Abstract
Background
Robotic surgery can enhance a surgeon’s laparoscopic skills through a magnified three-dimensional view and instruments with seven degrees of freedom compared to conventional laparoscopy.
Methods
This study reviewed a single surgeon’s experience of robotic liver resections in 30 consecutive patients, focusing on major hepatectomy. Clinicopathological characteristics and perioperative and short-term outcomes were analyzed.
Results
The mean age of the patients was 52.4 years and 14 were male. There were 21 malignant tumors and 9 benign lesions. There were 6 right hepatectomies, 14 left hepatectomies, 4 left lateral sectionectomies, 2 segmentectomies, and 4 wedge resections. The average operating time for the right and left hepatectomies was 724 min (range 648–812) and 518 min (range 315–763), respectively. The average estimated blood loss in the right and left hepatectomies was 629 ml (range 100–1500) and 328 ml (range 150–900), respectively. Four patients (14.8%) received perioperative transfusion. There were two conversions to open surgery (one right hepatectomy and one left hepatectomy). The overall complication rate was 43.3% (grade I, 5; grade II, 2; grade III, 6; grade IV, 0) and 40% in 20 patients who underwent major hepatectomy. Among the six (20.0%) grade III complications, a liver resection–related complication (bile leakage) occurred in two patients. The mean length of hospital stay was 11.7 days (range 5–46). There was no recurrence in the 13 patients with hepatocellular carcinoma during the median follow-up of 11 months (range 5–29).
Conclusions
From our experience, robotic liver resection seems to be a feasible and safe procedure, even for major hepatectomy. Robotic surgery can be considered a new advanced option for minimally invasive liver surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Safe liver surgery has a relatively short history. Until the 1980s, the operative mortality rate of liver resections was above 20% and was related mainly to massive hemorrhage [1]. The improved understanding of the liver’s anatomy and the advances in anesthesia and postoperative care have contributed to the safety of major liver resections, resulting in the current mortality rate of less than 5% in specialized centers [1, 2]. As liver surgery was considered to be no longer dangerous, a laparoscopic approach to liver resections began to be used by some experts in the mid-1990s [3–6]. The recent advances in laparoscopic devices and the development of liver parenchymal transection equipment have led to the increased performance of laparoscopic liver resection over the past several years [7].
The feasibility and safety of laparoscopic liver resections have been demonstrated by several recent studies [8, 9]. The best candidates for laparoscopic liver resection include only those with a surface lesion that can be removed by limited resection or left lateral sectionectomy according to the international consensus report [9]. However, laparoscopic major hepatectomy is being performed but only in a few centers. Obstacles to widespread use of laparoscopic liver resection, especially in major hepatectomy cases, may include the inherent limitations of laparoscopic surgery such as restricted movement of the instruments and the two-dimensional view. Therefore, a surgeon needs sufficient experience in laparoscopic surgery in addition to open liver surgery to perform laparoscopic liver resection. Another obstacle is that liver resection is a complex procedure and still has the potential risk of major bleeding during parenchymal transection.
Robotic surgical systems have been recently introduced to enhance a surgeon’s dexterity in the surgical field through a magnified three-dimensional view, instruments with seven degrees of freedom, and intuitive hand-control movements. After the practical benefits of the robotic system were demonstrated in urological and cardiac surgery, robotic surgery was frequently performed for gynecological and abdominal surgery [10, 11]. However, a few studies have been published about the technical feasibility and safety of robotic liver resections, especially in major hepatectomies [12–14]. In this study we report a single surgeon’s experience with robotic liver resection in 30 consecutive patients, focusing on major hepatectomy.
Methods
From November 2008 to April 2011, 30 patients underwent robotic liver resection using the da Vinci Surgical System® (Intuitive Surgical, Sunnyvale, CA) in Yonsei University Health System, Seoul, Korea. The indications of surgery did not differ from those of open liver resection. However, in the patients with hepatocellular carcinoma (HCC), both tumor characteristics and the underlying liver function were considered for surgery. The tumor should be a single lesion, 5 cm or less, and without radiologic vascular invasion. The liver function should correspond to Child A and there should be no clinically significant portal hypertension. When a right hepatectomy was planned for patients with chronic liver disease, the normal range of indocyanine green retention rate at 15 min (ICG R15 <10%) and residual liver volume ≥40% of the total liver volume were further considered as the indications for resection.
