Abstract
Wetlands of a large fluvial lake in the St. Lawrence River (Québec, Canada) were visited during 3 years (2004–2006) to collect macroinvertebrates across the belt of emergent vegetation. We tested the hypothesis that hydrology, river landscape, and local environment would explain variations in macroinvertebrates. The 66 taxa collected lake-wide comprised a few abundant but widespread groups (Malacostraca, Oligochaeta, Chironomidae, and Mollusca). Between 2004 and 2006, total abundance at 5 sites monitored annually fell by one order of magnitude, and taxa richness decreased from 19 to 12 taxa. Proportion of amphipods (Gammaridae) dropped sixfold whereas proportion of annelids (Oligochaetes) rose ninefold. The impoverishment of macroinvertebrates coincided with low summer water levels in 2005 and 2006, resulting in the periodic emersion of up-slope sites. Spatial differences in macroinvertebrate communities were less important than inter-annual differences, owing to large variability among sites. Patterns in macroinvertebrate communities were related to water depth, vegetation, and local changes in sediments (31% of variance explained). Gammaridae (Malacostraca) were strongly associated with down-slope sites, whereas Oligochaeta (Annelida) dominated in up-slope sites. Inter-annual changes in water level had major effects on macroinvertebrate communities in Lake Saint-Pierre, above and beyond other environmental variables.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Understanding how environmental factors drive the abundance and composition of biotic assemblages is a prerequisite for effective environmental assessment and management of large rivers and streams (Mykrä et al., 2008a, b; Habersack et al., 2014). Littoral macroinvertebrates are important in lake and river food webs, as a food for littoral fish (Magnin et al., 1978; Johnson & Dropkin, 1993) and waterbirds (Gammonley & Laubhan, 2002; Merritt et al., 2002). Macrobenthos has been used worldwide for biomonitoring water or sediment quality in rivers (Reynoldson et al., 2001; Armanini et al., 2011; Weigel & Dimick, 2011), streams (Chessman et al., 2007; Mykrä et al., 2008a, b), and lakes (Rosenberg & Resh, 1993; Pinel-Alloul et al., 1996; Bailey et al., 2004). In Canada, macroinvertebrate communities are used to evaluate the environmental quality of coastal wetlands in the Great Lakes (Burton et al., 1999; Kashian & Burton 2000) and large rivers (Reynoldson et al., 1997, 2001; Tall et al., 2008), and are the basis of the Canadian Aquatic Biomonitoring Network (CABIN) (Environment Canada, 2008).
Despite the major ecological role played by macroinvertebrates, the environmental factors controlling their distribution in wetlands are not yet clearly understood. Consistent patterns are lacking, and interactions can be complex and difficult to predict (Batzer et al., 1999; Batzer, 2013). We lack an understanding of the relative importance of different stressors in determining macroinvertebrate communities in shallow riparian zones (Burton et al., 2002).
St. Lawrence River wetlands support abundant and diverse epiphytic macroinvertebrates (Cremona et al., 2008; Tall et al., 2008; Tessier et al., 2008; Tourville-Poirier et al., 2010). However, these communities are affected by many human influences, including alteration of hydrological regime (Hudon, 1997; Hudon et al., 2005), water and sediment contamination (Carignan et al., 1994; Filion & Morin, 2000), nutrient inputs from municipal effluents and tributaries draining agricultural lands (Hudon & Carignan, 2008), commercial navigation and dredging (Morin & Coté, 2003), and invasion of exotic species (de Lafontaine & Costan, 2002).
The hydrological regime of the St. Lawrence River includes the frequency and duration of floods and great changes in flow. These characteristics determine sedimentation and erosion and affect plant zonation on riverbanks exposed to different sedimentary/erosion regimes (Cabezas et al., 2008). Hydrological regime affects macroinvertebrates either directly through water drawdown or flooding, or indirectly by changes in littoral habitats (Heino, 2000; Cabezas et al., 2008, 2009). Indeed, cycles of droughts and floods favor tolerant species, and reduce macrobenthos diversity and abundance (Batzer et al., 1999; Cabezas et al., 2008), but also affect wetland vegetation and sediments. Low water levels coincide with high water temperature, especially in shallow water (Hudon et al., 2010), induce a shift in plant assemblages from submerged to emergent plants (Hudon, 1997), and result in less diverse and less abundant macroinvertebrate communities (Cremona et al., 2008; Tessier et al., 2008). Conversely, flood levels affected macroinvertebrate communities more strongly than wetland vegetation in the Great Lakes, because macroinvertebrate density and diversity decreased and shifted upslope with high-water conditions, whereas plant zonation remained relatively unchanged (Gathman & Burton, 2011).
In the St. Lawrence River, different macroinvertebrate communities and indicator taxa have been associated with sediment contamination (Pinel-Alloul et al., 1996; Filion & Morin, 2000; Masson et al., 2010). Local nutrient inputs from municipal effluents and agricultural tributaries also modify macroinvertebrate assemblages in emergent marshes (Tall et al., 2008). However, in many rivers and streams, habitat conditions exert a stronger effect on macroinvertebrates than sediment contamination (Griffiths, 1991; Clements et al., 1992; Gower et al., 1994). These observations led us to hypothesize that macroinvertebrate communities are primarily structured by the hydrological regime, which interacts with river landscape features and environmental (physical, chemical and biological) conditions at each site.
This study examines lake-wide distribution and composition of macroinvertebrate communities in emergent vegetation of low marsh wetlands in Lake Saint-Pierre, a large and shallow fluvial lake of the St. Lawrence River (Quebec, Canada). First, we repeatedly sampled macroinvertebrate communities at 5 sites over 3 years showing widely different hydrological regimes. Second, we analyzed the macroinvertebrate communities from 54 sites subjected to a wide range of exposure (current and wind) and water quality. Finally, we identified the subset of environmental variables best explaining the composition of macroinvertebrate communities in the highly heterogeneous system of Lake Saint-Pierre. This information improves our understanding of the factors controlling macroinvertebrate abundance and composition, which in turn support the trophic network and fish production in the fluvial lakes of the St. Lawrence River.
Methods
Study area
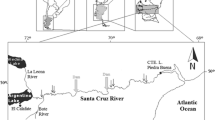
The study took place in Lake Saint-Pierre (LSP) (46º10′N; 72º50′W), the largest (402 km2) fluvial lake of the St. Lawrence River. This broadening of the river includes an archipelago of over 100 islands located upstream of an open water body reaching a width of 12 km (Fig. 1). The main body of LSP comprises two shallow (depth < 4 m) basins located to the north and south of an 11.3-m-deep man-made commercial navigation channel. The morphology of LSP gives great variation in its habitats. For example, narrow channels within the LSP archipelago are sheltered from wind and from the resulting erosion by waves and water surge (seiche effect), yet can be exposed to faster currents than sites located in the lake itself. Within the open lake, the north shore is more exposed to the dominant southwesterly winds than the south shore. Accordingly, sediment types also differ from temporary sand and silty sand in nearshore areas to postglacial clay in fast-flowing central areas subjected to erosion, with little net deposition (Rondeau et al., 2000) (Fig. 1).
