Abstract
Urban rivers face sustained anthropogenic pressures limiting biodiversity. Yet, urban waterways such as the Detroit River are important habitat in supporting regional diversity. The Detroit River is a Great Lakes Area of Concern where conservation and restoration efforts prioritize improved biological and habitat integrity in the connecting channel. This study explores benthic macroinvertebrate in submerged aquatic vegetation across five mainstem channel wetlands and two tributary sites of the Canadian wetlands to describe spatial patterns and diversity. We first examine inter-wetland differences between five mainstem wetlands by hierarchical cluster analysis, NMDS and PERMANOVA, identifying two mainstem groups: one comprising of two middle reach wetlands (Detroit River Marshes and Grass Island), the second showed similarities among wetlands across all reaches (Turkey Creek, River Canard and Peche Island). The latter groupings shared similar habitat characteristics, deeper and finer grain-sizes, and functional feeding group characteristics - low abundances of shredders. Second objective, we perform an intra-wetland comparison for Turkey Creek and River Canard to analyze for differences along tributaries. At neither River Canard nor Turkey Creek we observed significant tributary influence on mainstem communities but had found the Turkey Creek tributary communities significantly differed from the channel communities. Diversity metrics and Hilsenhoff Biotic Index illustrate strained benthic communities across the river. We had also found water quality to be consistently moderately degraded. Our findings differ from prior analyses within emergent vegetation that indicate variable water quality conditions between mainstem and tributary and non-impaired macroinvertebrate communities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Laurentian Great Lakes supports a growing 34 million people and are a biodiversity hot spot for 2200 aquatic taxa where 1433 are benthic invertebrates (Trebitz et al. 2019; Fergen et al. 2022). Benthic macroinvertebrates strongly influence nutrient and energy transfers from sediment to the water column and higher trophic organisms contributing to benthic-pelagic coupling (Grimm 1988; Wallace and Webster 1996; Demars et al. 2021). Anthropogenic stressors alter benthic community structures by altering hydraulic regimes, physical substrate characteristics (e.g., siltation of formerly granular substrates), altering oxygenation status and oxygen penetration within sediments and contribute to toxicity via accumulation of toxic chemicals in sediment (Buss et al. 2004; Larsen et al. 2011; Scavia et al. 2014; Desrosiers et al. 2018). Many of the above stressors contribute to chronic effects such as reduced growth and reproduction and acute effects such as mortality leading to loss of sensitive species (reduced biodiversity) and in some cases proliferation of a limited number of tolerant species (reduced species evenness) (Evans-White et al. 2009; Ligeiro et al. 2013; Barnum et al. 2017).
Benthic macroinvertebrates are used in biomonitoring and bioassessments as environmental tolerances have been extensively studied and developed into indices (Resh et al. 1995; Reynoldson et al. 1995; Townsend et al. 1997) and established to respond to water and sediment quality conditions (Lock and Goethals 2011; Beermann et al. 2018; Akyildiz and Duran 2021). The Hilsenhoff Biotic Index (HBI), developed in streams and used in river systems, estimates water quality and degree of organic pollution quantitatively utilizing macroinvertebrate taxonomic data (Hilsenhoff 1998; Gao et al. 2023). A common indicator group, Ephemeroptera, Plecoptera, and Trichoptera (EPT), are sensitive to contaminants, changes in organic loads, nutrients, and biological oxygen demand. The exclusion of sensitive species alters communities toward more tolerant ones (Chun et al. 2017; Saari et al. 2018). Pollution tolerant species are less desirable in supporting aquatic food webs due to bioaccumulation in tissues and low net energy (Bendell-Young 1999; Marcarelli et al. 2011; Bertoli et al. 2021; Das et al. 2023). Although invertebrates have been demonstrated to respond to disturbances, it can be difficult to assess highly impacted sites where diversity and indicator species are low (Ostermiller and Hawkins 2004). Species traits, such as functional feeding groups (FFGs) are available to gain further insights into functioning and the processing of energy in aquatic ecosystems (Cummins et al. 2005; Merritt et al. 2017). Several studies demonstrated relationships with FFGs responses to environmental perturbations. Typically, collectors (filters and gatherers) are pollution-tolerant whereas shredders and scrapers demonstrate environmental sensitivities (Carlisle and Clements 2005; Pastorino et al. 2020; Yaagoubi et al. 2023). Diversity-based tools involve niche partitioning as a central concept – the use of assigning taxonomic units to environmental gradients. Metacommunity analysis seeks to broaden niche-based approaches to consider traits, such as dispersal; regional trait databases are in development and providing insights into community structuring for management (Poff et al. 2006; Schmidt-Kloiber and Hering 2015; Sarremejane et al. 2020; Ao et al. 2022).
Species traits such as mobility (drift, crawling, swimming, flight) and species interactions (competition and predation) also affects community compositions (Mackay 1992; Kelly et al. 2006; Bonada et al. 2007). Community assemblages are influenced by habitat characteristics such as water depth, velocity, temperature, food sources, flow rates and substrate (Mackay 1992; Collier 1995; Buss et al. 2004; Jonsson et al. 2017; Demi et al. 2019). Many studies have analyzed community composition, particularly the Laurentian Great Lakes, yet studies on connecting channels are limited (Uzarski et al. 2017; Wick et al. 2019). These channels have a significant impact on biodiversity and consequences for management in the Great Lakes region by providing habitat and lake linkages for native and introduced species (Tucker et al. 2020). Connecting channels can have simplified habitats relative to multi-order streams and are characterized by high flow rates contributed almost entirely by the upstream water body along with minor water inputs stemming from smaller tributary inputs. Habitat complexity is contributed by depth transitions around navigational channels, islands, and shoreline features such as small embayments and/or areas receiving tributary inputs that support submerged wetlands. Our study examines diversity and functional feeding groups of submerged Detroit River wetlands (Canada) to gain insight into community patterns in this Great Lakes connecting channel and Great Lakes Area of Concern to determine whether tributary-associated wetlands support different communities compared to wetlands present in the main channel and embayments.
The Detroit River receives > 98% of its flow from the upstream Lake St. Clair. Water quality tends to remain consistent along most Canadian reaches before entering Lake Erie. Furthermore, deep, cooler, high velocity waters flowing through navigation channels throughout the river length have, for the most part, maintained hydraulic separation between nearshore water masses along Canadian and U.S. shorelines reducing the impact of U.S. legacy and point sources inputs on Canadian nearshore habitats (Drouillard et al. 2006). Sediment contamination in Canadian jurisdiction have been declining and are at suitable thresholds for benthos (Szalinska et al. 2013; DRCC 2020). However, there remain some concerns about localized impacts to water quality and potential implications to ecosystem indicators in Canadian nearshore areas subject to inputs from tributaries receiving urban and agricultural stressors. As tributaries join into rivers, areas of confluence can create physiochemical gradients depending on flow characteristics. These areas can increase biodiversity by creating more heterogeneity in a habitat. However, in stressed habitats benthic communities may be negatively impacted (Hayward et al. 2022).
Environment and Climate Change Canada (ECCC) routinely conducts biotic and water quality assessments at Canadian wetlands present in the upper, middle and lower reaches of the channel and two major tributaries, River Canard and Turkey Creek (ECCC 2022). Water quality ranges from good to severely degraded, with the two tributaries being moderately or severely degraded according to ECCC’s water quality index (Chow-Fraser 2006; ECCC 2022). Despite poor water quality index rankings, assessment of macroinvertebrate integrity at Turkey Creek and River Canard tributaries did not indicate marked impairment relative to other monitored Detroit River wetlands within the channel. However, ECCC’s surveys of macroinvertebrate communities are restricted to areas of emergent vegetation close to the shoreline (ECCC 2022). The submerged aquatic vegetation (SAV) sections that form large continuous beds connected with these marshes are non-wadable depths, located within the mainstem - upstream and downstream of the tributary discharge points. In this study, mainstem SAV beds adjacent to the tributary are referred to as mainstem-tributary (MT) wetlands, at Turkey Creek and River Canard. Non-tributary (NT) submerged wetland beds are Peche Island, Grass Island and Detroit River Marshes (DRM).
We conducted a community survey of Detroit River macroinvertebrates with two main objectives. First, is to examine whether five mainstem wetlands vary in composition such that mainstem-tributary wetlands differ most from non-tributary wetlands. It is predicted that mainstem-tributary wetlands will differ as these areas receive inputs from tributaries with longstanding water quality issues and can cause changes in habitat (Svendsen et al. 2009). The second objective is concentrated on the mainstem-tributary wetlands, and to investigate whether sampling nearby tributaries is representative of channel communities in submerged vegetation. Wetlands were delineated upstream and downstream sections to test for community differences to tributary communities. We predict that given longstanding water quality differences between mainstem and tributaries, communities will vary between the tributary and adjacent mainstem communities. Additionally, components of community diversity are explored to describe diversity found within the Great Lakes Area of Concern.