Patient demographics, perioperative data, and postoperative monitoring, including complications, were recorded in the database. All patients except those who had only a limited liver resection underwent computed tomography (CT) at 5 days postoperatively to assess any abnormal findings in the abdominal cavity. The complications were prospectively evaluated using the modified Clavien system [15]. The patients were informed of the innovative nature of the robotic system and provided written informed consent. The study protocol was approved by the Institutional Review Board of Severance Hospital in Yonsei University Health System, Seoul, Korea.
Surgical technique
Port placement and operating room setup
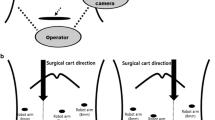
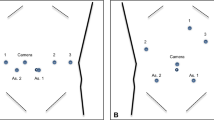
The patient was placed supine in a 15° reverse Trendelenburg position. Five ports were used: two 12-mm ports and three 8-mm robotic arm ports in all procedures. First, a 12-mm trocar was inserted into the umbilicus using an open technique and a pneumoperitoneum of 12 mmHg was established. A 30° robotic camera was inserted through the 12-mm umbilical port and laparoscopic abdominal exploration was performed. An additional four ports were introduced under the view of the laparoscope. For a right hepatectomy, the 12-mm camera port was introduced in the right paraumbilical area because the camera port is usually located in the middle of the target anatomy. The main working ports, the first (left) and second (right) robotic arm ports, were placed in the left and right upper quadrant area, respectively. The assistant port was selected based on both accessibility to the port with instrument arms in place and the feasibility of clipping, suction, intraoperative ultrasound, and vascular stapling during the procedure. Therefore, the umbilical port was used by an assistant surgeon. The third robotic arm port was placed near the left anterior axillary line and usually was used for exposure and retraction. The surgical cart was positioned over the patient’s head because it is generally in line with the working axis. An assistant and a nurse were positioned at the left and right sides of the patient, respectively. The anesthesiologist was positioned at the left side of the patient (Fig. 1A).
For a left hepatectomy, the patient position, port, and surgical cart placements were similar to those of a right hepatectomy. However, the camera port was placed at the umbilicus because the center of the target anatomy is medially shifted compared to that of a right hepatectomy. Therefore, the assistant port was placed at the right paraumbilical area and the assistant was positioned at the left side of the patient (Fig. 1B). For a left lateral sectionectomy, the port placement and operating room setup were identical to those of a left hepatectomy.
Right hepatectomy
The round and falciform ligaments were divided with harmonic curved shears and the anterior half of the coronary ligament was sectioned with a permanent cautery hook. Then, the third robotic arm retracted the right liver upward to expose the right triangular ligament and the hepatorenal attachment, which were divided using a cautery hook and Maryland bipolar forceps. The third robotic arm needed to be repositioned in a step-by-step manner to further expose the dissecting plane. The right side of the inferior vena cava (IVC) was dissected at the caudate process of the caudate lobe in a caudal-to-cephalad direction. The short hepatic veins were gently dissected and ligated using a Hem-o-lok clip. The IVC ligament was identified at the upper part and ligated with the Hem-o-lok clip. The dissection proceeded until the inferior aspect of the right hepatic vein was identified.
The hilum was exposed using the third robotic arm by retracting the inferior surface of the liver. The right hepatic artery was identified at the right side of the common bile duct and divided after applying Hem-o-lok clips. After the small branches to the caudate lobe were divided, the right portal vein was encircled with umbilical tape and then divided between the Hem-o-lok clips. The remaining right bile duct in the hilum was divided during parenchymal transection.
The parenchymal transection line was marked with a cautery hook along the ischemic demarcation line. Intraoperative ultrasound was performed to confirm the location of the tumor and to check the surgical resection margins. The images of the intraoperative ultrasound were transferred to the surgeon’s console using the Tilepro® (Intuitive Surgical) program, which is software for multiple-image display. For steady exposure of the parenchymal transection plane, we used rubber band. One end of each rubber band was anchored with stay stitches at the right and left resection margins. The other end was pulled out through the 2-mm trocars and fixed outside with appropriate tension (Fig. 2). The parenchymal transection was carried out using harmonic curved shears and Maryland bipolar forceps. Small portal pedicles and hepatic vein branches were well controlled by harmonic curved shears and bipolar forceps. However, medium- to large-sized hepatic vein branches such as segment 5 and segment 8 draining veins were carefully dissected and divided after clipping. The superior and inferior sides of the hilar plate were exposed and the hilar plate, including the right bile duct, was encircled with a piece of umbilical tape. The right bile duct was sectioned after applying Hem-o-lok clips. The parenchyma was gradually dissected toward the direction of the right hepatic vein between the caudate process and the paracaval portion of the caudate lobe. Finally, the right hepatic vein was identified and sectioned using a vascular endo-GIA (Fig. 3).
