Abstract
Water resources in many regions are stressed by impairments resulting from climate change, population growth and urbanization. In the United States (US), water quality criteria (WQC) and standards (WQS) were established to protect surface waters and associated designated uses, including aquatic life. In inland waters of the south central US, for example, depressed dissolved oxygen (DO) consistently results in impaired aquatic systems due to noncompliance with DO WQC and WQS. In the present study, we systematically examined currently available DO threshold data for freshwater fish and invertebrates and performed probabilistic aquatic hazard assessments with low DO toxicity data that were used to derive the US Environmental Protection Agency’s (EPA) Ambient Water Quality Criteria (AWQC) for DO and newly published information. Aquatic hazard assessments predicted acute invertebrate DO thresholds for Ephemeroptera, Plecoptera, or Trichoptera (EPT) taxa and species inhabiting lotic systems to be more sensitive than fish. For example, these organisms were predicted to have acute low DO toxicity thresholds exceeding the US EPA guidelines 17, 26, 31 and 38% and 13, 24, 30 and 39% of the time at 8.0, 5.0, 4.0 and 3.0 mg DO/L, respectively. Based on our analysis, it appears possible that low DO effects to freshwater organisms have been underestimated. We also identified influences of temperature on low DO thresholds and pronounced differences in implementation and assessment of the US EPA AWQC among habitats, seasons, and geographic regions. These results suggest some implemented DO guidelines may adversely affect the survival, growth, and reproduction of freshwater aquatic organisms in a region susceptible to climate change and rapid population growth. Given the global decline of species, particularly invertebrates, low DO threshold information, including sublethal (e.g., reproduction, behavior) responses, for additional species (e.g., mollusks, other invertebrates, warm water fish) across seasons, habitats, and life history stages using consistent experimental designs is needed to support more sustainable environmental assessment efforts and management of biodiversity protection goals in inland waters.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Freshwater systems can experience significant modification in response to climate change, population growth, and other anthropogenic stressors such as nutrient enrichment, contaminants of emerging concern, pH, and dissolved oxygen (DO). These alterations are particularly observed in arid to semi-arid regions (Brooks et al. 2006; Delorme 1982; Heathwaite 2010). Nutrient enrichment of freshwater systems due to anthropogenic point and nonpoint sources can indirectly result in depressed DO and, in extreme scenarios, hypoxic or anoxic conditions leading to poor water quality (Brooks et al. 2006; Delorme 1982; Heathwaite 2010; Valenti et al. 2011). In aquatic systems, an increase in temperature co-occurring with carbon dioxide accumulation can also exaggerate hypoxia due to elevated oxygen demand and lower oxygen solubility at high temperature (Brewer and Peltzer 2009; Pörtner 2010). Hypoxia in aquatic ecosystems is typically defined as low levels of DO from near maximum solubility to below 2 mg DO/L (Committee on Environment and Natural Resources 2003). Hypoxic conditions have occasionally occurred naturally in some systems, such as Lake Erie (Delorme 1982; Zhou et al. 2013) and the Chesapeake Bay (Cooper and Brush 1991; Committee on Environment and Natural Resources 2003). However, nutrient enrichment and increased organic matter due to anthropogenic activities has resulted in intensified magnitude, frequency, and duration of hypoxia and anoxia in freshwater and marine systems (Diaz and Breitburg 2009; Committee on Environment and Natural Resources 2003).
Low DO concentrations typically occur in hypolimnetic waters with high organic matter, poor circulation, defined stratification, or seasonal ice cover (Chambers et al. 1997; Diaz and Breitburg 2009). Depressed DO levels produce adverse effects on metabolic and behavioral processes in aquatic organisms. For example, moderate hypoxia (2 to 5 mg DO/L) can cause physiological or biochemical stress (e.g., hormonal responses, oxidative stress) in fish and invertebrates, while severe hypoxia can impact survival (mortality), growth, reproduction, and population trajectories of aquatic life (Brett and Blackburn 1981; Doudoroff and Shumway 1970). Unfortunately, though hypoxia has received much study in marine and coastal systems, depressed DO has received relatively limited attention in freshwater ecosystems over the past few decades (Pollock et al. 2007).
In the US, the 303(d) list (Section 303(d)) of the Clean Water Act (CWA) includes impaired surface waters that do not attain water quality standards (WQS). In states experiencing dramatic population growth and climate change, such as Texas, freshwater impoundments and tidally influenced rivers have been consistently listed on 303(d) lists due to noncompliance with DO water quality criteria (WQC) and standards (Brooks et al. 2008; Brooks et al. 2011). Reservoirs located in these arid to semi-arid regions are particularly prone to hypolimnetic and even metalimnetic hypoxia due to high loads of organic matter, droughts, withdrawal rates, and spatial variability (Brooks et al. 2011; Diaz and Breitburg 2009; Thornton et al. 1990). Though reservoir zones (e.g., riverine, transition, lacustrine) represent different aquatic habitats that should be considered during surface water quality assessment and management (Lind et al. 1993), various reservoir habitats are not routinely considered during surface water quality assessments of DO and other contaminants (Brooks et al. 2008; Brooks et al. 2011). Whether habitat-specific implementation and assessment of AWQC, including DO, differs among states and other geographic regions remains poorly described, but differing implementation practices can introduce uncertainty during surface water quality assessments and management activities.
The US CWA mandates states and authorized tribes to develop, implement, enforce, and periodically update WQC to protect designated uses of aquatic ecosystems. Based on the 1986 US EPA AWQC for DO, these WQC were intended to protect aquatic life uses and were predominantly dependent on available low DO toxicity data for growth impairment in cold and warm water fish (U.S. Environmental Protection Agency 1986; U.S. Environmental Protection Agency 2012). In 1986, the recommended freshwater DO AWQC were derived for the protection of no to slight (10%) growth/production impairment to fish populations because these DO concentrations were also expected to provide adequate protection for other aquatic organisms (i.e., invertebrates; U.S. Environmental Protection Agency 1986). Canada and UK published DO water quality guidelines after the US EPA in 1987 and 1992, respectively, with the UK specifically referencing both fresh and marine waters (Canadian Council of Ministers of the Environment 2001; Stiff et al. 1992). Similar to the US AWQC (U.S. Environmental Protection Agency 1986). Canada recommended DO criteria across different developmental stages, while the UK aquatic life criteria were categorized based on the fishery (e.g., salmonid, cyprinid, less-sensitive cyprinid). No revisions have occurred to the EPA AWQC since its initial publication 30 years ago; whether such criteria are protective of threatened and endangered species is largely understudied (Woods et al. 2010). However, DO is of particular importance because of the increased frequency of hypoxic events worldwide over the past few decades (Diaz 2001; Committee on Environment and Natural Resources 2003) and future projections of population growth, landscape modification, and climate change.