Study area and location of sampling stations in Lake Saint Pierre, divided into four regions based on site position in the Lake (L) or the Archipelago (A) (east and west of the dashed line, respectively), north (N) or south (S) of the 11-m-deep commercial navigation channel. The arrow shows flow direction. Within each region (LN, LS, AN, AS), site number is indicated for reference in the text. Symbols distinguish sites sampled in 2004 (circles), 2005 (triangles), 2006 (squares) or over more than 1 year (stars). Major types of sediment and the presence of aquatic vegetation are delimitated by different patterns
Depending on the position with respect to the plume of incoming tributaries, the different regions of LSP are exposed to waters of widely different qualities. Sites along the north shore are primarily influenced by waters originating from the Ottawa River and smaller tributaries (Maskinongé, du Loup and Yamachiche Rivers, Fig. 1), which drain woodlands, crop, and dairy farms and show large concentrations of suspended particulate matter, dissolved organic carbon, and nutrients. In contrast, sites in the southern part of the archipelago are exposed to transparent, high conductivity Great Lakes and Richelieu River waters. Finally, the south shore of the open lake is under the influence of colored, nutrient-rich waters from the Saint-François and Yamaska rivers (Fig. 1); the latter is known as one of the most agriculturally polluted in Quebec (St-Onge, 1999). To account for the natural heterogeneity in water masses of this large water body, sampling sites were selected within the four regions of LSP, including both the north and south shores of the archipelago and in the open lake area.
The large size, slow current and sloping shorelines of LSP favor the development of large expanses of emergent and submerged vegetation, which make it more closely related to a wetland than to a fast-flowing river. LSP wetlands cover an area of 18,350 ha representing over 70% of the St. Lawrence River freshwater marshes (Jean & Létourneau, 2014). Littoral vegetation is highly productive, macrophytes and attached epiphytes contributing together up to 70% of the lake primary productivity (Vis et al., 2007). Emergent plants in low marshes are the most abundant primary producers, comprising bulrushes (Schoenoplectus fluviatilis (Torr.) M.T. Strong, S. lacustris (L.) Palla, S. pungens (Vahl) Palla), broad-fruited bur-reed (Sparganium eurycarpum Engelm.), narrowleaf cattail (Typha angustifolia L.), and broad-leaved arrow-leaf (Sagittaria latifolia Willd.).
Seasonal and inter-annual variations in water level play a large role in structuring wetland vegetation, which exhibits considerable plasticity in response to hydrology; LSP wetlands shift from deep marshes during wet years to grass-dominated wet meadows during dry years (Hudon et al., 2005). Over the last decade, mean annual water discharge was 10 500 m3 s−1 and seasonal water level variation ranged from 1.31 to 2.26 m, including some years of extremely low water levels (Environment Canada, 2012). In addition to wetland vegetation structure, water level fluctuations have a broad range of effects on floodplain area (Hudon 1997), water quality (Hudon & Carignan, 2008), and temperature regime (Hudon et al., 2010) of LSP, all of which affect its overall carrying capacity for fish (Hudon et al., 2012).
Sampling design and environmental assessment
Sampling was carried out at 54 sites located in emergent vegetation of littoral LSP wetlands over three consecutive years (2004–2006) during September, coinciding with the period of maximum emergent vegetation biomass. Sampling sites were located along the north (N) and south (S) shores of the Sorel Archipelago (A) and of Lake Saint-Pierre (L) yielding a total of 63 macroinvertebrate samples among four sampling regions (AN, AS, LN, LS) (Fig. 1; Table 1). Four sites were sampled every year (AS2, AS3, LN13, LS3) and one (LN8) in 2005–2006 (Table 1).
Sampling sites were positioned throughout each region so as to cover the widest possible range of environmental conditions while maintaining a similar depth range and emergent plant cover. South shore sites were fewer (N = 11 in both the southern archipelago and lake regions) than north shore sites (N = 20 and 21 in northern archipelago and lake regions, respectively) (Table 1), owing to the small area of the archipelago located south of the navigation channel and the presence of a large restricted access zone belonging to the National Defense Department in the south-eastern lake region (Fig. 1). Sampling sites also reflected an upslope-to-downslope zonation of forested landscape, agricultural farmlands, swamps, marshes, and open water (Table 2). The sediment types were characterized by permanent deltaic sediments, temporary fluvial and littoral sediments, and post-glacial silts (Table 2; Fig. 1).
We evaluated the hydrological regime based on water level fluctuations among three sampling seasons (2004, 2005, and 2006), while accounting for seasonal differences (hydroperiods) and data for up to 14 days prior to sampling (Table 2). Hydroperiods (Winter November 26th−February 2nd; Spring February 3th−July 23th; Summer July 24th–September 1st; Fall September 2nd−November 25th) differ slightly from the astronomical seasons but they reflect more accurately the influence of climate on the hydraulic cycle of the St. Lawrence River (Marchant & de Lafontaine, 2003). Daily water levels (meters above sea level, International Great Lakes Datum of 1985, hereafter referred to as m asl, IGLD85) measured at Sorel (Gauging Station no. 15,930, Hydat 02OJ022, Fig. 1) were obtained for 1966–2006 from the Department of Fisheries and Oceans (DFO, 2007) (http://www.charts.gc.ca/index-eng.asp). Mean daily long-term (1966–2006) water level was calculated from the 40-year time-series. For each sampling year and sampling date at each site, we calculated the mean, minimum and maximum water level and the relative (%) variation in water level during each of the four hydroperiods (Table 2). We also determined average water levels (m asl, IGLD85) during periods of 1, 7, and 14 days prior to sampling, to account for short-term variability. Because all sites within a given year were sampled over a 2-week span, during which water level could vary by 10–30 cm, the short-term lake level history differed among sites, thus allowing us to separate the effects of “year” and “water level” at different sites.
Landscape features and land use around each sampling site within a 100 m radius (area: 31,420 m2) of each sampling site were derived from remote sensing imagery (IKONOS 2002, pixel size 4 × 4 m). This area was selected to represent the landscape features most likely to affect the relatively sedentary macroinvertebrate communities at each sampling site. Landscape analysis allowed us to assess the proportion of nine categories of wetlands and land-use classes (see Table 2) (Létourneau & Jean, 2006), the Simpson’s Index (H) of landscape feature diversity in wetlands, (spatial analysis and patch analysis from ArcGIS) and the types of sediment from laboratory analysis.
At each site, we measured water depth, conductivity, and pH using a Hydrolab surveyor 4a multiprobe at the time of macroinvertebrate sampling. Water quality (total and dissolved phosphorus, nitrate, chlorophyll a, total and dissolved organic carbon, suspended particles, water color, alkalinity) (Table 2) was assessed following standard protocols (Environment Canada, 2004). Dominant emergent macrophyte species were identified to characterize vegetation habitats using Fassett (2006). The upper 10–30 cm of sediment was collected with a modified core sampler for analyses of granulometry, composition, organic carbon and nitrogen contents, and metal contamination (Saskatchewan, 1993; Environment Canada, 2004). Trace metal concentrations were converted into anthropogenic enrichment ratios (measured/natural background concentration in non-contaminated sediment). Ratios above 1.0 indicate an anthropogenic or natural local enrichment (Environment Canada & MDDEP, 2007).