The main procedures of robotic right hepatectomy. A The right liver was mobilized and the short hepatic vein and vena cava ligament were dissected and sectioned. B The right hepatic artery was identified and divided. C The portal vein branch to the caudate lobe was divided after suture ligation. D After full mobilization, the right portal vein was sectioned. Parenchymal transection was then conducted. E The middle hepatic vein branch of segment 5 was dissected and divided. F Finally, the right hepatic vein was divided using a vascular endo-GIA
Left hepatectomy
The falciform, coronary, and left triangular ligaments were sectioned with harmonic curved shears and a cautery hook. After the lesser sac was exposed using the third robotic arm, it was dissected using harmonic shears. The lesser sac sometimes contains an accessory or replaced left hepatic artery, which should be ligated. When the lesser sac was sectioned as far as the left hepatic vein, the left liver was completely mobilized.
The hilar dissection and parenchymal transection were conducted as described for the right hepatectomy. The left bile duct was divided at the junction of the umbilical and transverse portion after applying Hem-o-lok clips during parenchymal transection. The left hepatic vein was divided by using a vascular linear stapler (Fig. 4).
The main procedures of robotic left hepatectomy. After mobilizing the left liver, the liver hilum was dissected. A The left hepatic artery was identified and divided. B The left portal vein was fully mobilized and then divided. C The segment 4b hepatic vein branch was carefully dissected and sectioned. D The left bile duct was divided at the junction of the umbilical and transverse portion during parenchymal transection. E The left hepatic vein was exposed and divided using a vascular endo-GIA. F The specimen was retrieved through a Pfannenstiel incision
Left lateral sectionectomy and other resections
For a left lateral sectionectomy, the left liver was mobilized as described for the left hepatectomy. The liver parenchyma was transected along the left side of the falciform ligament. During parenchymal transection, the round ligament was used as a handle to retract the liver toward the right. The parenchymal retraction method was the same as in major hepatectomy. The segment 2 and 3 pedicles were exposed intrahepatically using harmonic shears, bipolar forceps, and a hook, and were divided by either a separate ligation or a vascular stapler. The parenchymal transection continued until the left hepatic vein was exposed. The left hepatic vein also was divided using a vascular stapler.
Segmentectomy was performed using the Glissonian approach. Intraoperative ultrasound can help us find intrahepatic pedicles and follow the proper resection line. For a wedge resection, the resection margin was obtained with the help of intraoperative ultrasound.
Specimen retrieval
The specimen was placed in a big plastic bag. The area was irrigated and subtle bleeding at the cut liver surface and the dissection area was controlled. After the biliary fistula was checked using a dry surgical gauze, fibrin glue and Surgicel were applied to the cut surface of the liver. The robotic arms were undocked and the specimen was retrieved using a Pfannenstiel incision. In patients who had had a previous operation or a small specimen, the previous incision or the umbilical port was used for the retrieval of the specimen, respectively. The laparotomy incision was closed, and the pneumoperitoneum was made again. A drain was inserted in the operative field through an 8-mm robotic arm port under laparoscopic view.
Results
Patient demographics, indications, and type of resection
Patient characteristics and surgical indication are summarized in Table 1. The mean age of the patient group was 52.4 years and 14 patients were male. There were 21 malignant tumors, including 13 HCCs, 3 cholangiocellular carcinomas, and 5 liver metastases from the gastrointestinal tract, and 9 benign lesions, including 7 intrahepatic stones, 1 recurrent liver cyst, and 1 schwannoma. Of the 23 patients with malignant or benign tumors, 20 had a single lesion. Multiple lesions were observed in three patients with liver metastasis. Of the 27 resected lesions, 22 (81.5%) were located in inferior lateral segments (segments 2–6). The remaining five lesions were located in segment 8 (n = 3), segment 7 (n = 1), and segment 1 (n = 1). Right hepatectomy was performed in 6 patients, left hepatectomy in 14 patients, left lateral sectionectomy in 4 patients, segmentectomy in 2 patients, and wedge resection in 4 patients (Table 2). Seven patients underwent combined procedures such as colon resection (n = 2), radiofrequency ablation (n = 2), stomach resection (n = 1), fenestration of liver cyst (n = 1), and choledocholithotomy (n = 1).
