Abstract
Robot-assisted laparoscopic surgery is yet another modification of minimally invasive liver surgery. It is described as feasible and safe from the surgical point of view; however, oncological outcomes need to be adequately analysed to justify the use of this technique when resecting malignant liver tumours. We reviewed existing English medical literature on robot-assisted laparoscopic liver surgery. We analysed surgical outcomes and oncological outcomes. We analysed operative parameters including operative time, type of hepatectomy, blood loss, conversion rate, morbidity and mortality rates and length of stay. We also analysed oncological outcomes including completeness of resection (R status), recurrence, survival and follow-up data. A total of 582 patients undergoing robot-assisted laparoscopic liver surgery were analysed from 17 eligible publications. Only 5 publications reported survival data. The overall morbidity was 19% with 0.2% reported mortality. R0 resection was achieved in 96% of patients. Robotic liver surgery is feasible and safe with acceptable morbidity and oncological outcomes including resection margins. However, well-designed trials are required to provide evidence in terms of survival and disease-free intervals when performed for malignancy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Minimally invasive surgery is already established as the gold standard for many surgical procedures [1, 2]. A step further is robot-assisted laparoscopic surgery which provides several inbuilt advantages including a three-dimensional view and a wider range of movements at the tip of the instruments. Robotic surgery therefore has gained wide acceptance in difficult laparoscopic approaches and has unequivocally demonstrated benefit in highly specialized surgery such as prostatectomy and rectal resections in terms of reduced blood loss and transfusion requirements [3, 4].
Laparoscopic liver surgery has been implemented progressively over a more prolonged period of time, probably due to the difficult access, complex anatomy and difficult dissection when transecting the liver parenchyma. Despite all these factors, minimally invasive surgery for liver resections has been demonstrated to be feasible and safe in expert hands [5]. Part of the development of the minimally invasive approaches for liver surgery is the use of the robotic systems. Robot-assisted laparoscopic surgery has been successfully used for liver resection for several years and it is considered currently as a feasible and safe approach [6, 7].
Experience in this surgery is limited to a few centres worldwide and small case series. Additionally, oncological and long-term outcomes have not been adequately analysed. The aim of the current review is to analyse the outcomes of robot-assisted laparoscopic surgery in liver resections for malignant diseases.
Methods
Two independent authors (RDN and SV) performed a literature search (PubMed/MEDLINE and EMBASE) for originally published studies on robotic liver resection from January 1990 until December 2018. Used search terms included major MeSH terms “liver/hepatic neoplasm”, “Robot assisted liver resection”, “Robot assisted hepatectomy”, “Robot assisted laparoscopic liver resection”, “Robot assisted laparoscopic hepatectomy” and “robotics” in addition to the search phrases “robotic liver resection” OR “robotic hepatic resection” OR “robotic hepatec*”, “robotic surgery”, “hepatectomy” and “liver resection”.
All titles and abstracts were reviewed. Clearly irrelevant titles, duplicated series and case reports were excluded. Small cases series with fewer than 10 malignant cases reported were excluded as were single-centre series reporting duplicate data in separate manuscripts. Only studies reporting oncological data (at least one parameter on surgical margins or survival) were included in the analysis. Gallbladder cancer and hilar cholangiocarcinoma were excluded.
Demographic patient data (age, gender) were analysed along with American Society of Anaesthesiologists physical status classification (ASA grade) [8], body mass index (BMI) and indication for liver resection. Main surgical details collected were the type of resection, surgical technique, operative time, estimated blood loss and requirements for intraoperative transfusion, use of liver inflow occlusion, type of parenchymal transection, conversion rate, type of conversion and reason for conversion. Major hepatectomy was defined as any resection involving the removal of more than 3 contiguous liver segments. Minor liver resection was defined as the removal of 3 or fewer contiguous liver segments [9]. Main collected outcomes were postoperative mortality, percentage of optimal surgical resection (R0 resection based on “Residual Tumour classification” [10]), postoperative complications (collected based on Clavien-Dindo classification for surgical complications [11]), length of stay, recurrence rate, overall survival (OS) and disease-free survival (DFS). Post hepatectomy liver failure (PHLF) was defined using ISGLS criteria [12].
Results
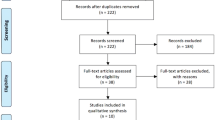
An initial literature search identified 2888 papers from which 73 eligible manuscripts were subsequently selected for detailed review. Finally, 17 studies were included for data extraction and analysis (Fig. 1) [13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28]. A total of 704 patients were included in this series for both benign and malignant diagnoses whilst there is reported data on 582 patients with malignant liver disease.