Methods
Site Description
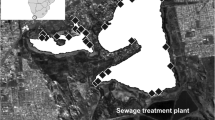
The Detroit River is a binational connecting channel in the Laurentian Great Lakes passing between the cities of Detroit, Michigan, USA and Windsor, Ontario, Canada. This connecting channel is also known as Waawiiatanong Ziibi in Anishinaabemowin, within the Traditional Territory of the Three Fires Confederacy of First Nations (Ojibwe, Odawa, and Potawatomi). The watershed drains 19,040km2 through 49% agricultural land and 21% urban land where Canadian landmass represents 79% of agricultural land and 17% of the urban (Scavia et al. 2019). Water levels are dependent on Lake St. Clair and Lake Erie levels as the river has a gradual slope. It flows southward 43.5 km from Lake St. Clair to Lake Erie with a discharge rate ranging from 4400m3s− 1 in the winter to 5,700 m3s− 1 in the summer (Derecki 1984). Sediment grain-size varies across the river as flow rates vary with changing depth, presence of islands and proximity to shorelines. Fine sediment (sand and silt) is common in the upper and middle reaches and very fine sediments (silt and clay) deposit mainly in the lower reach and in narrow canals. (Fallon and Horvath 1985; Wood 2004; Szalinska et al. 2013). The Canadian wetlands sampled within the Detroit River are Peche Island Marsh located in the upper reach, middle reach wetlands include Turkey Creek, Detroit River Marshes (DRM), and Grass Island and River Canard found in the lower reach (Fig. 1). Two tributary inlets were sampled at River Canard and Turkey Creek.
Sampling Design
Each wetland was sampled over the period of September 24th – October 3rd, 2019. Wetland boundaries were determined with the aid of the Essex Region Conservation Authority On-Line interactive mapping tool (http://ercamaps.countyofessex.ca/) using the Provincially Significant Wetland layer tool and Google Earth Pro. A total of 60 sites were sampled with 10 sites across mainstem wetlands (n = 5) and 5 sites per tributary (n = 2). Sample locations were assigned using a stratified random sampling design. Each mainstem wetland was overlayed with a grid (∼ 150 × 150 m) and sample locations assigned as the centre coordinate of 10 randomly selected grid cells. Mainstem-tributary wetlands were divided into 3 strata; two within the channel encompassing macrophyte bed positions upstream of the tributary input, the second strata downstream and the third within the tributary plume, each strata were sampled in five locations. These strata were delineated using Google Earth imagery and examining tributary plume extent dimensions across time using the historical imagery tool. Detailed maps of sample site locations at each of the five wetlands and tributaries are provided in Supplementary Information (Figures S1-S4).
Data Collection
Water quality data were collected using a Ruskin RBR profiler at each sample site to measure depth, conductivity, pH, temperature, and turbidity just above the sediment. Water quality was calculated as per Chow-Fraser (2006) according to the equation:
The associated qualitative descriptor has WQI values 0 to 1 as Good, 0 to -1 moderately degraded and <-1 as degraded (Chow-Fraser 2006). Bulk sediment for characterization was collected by petite ponar grab sampler for grain size distributions and loss on ignition derived sediment organic carbon content, referred to as Total Organic Carbon (TOC) in this study (Drouillard et al. 2006). Benthic macroinvertebrates were retrieved using triplicate grabs by petite ponar grab sampler (2.4 L), ensuring each accepted replicate came from a ponar grab that was at least ¾ full of sediment. Each sediment grab was washed on site through a 250 μm mesh and the sample preserved in a modified Kahles solution (10:5:1 v/v/v water:95% ethanol: formalin). One of the three samples were randomly selected for in-lab macroinvertebrate identification by dissection microscope.
Data Analyses
Differences between habitats were investigated via pairwise-PERMANOVA (Euclidean distance, Bonferroni-corrected) on depth and sediment characteristics (grain-sizes, and TOC). Forty-one benthic macroinvertebrate taxa were identified from 14,694 observed individuals mostly to genus or species with exception of Oligochaeta, Chironomidae, Nematoda, Rissooidea, and Turbellaria by the taxonomic keys: Freshwater Invertebrates: Keys to Nearctic Fauna (Thorp and Rogers 2016); An Introduction to the Aquatic Insects of North America (Merritt et al. 2008). Biodiversity surveys are limited by sampling efforts to capture very rare species (Cao et al. 2001) and this study was limited in its sampling effort on a per wetland basis to interpret differences in rare species abundances across wetlands. To reduce noise in the dataset (Gauch 1982), rare taxa (occurrence in < 10% of samples) were removed or where possible rare genera were combined to family level into a total of 28 taxa. This resulted in the removal of 26 individuals from 8 taxa that represented less than 3% of the relative abundance at each sample site (Resh et al. 2005). Individual species data was transformed to meet the assumptions of normality by an octave transformation of relative abundances (Preston 1948; Corkum et al. 1997).
Statistical analyses were performed by R-statistical software using vegan and adespatial packages (Oksanen et al. 2022, Dray et al. 2022), figures were generated in ggplot2 and pheatmap (Wickham 2016, Kolde 2019). Diversity metrics were calculated for each wetland and tributary for taxa richness, Pielou J’s evenness, EPT taxa (%), and Sorenson beta-diversity of nestedness and turnover. Beta-diversity was calculated by beta.div.comp function within the R adespatial package using default settings for relative abundance data (Legendre 2014). Benthic macroinvertebrate community data were first examined using heat maps accompanied with cluster analysis of a Bray-Curtis community dissimilarity matrix by agglomerative hierarchical clustering (stats package; function hclust), Ward’s method (Ward.D2) (Murtagh and Legendre 2014). Determining the number of optimal clusters was aided by NbClust (package and function) in R which provides results from 30 indices. While cubic clustering criterion supports two major clusters (Value Index: 7.2175), the weight of evidence supports three significant clusters by 9 indices value index: KL (4.022), Hartigan (2.4544), TrCovW (10596.97), Trace W (217.8027), Rubin (-0.1707), Silhouette (0.1697), Beale (-0.8409) and Ball (1036.522).
Community differences by wetland were visualized with non-metric multidimensional scaling (NMDS) ordination. PERMANOVA analyses with Bonferroni-corrected pairwise comparisons were then used to evaluate for community differences between the 5 mainstem wetlands (Bray-Curtis distances). We hypothesized that one or both mainstem tributary wetlands would deviate in their community structure from the 3 non-tributary wetlands. Next, the mainstem-tributary wetlands were examined independently to test for community differences with respect to tributary position (upstream, downstream, and tributary). Similar to mainstem-wetland contrasts, an NMDS ordination and PERMANOVA was performed to visualize and test for differences by position.
Hilsenhoff Biotic Index (HBI) values and FFGs were assigned under the following FFG classifications: collector-gatherer, collector-filterer, predator, parasite, scraper, and shredder. HBI Values and FFG designations were determined (Bode et al. 1996, 2002; Barbour et al. 1999). Significant differences among FFG relative abundances between wetlands and tributaries and differences were explored via PERMANOVA. For all PERMANOVA analysis on community composition and FFGs that bore significant differences, SIMPER analysis was performed using PAST Statistical Software (v. 4.03) with Bray-Curtis distances to provide insight into taxon influence on variation between communities (Clarke 1993; Hammer et al. 2001).
EPT taxa, Hyallela spp., and FFG count data associated with physiochemical properties of water quality and habitat characteristics using generalized linear models (GLMs). The distribution of the continuous data was non-normal (right-skewed), zero-inflated and overdispersed and not suitable for Gaussian or Poisson distribution – a gamma distribution hurdle model was utilized in this work as directed by Zuur & Ieno (2016). The hurdle model is compromised of two components, a Gamma GLM and Bernoulli GLM. In base R, presence/absence data was generated for the response variables for the binomial adjustment GLM (Bernoulli). The log link gamma model had response variables with zero entries removed which limits interpretation and sample size. Spearman rank-correlations coefficients were between the explanatory variables were assessed for values less than 0.50 to account for collinearity and minimize overfitting. In choosing the best model, the lowest Akaike Information Criterion (AIC) was selected. Models were generated for 13 taxa and 6 FFGs with 5 explanatory variable combinations. Models that had demonstrated significant associations were further analysed for the adjusted R2 (Tjur or Nagelkerke) and models were cross-validated with glmet::cv.glmnet function (Friedman et al. 2010) and mean squared error from subsetted training data. Relevant residual vs. fitted plots are provided as Figure S5.
Results Section
Environment
Mainstem-tributary sample sites were generally deeper with slightly higher conductivity and turbidity than non-tributary sample sites. Non-tributary wetland sites exhibited coarser grain sizes (Table S1). Mainstem-tributary wetlands, River Canard, the downstream positions were deeper with higher proportions of sand and reduced turbidity, TOC, and gravel (Table S2). Next, Turkey Creek’s downstream had lower turbidity and depth with similar grain distributions, while the tributary had overall higher conductivity, TOC, and was the shallowest of the three positions (Table S2). PERMANOVA on habitat characteristics between wetlands yielded differences (F4,44 = 4.67, p = 0.001), pairwise analysis revealed differences between Grass Island (NT) to Peche Island (NT) (F1,19 = 3.45, p = 0.030), Turkey Creek (MT) (F1,19 = 14.64, p = 0.002), and with River Canard (MT) (F1,19 = 6.80, p = 0.016). All wetlands and tributaries in the present study are considered moderately degraded with scores below zero, ranked in descending quality: Grass Island (-0.16), DRM (-0.35), Peche Island (-0.39) & Turkey Creek (-0.39), River Canard (MT) (-0.47), River Canard Tributary (-0.5), and Turkey Creek Tributary (-0.71).
Macroinvertebrate Community
Across sampled wetlands, twenty-eight taxa were identified from 14,694 individuals. Final taxonomic resolution belonged to 2 species, 10 genus, 13 families, 1 sub-class (Oligochaeta), 1 class (Turbellaria) and 1 phylum (Nematoda). The most common taxa observed were Chironomidae and Oligochaeta at 100% of sites, Nematoda (95%), Rissooidea (86.6%), Valvatidae (83.3%) and Dreissenidae (73.3%).
