Abstract
Background
Robotic liver surgery is a novel technique expanding the field of minimally invasive approaches. An increasing number of studies assess the outcomes of robotic liver resections (RLR). The aim of our meta-analysis is to provide an up-to-date comparison of RLR versus open liver resections (OLR), evaluating its safety and efficacy.
Materials and Methods
A systematic search of MEDLINE, Scopus, Google Scholar, Cochrane, and Clinicaltrials.gov for articles published from January 2000 until January 2022 was undertaken.
Results
Thirteen non-randomized retrospective and one prospective clinical study enlisting 1801 patients met our inclusion criteria, with 640 patients undergoing RLR and 1161 undergoing OLR. RLR resulted in significantly lower overall morbidity (p < 0.001), shorter length of hospital stay (p = 0.002), and less intraoperative blood loss (p < 0.001). Operative time was found to be significantly higher in the RLR group (p < 0.001). Blood transfusion requirements, R0 resection, and mortality rates presented no difference among the two groups. The cumulative rate of conversion was 5% in the RLR group.
Conclusion
The increasing experience in the implementation of the robot will undoubtedly generate more prospective randomized studies, necessary to assess its potential superiority over the traditional open approach, in a variety of hepatic lesions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The advances in the field of minimally invasive surgery (MIS), subsequently enriched the field of liver resections, traditionally correlated with the open surgical approach. To date laparoscopic liver resection (LLR) is acknowledged as a safe and efficient alternative to the traditional open liver resection (OLR) for either benign or malignant primary liver lesions, as well as metastases, especially colorectal liver metastases (CRLM) [1,2,3,4,5,6,7]. A minimally invasive approach diminishes surgical trauma, reduces postoperative pain, and provides the surgeon with amplified views of key abdominal structures and viewing angles (i.e., caudal view in liver resection) otherwise unknown in an open approach [8]. It is also proven by large series that LLR has the same outcomes, regarding postoperative morbidity and R0 resection rates, as the traditional approaches, especially for solitary lesions [9, 10].
However, several limitations are adherent to LLR. Restricted range of motion of the laparoscopic tools, inadequate access to the entire liver surface, and a steep learning curve compromise the wide adoption of LLR as a convenient alternative to OLR, especially for major resections and lesions located in the posterior segments of the liver [9, 11]. Robotic liver resections (RLR), on the other hand, fill the gap between optimal surgical technique and a minimally invasive procedure [12, 13]. Well bestowed advantages are human-independent stable three-dimensional vision and multi-axis hand-mimicking movements with tremor filtration [14]. They allow delicate manipulation of sensitive structures, mandatory for addressing the challenges of liver resection, which has led to the adoption of such an approach in a spanning field of surgical procedures.
Our aim is to provide a systematic review of the literature and meta-analysis comparing RLR to OLR for a plethora of hepatic lesions, suggesting it could be accounted for as a safe and efficient alternative.
Materials and Methods
Our study was designed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines based on the authors’ predetermined eligibility criteria [15]. All appropriate clinical studies comparing perioperative outcomes between robotic and open liver resections were considered eligible for inclusion in our meta-analysis. A comprehensive search of the MEDLINE (PubMed), Scopus, Google Scholar, and Cochrane CENTRAL Register for Controlled Trials and Clinicaltrials.gov was undertaken separately by three authors until January 2022 (PK, PD, and SK) with the objective of identifying studies comparing robotic to open liver resections, published in English language. The terms utilized included: “robot-assisted”, “robotic”, “liver resection”, “hepatectomy” combined with the Boolean operators AND/OR.
Studies reporting at least one postoperative outcome (operative time, estimated blood loss EBL, length of stay LOS, postoperative mortality, and morbidity) were considered eligible for inclusion. Exclusion criteria were (1) animal studies, (2) studies that included patients undergoing procedures other than resection (such as radiofrequency or microwave ablation), (3) studies including patients undergoing simultaneous resections (e.g., simultaneous colon and liver metastasis resection), (4) studies analyzing outcomes after hand-assisted or hybrid techniques, (5) non-comparative studies, and (6) duplicate studies. All articles deemed eligible for inclusion were subsequently reviewed by all authors and were selected for inclusion. The consensus from all authors resolved potential discordances in methodology, selection of articles, and statistical analysis.
