL-DNA and TR-DNA during long term culture and regeneration is reported in Solanum tuberosum [22]. Somaclonal variation among the Ri-transformed plants is reported in H. muticus [39].
In the present study, the Ri-transformed plants of T. indica were successfully acclimatised to polyhouse conditions with a success rate greater than 93 %. This is the first report of successful acclimatisation of Ri-transformed T. indica plants to soil. The plants showed retention of most of the transgenic morphological characteristics, and retained and expressed the rol genes suggesting genetic stability of the Ri-transformed plants even after 1 year of transfer to the field. However, wrinkled leaves and aerial roots were noted in very few plants. The majority of the leaves were expanded, such as those of normal plants, but smaller than untransformed plants. The retention of transgenic characteristics after greenhouse transfer is reported in a number of plants including Datura arborea [17], Limonium hybrid [27], and Kalanchoe blossfeldiana [10]. In D. arborea, some clones are reported to exhibit a plant height comparable to the control after 6 months in the greenhouse [17]. The absence of leaf wrinkling in Ri-transformed plants is reported in Pelargonium graveolens, although other transformed characters were retained after transfer to soil [37]. The disappearance of leaf wrinkling after transfer to soil has been observed in Plumbago rosea, although other characteristics such as the reduced leaf size and internode size were retained [36]. Morphological characterisation of Ri-transformed plants after 6 months of their growth in glass house is reported in Rauwolfia serpentina, where 90 % of these plants showed no phenotypic difference with the normal plants, while 10 % showed stumpy and poor growth [26].
A limited number of reports are available regarding analysis of secondary metabolites in the Ri-transformed plants and even fewer for Ri-transformed plants after field transfer, although extensive studies have been conducted with hairy root cultures of different plant species. In the present study, the long term cultures of Ri-transformed plants of T. indica were found to retain their capability to synthesise higher content of the alkaloid tylophorine compared to the non-transformed plants even after 1 year of field transfer. High capacity of secondary metabolite production stably maintained in clonal regenerants of Ri-transformed plants after field transfer is also reported in Ajuga reptans, although not after long term culture [44]. Saxena et al. [37] reported Ri-transformed plants of P. graveolens synthesising essential oils after 5 months of transfer to the soil. High reserpine productivity was also noted in R. serpentina after 6 months of glass house transfer [26]. Chromosome count in the long term cultures of Ri-transformed plants of T. indica was found to be 2n = 22. The normal diploid chromosome number in the wild type plants of this species is also reported to be 2n = 22 [32, 34, 35, 42]. Hence, no change in chromosome number was observed in the long term cultures of these Ri-transformed plants studied.
Therefore, from the present study we can conclude that the Ri-transformed plants of T. indica showed genetic stability after 6 years in vitro culture as well as after 1 year of field transfer.
References
Alpizar E, Dechamp E, Lapeyre-Montes F, Guilhaumon C, Bertrand B, Jourdan C, et al. Agrobacterium rhizogenes-transformed roots of Coffee (Coffea arabica): Conditions for long-term proliferation and morphological and molecular characterization. Ann Bot. 2008;101:929–40.
Bandyopadhyay M, Jha S, Tepfer D. Changes in morphological phenotypes and withanolide composition of Ri-transformed roots of Withania somnifera. Plant Cell Rep. 2007;26:599–609.
Bonhomme V, Laurain-Mattar D, Lacoux J, Fliniaux M, Dubreuil AJ. Tropane alkaloid production by hairy roots of Atropa belladonna obtained after transformation with Agrobacterium rhizogenes 15834 and Agrobacterium tumefaciens containing rol A, B, C genes only. J Biotechnol. 2000;81:151–8.
Chaudhuri KN, Ghosh B, Jha S. The root: a potential new source of competent cells for high-frequency regeneration in Tylophora indica. Plant Cell Rep. 2004;22:731–40.
Chaudhuri KN, Ghosh B, Tepfer D, Jha S. Genetic transformation of Tylophora indica with Agrobacterium rhizogenes A4: growth and tylophorine productivity in different transformed root clones. Plant Cell Rep. 2005;24:25–35.
Chaudhuri KN, Ghosh B, Tepfer D, Jha S. Spontaneous plant regeneration in transformed roots and calli from Tylophora indica: changes in morphological phenotype and tylophorine accumulation associated with transformation by Agrobacterium rhizogenes. Plant Cell Rep. 2006;25:1059–66.