Community diversity metrics are summarized in Tables 1 and taxon frequency tables by wetland are provided in Supplement 2. Across all mainstem wetlands (n = 50), Shannon H’ was 1.764 from an average taxa richness of 13.2. Pollution sensitive, EPT taxa (n = 8) made up 7.17% of individuals and were found at 60% of sample locations. Beta-diversity between Detroit River as a whole was primarily taxa turnover (0.736). Mainstem-tributary wetland sample sites (n = 20) were dominant in Chironomidae (0.403 ± 0.168), Oligochaeta (0.219 ± 0.094), Nematoda (0.126 ± 0.109) and Rissooidea (0.087 ± 0.092). Alpha diversity metrics show taxa richness was 11.5, moderately even 0.653 (Pielou J), Shannon diversity was poor (1.584) with EPT taxa in low abundance (3.7%). Beta-diversity was predominately represented by taxa turnover (67.3%).
Non-tributary sites (n = 30) were similarly dominant in Chironomidae (0.315 ± 0.022), Oligochaeta (0.228 ± 0.023) and Nematoda (0.076 ± 0.009), followed by a Trichopteran, Leptocerus spp. (0.061 ± 0.014). Taxa richness was higher (14.4) than MT wetlands (11.5), and assemblages were slightly more even (0.715), negligibly more favourable Shannon Diversity, 1.88, with higher EPT presence of 10.6%. Turnover contributed to 71.5% of beta diversity. Three taxa were unique to non-tributary wetlands, the platyhelminth Turbellaria; the sole Odonate taxa detected, Coenagrionidae; and the Trichopteran Nectopsyche spp.
Hilsenhoff Biotic Index scores below 6.50 are considered to have fair water quality, and the Detroit River and tributaries scored fairly poor (6.69) suggesting significant pollution impairing communities (Hilsenhoff 1987). HBI scores in descending quality are Turkey Creek (MT) (6.550), River Canard Tributary (6.610), DRM (6.619), River Canard (MT) (6.690), Turkey Creek Tributary (6.73), Grass Island (6.735), Peche Island (6.905). Functional feeding groups were dominant in collector-gatherers, followed by scrapers and parasites. Scrapers were found in low abundances at Peche Island (NT), River Canard (MT), and Turkey Creek (MT) (Fig. 2). Significant differences among wetland FFGs were found with Grass Island showing differences to Peche Island (F6,53 = 10.85, p = 0.017), Turkey Creek – mainstem (F1,19 = 3.261, p = 0.001) and tributary (F1,14 = 6.48, p = 0.0273). SIMPER indicates differences driven by collector-gatherers and shredders (Supplement 3).
Variable selection by Spearman correlation analysis reduced environmental variables to conductivity, turbidity, depth, TOC, silt for GLM models (Table S3). Nine models displayed significant values, however in cross-validation, mean standard error derived from training and test data were high for all but two presence/absence models for Caenis spp. and Hyalella spp. indicating depth as a significant predictor of their establishment. For the genus Caenis, regression considered depth, TOC and silt, the adjusted R2 value was reported to be weak at 0.11 and the AIC value was equal to 75.6. The effect of depth was statistically significant and negative (beta = -1.39, 95% CI [-2.91, -0.14], p = 0.047; Std. beta = -0.72, 95% CI [-1.18, 0.18]). The Hyalella spp. model considered conductivity and depth with a moderate Tjur’s R2 (0.21). The effect of depth is statistically significant and negative (beta = -2.40, 95% CI [-4.04, -1.03], p = 0.002; Std. beta = -1.24, 95% CI [-2.09, -0.53]). Table of regression parameters for all 9 models are provided in Table S4.
Between Wetland Benthic Community Contrasts
Cluster analysis of benthic communities by wetland shows there were three major clusters of communities, (Fig. 3, Figure S6). The first cluster indicates similarity between Grass Island (NT), DRM (NT), and Turkey Creek Tributary. The following two clusters stem from the same branch, one showing similarities between Peche Island (NT) and Turkey Creek (MT), and the second is predominately River Canard sites (MT and Tributary). River Canard mainstem sites are distributed among both clusters. Similar observations were generated according to the NMDS ordinations. In order to achieve an acceptable stress threshold (< 0.2) (Clarke 1993), the NMDS ordination was completed across four dimensions (stress: 0.134), Fig. 4 presents the ordination across the first 2 NMDS axes. 2-D plots across the remaining axes are provided in Supplementary (Figures S7-S11). Turkey Creek communities demonstrated moderate variability with sites resembling communities found at Peche Island whereas River Canard shown greater community variance with similarity to all wetland communities across NMDS1-NMDS2 (Fig. 4).
PERMANOVA indicated significant differences in macroinvertebrate community composition between the wetlands (F4,45 = 5.296, p = 0.001). Pairwise-comparisons (Bonferroni-corrected p-values) indicated Peche Island resembled Turkey Creek (p = 0.074) and River Canard (p = 0.056). All remaining between wetland comparisons were significantly different (p < 0.05) from one another. PERMANOVA and SIMPER results for all significantly different wetland pairs are provided in Supplement 3. It should be noted that the assumption for homogeneity of multivariate dispersions was not valid for the contrast between River Canard compared to DRM, Grass Island, and Turkey Creek. Therefore, River Canard taxonomically can only be compared to Peche Island with confidence by PERMANOVA (Supplement 3).
Heat map with cluster analysis of five mainstem wetland and two tributary communities annotated with wetland designation. (See Figure S6 for enlarged cluster dendrogram) (*denotes EPT taxa)
Between Strata Differences in Macroinvertebrate Communities at River Canard
Sample sites in the upstream and downstream strata of River Canard had similar taxa richness (11.8) and Pielou J’s evenness (0.658 and 0.668) with greater abundances of EPT taxa found at upstream (7.40%) compared downstream strata (3.82%). The tributary plume strata had the lowest taxa richness (9.8), highest Pielou J’s evenness 0.745, and EPT taxa comparable to upstream sites (7.57%). The most abundant upstream and tributary plume taxa were Chironomidae, Oligochaeta, Nematoda and Caenis spp. whereas the downstream was predominately Chironomidae, Oligochaeta, Rissooidea and Nematoda. Taxa turnover was lower in the downstream strata resulting in greater partitioning to nestedness at downstream (0.572) compared to upstream strata (0.285). Beta diversity at the tributary plume was predominately partitioned into taxa turnover (0.926) which was notably greater than upstream (0.715) and downstream (0.428) (Table 1). Richness within the tributary, sites TRC-5 and TRC-7 had the lowest taxa richness detected in this study. Three taxa were identified at TRC-05 (Chironomidae, Oligochaeta and Physa spp.) and five taxa were found at TRC-7 (Chironomidae, Oligochaeta, Nematoda, Sphaeriidae, and Caenis spp.).
NMDS ordination of benthic communities across River Canard strata (k = 3, stress = 0.061) showed a high degree of overlap in community ordination space (Fig. 5; Figures S12-S13). There were also no significant differences in macroinvertebrate community composition by taxa or FFGs by PERMANOVA (p > 0.05) detected across the three strata.
Between Strata Differences in Macroinvertebrate Communities at Turkey Creek
The most abundant taxa were similar across the three Turkey Creek strata and were dominated by Chironomidae, Oligochaeta, Nematoda, and Rissooidea. Diversity metrics at upstream and downstream strata demonstrated similarities with respects to richness (10.8 & 11.6), Pielou J’s evenness (0.617, 0.669) and low abundances of EPT (1.22% and 2.55%). Sites within the tributary plume had higher richness (16.4) and Pielou J evenness (0.750), EPT% (11.28). Beta diversity did not markedly vary and was primarily partitioned into turnover (0.712–0.735).
NMDS ordination of community structure at Turkey Creek (k = 3 stress = 0.077) showed overlap between the upstream and downstream strata of Turkey Creek that were distinctly separated from tributary plume on NMDS1-NMDS2 (Fig. 5) and NMDS1-NMDS3 (Figure S14-S15). PERMANOVA also revealed significant differences between the strata (F2,12 = 5.803, p = 0.001). Pairwise comparisons reaffirmed significant differences in the community structure between the tributary to upstream (F1,3 = 7.744, p = 0.024) and tributary to downstream (F1,3 = 8.14, p = 0.027) strata. Community composition in the upstream of Turkey Creek had the lowest Shannon H’ in this study with similar richness (10.8) to the downstream (11.6). The lowest EPT%, 1.22, was in the upstream of this wetland. It was observed sites TC-U3 and TC-U4 in the upper margins had slightly reduced richness (8–9 taxa). With exception to Leptocerus spp. found at TC-U3, taxa at sites can be described as pollution tolerant taxa, Chironomidae, Oligochaeta, Nematoda, Erpobdellidae and six Mollusca taxa. SIMPER analysis on taxa between Turkey Creek upstream to tributary indicate differences are largely represented by increased diversity and abundances at the tributary. Similarly, Turkey Creek downstream to Turkey Creek tributary indicate several taxa excluded or in low abundances in the downstream with more pronounced Helobdella spp. (Hirudinea) in the downstream (Supplement 3).
Distributions of FFGs between Turkey Creek strata held weak differences (non-significant) between the upstream and tributary (F1,3 = 3.700, p = 0.08) with Collector-Gatherers and Parasite groups carrying 53.11% of the dissimilarities (Supplement 3). The frequency of the collector-gatherer Chironomidae in the upstream portion was 0.493 and 0.263 in the tributary and the parasite, Nematoda, was also in higher abundances in the upstream, 0.166 to 0.066 in the tributary (Supplement 2).