Data Extraction and Management
Data extracted from eligible studies were inserted in Excel spreadsheets (Microsoft, Redmond, WA, USA). Data of interest included patient demographics, information on the size of lesion, perioperative outcomes and postoperative morbidity, and mortality.
Quality Assessment
The quality of all the included studies was assessed using the Methodological Index for Non-Randomized Studies (MINORS) [16]. This is a quality assessment tool, designed for estimating the methodological adequacy of non-randomized studies. The MINORS scale containing 12 items, each scored from 0 to 2, providing overall scores between 0 and 24. The methodological quality of the included studies was independently assessed by two reviewers (SK and PD). The choice of the MINORS scale was due to the fact that all of the studies included in our meta-analysis were non-randomized.
Statistical Analysis
The R©, version 3.3.2 (R Core Team, GNU GPL v2 License), R Studio, version 1.0.44 (RStudio, Inc. GNU Affero General Public License v3, Boston, MA, 2016), with the graphical user interface rBiostatistics.com alpha version (rBiostatistics.com, London, UK, 2017), was utilized as a tool for our meta-analyses. Risk ratio (RR) assessed dichotomous variables and mean difference assessed continuous variables. The inverse variance method was chosen for comparisons between dichotomous or continuous variables. The level of statistical significance was set at p value less than 0.05. The random effects model was used due the heterogeneity among studies. Statistical heterogeneity was assessed with the Higgin’s I2 statistic. When mean values and standard deviations (SD) were not mentioned, the equations proposed by Hozo et al. were used for calculation, and 95% confidence intervals (CI) were noted [17].
Results
Included Studies
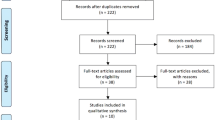
Our database search generated 2564 studies. After duplicate removal, 1105 records remained for screening, out of which 1067 were excluded due to irrelevance based on the title and abstract. Of the remaining 38 studies, most provided a comparison between laparoscopic and robotic hepatectomies or between minimally invasive liver resections and an open approach without differentiating laparoscopic and robotic procedures. Others contained non-comparative data from robotic liver resection series or referred to organs other than the liver. Fifteen reports were assessed for eligibility. A study from Lee et al. in 2016 was excluded, because there was an updated version of the study available from the same center containing patients up to 2019 [18] (Fig. 1). Thirteen non-randomized retrospective and one prospective study complied with our inclusion criteria, spanning from 2014 to 2022 (Table 1) [19,20,21,22,23,24,25,26,27,28,29,30,31]. The included studies were considered methodologically adequate according to MINORS scale, with scores ranging from 16 to 22. Most studies originated from Asia (5 out of 13), whereas 4 studies originated from Europe, 4 from America, and one originated from multiple countries.
Patient Characteristics
Out of 1801 patients in total, 640 underwent RLR and 1161 underwent OLR (Table 1). Patients’ demographic factors between the two groups were comparable. The percentage of male patients undergoing RLR displayed no significant difference to those undergoing OLR (RLR: 59.8% (383/640) vs OLR: 64.5% (750/1161), RR: 0.97 95% CI 0.90–1.05, I2:0%, p: 0.95) [19,20,21,22,23,24,25,26,27,28,29,30,31,32]. The percentage of patients with an ASA score of III/IV, presented no statistically significant difference between the two groups (RLR: 47.9% (141/294) vs OLR: 34.6% (171/493), RR: 1.36 95% CI 0.87–2.14 p: 0.18, I2: 83% p < 0.01) [21, 22, 24,25,26,27,28,29], eliminating potential discordances in patient selection in favor of the robotic arm of the study. This was also indicated in the percentage of patients having undergone previous abdominal surgery, which was 19.8% for RLR versus 19.2% for OLR [22,23,24, 27, 29, 30].
Indications for Surgery
Indications for resection (Table 1) included both benign and malignant liver tumors, as well as benign medical conditions, e.g., hepatolithiasis. There were no differences in the number of tumors with a mean of 1.45 for RLR vs. 1.39 for OLR. Patients treated with the robot had significantly smaller lesions, as opposed to those treated with an open approach, with a mean difference of − 0.41 cm (95% CI − 0.76 to − 0.07 p = 0.01, I2: 0%, p = 0.86) [19, 21, 23,24,25, 27,28,29, 31, 32].