Whether more recently published low DO toxicity data could improve our understanding of the adverse effects of hypoxia in inland waters, and thus reduce uncertainty during surface water quality assessment and management efforts, is not understood. Thus, in the present study, we (1) examined the current status of historical (pre-1986) and more recent low DO toxicity data (post-1986) for freshwater fish and invertebrates, hypothesizing more recent data would differ from historical information; (2) employed probabilistic aquatic hazard assessments to determine the percent of species affected by low DO relative to WQC; and (3) identified whether implementation and assessment of DO WQC differs among freshwater habitats, seasons, and the south central geographic area of the US, a region susceptible to climate change and population growth. We further examined the relationship between temperature and low DO thresholds because increasing temperature decreases oxygen water solubility under conditions when metabolic demands increase with less oxygen availability and the US AWQC are based on water temperature (cold water vs. warm water) and fish (salmonid vs. nonsalmonid species).
Methods
Data collection
Acute and chronic toxicity data (lethal or effect concentrations, LC50s or EC50s) for low DO and corresponding experimental conditions (e.g., DO, pH, temperature) of freshwater fishes and invertebrates were collected from the peer-reviewed literatures and the US EPA AQWQC document (U.S. Environmental Protection Agency 1986). Acute toxicity endpoints included individual species’ LC50 (≤96 h, ≥96 h) values, while chronic endpoints included EC10 and EC50s for the effects of DO on growth (>96 h). For data quality consistency, toxicity data were selected using the following approach. Only published DO experiments that documented experimental designs and study procedures were used for further analyses. These study procedures included sufficient water renewals, clearly identified DO control methods (constant or declining DO), organismal conditions (species, size, weight, life stage, source, diet, acclimation period), daily water chemistry observations (DO, pH, temperature), adequate controls, at least initial and final mortality observations (with sufficient control survival), and statistically calculated standard toxicity values (LC50 or ECx) (Sprague 1973). DO treatment levels reported simply as values greater or less than a concentration were excluded from probabilistic analyses. In the present study, low DO toxicity refers to a calculated lower DO threshold for either decreased survival or growth of an organism. Toxicity data used for species sensitivity distributions (SSDs) are listed in supplementary information (Table S1). Fish growth data calculated from both laboratory and mesocosm studies were used in our analyses because they were explicitly included in the derivation of the 1986 AWQC for DO.
Aquatic hazard assessments
Geometric means were calculated for species LC50 or ECx values when study conditions within 1 °C, the same life stage, and multiple toxicity values were reported. When multiple LC50 or ECx values were available for the same species from studies at temperatures varying by greater than 1 °C or by life stage, these data values were separately included in taxa SSD development. Low DO toxicity values were selected to be inclusive of all available temperature conditions, life stages, and study designs. Toxicity data were first ranked in ascending order and assigned percentiles using the Weibull equation:
where j is the percent rank, i is the rank assigned to an acute (LC50) or chronic concentration (EC10 or EC50), n is the number of species examined, and n + 1 accounts for the assumption that there is always one less than all species tested (Posthuma et al. 2002). SSDs were then constructed following the procedures described in Wheeler et al. (2002), having log concentrations of toxicity values (LC or EC) as x-axis and the proportion of species being affected as y-axis (SigmaPlot Version 11.0 Systat Software, Inc., San Jose, CA, USA). Analyses of covariance (ANCOVA, SPSS, Chicago, IL, USA) were conducted to compare the slopes and intercepts of Weibull ranked probit normalized regression models of specific classified datasets (e.g., Ephemeroptera, Plecoptera, or Trichoptera (EPT) taxa vs. non-EPT taxa). Due to a variety of low DO toxicity data, which spanned five decades across multiple species (e.g., Hyalella azteca, Hexagenia limbata, Onchorynchus mykiss), different SSDs were generated (i.e., EPT, lotic habitat, pre-1986) for fish and invertebrates.
Probabilistic aquatic hazard assessments using developed SSDs were then performed to determine the percentage of toxicity thresholds (e.g., LC50, EC50) likely to be exceeded at the existing US EPA AWQC. Slopes and y-intercepts were extracted from SSD regression models and centile values were calculated (Microsoft Excel 2016 Microsoft Corp, Richmond, WA, USA) using the equation:
where the NORMSDIST returns the standard normal cumulative distribution function of a selected value, and b and a represent the slope and intercept, respectively, from the linear regression.
To quantify differences in SSDs, hazard concentrations (HC) at the 80th percentile (i.e., HC20 or 20% protection level) were calculated from each SSD. More common HC95 or HC90 (i.e., 95 or 90% protection level for DO, respectively) values were not compared in this study because over half of the SSDs contained less than 20 data values (minimum was 5) and would introduce higher uncertainty in such predictions (Grist et al. 2002; Wheeler et al. 2002). HC values derived from each dataset were calculated and compared to compute an HC ratio. When a ratio was greater than one, the dataset/species were considered sensitive to DO. HCs and their corresponding 95% confidence interval were computed by Monte Carlo simulation, following the log-normal procedure available in the SAS package (SAS 9.4, Cary, NC, USA), and were determined at 10th, 50th, 80th, 90th, 95th, and 99th centiles.
Temperature-dependent DO thresholds
To investigate potential temperature effects on DO thresholds of freshwater species, a comprehensive meta-analysis of various acute toxicity endpoints (LC50s and EC50s) for DO across multiple temperatures (n ≥ 3) was conducted for the data generated from an individual study. Linear regression was applied to fit relationships between temperature and acute toxicity endpoints (SigmaPlot 13.0, San Jose, CA, USA). To define the inherent effect of temperature on freshwater communities and populations, temperature-dependent SSDs (i.e., 15, 20, and 25 °C) were constructed for examining the effects of temperature on SSDs. To further quantitatively compare the differences among temperature-dependent SSDs for DO, the HC value and 95% CIs were computed for each SSD by Monte Carlo simulation, following the log-normal procedure available in SAS (SAS 9.4, Cary, NC, USA). To minimize the uncertainty caused by data quantity (n = 5), relative species sensitivities among temperatures for DO were compared on the basis of HC20 values. A linear regression function (y = a + b x) was also applied to fit these data (SigmaPlot 13.0, San Jose, CA, USA).