Discussion
Our results generally supported the first hypothesis that there are significant differences in community composition across the mainstem wetlands. However, these differences did not conform to the predication that mainstem-tributary wetlands differ most from the non-tributary wetlands and by extension differences were not observed between the upper reach (Peche Island), middle reach (DRM, Grass Island, and Turkey Creek) and the lower reach wetlands (River Canard). River Canard, Turkey Creek and Peche island bore the most similarities by taxa, FFGs and lower diversity than DRM and Grass Island communities. This differs from Harris (1999) community analysis in the St. Clair River, a Great Lakes connecting channel, that observed changes in community along the first 10 km followed by a consistent 18 families per site across the length of the channel, whereas we had found variable richness throughout the channel, the average richness was lowest at mainstem-tributary wetlands and Peche (11–13) and highest at mid-reach mainstem wetlands, DRM, and Grass (15). Importantly, Harris found community compositions had changed across years, which highlights a limitation in this study - a single year survey. The second hypothesis seeking community variation between the tributary plume, upstream mainstem and downstream mainstem was rejected River Canard and partly supported at the Turkey Creek wetland. At Turkey Creek, there were no significant differences between the upstream and downstream mainstem. However, there were clear differences between mainstem strata and the tributary communities. The tributaries at Turkey Creek are providing conditions more suitable in supporting diverse macroinvertebrate communities. At this wetland location, sampling the wadable portions within emergent and submergent zones of the tributary does not reflect communities within the mainstem. However, at River Canard communities within the tributary inlet were similar to the mainstem wetland communities.
In-situ water quality readings taken at the time of sample collections demonstrated no differences in water quality across mainstem wetlands. These differences did not correspond with expected patterns of water quality differences described by prior studies (ECCC 2022). Data from the present research showed all wetlands including tributaries to have WQI scores considered moderately degraded, whereas the ECCC data indicate large between-wetland differences in WQI ranging from good at NT wetlands (DRM, Grass Island, Peche Island) to degraded (Turkey Creek Tributary and River Canard Tributary).
The between-wetland study differences in water quality scores could be related to the timing of sampling (multiple years for ECCC and a single year for the present work), the channel may experience seasonal variation (Yang et al. 2021) but there were also differences in the spatial scope of sampling adopted by each study. In the present study, wetlands were defined by submerged macrophyte edge boundaries with all portions of the wetland bed (submerged and emergent vegetation areas in tributaries) having an equal chance of being sampled. In contrast, the ECCC approach applied a directed-sampling design with repeated sampling at specific locations taken across years. Importantly, the two studies differed in sampling coverage of each wetland. ECCC’s study focused on wadable portions of the wetland near emergent vegetation that restricted sampling efforts in closer proximity to shorelines. The present research incorporated a larger potential sampling area at each wetland by including deeper sections of submerged macrophyte beds. For the mainstem-tributary wetlands, this enabled separate delineation and sampling of upstream and downstream strata surrounding the tributary plumes that would have been missed in the ECCC’s sampling campaigns. Additionally, sediment retention in submerged aquatic vegetation can be poorer than emergent vegetation, contributing to lower scores found in our study (Gurnell and Bertoldi 2022).
Habitat characteristics produced some variation between mainstem wetlands, but many wetlands provided similar sediment characteristics and depth such as Peche (NT), River Canard (MT), and Turkey Creek (MT) (p = 1) whereas DRM (NT) and Grass (NT) were similar to one another, being shallower with coarser sediments. Interestingly, community composition partly resembled these relationships. River Canard was most similar to Peche Island whereas Turkey Creek was only similar to Peche Island. Shredders, contributors of fine particulate organic matter, were found in low abundances at these three wetlands which are common for areas with riparian zone impacts (Barbour et al. 1992). SIMPER shown that differences found between Turkey Creek (MT) to DRM (NT) and Grass Island (NT) are in the absence or low abundance of collector-gatherers Hyalella spp, Caenis spp. and the shredder Leptocerus spp. at Turkey Creek. Hyalella spp. absence or low abundance at Peche Island (NT), River Canard (MT), and Turkey Creek (MT) contributes to dissimilarities to DRM (NT), Grass Island (NT). Studies have shown low abundances of Hyalella spp. associated to agricultural land uses (Cooper et al. 2007; Altieri et al. 2022) and can be restricted to shallow depths (Limén et al. 2005). In the models generated in this study we found support for potential exclusion by depth for Hyalella spp. and Caenis spp. genera. Turkey Creek and Peche Island had pronounced abundances of Hirudinea taxa, Helobdella spp. (H. Stagnalis) and Erpobdellidae. Hirudinea can be indicators of stress due to their moderate tolerances and are regularly associated with polluted areas as Helobdella stagnalis demonstrates preference for areas with elevated organic nutrient pollution (Tavzes et al. 2006; Kazancı et al. 2015). In this study, the Peche Island community was not categorized as a tributary-impacted wetland. However, they bore more similarities in community composition, functional feeding groups and habitat properties than the remaining non-tributary wetlands. The wetland community at Peche is located at the opening of the Detroit River, nested within an island, and is upstream of major urban development. Prior studies monitoring Peche Island has curiously shown higher dissolved phosphorus concentrations than the upstream Lake St. Clair, such that there may be a localized effect of elevated dissolved phosphorus from southeastern Lake St. Clair agricultural tributaries onto the sediments. (Burniston et al. 2009; Colborne et al. 2019; ECCC 2022). The stressors at Peche Island may be more representative of agricultural signals from the upstream waters but the stressor sources in the Detroit River are varied and difficult to distinguish (Maguire et al. 2019). Communities at River Canard (MT) demonstrated similarity to all wetlands in the NMDS ordination but had marked multivariate dispersion compared to the other sites suggesting caution in interpreting PERMANOVA results for this wetland. However, cluster analysis had revealed River Canard sites to resemble Peche Island (NT) and Turkey Creek (MT) communities. Provided the similarities between the three wetlands with respects to habitat, community compositions, functional feeding groups, we do not observe significant differences between the river’s reaches so much as more localized variation across the river which supports differences between wetlands. This variation is likely influenced by species traits, stochastic processes, and slight differences in habitat characteristics, such as depth and grain-size. Tall et al. (2016) in a three-year study on macroinvertebrates in a St. Lawrence River Lake found that sites become more stable from wave energy as depths increase over a meter, a depth many of our sites had exceeded. They found water depth, water level change, and sediment characteristics (silts, nitrogen) to be significant influences on community compositions. The Great Lakes had experienced a period of high-water levels at the time of this study. Coastal wetlands in the Great Lakes were found to migrate closer to land with a decrease in inundation and extent, submerged beds had shown the least change (Anderson et al. 2023).
It is unlikely water quality conditions is further differentiating community composition, degraded water conditions were observed throughout the study, and Hilsenhoff Biotic Index demonstrates communities which are all under water quality stress and/or significant organic pollution. As an urban waterway, nutrients, metals, and organic pollutants are a long-term problem in the area despite significant remediation progress. As sediment contamination and water quality remains consistent, it is likely water and sediment quality is a consistent determinant of colonization capabilities in the connecting channel.
Tributaries have the potential to modify main channel communities with the introduction of sediments, nutrients, contaminants, and organic matter (Bruns et al. 1984; Kiffney et al. 2006; Wallis et al. 2009; Howell et al. 2012) or aid in dispersal between mainstems and tributaries as invertebrates can drift (Elliott 2003; Wilson and McTammany 2014). In seeking community variation with respects to upstream, downstream, or tributary position, we had not found significant differences between upstream and downstream portions. Similar to Milner et al. (2019) we had not found increases in diversity downstream of tributaries in contrast with studies of communities around tributaries that found increased diversity downstream of tributaries (Vinson 2001; Katano et al. 2007). The effect of tributaries on rivers is influenced by channel and valley morphology such as basin shape, network patterns, and relative size of tributary and mainstem widths (Benda et al. 2004; Kiffney et al. 2006).
River Canard is an agricultural tributary with long-term water quality issues associated with nutrients, turbidity, and conductivity. Previous studies identified a high degree of extinction debt of fish communities at River Canard, with this location being among the most severe in the Lake Erie region (Montgomery et al. 2020). The River Canard downstream strata had the most nested beta-diversity. The elevated degree of downstream nestedness may be indicative of a pressure, natural or anthropogenic, in the area that is contributing to more homogenized macroinvertebrate community compositions. Nested communities are generally interpreted as the effects of a differential local extinction resulting in subsets of species whose tolerances, recolonization, and dispersal capacities are congruent with conditions at the habitat (Cutler 1994). The downstream locations were deeper which may have contributed to altered communities as depth is a significant factor to community compositions (Nelson and Steinman 2013; Schummer et al. 2021). Distance to agricultural tributaries commonly correlates with gradients of elevated conductivity, turbidity, and nitrates in wetlands (Schock et al. 2014). Two tributary locations were marked by reduced taxa richness of 3–5 taxa; TRC-05 had only three taxa (Chironomidae, Oligochaeta and Physa spp.) and TRC-07 had five taxa detected (Chironomidae, Oligochaeta, Nematoda, Sphaeriidae and Caenis spp.). Physa spp. are a scavenger/scraper mollusc with preference for periphyton and benefit from elevated nutrients, (Stelzer and Lamberti 2001; Lombardo and Cooke 2002) characteristic of agricultural streams (Heathwaite et al. 1996). The mayfly, Caenis spp. is also a consumer of periphyton associated with good-to-moderately degraded habitats and are common to agricultural streams (Zumberge et al. 2003; Alhejoj et al. 2014). The remaining three tributary sample sites were located along nearshore plume zones of the tributary-mainstem junction and supported relatively diverse communities in terms of richness and EPT%. Nearshore conditions may provide more optimal conditions and communities than within the tributary inlet. However, more sample sites are needed to investigate unbalanced biodiversity at the tributary.