Perioperative data and postoperative complications
The average operating time of all 30 patients was 507 min (range 120–812). In patients who had a right or a left hepatectomy, the mean operating time was 724 and 518 min, respectively. The average estimated blood loss of all 30 patients was 343 ml (range 95–1,500). In patients who had a right or a left hepatectomy, the mean blood loss was 629 and 328 ml, respectively. Four patients (14.8%) had a perioperative transfusion. There were two conversions (6.7%) to open surgery (Table 3). The first open conversion occurred in the fourth case. The patient was diagnosed with intrahepatic cholangiocarcinoma and was going to undergo a left hepatectomy. During parenchymal transection, significant bleeding occurred from the liver parenchyma and was not effectively controlled. The operation was converted to minilaparotomy and the injured hepatic vein was securely ligated. The other conversion was a patient with an intrahepatic duct stone. Multiple stones were impacted in the proximal right bile duct. The recurrent cholangitis resulted in atrophy of the right liver. The hilar structures were unexpectedly adhered severely and the portal bifurcation shifted more to the right side. Individual dissection of the hilum was performed. However, it was difficult to dissect the right portal vein from the hilar structure. Because the safe preservation of the left portal and bile duct could not be guaranteed, the operation was converted to a limited laparotomy.
Complications occurred in 13 patients (43.3%). According to the modified Clavien system, five patients had grade I (16.6%), two had grade II (6.7%), and six had grade III (20.0%). Grade III complications consisted of biliary complication (n = 2), superficial wound infection (n = 2), umbilical port hernia (n = 1), and colon anastomotic leakage (n = 1). Among the seven patients who had combined procedures, three developed complications. The complication rate (42.9%) in these patients was comparable to that (43.5%) of the patients who received liver resection alone (p = 0.100) The median length of stay in the hospital was 11.7 days (range 5–46) (Table 4).
To assess a learning-curve effect, the operating time was analyzed according to each procedure in ten consecutive patients who received left hepatectomy alone (Fig. 5). The most time-consuming procedure was the parenchymal transection. The total operating time and total console time had gradually decreased after the seventh case. The total operating time had decreased to 5 h in the last case.
Short-term results in patients with malignancy
Twenty-one patients underwent robotic liver resection to treat malignant diseases, including 13 HCCs, 5 liver metastases, and 3 intrahepatic cholangiocarcinomas. The clinicopathological characteristics and short-term outcomes in patients with HCC are summarized in Table 5. All lesions were single and the mean diameter was 3.1 cm (range 0.8–5.0). The mean surgical free margin was 2.1 cm (range 0.1–3.5) and 12 patients (92.3%) underwent anatomic resections of the tumor. Only one patient received a perioperative transfusion. On the pathologic examination, eight patients (61.5%) were found to have microscopic vascular invasions and five patients (38.5%) had cirrhosis. There was no recurrence during the median follow-up of 11 months (range 5–29).
The origins of liver metastases were colorectal cancer (n = 4) and small bowel cancer (n = 1). Three patients with colorectal liver metastases underwent liver and colon resections simultaneously. Two patients underwent additional radiofrequency ablations at the same time. Of these five patients, one developed recurrence at 5 months after resection. The remaining four patients maintained disease-free status at the median follow-up of 12 months (range 3–22). Three patients with intrahepatic cholangiocarcinoma underwent left hepatectomy and sampling of lymph nodes at the hepatoduodenal ligament. One patient was found to have the metastasis in the lymph nodes and received adjuvant postoperative chemotherapy. All three patients maintained disease-free status during the median follow-up of 21 months (range 3–22).
Discussion
Our study demonstrated the technical feasibility of robotic liver resection, especially in major hepatectomy, which was performed on 20 consecutive patients. Laparoscopic major hepatectomy has been challenged and performed limitedly in a few expert centers. The conversion rate of laparoscopic major hepatectomy to open has been reported to be 15-20% [7–9]. In this study, the conversion rate was 6.7% in all 30 patients and 10% in the 20 patients who had a major hepatectomy.