Geographic- and habitat-specific DO water quality criteria and standards
The south central region of the US is characterized by diverse watersheds, urbanization, population growth, and appreciable annual rainfall gradients. For example, annual rainfall in Texas spans over 114 cm per year from west to east, and contains three of the top ten largest and fastest growing metropolitan areas in the US (Dallas/Ft. Worth, Houston, San Antonio) and are thus potentially representative of other regions experiencing climate change and population growth. Subsequently, WQC for DO in the south central US, which corresponded to states in US EPA Region 6 (Arkansas, Louisiana, New Mexico, Oklahoma, Texas), were examined to determine whether habitat and geographic differences in implementation and assessment of WQC and WQS exist (Texas Commision on Environmental Quality 2010; Arkansas Pollution Control and Ecology Commission 2011; Louisiana Department of Environmental Quality 2012; New Mexico Environment Department 2000; Oklahoma Water Resources Board 2007).
Results
Freshwater invertebrate and fish thresholds to low DO
The majority of studies reporting standard lethality thresholds were conducted with invertebrates prior to the US EPA AWQC (pre-1986). Additionally, a large amount of low DO toxicity data published pre-1986 included chronic data for growth studies of multiple cold water (e.g., chinook salmon: Oncorhynchus kisutch) and warm water (e.g., largemouth bass: Micropterus salmoides) fish. Acute and chronic DO toxicity data for fishes and invertebrates published over the last five plus decades with over 70 different fish and invertebrate species are provided in supplementary information (Table S1). Prior to publication of the AWQC in 1986, no standard calculated fish toxicity values (e.g., LC50) were found (Table S1) because the majority of these historical studies reported the percent mortality at some DO treatment level(s). However, recent publications have derived DO LC50 values for a variety of fish species including rainbow trout (Oncorhynchus mykiss), suckers (Deltistes luxatus and Chasmistes brevirostris), common smelt (Retropinna retropinna), inanga (Galaxias maculatus), common bully (Gobiomorphus cotidianus), short-finned eel (Anguilla australis), shiner (Notropis topeka), and catfish (Rhamdia quelen) (Table S1). Acute and chronic invertebrate LC50 values published pre-1986 ranged from 0.03 (Jacob et al. 1984; Sprague et al., 1963) to 8.75 (Jacob et al. 1984) mg DO/L and 4.50 (Nebeker et al. 1992) to 5.00 (Nebeker et al. 1992) mg DO/L, respectively, while more recent (post-1986) acute and chronic invertebrate LC50 values ranged from 0.51 (Nebeker et al. 1992) to 1.95 (Nebeker et al. 1996) mg DO/L and 0.49 (Nebeker et al. 1992) to 2.00 (Nebeker et al. 1996) mg DO/L, respectively (Table S1). Only 8 and 13 acute DO toxicity values were published since 1986 for freshwater invertebrates and fish, respectively, and one chronic invertebrate DO toxicity value has been published since 1986. Such studies of invertebrate species largely focused on organisms from lentic and lotic habitats. Specifically, those species in both lotic and lentic habitats comprised ~58% of the available low DO toxicity data for invertebrates and represented the most robust invertebrate data set we examined. Similarly, fish DO toxicity data, which included mainly growth studies, were mainly comprised by species inhabiting cold waters (optimal temperature ~11 °C).
Aquatic hazard assessments
Nine invertebrate and five fish SSDs were generated using acute and chronic DO toxicity data. The dataset with the largest and smallest range of LC50 values were 8.72 and 1.44 mg DO/L (Table 1). Because pre-1986 data on acute DO toxicity only was identified for invertebrates, this dataset was compared to all available acute invertebrate and fish DO toxicity data published pre- and post-1986 and was significantly different from each other with the pre-1986 dataset more sensitive (Fig. 1a; ANCOVA, slope p > 0.217; y-int p < 0.001). These distributions differed at in the middle of the SSD and converged at the lower and upper end. Both datasets were dominated by invertebrates, with the latter dataset encompassing acute fish DO toxicity data and invertebrate data. This acute dataset including both invertebrate and fish DO toxicity data is comprised of 86.5 and 13.5% invertebrate and fish values, respectively. The datasets containing acute invertebrate DO toxicity data include some taxonomic diversity (Table 1) but those SSDs including all available invertebrate data were dominated by EPT taxa compared to non-EPT taxa (~3-fold difference in n and no. of species).
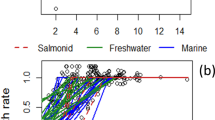
Aquatic hazard assessments of invertebrate and fish dissolved oxygen (DO) thresholds from both acute and chronic lethality (LC50) and chronic fish growth (EC) toxicity studies. a All acute (2–96 h) invertebrate toxicity data (LC50s) relative to those classified as published pre and post the U.S. Environmental Protection Agency (1986). b Acute lotic, lentic, and lotic and lentic invertebrate (LC50s). c Acute invertebrate data (LC50s) divided into the orders (taxa) Ephemeroptera, Plecoptera, and Trichoptera (EPT) or non-EPT taxa. d Acute and chronic invertebrate toxicity data (LC50s). e Chronic warm and cold water fish growth effect concentrations (EC50) relative to acute fish acute toxicity data (LC50). f Acute fish and invertebrate toxicity data (LC50) relative to acute invertebrate or acute fish toxicity data (LC50). Vertical lines (left to right) represent water quality criteria for DO subcategory high aquatic life use, commonly assigned to water bodies in Texas, where 24-h DO minimum are not to extend beyond 8 h (3 mg/L, dotted) and the 24-h mean minimum (5 mg/L, long dash) cannot be exceeded over 24 h (Texas Commision on Environmental Quality 2010)
When invertebrates were classified based on lentic or lotic habitats (Merritt and Cummins 1996), our results indicated all three SSDs were significantly different from each other (ANCOVA; lentic and lotic vs lentic p < 0.014; lotic vs lentic p < 0.008; both vs lotic, p < 0.001). Lentic and lotic datasets contained 71.4 and 92.6% EPT taxa. When we then classified acute invertebrate datasets by EPT and non-EPT taxa, our results indicated that the EPT and non-EPT taxa SSDs were also significantly different. Additionally, acute and chronic lethality (LC50) SSDs were significantly different (ANCOVA, p < 0.001), though the slopes were not (p > 0.762). As mentioned above, the pre-1986 dataset, which included only acute invertebrate DO toxicity data, was significantly different from both invertebrate and fish DO toxicity data; our results revealed acute invertebrate and acute fish SSDs were also significantly different (ANCOVA, p < 0.001). Acute fish DO toxicity data were dominated by warm water species with one acute cold water LC50 value identified for rainbow trout.