Chomcznski P, Sacchi N. Single step method of RNA isolation by acid guanidinium thiocyanate phenol chloroform extraction. Ann Biochem. 1987;162:156–9.
Chopra IC, Chopra RN, Nayar SL. Glossary of Indian medicinal plants. New Delhi: National Institute of Science Communication; 1996.
Christensen B, Sriskandarajah S, Serek M, Müller R. Transformation of Kalanchoe blossfeldiana with rol-genes is useful in molecular breeding towards compact growth. Plant Cell Rep. 2008;27:1485–95.
Christensen B, Sriskandarajah S, Müller R. Biomass distribution in Kalanchoe blossfeldiana transformed with rol-genes of Agrobacterium rhizogenes. Hort Sci. 2009;44(5):1233–7.
Christey MC. Use of ri-mediated transformation for production of transgenic plants. In Vitro Cell Dev Biol Plant. 2001;37:687–700.
Christey MC. Transgenic crop plants using Agrobacterium rhizogenes-mediated transformation. In: Doran PM, editor. Hairy roots: Culture and applications. Amsterdam: Harwood Academic Publishers; 1997. p. 99–110.
Dellaporta SL, Woods J, Hicks JB. A plant DNA minipreparation: version 2. Plant Mol Biol Rep. 1983;1:19–22.
Donaldson GR, Atkinson MR, Murray AW. Inhibition of protein synthesis in Ehrlich ascites-tumor cells by the phenanthrene alkaloids tylophorine, tylocrebrine and cryptopleurine. Biochem Biophys Res Commun. 1968;31:104–9.
Fladung M. Transformation of diploid and tetraploid potato clones with the rolC gene of Agrobacterium rhizogenes and characterization of transgenic plants. Plant Breed. 1990;104:295–304.
Gellert E. The indolizidine alkaloids. J Nat Prod. 1982;45:50–73.
Giovannini A, Pecchioni N, Rabaglio M, Allavena A. Characterization of ornamental Datura plants transformed by Agrobacterium rhizogenes. In Vitro Cell Dev Biol Plant. 1997;33:101–6.
Gopalakrishnan C, Shankaranarayan D, Nazimudeen SK, Kameswaran L. Effect of tylophorine, a major alkaloid of Tylophora indica, on immunopathological and inflammatory reactions. Indian J Med Res. 1980;71:940–8.
Gupta AK, Tandon N, Sharma M. Quality standards of Indian medicinal plants. Vol.1. Indian Council of Medical Research; 2003.
Gupta R, Datta A, Shri R. Extraction process optimization of tylophorine from Tylophora asthmatica Wight & Arn. Pharmacogn J. 2012;4(28):19–23.
Gutièrrez-Pesce P, Taylor K, Muleo R, Rugini E. Somatic embryogenesis and shoot regeneration from transgenic roots of the cherry rootstock Colt (Prunus avium x P. pseudocerasus) mediated by pRi 1855 T-DNA of Agrobacterium rhizogenes. Plant Cell Rep. 1998;17:574–80.
Hänisch TCCH, Loonen AEHM, Ottaviani MR, Ennik L, van Eldik G, Stiekema WJ. Frequent spontaneous deletions of Ri T-DNA in Agrobacterium rhizogenes transformed potato roots and regenerated plants. Plant Mol Biol. 1990;14:735–41.
Kamada H, Saitou T, Harada H. No requirement of vernalization for flower formation in Ri-transformed Cichorium plants. Plant Tiss Cult Lett. 1992;9:206–8.
Kirtikar KR, Basu BD. Indian medicinal plants, vol. 3rd volume. New Delhi: Periodic Experts Book Agency; 1991. p. 1631–2.
Majumdar S, Garai S, Jha S. Genetic transformation of Bacopa monnieri by wild type strains of Agrobacterium rhizogenes stimulates production of bacopa saponins in transformed calli and plants. Plant Cell Rep. 2011;30(5):941–54.
Mehrotra S, Goel MK, Rahman LU, Kukreja AK. Molecular and chemical characterization of plants regenerated from Ri-mediated hairy root cultures of Rauwolfia serpentina. Plant Cell Tissue Org Cult. 2013. doi:10.1007/s11240-013-0302-6.
Mercuri A, Bruna S, Benedetti LD, Burchi G, Schiva T. Modification of plant architecture in Limonium spp. induced by rol genes. Plant Cell Tissue Org Cult. 2001;65:247–53.
Murashige T, Skoog F. A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol Plant. 1962;15:473–97.