Macroinvertebrate sampling and analysis
Macroinvertebrates were collected in emergent vegetation using kick sampling with a rectangular net (length 45.7 cm; width 25.4 cm; depth 25.4 cm; 500-µm mesh size) following the CABIN protocol (Environment Canada, 2008). At each site on each date, a single macroinvertebrate sample was collected (see Table 1) by the same person following concentric circles for 3 min in shallow water (0.1–0.95 m). Macroinvertebrates and plant debris were preserved in 10% buffered formalin for 72 h. In the laboratory, samples were washed with tap water and put in a 70% ethanol solution. To reduce sorting time, we used a Marchant Box sub-sampler (Marchant, 1989) to fractionate each sample into similar aliquots (n = 10–15). As recommended in bioassessment protocols (Reynoldson et al., 2001; Environment Canada, 2002, 2010; Feio et al., 2006; Chessman et al., 2007; Jones, 2008; Environment Canada, 2010; Neeson et al., 2013), macroinvertebrates were identified to the family level for most groups (Annelida, Insecta, Malacostraca, Mollusca) using Merritt & Cummins (1996) and Smith (2001). The Oligochaeta, Polychaeta, Arachnida, Branchiopoda, and Cnidaria were sorted at the class level. Microscopic (Cladocera, Rotifera, Copepoda, Nematoda, Nemerta, Ostracoda) and terrestrial (spiders, earth worms) invertebrates were not counted. We counted a minimum of 300 individuals per sample, as suggested by Environment Canada (2002), and analyzed at least half of the total sample to get good estimates of macroinvertebrate taxa richness. More details on sampling and analysis were presented by Tall et al. (2008).
Since the kick-net samples could only be collected in water <1 m deep and that water levels varied on a day-to-day basis, we calculated the height of each site above sea level, by subtracting water depth measured at the time of sampling from the water level reported at Sorel on the same date. Site elevation was then used in conjunction with daily water level variations to calculate daily water depth at each site over the previous summer season (from May 1st to the date of sampling), and to assess the number of days each site was out of the water or exposed to a depth of water <10 cm (Table S1, supplemental information).
Statistical analysis
Inter-annual differences in total invertebrate abundance, taxon richness, and percent taxonomic composition were contrasted at the 5 sites (AS2, AS3, LN8, LN13, and LS3, Table 1) that were repeatedly sampled in 2004–2006, using one-way ANOVAs. Spatial differences were assessed by dividing all samples (N = 63) into 4 regions according to their location in LSP archipelago or main lake, along the north or south shore, using one-way ANOVAs. Total macroinvertebrate abundance data were log10 transformed to ensure homoscedasticity.
To determine the environmental variables that significantly (P < 0.05) contributed to changes in macroinvertebrate community, we performed a forward selection procedure on the full environmental matrix (63 samples × 83 environmental variables; see Table 2). The eleven variables selected (identified in bold in Table 2) were then used in a redundancy analysis (RDA) to explain variation in macroinvertebrate community structure. The 66 identified taxa were lumped together at a coarser taxonomic level when occurrences were low (under 5%) (See taxon groups in Table 3), yielding a total of 34 taxonomic groups that were used in the analysis. RDA served to estimate the relationships between the environmental matrix (63 samples × 11 environmental variables) and the macroinvertebrate community matrix (63 samples × 34 taxon groups). We applied Hellinger’s transformation to the macroinvertebrate abundance matrix, which contained many zeros, as recommended by Legendre & Gallagher (2001). Variance explained by the two first canonical axes was tested by permutation using Monte Carlo unrestricted 999 permutation tests (Legendre & Legendre, 1998). Finally, to compare the relative influence of hydrology, landscape, and environmental conditions in explaining spatial variation in macroinvertebrate communities, we used partial redundancy analysis (Legendre & Legendre, 1998). All analyses were performed using the R package (Ihaka & Gentleman, 1996; R Development Core Team, 2012).
The relationships between macroinvertebrate assemblages and the number of days each site was exposed to air or to shallow (<10 cm) water were assessed using parametric (Pearson r) correlations with log10-transformed macroinvertebrate abundance and taxonomic richness data (Table S2, supplemental information).
Results
Hydrology, landscape, and environment
Over the 3-year sampling period, water level at Sorel followed a characteristic pattern with alternating floods in the spring months and low water levels in the summer and fall (Fig. 2). Daily level averaged 4.48 m asl (Table 2). Of the 3 years, 2005 showed the largest amplitude of water level, with the highest maximum flood level of 6.78 m asl during the spring, after which water level dropped to the lowest minimum value of 3.88 m asl during the summer and fall (Fig. 2; Table 2).
Daily (May 2004–November 2006, full line) and mean long-term (1966–2006, dotted line) water level variations (m above sea level, IGLD85 datum) at Sorel (Gauging station 02OJ022). The duration of each hydroperiod is indicated above the X-axis, as follows: Spring (from February 3rd to July 23th), Summer (“Su” from July 24th to September 1st), Fall (from September 2nd to November 25th) and Winter (from November 26th to February 2nd). For each year, the elevation of individual sampling sites (horizontal bars) is indicated with respect to water level fluctuations, with sites constantly submerged between May 1st and sampling date (below the horizontal bar) and sites periodically out of the water shown above the horizontal bar)
Hydrology in Lake Saint-Pierre followed a strong seasonal pattern (Fig. 2). Maximum water levels (>5.5 m asl) occurred during spring. Summer had low water levels, generally <4.5 m asl. In general, water level during the sampling years (2004–2006) was below the average water level observed over the last 40 years (1966–2006), with extreme low levels occurring in the summer and fall of 2005 (Fig. 2). During the fall, water level decreased in 2004 and increased in 2005 and 2006. There were notable differences in water level between years and seasons: 2004 had the highest water level during summer and the lowest in spring and late fall; 2005 had the highest water levels in spring and the lowest in summer, while 2006 the highest water levels in fall. These inter-annual changes are reflected in the range of variation in site emersion across sampling years (Table S1, supplemental information). Water level variation was smaller during winter and summer (24.7–32% on average) than during spring and fall (45.6–49.9% on average) (Table 2).
Site elevation above sea level was 3.94 m on average and ranged from 3.44 to 4.32 m among sites (Table S1, supplemental information). The six sites sampled in 2004 were always underwater (Fig. 2) but two of them had shallow depths (<10 cm) for 2 and 7 days (Fig. 2). In contrast, more than half of the sites sampled in 2005 (18/29) were periodically dry in the weeks prior to sampling, including 9 sites for more than 10 days. In 2006, only 3 sites from 28 emerged above the waterline, in two cases for more than 10 days (Fig. 2).
At the time of sampling, water depth was on average less than 0.5 m but ranged from 0.1 to 0.95 m across sites (Table 2). Water quality showed a wide range of variation with maximum values for water conductivity (796 µS cm−1), suspended particles (672 mg l−1), alkalinity (3 mequiv. l−1), color (6110 Pt/Co), and turbidity (911 NTU) probably caused by short-term water pulses from farmland tributaries (Table 2). Vegetation was dominated by four emergent macrophyte species, each of them covering on average 7–19% of the area surrounding sampling sites. Sediment grain size was also highly variable (Phi 2.4–9.2); sediment had low organic carbon and nitrogen content and was mainly composed of fine sand, silt, and clay. Most of the mean enrichment ratios of metals in sediment were around or slightly above a threshold value of 1, indicating small anthropogenic input of contaminants in sediments.