Operative Outcomes
The cumulative conversion rate to OLR was 5% (32/640) ranging between 0 and 10% (Table 1) [20, 22,23,24,25,26, 28, 30, 31]. Major hepatectomies among the two groups accounted for 41.2% (260/630) in the RLR group and 35.2% (390/1108) in the OLR group (Table 1) [19, 20, 22, 24,25,26,27,28,29,30, 32]. The R0 resection rates were similar among the two groups, being 88.1% (357/405) for the RLR group and 91.7% (655/714) for the OLR group respectively (RR: 1.00 p: 0.9, 95% CI 0.96–1.04, I2: 58% p: 0.01) (Fig. 2a) [20, 22, 23, 25,26,27,28, 31, 32]. Intraoperative transfusion was necessary in 10.6% (43/404) patients undergoing RLR and in 18.9% (93/492) patients undergoing OLR (RR: 0.72 p: 0.37, 95% CI: 0.35–1.47), with a significant heterogeneity present among the included studies (I2: 67%, p < 0.01) [20, 22, 23, 25,26,27,28, 31]. EBL showed a statistically significant mean difference of − 182.4 ml in favor of the RLR group (p < 0.001, 95% CI: − 283 to − 81.7) with a significant heterogeneity among the studies (I2: 92%, p < 0.01) (Fig. 2b) [20, 22,23,24,25,26, 28, 30, 31]. On the contrary, RLR proved to be significantly more time-consuming than OLR, displaying a mean difference of − 58.8 min (p: 0.003, 95% CI: 19.4 to 98.3) and a high heterogeneity (I2: 93%, p < 0.01) (Fig. 2c) [20, 22,23,24,25,26, 28, 30,31,32].
Postoperative Outcomes
Robotic hepatectomy presented with a postoperative mortality rate of 0.4% (3/624) over 0.69% (8/1145) for open hepatectomy (RR: 0.54 p: 0.32, 95% CI: 0.16–1.82, I2: 0%, p: 0.81) [19,20,21,22,23,24,25,26,27,28,29,30,31]. Minor morbidity rates (Clavien-Dindo I–II) were significantly lower in the RLR group, with 13.7% (81/589) RLR presenting with a minor complication over 25% (254/1016) OLR (RR: 0.68 p < 0.001, 95% CI: 0.54–0.85, I2: 0% p: 0.95) (Fig. 3a) [20,21,22,23,24,25,26,27,28,29,30,31]. On the other hand, major morbidity rates (Clavien-Dindo III–IV) were similar, being 4.5% (29/640) for the RLR group over 6.8% (79/1161) for the OLR specifically (RR: 0.69 p: 0.09, 95% CI: 0.45–1.07, I2: 0%, p: 0.61) (Fig. 3a) [20, 22, 23, 25,26,27,28, 31, 32]. Overall morbidity rates found to be significantly lower in the RLR group with 19% (112/589) over 32.1% (327/1016) for the OLR (RR: 0.70 p < 0.001, 95% CI: 0.56–0.86, I2: 13%, p: 0.32) (Fig. 3c). Bile leakage occurred in 2.2% (14/617) RLR patients and 3.3% (38/1130) OLR patients, presenting no significant difference (RR: 0.76 p: 0.3, CI 95%: 0.40–1.43, I2: 0%, p: 0.68) [20,21,22,23,24,25,26,27,28,29,30,31,32]. RLR was also associated with a lower length of stay, with a mean difference of − 2.74 days (p < 0.001), (95% CI: − 4.20 to − 1.28), and a high heterogeneity among the included studies (I2: 93%, p < 0.01) (Fig. 4) [20, 22, 23, 25,26,27,28, 31,32,33].
Discussion
Based on our analysis, RLR constitutes a safe and efficient alternative to OLR. The robotic approach surpasses the traditional open liver resection in terms of short-term outcomes. Overall morbidity rates, length of stay, and estimated blood loss were significantly lower in the RLR group. Nonetheless, RLR requires more time to perform. Negative resection margin (R0) and postoperative mortality rates showed no significant difference, which in addition to the small rate of conversion support its efficacy and safety.
Whereas major complication (Clavien-Dindo III–IV) rate between the two approaches showed no difference, the RLR group had significantly fewer minor complications (Clavien-Dindo I–II), 13.7% over 25%, a result evident in overall morbidity rates also. In conjunction to the later, a lower complication rate could be associated with shorter LOS, hence an earlier induction of chemotherapy, which could eventuate in favorable oncologic outcomes [34]. Additionally, shorter LOS, earlier amputation, and reduced analgesic requirements result in reduced total cost per capita, outweighing the higher perioperative cost of the robot [35]. The cost-effectiveness analysis of Daskalaki et al. and Sham et al. confirms this hypothesis [22, 29].