When HCs were computed from each dataset and HC80s compared based on the minimum data values in several SSDs, the HC80 ratios for acute DO toxicity involving invertebrate from pre-1986 were consistently greater than one, suggesting that invertebrate mortality thresholds were more sensitive than fish. Specifically, the acute invertebrate pre-1986 DO toxicity SSD was more sensitive than the SSD including the most recent acute invertebrate data and the predicted HC80 was 10.6% lower following the addition of newly published data because these toxicity values fell within the bottom half of the distribution (Fig. S1). This is reflected by the acute invertebrate to fish HC ratio of 2.3, again suggesting that invertebrates are twice as sensitive to decreases in DO as fish. Increasing the exposure duration to low DO increased sensitivity, which was reflected in the chronic to acute invertebrate lethality HC ratio of 1.9. Further, EPT taxa were ~2.5× more sensitive than non-EPT taxa. However, ratios comparing fish growth (EC10) to acute invertebrate (LC50) were consistently greater than one regardless of habitat (cold or warm water, lotic or lentic), suggesting fish growth responses are more sensitive to DO than invertebrate mortality.
Aquatic hazard assessments predicted 14, 23, 28, and 35% of acute low DO toxicity values pre-1986 exceed the existing US EPA AWQC at 8.0, 5.0, 4.0, and 3.0 mg DO/L. When this dataset included the newly published invertebrate and fish DO toxicity values, the results predicted 7, 15, 20, and 28% of species to be adversely affected at same DO concentrations, respectively. In comparison, including acute fish DO toxicity data with the acute invertebrate data decreased the percent exceeded by 4–5% and again indicated, based on the data available, that freshwater invertebrates are more susceptible to DO than fish. When the acute invertebrate datasets were then classified based on habitat types, those species inhabiting lotic environments were predicted to be more adversely affected at 8.0, 5.0, 4.0, and 3.0 mg DO/L (13, 24, 30, and 39%) than those species inhabiting lentic or both lentic and lotic habitats (1–29 to 13–30%, respectively; Fig. 1b; Table 1). Interestingly, a similar percentages of species affected were predicted for those invertebrates divided to EPT (17, 26, 31, and 38%) and non-EPT taxa (5, 10, 14, and 19%) at the same DO concentrations (Fig. 1c; Table 1).
Though there were only five chronic invertebrate DO toxicity values, encompassing 7–30-day exposure durations, 22, 33, 39, and 45% of species were predicted to be affected at 8.0, 5.0, 4.0, and 3.0 mg DO/L (Fig. 1d, Table 1). Compared to predicted acute DO thresholds for aquatic invertebrates, there was an increase of ~10% in the number of affected invertebrates when exposure duration was greater than 7 days. The percent of acute fish DO toxicity values exceeding the US EPA AWQC at 8.0, 5.0, 4.0, and 3.0 mg/L (~0, 2, 5, and 11%, respectively; Fig. 1e, Table 1) were low compared to invertebrates. Again, such predictions were based on post-1986 acute fish toxicity values (LC50s) and could not be compared to DO toxicity values available pre-1986 because standard toxicity values were not identified. Using the cold water fish growth EC10 values, results indicated 23, 48, 61, and 76% of fish to be adversely affected at 8.0, 5.0, 4.0, and 3.0 mg DO/L, respectively (Fig. 1e, Table 1). Similarly, 2, 48, 83, and 99% of fishes are predicted to be adversely affected at the same DO levels, using the warm water fish growth EC10 values, although only five values were available from four different species.
Temperature-dependent DO thresholds
Temperature is an important factor in chemical-induced toxicity, which typically increases with increasing temperature. To examine potential errors and variability associated with different experimental conditions, we systematically examined low DO toxicity data from individual studies at multiple temperatures. There were 3 of 9 invertebrate (LC50) and 1 of 2 fish (EC90, EC50 growth) cases following a positive linear relationship, indicating low DO toxicity increased with elevating temperatures (Fig. S2A and S3). Three acute temperature-dependent SSDs (15, 20, 25 °C) were also constructed using five similarly available low DO toxicity values for species within the order Ephemeroptera (Fig. S2B). When temperature-dependent HC80 values were considered, an insignificant yet positive relationship between temperature and HC80 values was observed, which suggests low DO toxicity may be expected to increase with increasing surface water temperatures (Fig. S2C).
Geographic- and habitat-specific DO water quality criteria and standards
States in the south central US were found to have diverging surface water quality assessment approaches that varied by habitat, time of the year, and aquatic life use designations relative to AWQC (Table 2). In the state of Texas, for example, historical high and limited aquatic life use WQC consisted of a 24-h mean and absolute minimum DO WQC at 5 and 3 mg DO/L, respectively, for reservoir systems, and were dependent on the type of waterbody (stream, tidally influenced river; Texas Commission on Environmental Quality 2003). These WQS were revised in 2010 to implement DO concentrations based on aquatic life use (Texas Commision on Environmental Quality 2010). To compare different implementation practices within the south central US, we found the number of different WQC and standards per state ranged between 1 and 41, not including site-specific criteria within states of Arkansas, Louisiana, New Mexico, Oklahoma, and Texas. Subsequently, we compared the number of early life stage (ELS) and other life stage (OLS) WQC or WQS recommended for cold and warm water fish species within this region and found the number of states with these distinct criteria ranged from 0 to 4 and 0 to 36, respectively (Table 3). All six states have derived DO criteria for warm water species but only three of the six states (Arkansas, New Mexico, Oklahoma) appear to have derived criteria for cold water fishes due to inherent habitat differences. Arkansas has the most distinctly different DO WQC (41) accounting for ELS and OLS, while Louisiana has apparently derived the fewest criterion values (Table 3).