Macroinvertebrate community
Overall, 66 macroinvertebrate taxa comprising 56,906 individuals were collected lake-wide (Table 3). Macroinvertebrate communities were dominated by Gammaridae (Amphipoda), which represented half of the total macroinvertebrate abundance. Other important groups included Oligochaeta (Annelida), Asellidae (Isopoda), Chironomidae (Diptera), Caenidae (Ephemeroptera), Corixidae (Hemiptera), Arachnida, Pisidiidae (Bivalvia), and Planorbidae (Gastropoda). These taxa showed the highest abundances and occurrences and, taken together, represented 89% of all macroinvertebrates collected during our 3-year survey. Macroinvertebrate taxa richness ranged from 7 to 23 taxa across the 54 sampling sites.
Variations among years at 5 sites
Between 2004 and 2006, total invertebrate abundance dropped by one order of magnitude at all of the 5 sites that were monitored every year (Table 4A; Fig. 3A). On average, taxon richness also showed a significant decrease between 2004 and subsequent years (Fig. 3B), from 19.5 to fewer than 13 taxa (Table 4A). Examination of the taxonomic composition at these 5 sites revealed a sixfold drop in the proportion of Malacostraca (mostly Gammaridae (Amphipoda)) and a ninefold rise in annelids (oligochaetes) between 2004 and 2006 (Fig. 3C, Table 4A). Relative abundance of mollusks and chironomids did not show significant differences among years (Table 4A) although the former group tended to increase with time (Fig. 3C). The same trends and significant differences were recorded at all 5 sites, in spite of their wide geographical dispersion in three of the four regions (Fig. 1).
Between-year comparison of total macroinvertebrate abundance (A), taxa richness (B), and taxonomic composition (C) at 5 sites repeatedly sampled in 2004, 2005 and 2006. Individual sites were located in the southern Archipelago (AS2, AS3), northern lake (LN8—sampled 2005 and 2006 only, LN13) and southern lake (LS3) regions, as identified by stars in Fig. 1. See Table 4A for ANOVA results
Differences in macroinvertebrate community composition also coincided with the inter-annual differences in emersion conditions among years, which were clearly visible for the 5 sites sampled over multiple years. In the southern archipelago region, site AS2 was always under water in 2004 and 2006 and emerged intermittently for 14 days in 2005. This site was also under <10 cm of water for 19 and 20 days in 2005 and 2006. Site AS3 was always underwater in 2004 and 2006 and was exposed to air (4 days) or under <10 cm of water (7 days) for short periods in 2005. In the north lake region, site LN8 emerged above water or had only very shallow water for considerably longer periods (18 and 23 days, respectively) in 2005 than in 2006 (2 days in water <10 cm-deep). Site LN13 also was either dry (14 days) or in shallow water (22 days) in 2005 but always submerged in 2004 and 2006, with only 7 days of shallow water exposure. In contrast to the general inter-annual pattern indicating more emersion in 2005 than 2004 and 2006, site LS3 was always under water in 2004 and 2005 but was sporadically above water for 25 days prior to sampling in 2006.
Variations among regions
Regional differences in the macroinvertebrate communities were far less important than inter-annual differences, owing to the large level of variability within region (Fig. 4). Macroinvertebrate abundances were highly variable among sites, with maxima higher than 10,000 individuals and minima lower than 100 individuals. Accordingly, no significant difference was observed in total macroinvertebrate abundance and taxon richness among the four regions of LSP. However, regardless of the shore, sites located in the archipelago supported a two-fold higher proportion of Mollusca than lake sites (Table 4B).
Comparison of total macroinvertebrate abundance (A), taxa richness (B), and taxonomic composition (C) among samples collected at sites located within four regions of Lake Saint-Pierre, representing the north and south shores of the archipelago and main lake. For each box-plot, the mean and 25–75% percentiles (gray box), 95% confidence interval and outliers (black dots) are indicated. Numbers of samples (N) for each box-plot are specified for each group. See Table 4B for ANOVA results
Modeling of macroinvertebrate–environment relationships
Variations of the macroinvertebrate communities among sites and years were best explained by a subset of eleven environmental variables, accounting together for 31% of the total variance of the data set (Table 5; Fig. 5). Water depth (R 2 12%) and three hydrological variables (water level drop 1 and 14 days prior to sampling, and maximum spring water level) accounted for most of the explanatory power (Table 5). Water depth at the time of sampling can be considered as a surrogate variable indicating low-elevation sites with a low number of episodes out of the water. Indeed, a negative correlation was observed between water depth and site elevation above sea level (Pearson r = −0.33, P = 0.01) and the number of days when sites were exposed or under <10 cm of water (Pearson r = −0.50 and −0.66, respectively; P = 0.001). An additional 12% was explained by various habitat and landscape variables (low marsh area, organic nitrogen in the sediment and fluvial sediments, Typha cover, water pH) each of which explained 2–3% of the total variation in macroinvertebrates.
Redundancy analysis (RDA) plots representing A the contributions of the 11 environmental variables selected at each scale in the model and B the distribution of the macroinvertebrate taxa in the ordination plan. Sampling sites were represented in different ordination plots for 2004 (C), 2005 (D), and 2006 (E). Adjusted R 2 are given for axis 1 and 2 with P < 0.0001
The first axis of the RDA model explained 21.7% of the total variation (Fig. 5A) with opposing up-slope swamp sites, mainly submerged during maximum spring flooding (negative side) to deeper down-slope (generally submerged) sites located on littoral sediments and post-glacial silts (positive side). The second axis (6.4% of total variance) contrasted sites located in low marsh subjected to short-term water level fluctuation (positive side) with sites exposed to more alkaline waters (negative side).
Macroinvertebrate taxa distribution within the RDA (Fig. 5B) revealed a strong association along the first axis of Gammaridae (Malacostraca) with down-slope deeper sites characterized by post-glacial silt. At the opposite end of axis 1, Oligochaeta (Annelida) dominated in up-slope sites located in shallow waters with shrub swamp, and cattail-dominated marshes. Other macroinvertebrate groups such as Caenidae (Ephemeroptera), Asellidae (Isopoda), and Planorbidae (Mollusca) were associated with low marsh habitats in fluvial sediments, whereas Pisidiidae (Bivalvia) were found in alkaline waters.
The distribution of sampling sites along the first two axes of the RDA (Fig. 5) showed more important differences among years than among regions/shores. In 2004, all sites were located in down-slope deep waters and supported large densities of Gammaridae (Malacostraca) and Caenidae (Ephemeroptera) (Fig. 5B and C). In 2005, most archipelago sites were clustered on the left side of the ordination, coinciding with up-slope shallow water habitats supporting communities dominated by Oligochaeta (Annelida). In contrast, lake sites were more dispersed in the ordination space (Fig. 5D). In 2006, most of the sites from the south shore of the lake clustered in the upper right quadrant, characterized by deep open water and low marsh and were dominated by Asellidae (Malacostraca). Sites from the north shore of the lake, located in the lower quadrants of the ordination, were dominated either by Oligochaeta (Annelida) (left) or Gammaridae (Malacostraca) (right) (Fig. 5E).
Overall, macroinvertebrate taxonomic composition tended to follow the same trends as those documented for the 5 sites. Gammaridae and Asellidae (Malacostraca) tended to be more frequent in 2004 and 2006 whereas the Oligochaeta (Annelida), Chironomidae (Diptera), and Planorbidae (Mollusca) were more common in 2005.