The robotic surgical system has infiltrated the field of liver surgery. Many studies compare its safety, efficacy, and cost-effectiveness over open or laparoscopic approaches, with a growing number of liver surgeons implementing this new technique in complex liver resections. An international consensus statement on robotic hepatectomy surgery was issued in 2018 and sought to address the controversies related to the implementation of the robotic surgical system, thus proceeding to seven statements [36]. They concluded that RLR is safe and feasible compared to OLR, with a lower complication rate and less EBL, without sacrificing oncologic outcomes. Overall survival, recurrence, and radical resection rate had no significant differences. Compared to LLR, it is a safe and feasible alternative, with comparable EBL, complication rate, and oncological outcomes, though requiring more operative time and has a higher cost [36]. Their comparison included both minor and major hepatectomies.
A previously published meta-analysis, by Machairas et al. comparing ten non-randomized retrospective clinical studies with 1248 patients in total, demonstrated that RLR is associated with lower overall morbidity rates and shorter hospital stay [12]. Operative time was higher during RLR. The authors reported similar outcomes to OLR regarding blood loss, blood transfusion requirements, margin-free resection, and mortality. The conversion rate was 4.6%, a finding in accordance with our reported result.
During the 2nd International Consensus Conference on Laparoscopic Liver Resection (ICCLLR), held in 2014 in Morioka, Japan, the benefits of LLR were discussed among the experts in the field. The magnified views, the exposure of sensitive structures along the hilar plate, a caudal to cephalad view, and the effect of pneumoperitoneum on minor hemorrhage are well bestowed advantages of laparoscopy. But these benefits get overshadowed by some technical limitations, as inadequate range of motion due to stiffness of the laparoscopic tools and limited access to the entire liver surface, especially the posterior segments and a steep learning curve. The robot overcomes these limitations, providing three-dimensional view, hand filter tremor, motion scaling, and multi-axis freedom of movement [37]. The steep learning curve of laparoscopy is also flattened out by the initiation of the robot, which requires less effort for complex liver resections and the precise manipulation of vascular structures, without a difference in the conversion rate [38, 39]. In the fore coming years, as the experience of liver surgeons with the robot will increase, more favorable results will be probably presented in the literature [38].
The high cost of the implementation of the robot remains its greatest disadvantage. It compromises its cost-effectiveness even in prostatectomy where its superiority over an open approach is undoubted [40]. Furthermore, it is widely demonstrated that postoperative morbidity has a significant impact on in-hospital costs [41]. As reported by the published data in the case of RLR, the lower morbidity rate, and as a result, shorter length of stay, less postoperative analgesic requirements, and earlier amputation may counterbalance the increased cost of the procedure [42]. These findings are supported by a retrospective non-randomized cost-effectiveness analysis by Daskalaki et al. The authors compared the cost of robotic over open liver resections, concluding that the average total cost, including readmission, was lower for robotic surgery (37,518$ RLR vs. 41,948$ OLR) mainly due to less overall morbidity and ICU and hospital stay, when performed by an experienced surgeon [22]. On the other hand, in a 2020 systematic review and meta-analysis by Ziogas et al. comparing laparoscopy with robotic major hepatectomies though no statistically significant differences regarding perioperative outcomes were demonstrated, implementation of the robot resulted in higher costs [43]. This was attributed to the higher capital costs of the robotic platform, the annual maintenance cost, and the operating room total surgical supplies, doubting its cost-effectiveness especially in low-volume centers.
Despite the cost remaining an obstacle, especially for low-volume liver centers and low-income countries, a recent systematic review of 2021 presented a less steep learning curve for RLR over LLR. An analysis of 40 retrospective studies showed a smaller number of procedures necessary for technical competency in RLR vs LLR, being 25 (range 16–50) over 50 (range 25–58) respectively, with a year-on-year reduction in the number of procedures necessary for manual competency [44]. Chen et al. and Efanov et al. confirm these findings [38, 45]. They reported 15 and 16 low and intermediate difficulty RLR, over 25 and 29 LLR respectively, for surgeons to be able to proceed to more complex resections.