To assess the quality and safety of the operation, perioperative data and postoperative complications were prospectively collected, although it was reviewed retrospectively. The mean amount of blood loss was 343 ml and a perioperative transfusion was needed in only four patients (13.3%). Although the overall complication rate was 43.3%, grade III complications occurred in only six patients (20%). In a recent worldwide review of data collected on laparoscopic liver resection in 2,804 patients, the overall morbidity rate was 10.5%, which seems to be lower than that of our study [7]. However, major hepatectomies were only 13% of all the procedures in that review. In addition, there is a possibility that the complications were underestimated because the data were collected retrospectively. The liver resection–related grade III complications, which were biliary complications, occurred in only two patients (6.6%) in our series. Therefore, our study demonstrated that robotic liver resection is not only a feasible but also a safe procedure.
Laparoscopic liver resection, especially for major hepatectomy, is a challenging procedure because of several inherent limitations, including more complex spatial relationships between the liver’s anatomy and port placement, the restricted movements and the fulcrum effect of rigid instrument shafts and trocars [16, 17], and the two-dimensional laparoscopic view. The robotic system has been developed to overcome these laparoscopic limitations [10, 18]. An articulating instrument with seven degrees of freedom and a magnified three-dimensional view can improve a surgeon’s techniques in all the procedures of minimally invasive liver surgery. The intuitive translation of the surgeon’s hands and wrists into precise and nontremulous instrument movements eliminates the fulcrum effect and the difficulties in handling rigid instruments encountered during conventional laparoscopy. Therefore, the robotic system enables the surgeon to conduct a minimally invasive liver resection using the same techniques as used in open liver surgery. These advantages of the robotic system can be maximized in performing major hepatectomy. The dissection of the liver hilum requires delicate handling of major vessels and sometimes suture ligations to divide small portal vein branches; both maneuvers are difficult to perform laparoscopically. We could have dissected the liver hilum and safely divided the individual vessels in most of the patients who had a major hepatectomy, although there was one conversion during hilar dissection in a right hepatectomy due to a severe hilar adhesion and anatomic deformation.
Right liver mobilization is more challenging than that of the left liver because of the anatomic location and large volume. Various kinds of laparoscopic methods for mobilizing the right liver have been introduced, including the conventional approach [19], the anterior approach [20], the approach with the left lateral decubitus position [21], and the hand-assisted method [22]. A stable camera platform under a surgeon’s direct control and the capability of locking the instruments for stable exposure and retraction as well as wristed instruments provided easier mobilization of the right liver, which is the third robotic arm lifting method introduced by Giulianotti et al. [12]. This method can make sufficient working space in the posterior side of the right liver and allow safe dissection of the hepatocaval plane. In our six consecutive patients, right liver mobilization and dissection of the IVC were safely conducted using this method.
Parenchymal transection is one of the most challenging procedures in laparoscopic liver resection. Significant bleeding during parenchymal transection is the most common reason for conversion to open liver resection [8]. The stable and full exposure of the parenchymal transection plane is the key to a successful procedure. As introduced by Giulianotti et al. [12], we used the third robotic arm to laterally retract the left liver in the initial three cases. Because the robotic system does not have tactile feedback, we used a rubber band, which was fixed by stay stitches at the left resection margin, to prevent avulsion injury from inappropriate retraction. In the third case, the operation was converted to open surgery because significant bleeding was not controlled during parenchymal transection in left hepatectomy. The maintenance of low central venous pressure and meticulous parenchymal dissection are needed to prevent bleeding during parenchymal transection; however, a little bleeding may be an inevitable event in liver resection. The use of only two robotic arms may not be sufficient for safe parenchymal transection. Therefore, we started using the rubber band retraction method starting with the fourth case. This method allowed the simultaneous use of all three robotic arms during parenchymal transection. The third robotic arm can be used either to compress a bleeding site or to further expose the optimal transection field. Another advantage of this method is that the elastic power of the rubber band can automatically expose the parenchymal transection plane. From the fourth case on there has been no conversion due to bleeding during parenchymal transection in our series. This method can be also useful for inexperienced laparoscopic surgeons while performing laparoscopic parenchymal transection.
The improved performance using the robotic system compared to conventional laparoscopy has been demonstrated in well-designed recent studies [23–25]. They suggested that the robotic devices can shorten the learning curve of difficult laparoscopic procedures for inexperienced laparoscopic surgeons and enable expertise to conduct more complex laparoscopic procedures easily. In clinical practice, the robotic system has broadened the indications of minimally invasive surgery into the more complex liver surgeries such as major hepatectomy with biliary reconstruction and two-stage hepatectomy [13, 26, 27]. As for our series, the operator succeeded in performing robotic major hepatectomies without any experience with laparoscopic major liver resections. Thus, the robotic system may enable a surgeon with insufficient experience with laparoscopic surgery to perform the more complex laparoscopic procedures.