Discussion
Our 3-year survey, and previous studies in Lake Saint-Pierre (Tall et al., 2008; Tessier et al., 2008; Tourville-Poirier et al., 2010), indicate that cumulative taxonomic richness of macroinvertebrates in the St. Lawrence River is in the same range (48–66 taxa) as found in the Great Lakes coastal wetlands (Kashian & Burton, 2000; Cooper et al., 2007; Gathman & Burton, 2011) and in other large rivers in New Zealand (Collier & Lill, 2008), USA (Strayer et al., 2006), and China (Pan et al., 2011). Macroinvertebrate communities are dominated by a few relatively abundant and widespread taxa: four groups (Malacostraca, Oligochaeta, Chironomidae, and Mollusca) accounted for most of the taxa richness and abundance.
Comparison of macroinvertebrate communities at the same 5 sites sampled over the course of 3 years revealed significant drops in total abundance, taxon richness and changes in composition, under the unequivocal control of prevailing hydrological conditions. In the St. Lawrence River, hydrological factors appear as primary drivers of variation in macroinvertebrate communities, well ahead of morphological (exposure to wind, waves, and current at sites from different regions) and water quality variables. Water depth (12% of the total variation alone) and three hydrological variables (for an additional 12%) were the most meaningful variables explaining variability in macroinvertebrate communities in St. Lawrence River wetlands. Water level at sampling sites can be considered as a proxy reflecting the up-slope/down-slope elevation gradient, which is also well correlated with wave energy, organic sediment deposition and vegetation habitats, as observed in the Great Lakes coastal wetlands (Cooper et al., 2007). Maximum spring water level and water level variation over the 14 days prior to sampling were also major factors explaining macroinvertebrate community variation. Water level fluctuations are recognized as important drivers of both vegetation habitats and associated macroinvertebrate communities and their structure in wetlands of the Great Lakes (Burton et al., 2002). In Lake Saint-Pierre, seasonal water level fluctuations are important (Hudon, 1997) and affect emergent plant and macroinvertebrate distributions (Tall et al., 2008; Tessier et al., 2008).
As observed in the Great Lakes, macroinvertebrate taxa distribution in emergent marsh in the St. Lawrence varied primarily with up-slope down-slope gradients in flooding regime and water level, and secondarily with sediment and vegetation types along the wetland continuum (Euliss et al., 2004; Gathman & Burton, 2011). At the upper edge of the wetland continuum, very shallow (<10 cm) up-slope sites are flooded during spring and are subjected to large fluctuations in water level, including dry periods during summer. Such shallow-water habitats are also likely to experience the effects of wave erosion, wind-induced seiches, and episodic extreme water temperature. Indeed, water temperatures >25°C were experienced at shallow-water sites during a period of rapidly falling levels coinciding with hot, sunny weather, leading to massive carp mortality (Hudon et al. 2010). In the St. Lawrence River, up-slope, unstable environments are colonized and dominated by resistant endobenthic specialists such as Oligochaeta (Annelida), Chironomidae (Diptera), and Planorbidae and Physidae (Mollusca). These taxa can readily colonize newly flooded habitat during spring since they can be moved passively along the shore slope with water-level changes and can sustain low-oxygen and organic-rich conditions.
At the lower end of the shoreline continuum, down-slope sites (up to 1 m water depth) are relatively deep-water, stable habitats, less affected by wave energy and water level fluctuations than the up-slope sites. Deep marsh habitat supports robust perennial vegetation which further stabilizes the habitat substratum and favors the accumulation of organic, nitrogen-rich sediment. Such constantly flooded down-slope habitats are dominated by time-lagged responders such as Gammaridae and Asellidae (Malacostraca) which feed on vegetal organic matter and detritus in wave-protected fringing wetlands (Burton et al., 2002; Cooper et al., 2007). In summary, the differential response of macroinvertebrate taxa to a range of water depths and associated environmental conditions could explain the opposite distribution pattern observed for Oligochaeta (Annelida) and Gammaridae (Malacostraca) along the up-slope/down-slope elevation gradient in the St. Lawrence wetlands.
Other abundant taxa such as the Chironomidae (Diptera), Caenidae (Ephemeroptera), Asellidae (Malacostraca), Pisidiidae, and Planorbidae (Mollusca) showed different preferential habitats across wetland vegetation and water masses. These taxa have been sorted according to their responses to environmental gradients in Great Lakes coastal wetlands (Gathman & Burton, 2011). Past studies in Lake Saint-Pierre (Tall et al., 2008) have shown Gammaridae (Malacostraca) and oligochaetes (Annelida) to discriminate between reference fluvial sites and sites chronically exposed to the plume of farmland tributaries. The absence or low abundance of other taxon groups such as insect larvae (Ephemeroptera other than Caenidae, Trichoptera, Coleoptera) was another indication of a benthic fauna characteristic of impacted wetlands in the Great Lakes region (Kashian & Burton, 2000).
In our study, comparisons among many sites in different regions of LSP, using univariate metrics, such as taxa richness and total abundance, showed highly significant differences among years when the same sites were sampled over three years of widely different hydrological conditions. This is in contrast with other biomonitoring studies reporting that single measures lacked the sensitivity to detect changes in macroinvertebrate communities owing to high variability among sites (Flinn et al., 2005, 2008; Meyer & Whiles, 2008; Masson et al., 2010). On average, lake-wide macroinvertebrate abundance (370–1200 ind./sample) and taxa richness (13–16 taxa/sample) were highly variable among LSP sites but were within the range of values reported for other large rivers (Collier & Lill, 2008; Gallardo et al., 2008).
Multivariate metrics such as macroinvertebrate assemblages (taxa presence/absence or relative abundance) are also efficient tools for assessing changes in benthic fauna (this study, Tall et al., 2008; Masson et al., 2010). Macroinvertebrate taxa composition in the St. Lawrence wetlands responded to environmental gradients related to water depth, water level fluctuations, vegetation habitats, and local conditions. These findings are also consistent with other studies on benthic fauna in rivers, which have shown that multiple environmental variables explain major macroinvertebrate assemblages and diversity patterns (Gallardo et al., 2008; Hugues et al., 2008; Skoulikidis et al., 2009).
Our study also showed minor, yet significant effects of landscape and morphological features as factors modulating variations in macroinvertebrate communities. They included the organic nitrogen content in the sediment, the occurrence of low marsh vegetation dominated by cattail (Typha), and fluvial sediments. The following variables most likely integrate environmental factors that affect macroinvertebrate habitats: sediment grain size, compaction or mobility, vegetation structure and current regime (Strayer et al., 2006; Strayer & Malcom, 2007). They are also indirectly related to hydrological conditions, because water level variations determined the distribution of emergent plants in the St. Lawrence wetlands (Hudon, 1997). Organic nitrogen in sediment may also reflect the effect of agricultural input of nitrogen (Gallardo et al., 2008).