For Turkey Creek, the tributary plume had significantly different macroinvertebrate composition compared to benthic composition of the upstream and downstream strata of this wetland. The tributary had the highest taxa richness, evenness, Shannon diversity (H’), and EPT% in this study. EPT taxa were in low abundance throughout the mainstem wetland (1.83% as a whole), EPT taxa in the upstream and downstream strata were 1.22% and 2.55%, respectively, compared to 11.27% in the tributary plume; the latter being comparable to the non-tributary impacted wetlands of the Detroit River. Overall, EPT% was low in this study (Qu et al. 2023), low prevalence of EPT taxon richness is expected for an urban tributary as they are prone to fluctuating hydrologic regimes and contaminated sediments which can reduce their richness (Konrad and Booth 2005). Yet, urbanized areas have a range of habitat types that can resemble natural areas in flow velocities and conditions supporting richness (Goertzen et al. 2022).
In contrast to the tributary, the upstream and downstream strata of Turkey Creek had taxa dominated by pollution-tolerant taxa including Chironomidae, Oligochaeta, Nematoda, and Rissooidea and were lacking or lower in relative abundances of sensitive taxa compared to the tributary strata. This suggests that benthic communities at the Turkey Creek wetland are compromised, but that the stressor does not appear to derive from the tributary input but rather from proximate stressor sources in immediately upstream portions of the Detroit River.
The most upstream portion of Turkey Creek is within 600 m of a salt-mine and 4 km of a wastewater treatment plant (WWTP). WWTPs can be a source of additional inorganic nutrients, metals, and other contaminants (Rosario-Ortiz et al. 2007; VanDrecht et al. 2009; Zamora-Ledezma et al. 2021). Mining activities involve altered topography by the removal of topsoil and vegetation and are areas of compacted soil due to industrial activity, which are all detrimental characteristics contributing to storm run-off (Negley and Eshleman 2006). Water quality measurements at Turkey Creek were broadly consistent with measured values at other wetland sites of the Detroit River. This however does not negate the possibility of periodic inputs related to storm events, where the effects of run off or wind-dispersal can impair water quality over short time scales (Chen and Chang 2019; Delpla et al. 2023).
Macroinvertebrate communities strongly associate with submerged macrophyte beds in terms of macrophyte species compositions, coverage, and by creating habitat complexity (Berg et al. 1997; Cheruvelil et al. 2002; Thorp et al. 2006; González-Ortiz et al. 2016). Agricultural and urban stressors both contribute nutrients, metals, and persistent organic pollutants (Maguire et al. 2019). These complex stressors can alter SAV macrophyte composition and abundance and shift FFGs in macroinvertebrate communities (Kolada 2010; Altieri et al. 2022). In our community survey, Odonates were not found at mainstem-tributary wetlands and a single individual was found at Peche Island wetland. Vegetation cover and diversity in SAV beds can constrain Odonate populations strongly in urban landscapes due to fragmentation and human disturbances functioning as barriers (Watts et al. 2004; Sato et al. 2008). Jeanmougin et al. (2014), identified percent coverage of SAV as a primary driver in supporting robust Odonate diversity in urban ponds. A limitation in our study was not including an assessment of the integrity of SAV beds, which is commonly conducted in Great Lakes management toward restoration of fish habitat (Grabas et al. 2012; Midwood et al. 2021; ECCC 2022). However, Detroit River SAV beds are frequently qualitatively described as fair to good considering SAV species traits prioritizing water quality (turbidity tolerance, nutrient tolerances and algae) and percent coverage. These assessments find coverage often below 6% (ECCC 2022). Light attenuation in the Detroit River may be affecting SAV vegetation, best predicted by chlorophyll-a and turbidity measures (Scannell, 2023) which affects establishment depths and biomass (Hudon et al. 2000).
The quality of SAV habitat for aquatic organisms, such as fish, must reflect conditions suitable to support diverse macroinvertebrate communities. Our study describes community impairment within SAV beds despite good communities found in emergent vegetation in biomonitoring programs. Monitoring of SAV bed quality is an important step toward habitat restoration in the region for fisheries and macroinvertebrate communities improve with long-term management of wetlands (Schad et al. 2020). SAV beds are often overlooked sources of carbon pools because their contributions are usually outweighed by other carbon sources. In systems, such as the Detroit River, with highly modified shorelines, SAV beds can be an energy source to support aquatic systems (Paice et al. 2016).
In summary, we had not found significant differences in water quality from measurements taken above the sediment in the submerged aquatic beds such that all wetlands and tributary inlets were considered poor in water quality. However, we observed differences in benthic macroinvertebrate communities between these wetlands and determined the communities to all be impaired. Both findings are inconsistent with findings by environmental agencies which sample shallower emergent vegetation, in fewer sample sites, using sweep-netting methods across multiple years. With respects to the Detroit River’s Area of Concern status in the Great Lakes, the Canadian benthic macroinvertebrates have been upgraded to non-impaired as sediment contamination had reached acceptable levels to support benthic communities and surveys indicate non-impaired communities in emergent vegetation. Quality fish habitat is an outstanding impairment within the Detroit River Area of Concern and submerged aquatic vegetation can provide critical habitat for fish species to hide, spawn, and find food resources supporting diverse communities (Miller et al. 2018). Our study demonstrates that a food resource, benthic macroinvertebrate communities, as poor within submerged vegetation that are overlooked using current sampling protocols.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Akyildiz GK, Duran M (2021) Evaluation of the impact of heterogeneous environmental pollutants on benthic macroinvertebrates and water quality by long-term monitoring of the buyuk menderes river basin. Environ Monit Assess 193:280. https://doi.org/10.1007/s10661-021-08981-8
Alhejoj I, Salameh E, Bandel K (2014) Mayflies (Order Ephemeroptera): an effective Indicator of Water bodies conditions in Jordan. Int J Sci Res Environ Sci 2:361–370. https://doi.org/10.12983/ijsres-2014-p0361-0370
Altieri P, Ocon C, Jensen R, Capítulo AR (2022) Effects of Agriculture and Hydrological changes on Macrophyte and Macroinvertebrate assemblages: a case study in Lowland Riverine wetlands of Argentina. Wetlands 42:48. https://doi.org/10.1007/s13157-022-01561-7
Anderson O, Harrison A, Heumann B et al (2023) The influence of extreme water levels on coastal wetland extent across the Laurentian Great Lakes. Sci Total Environ 885:163755. https://doi.org/10.1016/j.scitotenv.2023.163755
Ao S, Li X, Tian Z et al (2022) Harmonizing and Searching Macroinvertebrate Trait Information in Alpine streams: Method and Application–A case study in the three parallel Rivers Region, China. Front Ecol Evol 10:945824. https://doi.org/10.3389/fevo.2022.945824
Barbour MT, Graves CG, Plafkin JL et al (1992) Evaluation of EPA’s rapid bioassessment benthic metrics: Metric redundancy and variability among reference stream sites. Environ Toxicol Chem 11:437–449. https://doi.org/10.1002/etc.5620110401
Barbour M, Gerritsen J, Snyder B, Stribling J (1999) Rapid Bioassessment Protocols for Use in Streams and Wadeable Rivers: Periphyton, Benthic Macroinvertebrates and Fish, Second Edition
Barnum TR, Weller DE, Williams M (2017) Urbanization reduces and homogenizes trait diversity in stream macroinvertebrate communities. Ecol Appl 27:2428–2442. https://doi.org/10.1002/eap.1619
Beermann AJ, Elbrecht V, Karnatz S et al (2018) Multiple-stressor effects on stream macroinvertebrate communities: a mesocosm experiment manipulating salinity, fine sediment and flow velocity. Sci Total Environ 610:961–971. https://doi.org/10.1016/j.scitotenv.2017.08.084
Benda L, Andras K, Miller D, Bigelow P (2004) Confluence effects in rivers: interactions of basin scale, network geometry, and disturbance regimes. Water Resour Res. https://doi.org/10.1029/2003wr002583
Bendell-Young LI (1999) Application of a kinetic model of Bioaccumulation across a pH and salinity gradient for the prediction of Cadmium Uptake by the Sediment Dwelling Chironomidae. Environ Sci Technol 33:1501–1508. https://doi.org/10.1021/es980680u