Nilsson O, Olsson O. Getting to the root: the role of the Agrobacterium rhizogenes rol genes in the formation of hairy roots. Physiol Plant. 1997;100:463–73.
Roychowdhury D, Majumder A, Jha S. Agrobacterium rhizogenes-mediated transformation in medicinal plants: prospects and challenges. In: Chandra S et al., editors. Biotechnology for medicinal plants. Berlin Heidelberg: Springer; 2013. p. 29–68.
Rugini E, Caricato G, Muganu M, Taratufolo C, Camilli M, Cammilli C. Genetic stability and agronomic evaluation of six-year-old transgenic kiwi plants for rolABC and rolB gene. Acta Hort. 1997;447:609–10.
Samaddar T, Nath S, Halder M, Sil B, Roychowdhury D, Sen S, et al. Karyotype analysis of three important traditional Indian medicinal plants, Bacopa monnieri, Tylophora indica and Withania somnifera. Nucl. 2012;55(1):17–20.
Sambrook J, Russel DW. Molecular cloning: a laboratory manual. 3rd ed. Cold Spring Harbour: Cold Spring Harbour Press; 2001.
Sarkar AK, Datta N, Chatterjee U, Datta R. In IOPB chromosome number reports XLII. Taxon. 1973;25:631–49.
Sarkar AK, Datta N, Chatterjee U. In chromosome number reports LXVII. Taxon. 1980;29:347–67.
Satheeshkumar K, Jose B, Soniya EV, Seeni S. Isolation of morphovariants through plant regeneration in Agrobacterium rhizogenes induced hairy root cultures of Plumbago rosea L. Indian J Biotechnol. 2009;8(4):435–41.
Saxena G, Banerjee S, Rahman L, Verma PC, Mallavarapu GR, Kumar S. Rose-scented geranium (Pelargonium sp.) generated by Agrobacterium rhizogenes mediated Ri-insertion for improved essential oil quality. Plant Cell Tissue Org Cult. 2007;90:215–23.
Sevón N, Dräger B, Hiltunen R, Oksman-Caldentey KM. Characterization of transgenic plants derived from hairy roots of Hyoscyamus muticus. Plant Cell Rep. 1997;16:605–11.
Sevón N, Hiltunen R, Oksman-Caldentey KM. Somaclonal variation in transformed roots and protoplast-derived hairy root clones of Hyoscyamus muticus. Planta Med. 1998;64:37–41.
Slightom JL, Durand-Tardif M, Jouanin L, Tepfer D. Nucleotide sequence analysis of TL-DNA of Agrobacterium rhizogenes agropine type plasmid. J Biol Chem. 1986;261:108–21.
Sokal RR, Rohlf FJ. Introduction to biostatistics. New York: WH Freeman; 1987.
Sreedevi P, Namboodiri AN. In IOPB chromosome number reports LVI. Taxon. 1977;26:257–74.
Sun LY, Touraud G, Charbonnier C, Tepfer D. Modification of phenotype in Belgian endive (Cichorium intybus) through genetic transformation by Agrobacterium rhizogenes: conversion from biennial to annual flowering. Transgenic Res. 1991;1:14–22.
Tanaka N, Matsumoto T. Regenerants from Ajuga hairy roots with high productivity of 20-hydroxyecdysone. Plant Cell Rep. 1993;13:87–90.
Tepfer D. Genetic transformation of several species of higher plants by Agrobacterium rhizogenes: phenotypic consequences and sexual transmission of the transformed genotype and phenotype. Cell. 1984;37:959–67.
Villaine F, Casse-Delbart F. Independent induction of transformed roots by the TL and TR regions of the Ri plasmid of agropine type Agrobacterium rhizogenes. Mol Gen Genet. 1987;206:17–23.
Wang YM, Wang JB, Luo D, Jia JF. Regeneration of plants from callus cultures of roots induced by Agrobacterium rhizogenes on Alhagi pseudoalhagi. Cell Res. 2001;11(4):279–84.
White FF, Taylor BH, Huffman GA, Gordon MP, Nester EW. Molecular and genetic analysis of the transferred DNA regions of the root inducing plasmid of Agrobacterium rhizogenes. J Bacteriol. 1985;164:33–44.
Acknowledgement
Dipasree Roychowdhury thanks University Grants Commission, for the award of the RFSMS Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roychowdhury, D., Ghosh, B., Chaubey, B. et al. Genetic and morphological stability of six-year-old transgenic Tylophora indica plants. Nucleus 56, 81–89 (2013). https://doi.org/10.1007/s13237-013-0084-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13237-013-0084-6