Several limitations of the present study should be highlighted. All the included studies were retrospective, with a possibility of an inherent selection bias, and the vast majority originating from a single center and six out of thirteen being case-matched comparisons and focusing mainly on short-term outcomes. Furthermore, including studies with high heterogeneity among the lesions’ type, size, and location across the liver parenchyma, resulting in either minor or major resections, entails critical limitations influencing the results. Finally, surgeons’ familiarization with the robotic platform, with the majority of resections being done by experienced robotic surgeons, EBL, OT, and LOS, is a potential limitation ought to be considered.
We hereby suggest that RLR, except for being a safe and efficient alternative to OLR, has advantages on overall morbidity and length of hospital over the traditional open approach. The adoption of the robotic platform in a spanning range of surgical procedures, along with the surgeons’ accrual experience and manual competency in robotic liver resections, will generate further studies, as well as prospective randomized studies in large cohorts required to determine the value of this approach in clinical practice.
References
Nguyen KT, Gamblin TC, Geller DA. World review of laparoscopic liver resection-2,804 patients. Ann Surg. 2009;250:831–41. https://doi.org/10.1097/SLA.0b013e3181b0c4df.
Sotiropoulos GC, Prodromidou A, Kostakis ID, Machairas N. Meta-analysis of laparoscopic vs open liver resection for hepatocellular carcinoma. Updates Surg. 2017;69:291–311. https://doi.org/10.1007/s13304-017-0421-4.
Machairas N, Sotiropoulos GC. Laparoscopic liver surgery: yesterday, today and tomorrow. Hepatobiliary Surg Nutr. 2019;8:324–6. https://doi.org/10.21037/hbsn.2019.01.12
Machairas N, Papaconstantinou D, Stamopoulos P, Prodromidou A, Garoufalia Z, Spartalis E, et al. The emerging role of laparoscopic liver resection in the treatment of recurrent hepatocellular carcinoma: a systematic review. Anticancer Res 2018. 38:3181–6. https://doi.org/10.21873/anticanres.12582
Moris D, Tsilimigras DI, Machairas N, Merath K, Cerullo M, Hasemaki N, et al. Laparoscopic synchronous resection of colorectal cancer and liver metastases: a systematic review. J Surg Oncol. 2019;119:30–9. https://doi.org/10.1002/jso.25313.
Machairas N, Kostakis ID, Schizas D, Kykalos S, Nikiteas N, Sotiropoulos GC. Meta-analysis of laparoscopic versus open liver resection for intrahepatic cholangiocarcinoma. Updates Surg. 2021;73:59–68. https://doi.org/10.1007/s13304-020-00930-3.
Machairas N, Daskalakis K, Felekouras E, Alexandraki KI, Kaltsas G, Sotiropoulos GC. Currently available treatment options for neuroendocrine liver metastases. Ann Gastroenterol. 2021;34:130–41. https://doi.org/10.20524/aog.2021.0574
Ciria R, Cherqui D, Geller DA, Briceno J, Wakabayashi G. Comparative short-term benefits of laparoscopic liver resection: 9000 cases and climbing. Ann Surg. 2016;263:761–77. https://doi.org/10.1097/SLA.0000000000001413.
Wakabayashi G, Cherqui D, Geller DA, Buell JF, Kaneko H, Han HS, et al. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg. 2015;261:619–29. https://doi.org/10.1097/SLA.0000000000001184.
Dorovinis P, Machairas N, Kykalos S, Stamopoulos P, Vernadakis S, Sotiropoulos GC. Safety and efficacy of laparoscopic caudate lobectomy: a systematic review. J Clin Med. 2021;10. https://doi.org/10.3390/jcm10214907
Machairas N, Prodromidou A, Kostakis ID, Spartalis E, Sotiropoulos GC. Safety and efficacy of laparoscopic liver resection for lesions located on posterosuperior segments: a meta-analysis of short-term outcomes. Surg Laparosc Endosc Percutan Tech. 2018;28:203–8. https://doi.org/10.1097/SLE.0000000000000562.
Machairas N, Papaconstantinou D, Tsilimigras DI, Moris D, Prodromidou A, Paspala A, et al. Comparison between robotic and open liver resection: a systematic review and meta-analysis of short-term outcomes. Updates Surg. 2019;71:39–48. https://doi.org/10.1007/s13304-019-00629-0.
Ioannidis A, Machairas N, Koutserimpas C, Spartalis E, Konstantinidis M, Konstantinidis K. Evolution of robot-assisted general surgery in Greece and Cyprus. J Robot Surg. 2019;13:315–7. https://doi.org/10.1007/s11701-018-00901-2.