The lack of significant influence of water chemistry (12 variables) or of anthropogenic contamination ratios in the sediment (23 variables) was unexpected, especially given the wide range of values encountered within LSP regions and the poor quality of its major tributaries (Hudon & Carignan, 2008). Such lack of response may result in part from the instantaneous nature of water quality and temperature measurements, whereas macroinvertebrate communities, habitat features, and landscape variables integrate longer time spans. Other studies have documented macroinvertebrate responses to restored and natural wetlands degraded by agricultural practices (Meyer & Whiles, 2008) and to sediment quality along a contamination gradient (Masson et al., 2010). In our study, water pH was the only variable showing a significant positive correlation and that only with Pisiidae (Mollusca). High pH values may reflect intense primary production in the hard waters originating from the Great Lakes, which constitute a preferential habitat for these small bivalves. Small Pisidiidae were reported to be generally more abundant in natural than in restored wetlands (Meyer & Whiles, 2008).
Although our study did not measure submerged aquatic vegetation or vertebrate predators, biotic interactions may also be important drivers of the unaccounted variance in macroinvertebrate communities. Macrophyte structural complexity and composition have been shown to affect macrobenthos, insect and fish communities, and aquatic food-webs (Boström & Bondorff, 2000; McAbendroth et al., 2005; Willis et al., 2005; Matias et al., 2010; Cunha et al., 2012; Bolduc et al., 2015). Batzer (2013) also emphasized the importance of predation by fish and salamanders on wetland macroinvertebrates. Our study demonstrates the overwhelming effect of hydrological regime on macroinvertebrate communities in Lake Saint-Pierre, coinciding with a sharp drop in their abundance over a three-year period, which may be an underlying factor in the recent collapse in perch recruitment in Lake Saint-Pierre.
References
Armanini, D. G., N. Horrigan, W. A. Monk, D. L. Peters & D. J. Baird, 2011. Development of a benthic macroinvertebrate flow sensitivity index for Canadian rivers. River Research and Applications 27: 723–737.
Bailey, R. C., R. H. Norris & T. B. Reynoldson, 2004. Bioassessment of Freshwater Ecosystems: Using the Reference Condition Approach. Kluwer Academic Publishers, Boston.
Batzer, D. P., R. B. Rader & S. A. Wissinger (eds), 1999. Wetlands: Unique Habitats with Unique Invertebrate Species. Wiley, New York.
Batzer, D. P., 2013. The seemingly intractable ecological responses of invertebrates in North American wetlands: a review. Wetlands 33: 1–15.
Bolduc, P., A. Bertolo & B. Pinel-Alloul, 2015. Does submerged aquatic vegetation shape zooplankton community structure and functional diversity? A test with a shallow fluvial lake system. Hydrobiologia (Accepted October 2015).
Boström, C. & E. Bonsdorff, 2000. Zoobenthic community establishment and habitat complexity and the importance of seagrass shoot density, morphology and physical disturbance for faunal recruitment. Marine Ecology Progress Series 205: 123–138.
Burton, T. M., D. G. Uzarski, J. P. Gathman, J. A. Genet, B. E. Keas & C. A. Stricker, 1999. Development of a preliminary invertebrate index of biotic integrity for Lake Huron coastal wetlands. Wetlands 19: 869–882.
Burton, T. M., C. A. Stricker & D. G. Uzarski, 2002. Effects of plant composition and exposure to wave action on invertebrate habitat use of Lake Huron coastal wetlands. Lakes & Reservoirs: Research and Management 7: 255–269.
Cabezas, A., F. A. Comín, M. García, B. Gallardo, E. González & M. González, 2008. Effects of hydrological connectivity on the substrate and understory structure of riparian wetlands in the Middle Ebro River (NE Spain): implications for restoration and management. Aquatic Sciences 70: 361–376.
Cabezas, A., M. Garcìa, B. Gallardo, E. Gonzalez, M. Gonzalez-Sanchis & F. A. Comin, 2009. The effect of anthropogenic disturbance on the hydrochemical characteristics of riparian wetlands at the Middle Ebro River (NE Spain). Hydrobiologia 616: 101–117.
Carignan, R., S. Lorrain & K. Lum, 1994. A 50-year record of pollution by nutrients, trace metals and organic chemicals in the St. Lawrence River. Canadian Journal of Fisheries and Aquatic Sciences 51: 1088–1100.
Chessman, B. C., S. Williams & C. Besley, 2007. Bioassessment of streams with macroinvertebrates: effect of sampled habitat and taxonomic resolution. Journal of North American Benthological Society 26: 546–565.
Clements, W. H., D. S. Cherry & J. H. Van Hassel, 1992. Assessment of the impact of heavy metals on benthic communities in the Clinch River (Virginia): evaluation of an index of community sensitivity. Canadian Journal of Fisheries and aquatic Sciences 49: 1686–1694.
Collier, K. J. & A. Lill, 2008. Spatial patterns in the composition of shallow-water macroinvertebrate communities of a large New-Zealand river. New Zealand Journal of Marine and Freshwater Research 42: 129–141.
Cooper, M. J., D. G. Uzarski & T. M. Burton, 2007. Macroinvertebrate community composition in relation to anthropogenic disturbance, vegetation, and organic sediment depth in four Lake Michigan drowned river-mouth wetlands. Wetlands 27: 894–903.
Cremona, F., D. Planas & M. Lucotte, 2008. Biomass and composition of macroinvertebrate communities associated with different types of macrophyte architectures and habitats in a large fluvial lake. Archiv für Hydrobiologie 171: 119–130.
Cunha, E., S. Thomaz, R. Mormul, E. Cafofo & A. Bonaldo, 2012. Macrophyte structural complexity influences spider assemblage attributes in Wetlands. Wetlands 32: 369–377.
de Lafontaine, Y. & G. Constan, 2002. Introduction and transfer of alien aquatic species in the Great Lakes - St. Lawrence River drainage basin. In Renata, C., P. Nantel & E. Muckle-Jeffs (eds), Alien Invaders in Canada’s Waters, Wetlands, and Forests. Canadian Forest Service, Natural Resources Canada, Ottawa: 73–91.
Department of Fisheries and Oceans (DFO), 2007. Daily water level data (2004–2007) for Lake Saint-Pierre (Sorel, gauging station no. 15930, N 46,047139W 73,115694, 13,488m IGLD85).
Environment Canada, 2002. Revised Guidance for Sample Sorting and Sub-sampling Protocols for EEM Benthic Invertebrate Community Surveys. http://www.ec.gc.ca/eem/english/publications/web_publications/sub_sampling/default.cfm.
Environment Canada, 2004. Manuel des méthodes analytiques. Quality assurance and control manual. Environment Canada, St, Lawrence Centre.
Environment Canada, 2008. Canadian Aquatic Biomonitoring Network (CABIN)-Réseau canadien de Biosurveillance aquatique (RCBA). http://cabin.cciw.ca.
Environment Canada, 2010. Canadian Aquatic Biomonitoring Network - Laboratory methods: processing, taxonomy, and quality control of benthic macroinvertebrates samples.
Environment Canada, 2012. Water Survey Canada: Hydat database. Environment Canada, Ottawa, Ontario, Canada.
Environnement Canada & Ministère du Développement durable, de l’Environnement et des Parcs du Québec, 2007. Critères pour l’évaluation de la qualité des sédiments au Québec et cadres d’application : prévention, dragage et restauration.
Euliss, N. H., J. W. LaBaugh, L. H. Fredrickson, D. M. Mushet & M. K. Laubhan, 2004. The wetland continuum: a conceptual framework for interpreting biological studies. Wetlands 24: 448–458.