Patient demographic data is illustrated in Table 1. Mean age is 58.7 years old. Only 10 studies reported on ASA grade and 6 studies reported on BMI (range 16.7–45). The commonest indication for liver resection was HCC (45%), followed by colorectal liver metastases (CRC) (36%). The types of resection reported included any type of hepatectomy from wedge resections and segmentectomies to right trisectionectomies (Table 2).
Most reported series had a mix of benign and malignant diseases. Mean operative time was 277 min for all procedures. The mean blood loss was estimated at 250 ml (from negligible to a maximum of 3500 ml). Some series reported on the use of inflow occlusion during liver resection with a very variable range of times (from 0 to 166 min of occlusion time). There were a wide variety of instruments used for the parenchymal transection including ultrasonic and harmonic scalpels, diathermy and vascular staplers. Mean length of stay was 6.3 days ranging from 1 to 46.
Overall morbidity was 19% (135 patients) including all complications (grades 1–4 of Clavien-Dindo classification [11]). There were 3 cases reported of urinary bladder injury [30, 31]. There were 2 cases of postoperative mortality (0.2%) in the whole series.
Oncological outcomes, either from the surgical point of view or long-term results, are summarised in Table 3. Overall R0 resection rate was 96%. Only two of the studies reported an R0 resection rate inferior to 90% and there were no cases of R2 resection. Four of the 17 case series reported a 100% R0 resection rate. In 6 of the total 582 patients included in this analysis, conversion to an open procedure was required to maintain the oncological nature of the procedure (Table 2) [17, 29]. Only five papers analysed survival for their patients including OS and DFS for the different indications (HCC and CRC) (Table 3).
Discussion
This review illustrates the present position of robotic liver surgery. As most of the studies have concluded, robotic liver resection is feasible and safe. However, long-term data for oncological outcomes including overall survival and disease-free survival is still lacking [11, 32]. This may partly be due to the fact that robotic liver surgery is a recent development, and most case series are small and have included a mix of benign and malignant pathology.
Even where malignant cases have been analysed, the group is heterogeneous and hence no definite analysis of oncological outcomes has been done. Furthermore, most papers have focussed on immediate postoperative outcomes, with respect to morbidity and conversion aspects of robotic surgery. Hence, oncological and survival data has not been included in the majority of these publications.
The main surgical outcomes are properly described in all the included studies. The cumulative data demonstrated that there was a 0.2% reported mortality from robotic liver surgery. All the studies but one report no mortality. No mortality is of the greatest value but we understand that it might be related to the small cohort of patients and case selection. This data should be contrasted in the future, as, in case of confirmation, a reduction in the surgical mortality would promote the use of this surgical approach.
Reduction of the intraoperative blood loss is the most commonly reported advantage for robotic surgery [3, 4]. Most papers comparing robotic versus laparoscopic and/or open surgery agree that there is a significant reduction in blood loss in favour of the robotic approach. Benefits of reducing the amount of blood loss not only affect the actual haemodynamic response but also minimise the needs for transfusion and the associated risks. Data from this review suggests that this assessment is also applicable to robotic liver surgery. There is however limited data on results and the influence of inflow occlusion.
The mean surgical time in the series was 277 min. This is slightly higher than that reported for laparoscopic liver surgery (range 95–280 min) [5]. In this sense, there was no differentiation between a major and minor liver resection and a healthy vs cirrhotic liver. Only one paper described and analysed the data regarding the docking time and console time [33]. It is common knowledge that the perioperative preparation and logistics of robotic surgery, including anaesthetic strategies, increase the operative time. In this sense, the learning curve of this novel approach partially justifies this longer time [29].
Of all the complications reported 73% were minor complications and 27% were major complications, which are comparable to accepted complication rates for liver resection [5, 16]. However, this is the first review where we highlight a complication related only to the surgical approach. The presence of 3 cases of urinary bladder injury is of the most relevant consideration [30, 31]. There is, obviously, nothing reported of this complication in open liver surgery and only some series of laparoscopic pelvic surgery report data on bladder injuries but all of them during the surgical procedure [34, 35]. Gynaecological procedures describe up to 8% of iatrogenic bladder injuries [34] whilst in general surgery procedures such as bowel resections (including rectal resection), it occurred between 0.12 and 0.41% [35]. Robotic liver surgery therefore might represent an increment in the rate of urinary bladder injuries and this data needs to be confirmed. Damage during the specimen extraction could be justified because of the bigger size of the resected specimen as it has been described before during the retrieval of a laparoscopically resected kidney [36].