Bertoli M, Piazza G, Pastorino P et al (2021) Macrobenthic invertebrate energy densities and ecological status in freshwater watercourses (Friuli Venezia-Giulia, Northeast Italy). Aquat Ecol 55:501–518. https://doi.org/10.1007/s10452-021-09840-x
Bode R, Novak M, Abele L (1996) Quality assurance work plan for biological stream monitoring in New York State. NYS Department of Environmental Conservation, Albany, NY, United States of America
Bode R, Novak M, Abele M (2002) Quality assurance work plan for biological stream monitoring in New York State. Stream Biomonitoring Unit, Division of Water, NYS Dept. of Environmental Conservation., Albany, NY, United States of America
Bonada N, Dolédec S, Statzner B (2007) Taxonomic and biological trait differences of stream macroinvertebrate communities between mediterranean and temperate regions: implications for future climatic scenarios. Glob Change Biol 13:1658–1671. https://doi.org/10.1111/j.1365-2486.2007.01375.x
Bruns D, Minshall G, Cushing C et al (1984) Tributaries as modifiers of the river continuum concept—analysis by polar ordination and regression. Archive fur Hydrobiol 99:208–220
Burniston D, McCrea R, Klawunn P et al (2009) Detroit River Phosphorus Loading determination. Environment Canada, Water Quality Monitoring and Surveillance Office (Locator No. WQMS09-006. Contribution No. 09–757)
Buss DF, Baptista DF, Nessimian JL, Egler M (2004) Substrate specificity, environmental degradation and disturbance structuring macroinvertebrate assemblages in neotropical streams. Hydrobiologia 518:179–188. https://doi.org/10.1023/b:hydr.0000025067.66126.1c
Cao Y, Larsen DP, Thorne RS-J (2001) Rare species in multivariate analysis for bioassessment: some considerations. J North Am Benthological Soc 20:144–153. https://doi.org/10.2307/1468195
Carlisle DM, Clements WH (2005) Leaf litter breakdown, microbial respiration and shredder production in metal-polluted streams. Freshw Biol 50:380–390. https://doi.org/10.1111/j.1365-2427.2004.01323.x
Chen J, Chang H (2019) Dynamics of wet-season turbidity in relation to precipitation, discharge, and land cover in three urbanizing watersheds, Oregon. River Res Appl 35:892–904. https://doi.org/10.1002/rra.3487
Cheruvelil KS, Soranno PA, Madsen JD, Roberson MJ (2002) Plant architecture and epiphytic macroinvertebrate communities: the role of an exotic dissected macrophyte. J North Am Benthological Soc 21:261–277. https://doi.org/10.2307/1468414
Chow-Fraser (2006) Development of the Water Quality Index (WQI) to assess effects of basin-wide land-use alteration on coastal marshes of the Laurentian Great Lakes. In: Simon TP, Stewart PM (eds) Coastal wetlands of the Laurentian Great lakes: Health, Habitat, and indicators. Indiana Biological Survey, Bloomington, Indiana, pp 137–166
Chun S-P, Jun Y-C, Kim H-G et al (2017) Analysis and prediction of the spatial distribution of EPT (Ephemeroptera, Plecoptera, and Trichoptera) assemblages in the Han River watershed in Korea. J Asia Pac Entomol 20:613–625. https://doi.org/10.1016/j.aspen.2017.03.024
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Austral Ecol 18:117–143. https://doi.org/10.1111/j.1442-9993.1993.tb00438.x
Colborne SF, Maguire TJ, Mayer B et al (2019) Water and sediment as sources of phosphate in aquatic ecosystems: the Detroit River and its role in the Laurentian Great Lakes. Sci Total Environ 647:1594–1603. https://doi.org/10.1016/j.scitotenv.2018.08.029
Collier KJ (1995) Environmental factors affecting the taxonomic composition of aquatic macroinvertebrate communities in lowland waterways of Northland, New Zealand. N Z J Mar Freshwat Res 29:453–465. https://doi.org/10.1080/00288330.1995.9516679
Cooper MJ, Uzarski DG, Burton TM (2007) Macroinvertebrate community composition in relation to anthropogenic disturbance, vegetation, and organic sediment depth in four Lake Michigan drowned river-mouth wetlands. Wetlands 27:894–903. https://doi.org/10.1672/0277-5212(2007)27[894:mccirt]2.0.co;2
Corkum LD, Ciborowski JJH, Lazar R (1997) The distribution and contaminant burdens of adults of the burrowing mayfly, Hexagenia, in Lake Erie. J Great Lakes Res 23:383–390. https://doi.org/10.1016/s0380-1330(97)70920-1
Cummins KW, Merritt RW, Andrade PC (2005) The use of invertebrate functional groups to characterize ecosystem attributes in selected streams and rivers in south Brazil. Stud Neotropical Fauna Environ 40:69–89. https://doi.org/10.1080/01650520400025720
Cutler AH (1994) Nested biotas and biological conservation: metrics, mechanisms, and meaning of nestedness. Landsc Urban Plann 28:73–82. https://doi.org/10.1016/0169-2046(94)90045-0
Das S, Kar I, Patra AK (2023) Cadmium induced bioaccumulation, histopathology, gene regulation in fish and its amelioration– A review. J Trace Elem Med Biol 79:127202. https://doi.org/10.1016/j.jtemb.2023.127202
Delpla I, Bouchard C, Dorea C, Rodriguez MJ (2023) Assessment of rain event effects on source water quality degradation and subsequent water treatment operations. Sci Total Environ 866:161085. https://doi.org/10.1016/j.scitotenv.2022.161085
Demars BOL, Kemp JL, Marteau B et al (2021) Stream macroinvertebrates and Carbon Cycling in tangled food webs. Ecosystems 24:1944–1961. https://doi.org/10.1007/s10021-021-00626-8
Demi LM, Benstead JP, Rosemond AD, Maerz JC (2019) Experimental N and P additions alter stream macroinvertebrate community composition via taxon-level responses to shifts in detrital resource stoichiometry. Funct Ecol 33:855–867. https://doi.org/10.1111/1365-2435.13289
Derecki J (1984) Detroit River physical and hydraulic characteristics. National Oceans and Atmospheric Administration, Great Lakes Environmental Research Laboratory Contribution No. 417. Ann Arbor, Michigan, U.S.A.
Desrosiers M, Usseglio-polatera P, Archaimbault V et al (2018) Assessing anthropogenic pressure in the St. Lawrence River using traits of benthic macroinvertebrates. Sci Total Environ 649:233–246. https://doi.org/10.1016/j.scitotenv.2018.08.267
Dray S, Bauman D, Blanchet G et al (2022) adespatial: multivariate multiscale spatial analysis
DRCC (2020) Re-designation Report: Assessment of Benthos (BUI #6) in the Detroit River Canadian Area of Concern. 1–92
Drouillard KG, Tomczak M, Reitsma S, Haffner GD (2006) A river-wide survey of Polychlorinated Biphenyls (PCBs), Polycylic Aromatic Hydrocarbons (PAHs), and selected organochlorine pesticide residues in sediments of the Detroit River1999. J Great Lakes Res 32:209–226. https://doi.org/10.3394/0380-1330(2006)32[209:arsopb]2.0.co;2
ECCC (2022) Detroit River Area of Concern: Coastal Wetland Habitat Assessment Report 2021 Update. 1–23
Elliott JM (2003) A comparative study of the dispersal of 10 species of stream invertebrates. Freshw Biol 48:1652–1668. https://doi.org/10.1046/j.1365-2427.2003.01117.x
Evans-White MA, Dodds WK, Huggins DG, Baker DS (2009) Thresholds in macroinvertebrate biodiversity and stoichiometry across water-quality gradients in Central Plains (USA) streams. J North Am Benthological Soc 28:855–868. https://doi.org/10.1899/08-113.1
Fallon ME, Horvath FJ (1985) Preliminary Assessment of Contaminants in Soft sediments of the Detroit River. J Great Lakes Res 11:373–378. https://doi.org/10.1016/s0380-1330(85)71781-9
Fergen JT, Bergstrom RD, Twiss MR et al (2022) Updated census in the Laurentian Great Lakes Watershed: a framework for determining the relationship between the population and this aquatic resource. J Great Lakes Res 48:1337–1344. https://doi.org/10.1016/j.jglr.2022.03.004
Friedman J, Hastie T, Tibshirani R (2010) Regularization paths for generalized Linear models via Coordinate Descent. J Stat Softw 33:1–22. https://doi.org/10.18637/jss.v033.i01
Gao Q, Zhang Q, Zeng J et al (2023) Macroinvertebrate community structure, pollution tolerance, diversity and feeding functional groups in polluted urban rivers under different black and odorous levels. Ecol Ind 156:111148. https://doi.org/10.1016/j.ecolind.2023.111148
Gauch H (1982) Multivariate Analysis in Community Ecology. Cambridge University Press, Cambridge, UK
Goertzen D, Schneider A-K, Eggers TO, Suhling F (2022) Temporal changes of biodiversity in urban running waters– results of a twelve-year monitoring study. Basic Appl Ecol 58:74–87. https://doi.org/10.1016/j.baae.2021.11.007
González-Ortiz V, Egea LG, Jiménez‐Ramos R et al (2016) Submerged vegetation complexity modifies benthic infauna communities: the hidden role of the belowground system. Mar Ecol 37:543–552. https://doi.org/10.1111/maec.12292
Grabas GP, Blukacz-Richards EA, Pernanen S (2012) Development of a submerged aquatic vegetation community index of biotic integrity for use in Lake Ontario coastal wetlands. J Great Lakes Res 38:243–250. https://doi.org/10.1016/j.jglr.2012.02.014
Grimm NB (1988) Role of macroinvertebrates in Nitrogen Dynamics of a Desert Stream. Ecology 69:1884–1893. https://doi.org/10.2307/1941165
Gurnell AM, Bertoldi W (2022) The impact of plants on fine sediment storage within the active channels of gravel-bed rivers: a preliminary assessment. Hydrol Process. https://doi.org/10.1002/hyp.14637