The robotic system has some disadvantages with respect to technical aspects compared to the conventional laparoscopy. One is that patient repositioning and additional placement of ports are restricted because the surgical cart in the operative field would have to be unlocked [18, 28]. Another is that robotic surgery has limited options for port placement due to the bulky robotic arm. Therefore, principles should be followed to properly select the port sites. The camera port should be placed at the midline of the target anatomy and 15–20 cm apart from the target anatomy [28]. To minimize internal collisions, ports for robotic arms should be placed outside the triangular area that is formed by the camera port and both ends of the target anatomy. The robotic arm port should be placed at least 8 cm from the other port to minimize external collisions. In patients with a slim abdominal shape, the inferior border of the liver usually lies lower. The patient’s body shape should also be considered when deciding port placement. Unlike other robotic instruments, the harmonic curved shears do not have the EndoWrist function. To follow the proper transection plane, the port for the shears should be properly selected. In right or left hepatectomy, mounting the harmonic curved shears on the second robotic arm, which was placed at the right upper quadrant area, and the lower position of the active blade usually allowed the parenchymal transection plane to be followed properly. In some cases of major hepatectomy or other hepatectomies, the first robotic arm was also used for the harmonic shears according to the alignment of transection plane. By using these methods, three different transection planes could be produced in two cases of anterior segmentectomy.
The long operating time seems to be another discouraging finding of the robotic surgery in this study as well as other studies [13]. This may be the result of the additional set-up time needed, longer time for the assistant to switch the robotic instruments, relatively slow movement from one surgical field to another, and the learning-curve effect. The mean operating time of right or left hepatectomy in our series was actually longer than that of other reports. When the operating time for a left hepatectomy alone was analyzed, the time-limiting procedure was the parenchymal transection. As shown in Fig. 5, the total operating time, total console time, and parenchymal transection time decreased after the seventh case. With more accumulation of experience and technical refinement, the operating time is expected to further decrease. However, to demonstrate the learning-curve effect in robotic liver resection, surgical improvement should be assessed using operating time as well as estimated blood loss, complication rate, and conversion rate. The cumulative sum methodology is useful for evaluating the learning curve of this novel surgical technique [29, 30].
Although the robotic system allows for more complex liver surgery, one of the main limiting factors for the laparoscopic approach is tumor location [8, 9]. According to the international consensus conference, the optimal candidates for laparoscopic liver resection are those with single lesions in the peripheral liver segments (segments 2–6) [9]. In our series, two types of port placement enabled us to perform major hepatectomy, left lateral sectionectomy, segmentectomy of segment 4b and segment 5, and wedge resection of segment 6. All resections were restricted to the inferior lateral segments, except for major hepatectomies. Like the laparoscopic approach, the robotic system also has technical problems when it comes to resecting the posterior and superior segments. In accordance with previous studies [12, 31], in our series a right hepatectomy was the preferred procedure to remove the tumor located in the deep parenchyma of posterior and superior segments. In only one patient was the tumor, superficially located in segment 8, nonanatomically resected. In this patient, two main working ports were placed in the upper area, near the subcostal margin, to achieve the proper angle for parenchymal transection. Gumbs et al. [32] introduced the lateral laparoscopic approach to lesions in the posterior segments. Recently, Casciola et al. [14] demonstrated the feasibility of robotic liver resection for lesions in the posterosuperior segments. The optimal patient positioning and proper port placement as well as the improved dexterity of the robotic system could enable surgeons to more easily resect the posterior and superior segments.
The oncological results of laparoscopic cancer surgery have been demonstrated in previous studies. Currently, laparoscopic liver resection is recommended in patients with relatively early-stage cancer such as a single lesion less than 5 cm in diameter [9]. Recent comparative studies showed the comparable oncological results between laparoscopic and open liver resections [33–35]. In our study, all 13 patients with HCC had a single lesion less than 5 cm in diameter. All patients had a margin-negative resection and 12 of 13 patients (92.3 %) had an anatomic resection. Transfusion was performed in only one patient. There were no recurrences during the 11-month median follow-up. If the oncologic principles such as margin-negative curative resection, no direct manipulation of the tumor, more anatomic resection, and minimal blood loss are strictly followed, the same oncologic outcomes can be expected with robotic liver resection.