Appropriate case selection is important for achieving success in a new surgical procedure. Patient body habitus may be shown to create additional difficulties in planning a laparoscopic approach. There is no evidence however of any standard anatomy or physiognomy that could represent a contraindication for any type of surgery whilst there is evidence supporting that obese patients can receive laparoscopic liver surgery safely [37]. However, a more detailed analysis of BMI and its influence on outcomes following robotic liver surgery would be able to provide greater guidance on patient selection.
Only 6 studies were reported on BMI (range 16–40) [13, 23, 24, 26, 32, 33, 38]. This data includes a wide range of values, from 16 to 45, which would support the application of robotic liver surgery to patients irrespective of their BMI. Considering that the concept of robotic surgery is essentially a modification of the traditional laparoscopic approach, it is likely that the influence of BMI on laparoscopic surgery can be applied to the robotic approach and therefore be considered safe. There is however an alternative view put forward by Trachart et al. who concluded that higher BMI can be a risk factor for increased complications in robotic liver surgery [24].
The main outcomes of the current review are those related to the oncological results. Completeness of resection and the presence of negative surgical margins (R0 resection) are possibly the most important prognostic factor determining survival and recurrence following liver surgery and they have demonstrated to be relevant for the patient survival and therefore of the most interest in the full process of treatment [39, 40]. All series have reported on R status following robotic liver resection. Despite the small numbers, the initial results following robotic liver surgery seem equivalent to similar comparisons for open and laparoscopic surgery [5, 40]. Data reported for the long-term survival in terms of DFS and OS is reported separately for CRC and HCC and again is comparable to the literature available. However, well-designed prospective trials are needed to provide stronger evidence.
Special consideration should be given to a recent manuscript published by Khan and colleagues. It is a large retrospective multicentre analysis but it has not been included in the tables as it is a compilation of the multiple centres involved [41]. Most of the data reported the same was already included individually. This review suggests that whilst robotic surgery provides an equal chance as open and laparoscopic liver surgery of obtaining negative margins, no conclusions can be drawn on the long-term overall and disease-free survivals, as data is minimal.
Cost and economic implications of robotic surgery have to be assessed. Two papers reported on the economic data and cost implications [28, 32]. They both report higher costs for the robotic cases. Economic benefit of the minimally invasive approach is not based on the surgical procedure but in facilitating quicker recovery, shorter length of stay and earlier resumption to work. These advantages may thus neutralise and offset the absolute cost of the procedure, which may be higher. The shorter length of stay in the hospital and/or ITU may represent an economic compensation of the more expensive surgical procedure.
In summary, robot-assisted laparoscopic liver surgery for malignant diseases is feasible and safe. Current data suggests that it is an optimal approach for malignant liver tumours in terms of clearance of the resection margins. It may be used and employed in appropriate indications by experienced liver surgeons, trained to perform this procedure. Long-term survival data with respect to its overall oncological safety and efficacy is awaited.
References
Lee SW (2009) Laparoscopic procedures for colon and rectal cancer surgery. Clin Colon Rectal Surg 22(4):218–224
Sain AH (1996) Laparoscopic cholecystectomy is the current “gold standard” for the treatment of gallstone disease. Ann Surg 224(5):689–690