Giulianotti PC, Coratti A, Angelini M, Sbrana F, Cecconi S, Balestracci T, et al. Robotics in general surgery: personal experience in a large community hospital. Arch Surg. 2003;138:777–84. https://doi.org/10.1001/archsurg.138.7.777.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151:W65-94.
Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73:712–6.
Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. https://doi.org/10.1186/1471-2288-5-13.
Lee W, Park JH, Kim JY, Kwag SJ, Park T, Jeong SH, et al. Comparison of perioperative and oncologic outcomes between open and laparoscopic liver resection for intrahepatic cholangiocarcinoma. Surg Endosc. 2016;30:4835–40. https://doi.org/10.1007/s00464-016-4817-x.
Yang HY, Rho SY, Han DH, Choi JS, Choi GH. Robotic major liver resections: surgical outcomes compared with open major liver resections. Ann Hepatobiliary Pancreat Surg. 2021;25:8–17. https://doi.org/10.14701/ahbps.2021.25.1.8
Chen PD, Wu CY, Hu RH, Chou WH, Lai HS, Liang JT, et al. Robotic versus open hepatectomy for hepatocellular carcinoma: a matched comparison. Ann Surg Oncol. 2017;24:1021–8. https://doi.org/10.1245/s10434-016-5638-9.
Croner RS, Perrakis A, Hohenberger W, Brunner M. Robotic liver surgery for minor hepatic resections: a comparison with laparoscopic and open standard procedures. Langenbecks Arch Surg. 2016;401:707–14. https://doi.org/10.1007/s00423-016-1440-1.
Daskalaki D, Gonzalez-Heredia R, Brown M, Bianco FM, Tzvetanov I, Davis M, et al. Financial impact of the robotic approach in liver surgery: a comparative study of clinical outcomes and costs between the robotic and open technique in a single institution. J Laparoendosc Adv Surg Tech A. 2017;27:375–82. https://doi.org/10.1089/lap.2016.0576.
Kingham TP, Leung U, Kuk D, Gonen M, D’Angelica MI, Allen PJ, et al. Robotic liver resection: a case-matched comparison. World J Surg. 2016;40:1422–8. https://doi.org/10.1007/s00268-016-3446-9.
Lee KF, Chong C, Cheung S, Wong J, Fung A, Lok HT, et al. Robotic versus open hemihepatectomy: a propensity score-matched study. Surg Endosc. 2021;35:2316–23. https://doi.org/10.1007/s00464-020-07645-x.
Morel P, Jung M, Cornateanu S, Buehler L, Majno P, Toso C, et al. Robotic versus open liver resections: a case-matched comparison. Int J Med Robot. 2017;13. https://doi.org/10.1002/rcs.1800
Nota CL, Woo Y, Raoof M, Boerner T, Molenaar IQ, Choi GH, et al. Robotic versus open minor liver resections of the posterosuperior segments: a multinational, propensity score-matched study. Ann Surg Oncol. 2019;26:583–90. https://doi.org/10.1245/s10434-018-6928-1.
Patriti A, Cipriani F, Ratti F, Bartoli A, Ceccarelli G, Casciola L, et al. Robot-assisted versus open liver resection in the right posterior section. JSLS. 2014;18. https://doi.org/10.4293/JSLS.2014.00040
Pesi B, Bencini L, Moraldi L, Tofani F, Batignani G, Bechi P, et al. Robotic versus open liver resection in hepatocarcinoma: surgical and oncological outcomes. Surg Laparosc Endosc Percutan Tech. 2021;31:468–74. https://doi.org/10.1097/SLE.0000000000000904.
Sham JG, Richards MK, Seo YD, Pillarisetty VG, Yeung RS, Park JO. Efficacy and cost of robotic hepatectomy: is the robot cost-prohibitive? J Robot Surg. 2016;10:307–13. https://doi.org/10.1007/s11701-016-0598-4.
Shu J, Wang XJ, Li JW, Bie P, Chen J, Zheng SG. Robotic-assisted laparoscopic surgery for complex hepatolithiasis: a propensity score matching analysis. Surg Endosc. 2019;33:2539–47. https://doi.org/10.1007/s00464-018-6547-8.
Wang WH, Kuo KK, Wang SN, Lee KT. Oncological and surgical result of hepatoma after robot surgery. Surg Endosc. 2018;32:3918–24. https://doi.org/10.1007/s00464-018-6131-2.