Hammer Ø, Harper D, Ryan P, Statistics Software Package for Education and Data Analysis Øyvind, Hammer, David AT, Harper, Paul D (2001) Ryan Palaenontologia Electronica 4:1–9
Harris I (1999) Some factors affecting density and richness of invertebrate populations in the near-shore sediments of the St. Clair River in 1990–1995. Can Field-Nat 113:576–584
Hayward EE, Gillis PL, Bennett CJ et al (2022) Freshwater mussels in an impacted watershed: influences of pollution from point and non-point sources. Chemosphere 307:135966. https://doi.org/10.1016/j.chemosphere.2022.135966
Heathwaite AL, Johnes PJ, Peters NE (1996) Trends in nutrients. Hydrol Process 10:263–293. https://doi.org/10.1002/(sici)1099-1085(199602)10:2%3C263::aid-hyp441%3E3.0.co;2-k
Hilsenhoff WL (1987) An improved biotic index of organic stream pollution. Gt Lakes Entomol 20(1):31–39. https://doi.org/10.22543/0090-0222.1591
Hilsenhoff WL (1998) A modification of the Biotic Index of Organic Stream Pollution to remedy problems and Permit its Use throughout the year. Great Lakes Entomol. https://doi.org/10.22543/0090-0222.1944
Howell ET, Chomicki KM, Kaltenecker G (2012) Tributary discharge, lake circulation and lake biology as drivers of water quality in the Canadian Nearshore of Lake Ontario. J Great Lakes Res 38:47–61. https://doi.org/10.1016/j.jglr.2012.03.008
Hudon C, Lalonde S, Gagnon P (2000) Ranking the effects of site exposure, plant growth form, water depth, and transparency on aquatic plant biomass. Can J Fish Aquat Sci 57:31–42. https://doi.org/10.1139/f99-232
Jeanmougin M, Leprieur F, Loïs G, Clergeau P (2014) Fine-scale urbanization affects Odonata species diversity in ponds of a megacity (Paris, France). Acta Oecol 59:26–34. https://doi.org/10.1016/j.actao.2014.05.008
Jonsson M, Burrows RM, Lidman J et al (2017) Land use influences macroinvertebrate community composition in boreal headwaters through altered stream conditions. Ambio 46:311–323. https://doi.org/10.1007/s13280-016-0837-y
Katano I, Doi H, Houki A et al (2007) Changes in periphyton abundance and community structure with the dispersal of a caddisfly grazer, Micrasema Quadriloba. Limnology 8:219–226. https://doi.org/10.1007/s10201-007-0211-7
Kazancı N, Ekingen P, Dügel M, Türkmen G (2015) Hirudinea (Annelida) species and their ecological preferences in some running waters and lakes. Int J Environ Sci Technol 12:1087–1096. https://doi.org/10.1007/s13762-014-0574-3
Kelly DW, Bailey RJ, MacNeil C et al (2006) Invasion by the amphipod Gammarus pulex alters community composition of native freshwater macroinvertebrates. Divers Distrib 12:525–534. https://doi.org/10.1111/j.1366-9516.2006.00275.x
Kiffney PM, Greene CM, Hall JE, Davies JR (2006) Tributary streams create spatial discontinuities in habitat, biological productivity, and diversity in mainstem rivers. Can J Fish Aquat Sci 63:2518–2530. https://doi.org/10.1139/f06-138
Kolada A (2010) The use of aquatic vegetation in lake assessment: testing the sensitivity of macrophyte metrics to anthropogenic pressures and water quality. Hydrobiologia 656:133–147. https://doi.org/10.1007/s10750-010-0428-z
Kolde R (2019) pheatmap: pretty heatmaps
Konrad C, Booth D (2005) Hydrologic Changes in Urban Streams and Their Ecological Significance. American Fisheries Society Symposium 47:157–177
Larsen S, Pace G, Ormerod SJ (2011) Experimental effects of sediment deposition on the structure and function of macroinvertebrate assemblages in temperate streams. River Res Appl 27:257–267. https://doi.org/10.1002/rra.1361
Legendre P (2014) Interpreting the replacement and richness difference components of beta diversity. Glob Ecol Biogeogr 23:1324–1334. https://doi.org/10.1111/geb.12207
Ligeiro R, Hughes RM, Kaufmann PR et al (2013) Defining quantitative stream disturbance gradients and the additive role of habitat variation to explain macroinvertebrate taxa richness. Ecol Ind 25:45–57. https://doi.org/10.1016/j.ecolind.2012.09.004
Limén H, van Overdijk CDA, MacIsaac HJ (2005) Food partitioning between the amphipods Echinogammarus ischnus, Gammarus fasciatus, and Hyalella azteca as revealed by stable isotopes. J Great Lakes Res 31:97–104. https://doi.org/10.1016/s0380-1330(05)70241-0
Lock K, Goethals PLM (2011) Distribution and ecology of the mayflies (Ephemeroptera) of Flanders (Belgium). Ann De Limnologie - Int J Limnol 47:159–165. https://doi.org/10.1051/limn/2011011
Lombardo P, Cooke GD (2002) Consumption and preference of selected food types by two freshwater gastropod species. Fundamental Appl Limnol 155:667–685. https://doi.org/10.1127/archiv-hydrobiol/155/2002/667
Mackay RJ (1992) Colonization by Lotic Macroinvertebrates: a review of processes and patterns. Can J Fish Aquat Sci 49:617–628. https://doi.org/10.1139/f92-071
Maguire TJ, Spencer C, Grgicak-Mannion A et al (2019) Distinguishing point and non-point sources of dissolved nutrients, metals, and legacy contaminants in the Detroit River. Sci Total Environ 681:1–8. https://doi.org/10.1016/j.scitotenv.2019.04.311
Marcarelli AM, Baxter CV, Mineau MM, Hall RO (2011) Quantity and quality: unifying food web and ecosystem perspectives on the role of resource subsidies in freshwaters. Ecology 92:1215–1225. https://doi.org/10.1890/10-2240.1
Merritt R, Cummins K, Berg M (2008) Introduction to the aquatic insects of North America. Kendall/Hunt Publishing Co, Dubuque, Iowa
Merritt RW, Cummins KW, Berg MB (2017) Methods in Stream Ecology, 1 (Third Edition). Section C: community interactions. 413–433. https://doi.org/10.1016/b978-0-12-416558-8.00020-2
Midwood JD, Tang RWK, Doka SE, Costa JMG (2021) Comparison of approaches for modelling submerged aquatic vegetation in the Toronto and Region Area of concern. J Great Lakes Res 47:395–404. https://doi.org/10.1016/j.jglr.2020.08.019
Miller JW, Kocovsky PM, Wiegmann D, Miner JG (2018) Fish Community responses to submerged aquatic vegetation in Maumee Bay, Western Lake Erie. North Am J Fish Manag 38:623–629. https://doi.org/10.1002/nafm.10061
Milner VS, Yarnell SM, Peek RA (2019) The ecological importance of unregulated tributaries to macroinvertebrate diversity and community composition in a regulated river. Hydrobiologia 829:291–305. https://doi.org/10.1007/s10750-018-3840-4
Montgomery F, Reid SM, Mandrak NE (2020) Extinction debt of fishes in great lakes coastal wetlands. Biol Conserv 241:108386. https://doi.org/10.1016/j.biocon.2019.108386
Murtagh F, Legendre P (2014) Ward’s hierarchical agglomerative clustering method: which Algorithms Implement Ward’s Criterion? J Classif 31:274–295. https://doi.org/10.1007/s00357-014-9161-z
Negley TL, Eshleman KN (2006) Comparison of stormflow responses of surface-mined and forested watersheds in the Appalachian Mountains, USA. Hydrol Process 20:3467–3483. https://doi.org/10.1002/hyp.6148
Nelson WA, Steinman AD (2013) Changes in the benthic communities of Muskegon Lake, a great lakes area of concern. J Great Lakes Res 39:7–18. https://doi.org/10.1016/j.jglr.2012.12.016
Oksanen J, Simpson GL, Blanchet FG et al (2022) vegan: community ecology package
Ostermiller JD, Hawkins CP (2004) Effects of sampling error on bioassessments of stream ecosystems: application to RIVPACS-type models. J North Am Benthological Soc 33:363–382. https://doi.org/10.1899/0887-3593(2004)023%3C0363:eoseob%3E2.0.co;2
Paice RL, Chambers JM, Robson BJ (2016) Potential of submerged macrophytes to support food webs in lowland agricultural streams. Mar Freshw Res 68:549. https://doi.org/10.1071/mf15391
Pastorino P, Zaccaroni A, Doretto A et al (2020) Functional feeding groups of aquatic insects Influence Trace element Accumulation: findings for Filterers, scrapers and predators from the Po Basin. Biology 9:288. https://doi.org/10.3390/biology9090288
Poff NL, Olden JD, Vieira NKM et al (2006) Functional trait niches of north American lotic insects: traits-based ecological applications in light of phylogenetic relationships. J North Am Benthological Soc 33:730–755. https://doi.org/10.1899/0887-3593(2006)025[0730:ftnona]2.0.co;2
Preston FW (1948) The commonness, and rarity, of species. Ecology 29:254–283. https://doi.org/10.2307/1930989
Qu Y, Keller V, Bachiller-Jareno N et al (2023) Significant improvement in freshwater invertebrate biodiversity in all types of English rivers over the past 30 years. Sci Total Environ 905:167144. https://doi.org/10.1016/j.scitotenv.2023.167144
Resh V, Norris RH, Barbour MT (1995) Design and implementation of rapid assessment approaches for water resource monitoring using benthic macroinvertebrates. Austral Ecol 20:108–121. https://doi.org/10.1111/j.1442-9993.1995.tb00525.x
Resh VH, Bêche LA, McElravy EP (2005) How common are rare taxa in long-term benthic macroinvertebrate surveys? J North Am Benthological Soc 24:976–989. https://doi.org/10.1899/05-026.1
Reynoldson TB, Bailey R, Day K, Norris RH (1995) Biological guidelines for freshwater sediment based on BEnthic Assessment of SedimenT (the BEAST) using a multivariate approach for predicting biological state. Aust J Ecol 20:198–219. https://doi.org/10.1111/j.1442-9993.1995.tb00532.x