Novara G, Ficarra V, Mocellin S, Ahlering TE, Carroll PR, Graefen M, Guazzoni G, Menon M, Patel VR, Shariat SF, Tewari AK, van Poppel H, Zattoni F, Montorsi F, Mottrie A, Rosen RC, Wilson TG (2012) Systematic review and meta-analysis of studies reporting oncologic outcome after robot-assisted radical prostatectomy. Eur Urol 62(3):382–404
Yang Y, Wang F, Zhang P, Shi C, Zou Y, Qin H, Ma Y (2012) Robot-assisted versus conventional laparoscopic surgery for colorectal disease, focusing on rectal cancer: a meta-analysis. Ann Surg Oncol 19(12):3727–3736
Alkhalili E, Berber E (2014) Laparoscopic liver resection for malignancy: a review of the literature. World J Gastroenterol 20(37):13599–13606
Ho CM, Wakabayashi G, Nitta H, Ito N, Hasegawa Y, Takahara T (2013) Systematic review of robotic liver resection. Surg Endosc 27(3):732–739
Buell JF, Cherqui D, Geller DA, O'Rourke N, Iannitti D, Dagher I, Koffron AJ, Thomas M, Gayet B, Han HS, Wakabayashi G, Belli G, Kaneko H, Ker CG, Scatton O, Laurent A, Abdalla EK, Chaudhury P, Dutson E, Gamblin C, D'Angelica M, Nagorney D, Testa G, Labow D, Manas D, Poon RT, Nelson H, Martin R, Clary B, Pinson WC, Martinie J, Vauthey JN, Goldstein R, Roayaie S, Barlet D, Espat J, Abecassis M, Rees M, Fong Y, McMasters K, Broelsch C, Busuttil R, Belghiti J, Strasberg S, Chari RS, World Consensus Conference on Laparoscopic Surgery (2009) The international position on laparoscopic liver surgery: the Louisville statement, 2008. Ann Surg 250(5):825–830
Daabiss M (2011) American Society of Anaesthesiologists physical status classification. Indian J Anaesth 55(2):111–115
Reddy SK, Barbas AS, Turley RS, Steel JL, Tsung A, Marsh JW, Geller DA, Clary BM (2011) A standard definition of major hepatectomy: resection of four or more liver segments. HPB 13(7):494–502
Hermanek P, Wittekind C (1994) Residual tumor (R) classification and prognosis. Semin Surg Oncol 10(1):12–20
Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M (2009) The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 250(2):187–196
Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, Koch M, Makuuchi M, Dematteo RP, Christophi C, Banting S, Usatoff V, Nagino M, Maddern G, Hugh TJ, Vauthey JN, Greig P, Rees M, Yokoyama Y, Fan ST, Nimura Y, Figueras J, Capussotti L, Büchler MW, Weitz J (2011) Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 149(5):713–724
Casciola L, Patriti A, Ceccarelli G, Bartoli A, Ceribelli C, Spaziani A (2011) Robot-assisted parenchymal-sparing liver surgery including lesions located in the posterosuperior segments. Surg Endosc 25(12):3815–3824
Ceccarelli G, Andolfi E, Fontani A, Calise F, Rocca A, Giuliani A (2018) Robot-assisted liver surgery in a general surgery unit with a “Referral Centre Hub&Spoke Learning Program”. Early outcomes after our first 70 consecutive patients. Minerva Chir 73(5):460–468
Chandarana M, Patkar S, Tamhankar A, Garg S, Bhandare M, Goel M (2017) Robotic resections in hepatobiliary oncology - initial experience with Xi da Vinci system in India. Indian J Cancer 54(1):52–55
Choi GH, Choi SH, Kim SH, Hwang HK, Kang CM, Choi JS, Lee WJ (2012) Robotic liver resection: technique and results of 30 consecutive procedures. Surg Endosc 26(8):2247–2258
Giulianotti PC, Coratti A, Sbrana F, Addeo P, Bianco FM, Buchs NC, Annechiarico M, Benedetti E (2011) Robotic liver surgery: results for 70 resections. Surgery 149(1):29–39
Kingham TP, Leung U, Kuk D, Gonen M, D'Angelica MI, Allen PJ et al (2016) Robotic liver resection: a case-matched comparison. World J Surg 40(6):1422–1428
Lai EC, Tang CN (2016) Long-term survival analysis of robotic versus conventional laparoscopic hepatectomy for hepatocellular carcinoma: a comparative study. Surg Laparosc Endosc Percutan Tech 26(2):162–166
Lee KF, Cheung YS, Chong CC, Wong J, Fong AK, Lai PB (2016) Laparoscopic and robotic hepatectomy: experience from a single centre. ANZ J Surg 86(3):122–126
Marino MV, Gulotta G, Komorowski AL (2019) Fully robotic left hepatectomy for malignant tumor: technique and initial results. Updates Surg 71(1):129–135
Montalti R, Scuderi V, Patriti A, Vivarelli M, Troisi RI (2016) Robotic versus laparoscopic resections of posterosuperior segments of the liver: a propensity score-matched comparison. Surg Endosc 30(3):1004–1013