Discussion
Because the occurrence of worldwide hypoxia may increase in both freshwater (Watson et al. 2016) and marine environments (Brewer and Peltzer 2009; Pörtner 2010), the present study examined variability in acute and chronic DO thresholds among freshwater species. We found experimental designs and study protocols (e.g., experimental exposure temperatures, species life stages) for low DO toxicity studies to vary considerably among fish and invertebrates. For a number of studies, potential confounding factors could not be resolved in large part due to data paucity and experimental conditions. For example, Elshout et al. (2013) previously identified juvenile fish to have higher DO tolerances compared to adults based on LOEC values yet we could not identify adequate data to further explore such relationships in the present study. In addition, we were unable to conclude whether the DO toxicity data that formed the basis for derivation of the 1986 AWQC (U.S. Environmental Protection Agency 1986) were confounded by differences among wild and hatchery fish, seasonal conditions, acclimation to laboratory conditions, or by an exceptionally wide range of experimental conditions including varied feeding regimes and age of organisms. For example, time of the year is known to play an important role controlling food consumption and growth rate of both chinook and coho salmon were higher in June than July (and October for coho salmon) at temperatures near 18 °C (Warren et al. 1973).
As noted above, when additional acute toxicity data for invertebrates were incorporated in our analyses, the likelihood of encountering species threshold to DO above the recommended US EPA AWQC increased. Such observations are likely explained by the selection of species and experimental endpoints studied since 1986. As indicated by our DO sensitivity metrics (i.e., SSDs, HC ratios), invertebrates, particularly EPT and lotic taxa compared to non-EPT and lentic species, were more sensitive to acute DO than fish. EPT taxa are commonly used as sensitive bioindicators of environmental quality in aquatic ecosystems (Cairns and Pratt 1993) because these organisms are quite sensitive to reduced DO and other types of pollution, which tends to be correlated with specific habitat types (Jacobsen et al. 2003). Elevated DO sensitivity of invertebrates was also observed in a study of marine benthic organisms, especially crustaceans and mollusks (Vaquer-Sunyer and Duarte 2008). Based on the data availability examined in the present study, we observed lotic taxa to be more sensitive than lentic species, largely because EPT taxa primarily contributed to lotic distributions. Robust DO thresholds were not available for numerous benthic invertebrates and fish species, including threatened and endangered organisms. Thus, whether existing DO WQC and WQS are protective of most of these imperiled species has not been examined (Woods et al. 2010). Additional high-quality low DO toxicity data is needed for freshwater fish and invertebrates from lotic and lentic habitats to more effectively understand differences in DO sensitivity among freshwater organisms and support more sustainable environmental quality assessment and management.
When chronic data is not available for contaminants, acute-to-chronic (ACR) ratios have been used to predict sublethal responses from acute toxicity data. For hypoxia in freshwater systems, the DO concentration where ≥50% growth impairments occur has historically been reported to accompany the onset of fish mortality (Doudoroff and Shumway 1970; U.S. Environmental Protection Agency 1986). In the present study, we derived a novel ACR of 2.63 from the 80th percentile of warm water fish LC50 and warm water fish growth EC10 distributions (Fig. S4). This ratio supports the hypothesis that the sensitivity of fish growth and potentially other chronic responses occur at DO concentrations almost three times the LC50 value (Vaquer-Sunyer and Duarte 2008). An SSD-derived ACR from cold water fish or freshwater invertebrates could not be calculated due to a lack of available data. However, we derived an ACR of 3.8 for rainbow trout using a geometric LC50 and available EC10 data for growth. Prior to 1986, DO chronic toxicity data were mostly available for fish, especially those in the family Salmonidae based on economical and sociological reasons (U.S. Environmental Protection Agency 1986). Most of the studies used to derive the AWQC predominantly investigated growth along with some studies of embryonic development and swimming behavior. Most chronic DO toxicity studies prior to 1986 failed to include a full life cycle, examine both embryo and larval stages, or encompass an adequate period of post-larval feeding and growth (U.S. Environmental Protection Agency 1986). Further, studies of the effects of DO to cold water fish reproduction, fecundity, or fertility, which are important endpoints relevant to ecological risk assessment and management (Ankley et al. 2010; U.S. Environmental Protection Agency 1986), were also lacking. Prior to 1986, two studies were conducted with warm water fish that investigated the effects of DO on reproduction with fathead minnows and black crappie, but the quality of a life cycle experiment with fathead minnows is uncertain due to 50% mean larval survival in some experimental controls (U.S. Environmental Protection Agency 1986; Brungs 1971). Clearly, future studies are necessary to understand reproductive thresholds of DO to freshwater fish, amphibians, and invertebrates.
To assess the likelihood of acute DO hazards to freshwater communities, we performed aquatic hazard assessments, which indicated 7, 15, 20, and 28% of invertebrates, and fish are expected to be adversely affected at 8.0, 5.0, 4.0, and 3.0 mg DO/L, respectively (Fig. 1f). A similar assessment was conducted by Vaquer-Sunyer and Duarte (2008) with marine benthic organisms in which cumulative distributions were created utilizing median lethal concentrations, sublethal thresholds, and median lethal times that were classified by organism types ranging from echinoderms to fish. Vaquer-Sunyer and Duarte (2008) identified that the most sensitive groups of organisms exhibiting the highest LC50 and lowest LT50 90th percentiles were the crustaceans and molluscs, respectively. In the present study, including the acute warm and cold water fish LC50 values with the acute invertebrate LC50 values decreased the predicted affects by 4–5% and illustrated the invertebrate community was more sensitive to DO than fish. Conversely, fish exhibited the highest sublethal response to DO of the compiled marine benthic organisms, which included endpoints such as avoidance of hypoxic waters, behavior, and increased ventilation, which differs from the type of fish chronic toxicity data used in the current study. However, in both assessments, fish chronic responses were the most sensitive to DO. In the present study, the 50th and 90th percentile warm and cold water fish LC50 values were 1.26 and 4.01 mg DO/L, respectively, which are similar concentrations from two different water types. Freshwater to saltwater and vice versa toxicity extrapolations were previously investigated by Wheeler et al. (2002) who indicated differences in toxicity sensitivity depending on the chemical (e.g., ammonia, metals, pesticides, narcotics) that could be accounted for with an appropriate adjustment factor. Regardless, DO sensitivities across saltwater and freshwater organisms require further research to develop a comparative understanding of DO thresholds among fish and invertebrates, and support ecosystem protection goals related to biodiversity.