Rosario-Ortiz FL, Snyder SA, Suffet IH (eds) (2007) (Mel) Characterization of dissolved organic matter in drinking water sources impacted by multiple tributaries. Water Research 41:4115–4128. https://doi.org/10.1016/j.watres.2007.05.045
Saari GN, Wang Z, Brooks BW (2018) Revisiting inland hypoxia: diverse exceedances of dissolved oxygen thresholds for freshwater aquatic life. Environ Sci Pollut Res 25:3139–3150. https://doi.org/10.1007/s11356-017-8908-6
Sarremejane R, Cid N, Stubbington R et al (2020) DISPERSE, a trait database to assess the dispersal potential of European aquatic macroinvertebrates. Sci Data 7:386. https://doi.org/10.1038/s41597-020-00732-7
Sato M, Kohmatsu Y, Yuma M, Tsubaki Y (2008) Population genetic differentiation in three sympatric damselfiy species in a highly fragmented urban landscape. (Zygoptera: Coena… Odonatologica 2:131–144
Scannell J (2023) Assessment of water quality index, light attenuation and nutrient sequestering by submerged aquatic vegetation in the Detroit River. University of Windsor - Electronic Theses and Dissertations. 8958. https://scholar.uwindsor.ca/etd/8958
Scavia D, Allan JD, Arend KK et al (2014) Assessing and addressing the re-eutrophication of Lake Erie: Central basin hypoxia. J Great Lakes Res 40:226–246. https://doi.org/10.1016/j.jglr.2014.02.004
Scavia D, Bocaniov SA, Dagnew A et al (2019) St. Clair-Detroit River system: Phosphorus mass balance and implications for Lake Erie load reduction, monitoring, and climate change. J Great Lakes Res 45:40–49. https://doi.org/10.1016/j.jglr.2018.11.008
Schad AN, Kennedy JH, Dick GO, Dodd L (2020) Aquatic macroinvertebrate richness and diversity associated with native submerged aquatic vegetation plantings increases in longer-managed and wetland-channeled effluent constructed urban wetlands. Wetlands Ecol Manage 28:461–477. https://doi.org/10.1007/s11273-020-09724-1
Schmidt-Kloiber A, Hering D (2015) www.freshwaterecology.info– an online tool that unifies, standardises and codifies more than 20,000 European freshwater organisms and their ecological preferences. Ecol Ind 53:271–282. https://doi.org/10.1016/j.ecolind.2015.02.007
Schock NT, Murry BA, Uzarski DG (2014) Impacts of agricultural drainage outlets on Great Lakes coastal wetlands. Wetlands 34:297–307. https://doi.org/10.1007/s13157-013-0486-x
Schummer ML, Eason KM, Hodges TJ et al (2021) Response of aquatic macroinvertebrate density and diversity to wetland management and structure in the Montezuma Wetlands Complex, New York. J Great Lakes Res 47:875–883. https://doi.org/10.1016/j.jglr.2021.03.001
Stelzer RS, Lamberti GA (2001) Effects of N: P ratio and total nutrient concentration on stream periphyton community structure, biomass, and elemental composition. Limnol Oceanogr 46:356–367. https://doi.org/10.4319/lo.2001.46.2.0356
Svendsen KM, Renshaw CE, Magilligan FJ et al (2009) Flow and sediment regimes at tributary junctions on a regulated river: impact on sediment residence time and benthic macroinvertebrate communities. Hydrol Process 23:284–296. https://doi.org/10.1002/hyp.7144
Szalinska E, Grgicak-Mannion A, Haffner GD, Drouillard KG (2013) Assessment of decadal changes in sediment contamination in a large connecting channel (Detroit River, North America). Chemosphere 93:1773–1781. https://doi.org/10.1016/j.chemosphere.2013.06.009
Tall L, Armellin A, Pinel-Alloul B et al (2016) Effects of hydrological regime, landscape features, and environment on macroinvertebrates in St. Lawrence River wetlands. Hydrobiologia 778:221–241. https://doi.org/10.1007/s10750-015-2531-7
Tavzes B, Urbanič G, Toman MJ (2006) Biological and hydromorphological integrity of the small urban stream. Phys Chem Earth Parts A/B/C 31:1062–1074. https://doi.org/10.1016/j.pce.2006.07.009
Thorp J, Rogers D (2016) Keys to nearctic fauna. II, Thorp and Covich’s freshwater invertebrates. https://doi.org/10.1016/c2010-0-65589-1
Thorp JH, Thoms MC, Delong MD (2006) The riverine ecosystem synthesis: biocomplexity in river networks across space and time. River Res Appl 22:123–147. https://doi.org/10.1002/rra.901
Townsend CR, Scarsbrook MR, Dolédec S (1997) The intermediate disturbance hypothesis, refugia, and biodiversity in streams. Limnol Oceanogr 42:938–949. https://doi.org/10.4319/lo.1997.42.5.0938
Trebitz A, Sykes M, Barge J (2019) A reference inventory for aquatic fauna of the Laurentian Great Lakes. J Great Lakes Res 45:1036–1046. https://doi.org/10.1016/j.jglr.2019.10.004
Tucker A, Chadderton L, Annis G et al (2020) A framework for aquatic invasive species surveillance site selection and prioritization in the US waters of the Laurentian Great Lakes. Manage Biol Invasions 11:607–632. https://doi.org/10.3391/mbi.2020.11.3.17
Uzarski DG, Brady VJ, Cooper MJ et al (2017) Standardized measures of Coastal Wetland Condition: implementation at a Laurentian Great Lakes Basin-wide scale. Wetlands 37:15–32. https://doi.org/10.1007/s13157-016-0835-7
van den Berg MS, Coops H, Noordhuis R et al (1997) Macroinvertebrate communities in relation to submerged vegetation in two Chara-dominated lakes. Hydrobiologia 342–343:143–150. https://doi.org/10.1023/a:1017094013491
VanDrecht G, Bouwman AF, Harrison J, Knoop JM (2009) Global nitrogen and phosphate in urban wastewater for the period 1970 to 2050. Global biogeochemical cycles 23:n/a-n/a. https://doi.org/10.1029/2009gb003458
Vinson M (2001) Long-term dynamics of an invertebrate assemblage downstream from a large dam. Ecol Appl 3:711–730
Wallace JB, Webster JR (1996) The role of macroinvertebrates in Stream ecosystem function. Ann Rev Entomol 41:115–139. https://doi.org/10.1146/annurev.en.41.010196.000555
Wallis E, Nally RM, Lake S (2009) Do tributaries affect loads and fluxes of particulate organic matter, inorganic sediment and wood? Patterns in an upland river basin in south-eastern Australia. Hydrobiologia 636:307–317. https://doi.org/10.1007/s10750-009-9961-z
Watts PC, Rouquette JR, Saccheri IJ et al (2004) Molecular and ecological evidence for small-scale isolation by distance in an endangered damselfly, Coenagrion mercuriale. Mol Ecol 13:2931–2945. https://doi.org/10.1111/j.1365-294x.2004.02300.x
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer-Verlag, New York
Wick MJ, Angradi TR, Pawlowski M et al (2019) An assessment of water quality in two Great Lakes connecting channels. J Great Lakes Res 45:901–911. https://doi.org/10.1016/j.jglr.2019.08.001
Wilson MJ, McTammany ME (2014) Tributary and mainstem benthic macroinvertebrate communities linked by direct dispersal and indirect habitat alteration. Hydrobiologia 738:75–85. https://doi.org/10.1007/s10750-014-1920-7
Wood (2004) The Use of Benthic Macroinvertebrate Community Composition as a measure of Contaminant Induced stress in the sediments of the Detroit River. University of Windsor
Yaagoubi SE, Alami ME, Harrak R et al (2023) Assessment of functional feeding groups (FFG) structure of aquatic insects in North- western Rif - Morocco. Biodivers Data J 11:e104218. https://doi.org/10.3897/bdj.11.e104218
Yang S, Liang M, Qin Z et al (2021) A novel assessment considering spatial and temporal variations of water quality to identify pollution sources in urban rivers. Sci Rep 11:8714. https://doi.org/10.1038/s41598-021-87671-4
Zamora-Ledezma C, Negrete-Bolagay D, Figueroa F et al (2021) Heavy metal water pollution: a fresh look about hazards, novel and conventional remediation methods. Environ Technol Innov 22:101504. https://doi.org/10.1016/j.eti.2021.101504
Zumberge J, Lee K, Goldstein R (2003) Relation of Periphyton and Benthic Invertebrate communities to Environmental factors and land use at selected sites in Part of the Upper Mississippi River Basin, 1996-98. U.S. Department of the Interior, U.S. Geological Survey, Mounds View, Minnesota
Zuur A, Ieno E (2016) Beginner’s guide to zero-inflated models with R. Highland Statistics, Newburgh, United Kingdom
Acknowledgements
Thank you to the University of Windsor faculty and staff for your help and guidance, Catherine Febria, Alyssa Frazao, Jan Ciborowski and Rong Lou. Also, to Wetlands editor, Marinus L. Otte and the two anonymous reviewers for their kindness, helpful feedback, and insights.
Funding
This research was supported by NSERC Discovery Grant to K.G. Drouillard.
Author information
Authors and Affiliations
Contributions
Jessica Robson and Kenneth G. Drouillard contributed to the study conception, design, material preparation, data collection and analysis. The first draft of the manuscript was written by Jessica Robson. Kenneth G. Drouillard commented on versions of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Robson, J., Drouillard, K.G. Macroinvertebrate Diversity of Submerged Detroit River Coastal Wetlands. Wetlands 44, 74 (2024). https://doi.org/10.1007/s13157-024-01829-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13157-024-01829-0