Spampinato MG, Coratti A, Bianco L, Caniglia F, Laurenzi A, Puleo F, Ettorre GM, Boggi U (2014) Perioperative outcomes of laparoscopic and robot-assisted major hepatectomies: an Italian multi-institutional comparative study. Surg Endosc 28(10):2973–2979
Tranchart H, Ceribelli C, Ferretti S, Dagher I, Patriti A (2014) Traditional versus robot-assisted full laparoscopic liver resection: a matched-pair comparative study. World J Surg 38(11):2904–2909
Troisi RI, Patriti A, Montalti R, Casciola L (2013) Robot assistance in liver surgery: a real advantage over a fully laparoscopic approach? Results of a comparative bi-institutional analysis. Int J Med Rob Comput Assisted Surg 9(2):160–166
Tsung A, Geller DA, Sukato DC, Sabbaghian S, Tohme S, Steel J, Marsh W, Reddy SK, Bartlett DL (2014) Robotic versus laparoscopic hepatectomy: a matched comparison. Ann Surg 259(3):549–555
Wang WH, Kuo KK, Wang SN, Lee KT (2018) Oncological and surgical result of hepatoma after robot surgery. Surg Endosc 32(9):3918–3924
Yu YD, Kim KH, Jung DH, Namkoong JM, Yoon SY, Jung SW, Lee SK, Lee SG (2014) Robotic versus laparoscopic liver resection: a comparative study from a single center. Langenbeck's Arch Surg 399(8):1039–1045
Giulianotti PC, Sbrana F, Coratti A, Bianco FM, Addeo P, Buchs NC, Ayloo SM, Benedetti E (2011) Totally robotic right hepatectomy: surgical technique and outcomes. Arch Surg 146(7):844–850
Chan OC, Tang CN, Lai EC, Yang GP, Li MK (2011) Robotic hepatobiliary and pancreatic surgery: a cohort study. J Hepatobiliary Pancreat Sci 18(4):471–480
Lai EC, Yang GP, Tang CN (2013) Robot-assisted laparoscopic liver resection for hepatocellular carcinoma: short-term outcome. Am J Surg 205(6):697–702
Packiam V, Bartlett DL, Tohme S, Reddy S, Marsh JW, Geller DA, Tsung A (2012) Minimally invasive liver resection: robotic versus laparoscopic left lateral sectionectomy. J Gastrointest Surg 16(12):2233–2238
Kandil E, Noureldine SI, Saggi B, Buell JF (2013) Robotic liver resection: initial experience with three-arm robotic and single-port robotic technique. JSLS 17(1):56–62
Ostrzenski A, Ostrzenska KM (1998) Bladder injury during laparoscopic surgery. Obstet Gynecol Surv 53(3):175–180
Sawkar HP, Kim DY, Thum DJ, Zhao L, Cashy J, Bjurlin M, Bhalani V, Boller AM, Kundu S (2014) Frequency of lower urinary tract injury after gastrointestinal surgery in the nationwide inpatient sample database. Am Surg 80(12):1216–1221
Camargo AH, Rubenstein JN, Ershoff BD, Meng MV, Kane CJ, Stoller ML (2006) The effect of kidney morcellation on operative time, incision complications, and postoperative analgesia after laparoscopic nephrectomy. Int Braz J Urol 32(3):273–279 discussion 9-80
Nomi T, Fuks D, Ferraz JM, Kawaguchi Y, Nakajima Y, Gayet B (2015) Influence of body mass index on postoperative outcomes after laparoscopic liver resection. Surg Endosc 29:3647–3654
Patriti A, Ceccarelli G, Bartoli A, Spaziani A, Lapalorcia LM, Casciola L (2009) Laparoscopic and robot-assisted one-stage resection of colorectal cancer with synchronous liver metastases: a pilot study. J Hepato-Biliary-Pancreat Surg 16(4):450–457
Nuzzo G, Giuliante F, Ardito F, Vellone M, Giovannini I, Federico B, Vecchio FM (2008) Influence of surgical margin on type of recurrence after liver resection for colorectal metastases: a single-center experience. Surgery. 143(3):384–393
Giuliante F, Ardito F, Vellone M, Ranucci G, Federico B, Giovannini I, Nuzzo G (2009) Role of the surgeon as a variable in long-term survival after liver resection for colorectal metastases. J Surg Oncol 100(7):538–545
Khan S, Beard RE, Kingham PT, Fong Y, Boerner T, Martinie JB, Vrochides D, Buell JF, Berber E, Kahramangil B, Troisi RI, Vanlander A, Molinari M, Tsung A (2018) Long-term oncologic outcomes following robotic liver resections for primary hepatobiliary malignancies: a multicenter study. Ann Surg Oncol 25(9):2652–2660
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Diaz-Nieto, R., Vyas, S., Sharma, D. et al. Robotic Surgery for Malignant Liver Disease: a Systematic Review of Oncological and Surgical Outcomes. Indian J Surg Oncol 11, 565–572 (2020). https://doi.org/10.1007/s13193-019-00945-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13193-019-00945-2