It appears possible that DO effects to marine and freshwater organisms have been underestimated. For example, Vaquer-Sunyer and Duarte (2008) illustrated the effects of DO to marine benthic organisms were above the conventional 2 mg DO/L definition of hypoxia; such predictions for marine invertebrates are consistent with fish and invertebrate SSDs and corresponding 80th percentile values in the present study (Table 1). Further, Vaquer-Sunyer and Duarte (2008) predicted a 90th percentile median LC50 value for marine organisms of 4.59 mg DO/L, which is similar to the 80th percentile concentration of 4.64 mg DO/L predicted to adversely affect 20% of freshwater species from a community SSD for acute DO toxicity data to invertebrates and fish (Fig. 1). Therefore, DO concentrations approximately twice the 2 mg DO/L hypoxia threshold are expected to cause significant mortality in marine and freshwater organisms. These DO thresholds are in direct contrast to the 2.3 mg DO/L ASWQC-derived limit to avoid juvenile and adult mortality. Such a difference may be due to the small range of DO LC50 values available (~1.29) for both juvenile and adults used in the EPA saltwater criteria recommendations, while the LC50 range in our present study was 8.72. The conventional 2 mg DO/L threshold is commonly used to indicate the potential risk to fisheries, but again, to conserve diversity and avoid mortality events, higher DO levels are predicted necessary to maintain most aquatic life populations (Vaquer-Sunyer and Duarte 2008). In fact, the ASWQC recommends a general 4.8 mg DO/L level to prevent no more than a 25% chronic growth reduction in species (U.S. Environmental Protection Agency 2000). In comparison, this value is twice as high as our predicted 75th percentile values of 2.41 and 3.21 mg DO/L from warm and cold water fish growth (25% growth reduction) EC50 SSDs, respectively. Given the global decline of species, particularly invertebrates, during the Anthropocene (Dirzo et al. 2014), future studies are necessary to experimentally examine such predictions of DO thresholds for inland waters.
We further examined temperature influences on DO thresholds to freshwater organisms. Oxygen plays a critical role influencing acute temperature limits of organisms and relationships among temperature limits of physiological and biochemical pathways associated with the oxygen supply cascade. For example, the oxygen-limited thermal tolerance (OLTT) model describes physiological activities of ectotherms when exposed to various temperatures (Frederich and Pörtner 2000; Pörtner 2001). This model suggests that aquatic ectotherms, like fishes, generally live within a confined range of temperatures where they function aerobically without displaying any sign of stress (e.g., behavioral disorders). Beyond the optimum temperature range, however, ectotherms encounter a mismatch of energy demand and supply and eventually shift to anaerobic respiration at extreme high or low temperatures to increase energy supply for sustaining essential cellular and physiological functions (Pörtner 2010). When such changes in temperature and oxygen concentration are introduced, total metabolism, basal metabolism, and scope of activity of aquatic organisms decreases, while the frequency of locomotory acts and mechanical power decline (Svetlichny et al. 2000). Therefore, oxygen deficiency (e.g., hypoxia) within body tissues results in changes in growth, survival, reproduction and even population distribution and abundance under thermal stress (Perry et al. 2005; Pörtner 2010).
Previous studies have compared temperature-dependent chemical toxicity alone (Zhou et al. 2014) and between geological regions (e.g., temperate, tropical) to the same chemicals (Wang et al. 2014), but fewer studies have compared stressor-dependent toxicity to other abiotic factors such as pH (Wang et al. 2016). For the majority of ectotherms, their physiological performances (e.g., metabolism, appetite, behavior) follow a thermal curve and experience increased mortality when temperature deviates from optimum (Bao et al. 2008; Pörtner 2002; Schulte et al. 2011). While the derived US EPA AWQC were designed to be protective of high seasonal surface water temperatures, most organisms used to derive the criteria were studied at optimal thermal conditions during acute and chronic exposures. To examine temperature-dependent DO thresholds of freshwater organisms, a total of nine invertebrate and two fish species were found to be studied across at least three different temperatures within the same study (Fig. S2A and S3). For invertebrates, only five species emphemeropterans were studied across the same three temperatures (15, 20, and 25 °C). HC80 values for this temperature-dependent SSD were 3.94, 6.36, and 12.5 mg DO/L at 15, 20, and 25 °C, respectively. Clearly, the HC80 values increased with increasing temperature but, again, are only representative of five species within the order. Coho and chinook salmon were two additional species studied across more than three different temperatures (Fig. S3). A temperature-dependent DO toxicity relationship with growth was clearly observed in chinook salmon while the relationship was less clear for cohos. However, these studies were conducted with juveniles at different developmental stages, weights, diets, and months of the year that could confound the results. In fact, as mentioned above, time of year was observed to play an important role in controlling the food consumption and growth rate of both chinook and coho salmon (Warren et al. 1973). Additional research is clearly needed to understand the influences of temperature on DO thresholds of freshwater organisms, particularly when considering predictions of climate change.
Divergent implementation practice efforts in surface water quality assessment and management of DO was observed in a region characterized by diverse watersheds, experiencing population growth, and susceptible to climate change. We specifically observed surface water quality practices to differ across habitats, seasons, and aquatic life relative to the US EPA AWQC in the south central US (Table 2). For example, New Mexico and Oklahoma have derived criteria based on cold and warm water aquatic life/communities and both states even derive specific aquatic life/community values for cool water organisms (New Mexico Environment Department 2000; Oklahoma Water Resources Board 2007). Similarly, Arkansas derives DO WQS based on the presence of trout and by habitat categories specific to different stream watershed sizes throughout the state, and has specific DO standards derived for lakes and reservoirs (Arkansas Pollution Control and Ecology Commission 2011). Some states (Texas, Oklahoma, Arkansas) have specific seasonal DO criteria for at least the spring season (March to June depending on the state), yet routine monitoring for surface water quality parameters, including DO, largely occurs in summer months. Further, Louisiana does not have specific DO criteria for habitats or seasons (Louisiana Department of Environmental Quality 2012). Though the US EPA AWQC recommended instantaneous minimum DO concentrations to be achieved at all times (U.S. Environmental Protection Agency 1986), our analysis reveals some DO criteria within the south central US appear inadequate to prevent species from adverse mortality (Table 3). These observations are salient given challenges to develop, implement and enforce criteria and standards elsewhere in developed and developing countries. For example, as of 2014, 27 states within the US did not have numeric criteria for total nitrogen or phosphorus (Manuel 2014), despite influences of nutrient enrichment on surface water quality and the development of harmful algal blooms (Watson et al. 2015; Brooks et al. 2016). Given such nutrient enrichment, population growth, rising surface water temperatures, and potential climate-induced sensitivity of organisms (Heathwaite 2010; Hooper et al. 2013; Solomon et al. 2007), additional studies examining whether current DO criteria and standards are adequate to protect freshwater organisms across seasons, habitats, and life history stages are warranted.