Sucandy I, Shapera E, Syblis CC, Crespo K, Przetocki VA, Ross SB, et al. Propensity score matched comparison of robotic and open major hepatectomy for malignant liver tumors. Surg Endosc. 2022. https://doi.org/10.1007/s00464-021-08948-3.
Lee KF, Fong AK, Chong CC, Cheung SY, Wong J, Lai PB. Robotic liver resection for primary hepatolithiasis: is it beneficial? World J Surg. 2016;40:2490–6. https://doi.org/10.1007/s00268-016-3528-8.
Tohme S, Goswami J, Han K, Chidi AP, Geller DA, Reddy S, et al. Minimally invasive resection of colorectal cancer liver metastases leads to an earlier initiation of chemotherapy compared to open surgery. J Gastrointest Surg. 2015;19:2199–206. https://doi.org/10.1007/s11605-015-2962-5.
Ahmed EA, Montalti R, Nicolini D, Vincenzi P, Coletta M, Vecchi A, et al. Fast track program in liver resection: a PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore). 2016;95: e4154. https://doi.org/10.1097/MD.0000000000004154.
Liu R, Wakabayashi G, Kim HJ, Choi GH, Yiengpruksawan A, Fong Y, et al. International consensus statement on robotic hepatectomy surgery in 2018. World J Gastroenterol. 2019;25:1432–44. https://doi.org/10.3748/wjg.v25.i12.1432.
Salloum C, Lim C, Malek A, Compagnon P, Azoulay D. Robot-assisted laparoscopic liver resection: a review. J Visc Surg. 2016;153:447–56. https://doi.org/10.1016/j.jviscsurg.2016.08.005.
Efanov M, Alikhanov R, Tsvirkun V, Kazakov I, Melekhina O, Kim P, et al. Comparative analysis of learning curve in complex robot-assisted and laparoscopic liver resection. HPB (Oxford). 2017;19:818–24. https://doi.org/10.1016/j.hpb.2017.05.003.
Guan R, Chen Y, Yang K, Ma D, Gong X, Shen B, et al. Clinical efficacy of robot-assisted versus laparoscopic liver resection: a meta analysis. Asian J Surg. 2019;42:19–31. https://doi.org/10.1016/j.asjsur.2018.05.008.
Ahmed K, Ibrahim A, Wang TT, Khan N, Challacombe B, Khan MS, et al. Assessing the cost effectiveness of robotics in urological surgery - a systematic review. BJU Int. 2012;110:1544–56. https://doi.org/10.1111/j.1464-410X.2012.11015.x.
Raptis DA, Hanna T, Machairas N, Owen T, Davies D, Fusai GK. The economic burden of postoperative complications predicted by the Comprehensive Complication Index((R)) in patients undergoing elective major hepatopancreaticobiliary surgery for malignancy - a prospective cost analysis. In Vivo. 2021;35:1065–71. https://doi.org/10.21873/invivo.12351
Chen PD, Wu CY, Hu RH, Ho CM, Lee PH, Lai HS, et al. Robotic liver donor right hepatectomy: a pure, minimally invasive approach. Liver Transpl. 2016;22:1509–18. https://doi.org/10.1002/lt.24522.
Ziogas IA, Giannis D, Esagian SM, Economopoulos KP, Tohme S, Geller DA. Laparoscopic versus robotic major hepatectomy: a systematic review and meta-analysis. Surg Endosc. 2021;35:524–35. https://doi.org/10.1007/s00464-020-08008-2.
Chua D, Syn N, Koh YX, Goh BKP. Learning curves in minimally invasive hepatectomy: systematic review and meta-regression analysis. Br J Surg. 2021;108:351–8. https://doi.org/10.1093/bjs/znaa118.
Chen PD, Wu CY, Hu RH, Chen CN, Yuan RH, Liang JT, et al. Robotic major hepatectomy: is there a learning curve? Surgery. 2017;161:642–9. https://doi.org/10.1016/j.surg.2016.09.025.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Papadopoulou, K., Dorovinis, P., Kykalos, S. et al. Short-Term Outcomes After Robotic Versus Open Liver Resection: A Systematic Review and Meta-analysis. J Gastrointest Canc 54, 237–246 (2023). https://doi.org/10.1007/s12029-022-00810-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12029-022-00810-6