In conclusion, robotic liver resection seems to be a feasible and safe procedure, particularly for major hepatectomy, from our initial data. There were lower conversion rate, minimal blood loss, lower rate of perioperative transfusion, and a postoperative complication rate comparable to that of laparoscopy. Although a longer operating time was found to be the main drawback, it has decreased over time and can be expected to further decrease with more experience. Therefore, the robotic system should be considered a new technical option for minimally invasive liver surgery. Although the maximum benefits of the robotic system seem to come with more complex hepatectomy, the cost effectiveness and actual benefits of the robotic system in minimally invasive liver surgery should be demonstrated in large prospective comparative studies.
References
Fortner JG, Blumgart LH (2001) A historic perspective of liver surgery for tumors at the end of the millennium. J Am Coll Surg 193:210–222
Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, Corvera C, Weber S, Blumgart LH (2002) Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg 236:397–406 discussion 406–397
Rau HG, Meyer G, Cohnert TU, Schardey HM, Jauch K, Schildberg FW (1995) Laparoscopic liver resection with the water-jet dissector. Surg Endosc 9:1009–1012
Cuesta MA, Meijer S, Paul MA, de Brauw LM (1995) Limited laparoscopic liver resection of benign tumors guided by laparoscopic ultrasonography: report of two cases. Surg Laparosc Endosc 5:396–401
Kaneko H, Takagi S, Shiba T (1996) Laparoscopic partial hepatectomy and left lateral segmentectomy: technique and results of a clinical series. Surgery 120:468–475
Gugenheim J, Mazza D, Katkhouda N, Goubaux B, Mouiel J (1996) Laparoscopic resection of solid liver tumours. Br J Surg 83:334–335
Nguyen KT, Gamblin TC, Geller DA (2009) World review of laparoscopic liver resection-2,804 patients. Ann Surg 250:831–841
Vigano L, Tayar C, Laurent A, Cherqui D (2009) Laparoscopic liver resection: a systematic review. J Hepatobiliary Pancreat Surg 16:410–421
Buell JF, Cherqui D, Geller DA, O’Rourke N, Iannitti D, Dagher I, Koffron AJ, Thomas M, Gayet B, Han HS, Wakabayashi G, Belli G, Kaneko H, Ker CG, Scatton O, Laurent A, Abdalla EK, Chaudhury P, Dutson E, Gamblin C, D’Angelica M, Nagorney D, Testa G, Labow D, Manas D, Poon RT, Nelson H, Martin R, Clary B, Pinson WC, Martinie J, Vauthey JN, Goldstein R, Roayaie S, Barlet D, Espat J, Abecassis M, Rees M, Fong Y, McMasters KM, Broelsch C, Busuttil R, Belghiti J, Strasberg S, Chari RS (2009) The international position on laparoscopic liver surgery: the Louisville Statement, 2008. Ann Surg 250:825–830
Hanly EJ, Talamini MA (2004) Robotic abdominal surgery. Am J Surg 188:19S–26S
Song J, Oh SJ, Kang WH, Hyung WJ, Choi SH, Noh SH (2009) Robot-assisted gastrectomy with lymph node dissection for gastric cancer: lessons learned from an initial 100 consecutive procedures. Ann Surg 249:927–932
Giulianotti PC, Coratti A, Sbrana F, Addeo P, Bianco FM, Buchs NC, Annechiarico M, Benedetti E (2010) Robotic liver surgery: results for 70 resections. Surgery 149:29–39
Ji WB, Wang HG, Zhao ZM, Duan WD, Lu F, Dong JH (2011) Robotic-assisted laparoscopic anatomic hepatectomy in China: initial experience. Ann Surg 253:342–348
Casciola L, Patriti A, Ceccarelli G, Bartoli A, Ceribelli C, Spaziani A (2011) Robot-assisted parenchymal-sparing liver surgery including lesions located in the posterosuperior segments. Surg Endosc 25(12):3815–3824
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6,336 patients and results of a survey. Ann Surg 240:205–213
Crothers IR, Gallagher AG, McClure N, James DT, McGuigan J (1999) Experienced laparoscopic surgeons are automated to the “fulcrum effect”: an ergonomic demonstration. Endoscopy 31:365–369
Vigano L, Laurent A, Tayar C, Tomatis M, Ponti A, Cherqui D (2009) The learning curve in laparoscopic liver resection: improved feasibility and reproducibility. Ann Surg 250:772–782