References
Ankley G, Bennett RS, Erickson RJ, Hoff DJ, Hornung MW, Johnson RD, Mount DR, Nichols JW, Russom CL, Schmieder PK, Serrrano JA, Tietge JE, Villeneuve DL (2010) Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environ Toxicol Chem 29:730–741
Arkansas Pollution Control and Ecology Commission (2011) Regulation establishing water quality standards for surface waters of the state of Arkansas. 014.00–002 regulation no. 2. Arkansas Pollution Control and Ecology Commission, Little Rock, p 124
Bao VWW, Koutsaftis A, Leung KMY (2008) Temperature-dependent toxicities of chlorothalonil and copper pyrithione to the marine copepod Tigriopus japonicus and dinoflagellate Pyrocystis lunula. Australas J Ecotoxicol 14:45–54
Brett J, Blackburn J (1981) Oxygen requirements for growth of your coho salmon (Orconhynchus kisutch) and sockey (O. nerka) salmon at 15 degrees Celcius. Can J Fish Aquat Sci 38:399–404
Brewer PG, Peltzer ET (2009) Limits to marine life. Science 324:347–348
Brooks BW, Riley TM, Taylor RD (2006) Water quality of effluent-dominated ecosystems: ecotoxicological, hydrological, and management considerations. Hydrobiologia 556:365–379
Brooks BW, Scott JT, Forbes MG, Valenti TW, Stanley JK, Doyle RD, Dean KE, Patek J, Palachek RM, Taylor RD, Koenig L (2008) Reservoir zonation and water quality: science, management and regulations. LakeLine 28:39–43
Brooks BW, Valenti TW, Cook-Lindsay BA, Forbes MG, Scott JT, Stanley JK, Doyle RD (2011) Influence of climate change on reservoir water quality assessment and management: effects of reduced inflows on Diel pH and site-specific contaminant hazards. In: Linkov I, Bridges TS (eds) Climate: global change and local adaptation. NATO Science for Peace and Security Series C: Environmental Security, Springer, New York, pp 491–522
Brooks BW, Lazorchak JM, Howard MDA, Johnson MV, Morton SL, Perkins DAK, Reavie ED, Scott GI, Smith SA, Steevens JA (2016) Are harmful algal blooms becoming the greatest inland water quality threat to public health and aquatic ecosystems?. Environ Toxicol Chem 35:6–13
Brungs WA (1971) Chronic effects of low dissolved oxygen concentrations on the fathead minnow (Pimephales promelas). J Fish Res Board Can 28:1119–1123
Cairns J Jr, Pratt JR (1993) A history of biological monitoring using benthic macroinvertebrates. In: Rosenberg DM, Resh VH (eds) Freshwater biomonitoring and benthic macroinvertebrates. Chapman and Hall, New York, pp 10–27
Canadian Council of Ministers of the Environment (2001) Introduction. Updated. In: Canadian environmental quality guidelines, 1999. Canadian Council of Ministers of the Environment, Winnipeg, p 4
Chambers PA, Scrimgeour GJ, Pietroniro A (1997) Winter oxygen conditions in ice-covered rivers: the impact of pulp mill and municipal effluents. Can J Fish Aquat Sci 54:2796–2806
Committee on Environment and Natural Resources (2003) An assessment of coastal hypoxia and eutophication in U.S. waters. National Science Technology Council Committee on Environment and Natural Resources, Washington, DC, p 74
Cooper SR, Brush GS (1991) Long-term history of Chesapeake Bay anoxia. Science 254:992–996
Delorme L (1982) Lake Erie oxygen; the prehistoric record. Can J Fish Aquat Sci 39:1021–1029
Diaz RJ (2001) Overview of hypoxia around the world. J Environ Qual 30:275–281
Diaz RJ, Breitburg DL (2009) The hypoxic environment. Fish Physiol 27:1–23
Dirzo R, Young HS, Galetti M, Ceballos G, Isaac NJB, Collen B (2014) Defaunation in the Anthropocene. Science 345:401–406
Doudoroff O, Shumway DL (1970) Dissolved oxygen requirements of freshwater fishes vol FAO technical paper no. 86. Food Agriculture Organization, United Nations, Rome
Elshout PMF, Dionisio Pires LM, Leuven RSEW, Wendelaar Bonga SE, Hendriks AJ (2013) Low oxygen tolerance of different life stages of temperate freshwater fish species. J Fish Biol 83:190–206
Frederich M, Pörtner HO (2000) Oxygen limitation of thermal tolerance defined by cardiac and ventilatory performance in spider crab, Maja squinado. Am J Phys 279:R1531–R1538
Grist EP, Leung KM, Wheeler JR, Crane M (2002) Better bootstrap estimation of hazardous concentration thresholds for aquatic assemblages. Environ Toxicol Chem 21:1515–1524
Heathwaite AL (2010) Multiple stressors on water availability at global to catchment scales: understanding human impact on nutrient cycles to protect water quality and water availability in the long term. Fresh Biol 55:241–257
Hooper MJ, Ankley GT, Cristol DA, Maryoung LA, Noyes PD, Pinkerton KE (2013) Interactions between chemical and climate stressors: a role for mechanistic toxicology in assessing climate change risks. Environ Toxicol Chem 32:32–48
Jacob U, Hendrik W, Reinhard K (1984) Aquatic insect larvae as indicators of limiting minimal contents of dissolved oxygen‐part II. Aquat Insect 6:185–190
Jacobsen D, Rostgaard S, Vásconez JJ (2003) Are macroinvertebrates in high altitude streams affected by oxygen deficiency? Freshw Biol 48:2025–2032
Lind OT, Terrell TT, Kimmel BL (1993) Problems in reservoir trophic-state classification and implications for reservoir management. Comparative Reservoir Limnology and Water Quality Management. Springer, New York, pp 57–67
Louisiana Department of Environmental Quality (2012) Water pollution control. Title 33 Environmental quality park IX water quality subpart 1. Louisiana Department of Environmental Quality, Baton Rouge, p 398
Manuel J (2014) Nutrient pollution: a persistent threat to waterways. Environ Health Perspect 122:A304
Merritt RW, Cummins KW (1996) An introduction to the aquatic insects of North America. Kendall Hunt
Nebeker AV, Dominguez SE, Chapman GA, Onjukka ST, Stevens DG (1992) Effects of low dissolved oxygen on survival, growth and reproduction of Daphnia, Hyalella and Gammarus. Environ Toxicol Chem 11:373–379
Nebeker AV, Onjukka ST, Stevens DG, Chapman GA (1996) Effect of low dissolved oxygen on aquatic life stages of the caddisfly Clistoronia magnifica (Limnephilidae). Arch Environ Con Tox 31:453–458