Fassett, N. C., 2006. A Manual of Aquatic Plants. University of Wisconsin Press, 2ème édition, 416 p.
Feio, M. J., T. B. Reynoldson & M. A. S. Graça, 2006. The influence of taxonomic level on the performance of a predictive model for water quality assessment. Canadian Journal of Fisheries and Aquatic Sciences 63: 367–376.
Filion, A. & A. Morin, 2000. Effects of local sources on metal concentrations in littoral sediments and aquatic macroinvertebrates of the St. Lawrence River, near Cornwall, Ontario. Canadian Journal of Fisheries and Aquatic Sciences 57(Suppl. 1): 113–125.
Flinn, M. B., M. R. Whiles, S. R. Adams & J. E. Garvey, 2005. Macroinvertebrate and zooplankton responses to emergent plant production in upper Mississippi River floodplain wetlands. Archives für Hydrobiologie 162(2): 187–210.
Flinn, M. B., S. R. Adams, M. R. Whiles & J. E. Garvey, 2008. Biological responses to constrasting hydrology in backwaters of Upper Mississippi River Navigation pool 25. Environmental Management 41: 468–486.
Gallardo, B., M. Garcias, A. Cabezas, E. Gonzalez, C. Ciancarelli, M. Gonzalez-Sanchís & F. A. Comin, 2008. Macroinvertebrate patterns along environmental gradients and hydrological connectivity within a regulated river-floodplain. Aquatic Sciences 70: 248–258.
Gammonley, J. H. & M. K. Laubhan, 2002. Patterns of food abundance for breeding waterbirds in the San Luis Valley of Colorado. Wetlands 22: 499–508.
Gathman, J. P. & T. M. Burton, 2011. A Great Lakes wetland invertebrate community gradient: relative influence of flooding regime and vegetation zonation. Wetlands 31: 329–341.
Gower, A. M., G. Myers, M. Kent & M. E. Foulkes, 1994. Relationships between macroinvertebrate communities and environmental variables in metal-contaminated streams in south-west England. Freshwater Biology 32: 199–221.
Griffiths, R. W., 1991. Environmental quality assessment of the St. Clair River as reflected by the distribution of benthic macroinvertebrates in 1985. Hydrobiologia 219: 143–164.
Habersack, H., D. Haspel, S. Muhar & H. Waidbacher, 2014. Impacts of human activities on biodiversity of large rivers. World’s Large Rivers Conference. Hydrobiologia 729: 1–2.
Heino, J., 2000. Lentic macroinvertebrate assemblage structure along gradients in spatial heterogeneity, habitat size and water chemistry. Hydrobiologia 418: 229–242.
Hudon, C., 1997. Impact of water level fluctuations on St. Lawrence River aquatic vegetation. Canadian Journal of Fisheries and Aquatic Sciences 54: 2853–2865.
Hudon, C. & R. Carignan, 2008. Cumulative impacts of hydrology and human activities on water quality in the St. Lawrence River (Lake Saint-Pierre, Quebec, Canada). Canadian Journal of Fisheries and Aquatic Sciences 65: 1165–1180.
Hudon, C., P. Gagnon, J.-P. Amyot, G. Létourneau, M. Jean, C. Plante, D. Rioux & M. Deschênes, 2005. Historical changes in herbaceous wetland distribution induced by hydrological conditions in Lake Saint-Pierre (St. Lawrence River, Quebec, Canada). Hydrobiologia 539: 205–224.
Hudon, C., A. Armellin, P. Gagnon & A. Patoine, 2010. Variations of water temperature and level in the St. Lawrence River (Quebec, Canada): effects on three common fish species. Hydrobiologia 647: 145–161.
Hudon, C., A. Cattaneo, A.-M. Tourville Poirier, P. Brodeur, P. Dumont, Y. Mailhot, J.-P. Amyot, S.-P. Despatie & Y. de Lafontaine, 2012. Oligotrophication from wetland epuration alters the riverine trophic network and carrying capacity for fish. Aquatic Sciences 74: 495–511.
Hugues, S., T. Ferreira & R. V. Cortes, 2008. Hierarchical spatial patterns and drivers of change in benthic macroinvertebrate communities in an intermittent Mediterranean river. Aquatic Conservation: Marine and Freshwater Ecosystems 18: 742–760.
Ihaka, R. & R. Gentleman, 1996. R: a language for data analysis and graphics. Journal of computational and graphical statistics 5: 299–314.
Jean, M. & G. Létourneau, 2014. Freshwater Wetlands, Third Edition. St. Lawrence Action Plan, Monitoring the State of the St. Lawrence. http://plantstlaurent.qc.ca/en/state_monitoring/monitoring_sheets/monitoring_the_state_of_the_st_lawrence_river.html#c2411. Accessed on 26 March 2015.
Johnson, J. H. & D. S. Dropkin, 1993. Diel variation in diet composition of a riverine fish community. Hydrobiologia 271: 149–158.
Jones, C., 2008. Taxonomic sufficiency: the influence of taxonomic resolution on freshwater bioassessments using benthic macroinvertebrates. Environment Review 16: 45–69.
Kashian, D. R. & T. M. Burton, 2000. A comparison of macroinvertebrates of two Great Lakes coastal wetlands: testing potential metrics for an index of ecological integrity. Journal of Great Lakes Research 26: 460–481.
Legendre, P. & E. D. Gallagher, 2001. Ecologically meaningful transformations for ordination of species data. Oecologia 129: 271–280.
Legendre, P. & L. Legendre, 1998. Numerical Ecology. Second English Edition. Elsevier Scientific Publishing Company, Amsterdam: 853 pp.
Létourneau, G. & M. Jean, 2006. Cartographie par télédétection des milieux humides du Saint-Laurent (2002). Environnement Canada, Direction générale des sciences et de la technologie, Monitoring et surveillance de la qualité de l’eau au Québec. Rapport scientifique et technique ST-239.
Magnin, E., E. Murawska & A.-M. Clément, 1978. Régime alimentaire de sept poissons littoraux de la Grande Anse de l’île Perrot, sur le lac Saint-Louis, près de Montréal, Québec. Naturaliste Canadien 105: 89–101.
Marchand, F. & Y. De Lafontaine, 2003. The impact of hydrological variation on the seasonal occurrence and migratory timing of freshwater fish species in the lower St. Lawrence River. Technical Report to International Joint Commission from St. Lawrence Centre, Environment Canada, 105 McGill Street, 7th Floor, Montréal, Québec Canada.
Marchant, R., 1989. A sub-sampler for samples of benthic invertebrates. Bulletin of the Austrialian Society of Limnology 12: 49–52.
Masson, S., M. Desrosiers, B. Pinel-Alloul & L. Martel, 2010. Relating macroinvertebrate community structure to environmental characteristics and sediment contamination at the scale of the St. Lawrence River. Hydrobiologia 647: 35–50.
Matias, M. G., A. J. Underwood, D. F. Hochuli & R. A. Coleman, 2010. Independent effects of patch size and structural complexity on diversity of benthic macroinvertebrates. Ecology 91: 1908–1915.
McAbendroth, L., P. M. Ramsay, A. Foggo, S. D. Rundle & D. T. Bilton, 2005. Does macrophyte fractal complexity drive invertebrate diversity, biomass and body size distributions? Oikos 111: 279–290.