Idrees K, Bartlett DL (2010) Robotic liver surgery. Surg Clin North Am 90:761–774
Gayet B, Cavaliere D, Vibert E, Perniceni T, Levard H, Denet C, Christidis C, Blain A, Mal F (2007) Totally laparoscopic right hepatectomy. Am J Surg 194:685–689
Bryant R, Laurent A, Tayar C, Cherqui D (2009) Laparoscopic liver resection: understanding its role in current practice: the Henri Mondor Hospital experience. Ann Surg 250:103–111
Buell JF, Thomas MT, Rudich S, Marvin M, Nagubandi R, Ravindra KV, Brock G, McMasters KM (2008) Experience with more than 500 minimally invasive hepatic procedures. Ann Surg 248:475–486
Koffron AJ, Auffenberg G, Kung R, Abecassis M (2007) Evaluation of 300 minimally invasive liver resections at a single institution: less is more. Ann Surg 246:385–392 discussion 392-384
Chandra V, Nehra D, Parent R, Woo R, Reyes R, Hernandez-Boussard T, Dutta S (2011) A comparison of laparoscopic and robotic assisted suturing performance by experts and novices. Surgery 147:830–839
Stefanidis D, Wang F, Korndorffer JR Jr, Dunne JB, Scott DJ (2011) Robotic assistance improves intracorporeal suturing performance and safety in the operating room while decreasing operator workload. Surg Endosc 24:377–382
Kenngott HG, Muller-Stich BP, Reiter MA, Rassweiler J, Gutt CN (2008) Robotic suturing: technique and benefit in advanced laparoscopic surgery. Minim Invasive Ther Allied Technol 17:160–167
Giulianotti PC, Sbrana F, Bianco FM, Addeo P (2010) Robot-assisted laparoscopic extended right hepatectomy with biliary reconstruction. J Laparoendosc Adv Surg Tech A 20:159–163
Patriti A, Ceccarelli G, Bartoli A, Spaziani A, Lapalorcia LM, Casciola L (2009) Laparoscopic and robot-assisted one-stage resection of colorectal cancer with synchronous liver metastases: a pilot study. J Hepatobiliary Pancreat Surg 16:450–457
Giulianotti PC, Coratti A, Angelini M, Sbrana F, Cecconi S, Balestracci T, Caravaglios G (2003) Robotics in general surgery: personal experience in a large community hospital. Arch Surg 138:777–784
Wohl H (1977) The CUSUM plot: its utility in the analysis of clinical data. N Engl J Med 296:1044–1045
Bokhari MB, Patel CB, Ramos-Valadez DI, Ragupathi M, Haas EM (2011) Learning curve for robotic-assisted laparoscopic colorectal surgery. Surg Endosc 25:855–860
Cho JY, Han HS, Yoon YS, Shin SH (2008) Feasibility of laparoscopic liver resection for tumors located in the posterosuperior segments of the liver, with a special reference to overcoming current limitations on tumor location. Surgery 144:32–38
Gumbs AA, Gayet B (2008) Video: the lateral laparoscopic approach to lesions in the posterior segments. J Gastrointest Surg 12:1154
Belli G, Limongelli P, Fantini C, D’Agostino A, Cioffi L, Belli A, Russo G (2009) Laparoscopic and open treatment of hepatocellular carcinoma in patients with cirrhosis. Br J Surg 96:1041–1048
Tranchart H, Di Giuro G, Lainas P, Roudie J, Agostini H, Franco D, Dagher I (2010) Laparoscopic resection for hepatocellular carcinoma: a matched-pair comparative study. Surg Endosc 24:1170–1176
Aldrighetti L, Guzzetti E, Pulitano C, Cipriani F, Catena M, Paganelli M, Ferla G (2010) Case-matched analysis of totally laparoscopic versus open liver resection for HCC: short and middle term results. J Surg Oncol 102:82–86
Acknowledgments
The authors thank Mr. Dong-Su Jang, Research Assistant, Department of Anatomy, Yonsei University College of Medicine, Seoul, Korea, for his help with the figures. This study was supported by a faculty research grant from the Yonsei University College of Medicine for 2008 (6-2008-0128).
Disclosures
Gi Hong Choi, Sung Hoon Choi, Sung Hoon Kim, Ho Kyoung Hwang, Chang Moo Kang, Jin Sub Choi, and Woo Jung Lee have no conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Choi, G.H., Choi, S.H., Kim, S.H. et al. Robotic liver resection: technique and results of 30 consecutive procedures. Surg Endosc 26, 2247–2258 (2012). https://doi.org/10.1007/s00464-012-2168-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-012-2168-9