New Mexico Environment Department (2000) Water quality part: standards for interstate and intrastate surface waters. Title 20 Environment protection. New Mexico Environment Department, Santa Fe, p 48
Oklahoma Water Resources Board (2007) Oklahoma’s water quality standards. OAC Title 785 chapter 45. Oklahoma Water Resources Board, Oklahoma City, p 114
Perry AL, Low PJ, Ellis JR, Reynolds JD (2005) Climate change and distribution shifts in marine fishes. Science 308:1912–1915
Pollock MS, Clarke LMJ, Dube MG (2007) The effects of hypoxia on fishes: from ecological relevance to physiological effects. Environ Rev 15:1–14
Pörtner HO (2001) Climate change and temperature-dependent biogeography:oxygen limitation of thermal tolerance in animals. Naturwissenschaften 88:137–146
Pörtner HO (2002) Climate variations and the physiological basis of temperature dependent biogeography: systemic to molecular hierarchy of thermal tolerance in animals. Comp Biochem Physiol A Mol Integr Physiol 132:739–761
Pörtner H-O (2010) Oxygen-and capacity-limitation of thermal tolerance: a matrix for integrating climate-related stressor effects in marine ecosystems. J Exp Biol 213:881–893
Posthuma L, Traas TP, Sutter II GW (eds) (2002) Species sensitivity distributions in ecotoxicology. Lewis, Boca Raton
Schulte PM, Healy TM, Fangue NA (2011) Thermal performance curves, phenotypic plasticity, and the time scales of temperature exposure. Integr Comp Biol 51:691–702
Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL (2007) Contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge, p 996
Sprague JB (1963) Resistance of four freshwater crustaceans to lethal high temperature and low oxygen. J Fish Res Board Can 20:387–415
Sprague JB (1973) The ABC’s of pollutant bioassay using fish. ASTM International 528:6–30
Stiff MJ, Cartwright NG, Crane RI (1992) Environmental quality standards for dissolved oxygen. National Rivers Authority, Almondsbury Bristol, p 58
Svetlichny LS, Hubareva ES, Erkan F, Gucu AC (2000) Physiological and behavioral aspects of Calanus euxinus females (Copepoda: Calanoida) during vertical migration across temperature and oxygen gradients. Mar Biol 137:963–971
Texas Commission on Environmental Quality (2003) Guidance for assessing Texas surface and finished drinking water quality data, 2004. Surface Water Quality Monitoring Program, Texas Commission on Environmental Quality, Austin, TX
Texas Commision on Environmental Quality (2010) Chapter 307—Texas surface water quality standards. Texas Commision on Environmental Quality 307:1–307.10
Thornton KW, Kimmel BL, Payne FE (1990) Reservoir limnology: ecological perspectives. John Wiley & Sons, Inc., New York, p 256
U.S. Environmental Protection Agency (1986) Ambient water quality criteria for dissolved oxygen. EPA-440/5–86-003. Environmental Protection Agency, Washington, DC, p 46
U.S. Environmental Protection Agency (2000) Ambient aquatic life water quality criteria for dissolved oxygen (saltwater): Cape Cod to Cape Hatteras. EPA-822-R-00-012. Environmental Protection Agency, Washington, DC p 49
U.S. Environmental Protection Agency (2012) National Coastal Condition Report IV. EPA-842-R-10-003. Environmental Protection Agency, Washington, DC, p 368
Valenti TW, Taylor JM, Back JA, King RS, Brooks BW (2011) Influence of drought and total phosphorus on diel pH in wadeable streams: implications for ecological risk assessment of ionizable contaminants. Integr Enviro Assess Manage 7:636–647
Vaquer-Sunyer R, Duarte CM (2008) Thresholds of hypoxia for marine biodiversity. Proc Natl Acad Sci U S A 105:15452–15457
Wang Z, Kwok KW, Lui GC, Zhou G-J, Lee J-S, Lam MH, Leung KM (2014) The difference between temperate and tropical saltwater species’ acute sensitivity to chemicals is relatively small. Chemosphere 105:31–43
Wang Z, Meador JP, Leung KM (2016) Metal toxicity to freshwater organisms as a function of pH: a meta-analysis. Chemosphere 144:1544–1552
Warren C, Doudoroff P, Shumway D (1973) Development of dissolved oxygen criteria for freshwater fish. EPA-R3–73-019. Ecological Research Series Report, Washington, DC, USA, p 121
Watson SB, Whitton BA, Higgins SN, Paerl HW, Brooks BW, Wehr J (2015) Harmful Algal Blooms. In: J Wehr, RG Sheath, JP Kociolek (Eds). Freshwater Algae of North America: Ecology and Classification, 2nd Edition. Academic Press, Amsterdam. pp. 871-918.Wheeler JR, Grist EPM, Leung KMY, Morritt D, Crane M (2002) Species sensitivity distributions: data and model choice. Marine Poll Bull 45:192–202
Watson SB, Miller C, Arhonditsis G, Boyer GL, Carmichael W, Charlton MN, Confesor R, Depew DC, Höök TO, Ludsin SA, Matisoff G (2016) The re-eutrophication of Lake Erie: harmful algal blooms and hypoxia. Harmful Algae 56:44–66
Woods HA, Poteet MF, Hitchings PD, Brain RA, Brooks BW (2010) Conservation physiology of the Plethodontid salamanders Eurycea nana and E. sosorum: response to declining dissolved oxygen. Copeia 2010(4):540–553
Zhou Y, Obenour DR, Scavia D, Johengen TH, Michalak AM (2013) Spatial and temporal trends in Lake Erie hypoxia, 1987-2007. Environ Sci Technol 47:899–905
Zhou G-J, Wang Z, Lau ETC, Xu X-R, Leung KMY (2014) Can we predict temperature-dependent chemical toxicity to marine organisms and set appropriate water quality guidelines for protecting marine ecosystems under different thermal scenarios? Mar Pollut Bull 87:11–21
Acknowledgements
This research was supported by a U.S. Environmental Protection Agency STAR Fellowship (FP-91767201-0) and the C. Gus Glasscock Jr. Endowed Fund for Excellence in Environmental Sciences to GNS.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Saari, G.N., Wang, Z. & Brooks, B.W. Revisiting inland hypoxia: diverse exceedances of dissolved oxygen thresholds for freshwater aquatic life. Environ Sci Pollut Res 25, 3139–3150 (2018). https://doi.org/10.1007/s11356-017-8908-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-8908-6