Merritt, R. W. & K. W. Cummins, 1996. Introduction to the Aquatic Insects of North America, 3rd ed. Kendall/Hunt Publishing Company, Dubuque: 862.
Merritt, R. W., M. E. Benbow & P. L. Hudson, 2002. Wetland macroinvertebrates of Prentiss bay. Lake Huron, Michigan: diversity and functional group composition. Great Lakes Entomologist 35: 149–160.
Meyer, C. K. & M. R. Whiles, 2008. Macroinvertebrate communities in restored and natural Platte River slough wetlands. Journal of the North Benthological Society 27: 626–639.
Morin, J. & J.-P. Côté, 2003. Modifications anthropiques sur 150 ans au lac Saint-Pierre: une fenêtre sur les transformations de l’écosystème du Saint-Laurent. Vertigo - La revue en sciences de l’environnement 4: 132–141.
Mykrä, H., J. Aroviita, J. Kotanen, H. Hämäläinen & T. Muotka, 2008a. Predicting the stream macroinvertebrate fauna across regional scales: influence of geographical extent on model performance. Journal of North American Benthological Society 27: 705–716.
Mykrä, H., J. Aroviita, H. Hämäläinen, J. Kotanen, K.-M. Vuori & T. Muotka, 2008b. Assessing stream condition using macroinvertebrates and macrophytes: concordance of community responses to human impact. Fundamental and Applied Limnology 172(3): 191–203.
Neeson, T. M., I. Van Rijn & Y. Mandelik, 2013. How taxonomic diversity, community structure, and simple size determine the reliability of higher taxon surrogates. Ecological Applications 23: 1216–1225.
Pan, Bao-Zhu, Hai-Jun Wang, Xiao-Min Liang & Hong-Zhu Wang, 2011. Macrobenthos in Yangtze floodplain lakes: patterns of density, biomass, and production in relation to river connectivity. Journal of North American Benthological Society 30: 589–602.
Pinel-Alloul, B., G. Méthot, L. Lapierre & A. Willsie, 1996. Macroinvertebrate community as a biological indicator of ecological and toxicological factors in Lake Saint-François (Québec). Environmental Pollution 91: 65–87.
R Development Core Team, 2012. R: a language and environment for statistical computing. Austria, Vienna.
Reynoldson, T. B., D. M. Rosenberg & V. H. Resh, 2001. Comparison of methods predicting invertebrate assemblages for biomonitoring in the Fraser River catchment, British Columbia. Canadian Journal of Fisheries and Aquatic Sciences 58: 1395–1410.
Reynoldson, T. B., R. H. Norris, V. H. Resh, K. E. Day & D. M. Rosenberg, 1997. The reference condition: a comparison of multimetric and multivariate approaches to assess water-quality impairment using benthic macroinvertebrates. Journal of North American Benthological Society 16: 833–852.
Rondeau, B., D. Cossa, P. Gagnon & L. Bilodeau, 2000. Budget and sources of suspended sediment transported in the St. Lawrence River, Canada. Hydrological Processes 14: 21–36.
Rosenberg, D. M. & V. H. Resh, 1993. Freshwater biomonitoring and benthic macroinvertebrates. Chapman and Hall, London.
Saskatchewan, 1993. Standard Test Procedures Manual: Mechanical Analysis. SPT 205-10, Hydrometer. Saskatchewan Highways and Transportation. 8 pp.
Skoulikidis, N Th, I. Karaouzas & K. C. Gritzalis, 2009. Identifying key environmental variables structuring benthic fauna for establishing a biotic typology for Greek running waters. Limnologica 39: 56–66.
Smith, D. G., 2001. Pennak’s Freshwater Invertebrates of the United States: Porifera to Crustacea, 4th ed. Wiley, New York: 638.
St-Onge, J. 1999. Le bassin de la rivière Yamaska: les communautés benthiques et l’intégrité biotique du milieu, section 5 dans ministère de l’Environnement (ed.), Le bassin de la rivière Yamaska: état de l’écosystème aquatique. Québec, Direction des écosystèmes aquatiques. Rapport EA-14.
Strayer, D. L. & H. M. Malcom, 2007. Submerged vegetation as habitat for invertebrates in the Hudson River Estuary. Estuaries and Coasts 30: 253–264.
Strayer, D. L., H. M. Malcom, R. E. Bell, S. M. Carbotte & F. O. Nitsche, 2006. Using geophysical information to define benthic habitats in a large river. Freshwater Biology 51: 25–38.
Tall, L., G. Méthot, A. Armellin & B. Pinel-Alloul, 2008. Bioassessment of benthic macroinvertebrates in wetlands habitats of Lake Saint-Pierre (St. Lawrence River). Journal of Great Lakes Research 34: 599–614.
Tessier, C., A. Cattaneo, B. Pinel-Alloul, C. Hudon & D. Borcard, 2008. Invertebrate communities associated with metaphyton and emergent and submerged macrophytes in a large river. Aquatic Sciences 70: 10–20.
Tourville-Poirier, A.-M., A. Cattaneo & C. Hudon, 2010. Benthic cyanobacteria and filamentous chlorophytes affect macroinvertebrate assemblages in a large fluvial lake. Journal of the North American Benthological Society 29: 737–749.
Vis, C., C. Hudon, R. Carignan & P. Gagnon, 2007. Spatial analysis of production of macrophytes, phytoplankton and epiphyton in a large river system under different water-level conditions. Ecosystems 10: 293–310.
Weigel, B. M. & J. J. Dimick, 2011. Development, validation, and application of a macroinvertebrate-based Index of Biotic Integrity for nonwadeable rivers of Wisconsin. Journal of the North American Benthological Society 30: 665–679.
Willis, S. C., K. O. Winemiller & H. Lopez-Fernandez, 2005. Habitat structural complexity and morphological diversity of fish assemblages in a Neotropical floodplain river. Oecologia 142: 284–295.
Acknowledgments
The research was supported through funding to B. Pinel-Alloul from NSERC (Discovery Subvention), FQRNT, (GRIL, Groupe de recherche interuniversitaire en limnologie et environnement aquatique), and Environment Canada (research contract). Financial support for fieldwork in Lake Saint-Pierre, laboratory analysis (water analysis, sediment grain size and metal contamination), and determination of landscape features was provided by the St. Lawrence Action Plan and Environment Canada. Laure Tall received a grant from the Science Horizon Program of Environment Canada. We are indebted to Anita Rogic, Jean Christian Laforge and Anne-Marie Bouchard for taxa sorting and group classification. We thank Louise Cloutier for valuable assistance in taxa identification, Pierre Legendre and Daniel Borcard for help in statistical analysis, Caroline Savage for landscape analysis, François Boudreault for mapping, and Claude Lessard and Germain Brault for field technical support. Editorial comments made by Brian Moss on the revised version of the manuscript are acknowledged with thanks.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: M. Beklioğlu, M. Meerhoff, T. A. Davidson, K. A. Ger, K. E. Havens & B. Moss / Shallow Lakes in a Fast Changing World
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tall, L., Armellin, A., Pinel-Alloul, B. et al. Effects of hydrological regime, landscape features, and environment on macroinvertebrates in St. Lawrence River wetlands. Hydrobiologia 778, 221–241 (2016). https://doi.org/10.1007/s10750-015-2531-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-015-2531-7