Abstract
The aim of this study was to investigate the effects of agricultural land use and periods of hydrological variability on the environmental variables, as well as macrophyte and macroinvertebrate assemblages in lowland riverine wetlands. As a case study, we compared two periurban wetlands with intensive agricultural land use against two others with extensive livestock, considered references for the region during a normal and a dry flow period. Nutrient concentrations were significantly higher in agricultural riverine wetlands (total phosphorus and total nitrogen 30% higher). These wetlands exhibited higher relative coverage of floating anchored macrophytes and the absence of submerged vegetation. They showed significantly lower taxonomic richness and 40% lower density of macroinvertebrates, and a higher relative abundance of scrapers and predators. Wetlands of both land uses had a lower total density of macroinvertebrates and a higher abundance of tolerant desiccation taxa in the dry period. Particular differences between land uses, such as lower dissolved oxygen concentrations and lower macroinvertebrate diversity in agricultural wetlands, were found during the dry period. These findings indicate that the differences between land uses seemed to increase during the aforementioned period. Further studies in riverine wetlands of both land uses must be carried on to generalize the results found. Despite this limitation, this study provides evidence of the effects of the surrounding landscape and hydrologic periods in the environmental characteristics, as well as the macrophyte and macroinvertebrate assemblages of the riverine wetlands studied.
Resumen
El objetivo del estudio fue investigar los efectos del uso del suelo agrícola y de diferentes periodos hidrológicos en las variables ambientales, así como en el ensamble de macrófitas y macroinvertebrados en bañados de desborde fluvial. Como caso de estudio, comparamos dos humedales periurbanos con uso del suelo agrícola intensivo con otros dos con ganadería extensiva, considerados de referencia para la región, durante un periodo de caudal normal y otro de sequía. Las concentraciones de nutrientes resultaron significativamente mayores en los bañados de desborde fluvial agrícolas (fósforo y nitrógeno total 30% mayores). Estos humedales exhibieron una mayor cobertura relativa de macrófitas flotantes arraigadas y ausencia de vegetación sumergida. Además mostraron una riqueza taxonómica de macroinvertebrados significativamente menor y 40% menor densidad; por otro lado, presentaron una mayor abundancia relativa de raspadores y predadores. Durante el periodo de sequía, los humedales de ambos usos del suelo, mostraron una menor densidad de macroinvertebrados y una mayor abundancia de taxa tolerantes a la desecación. Se encontraron diferencias particulares entre usos del suelo como concentraciones menores de oxígeno disuelto y menor diversidad de macroinvertebrados en bañados agrícolas durante el período de sequía. Estos resultados indican que las diferencias entre usos del suelo parecieron aumentar durante el periodo mencionado. Para la generalización de los resultados presentados son necesarios estudios adicionales en bañados de desborde fluvial. A pesar de esta limitación, este estudio proporciona evidencia de los efectos del paisaje circundante y de los períodos hidrológicos en las características ambientales y en los ensambles de macrófitas y macroinvertebrados de los bañados de desborde fluvial estudiados.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Wetlands are globally recognized as essential due to the many ecological functions and services that they provide to human society (Dixon and Wood 2003). They provide water quality protection (Verhoeven et al. 2006; Daneshvar et al. 2017), climate change mitigation, flood control, and drought prevention (Mitsch and Gosselink 2000; Zedler and Kercher 2005). They are also an important reservoir of flora and fauna (Sileshi et al. 2020; Gupta et al. 2020). Around the world, wetlands are subjected to several types of threats, both anthropogenic and natural. Agriculture is currently considered the main driver of wetland loss or degradation globally (Davidson 2014; Everard and Wood 2017).
The replacement of natural ecosystems by agricultural land use produces a homogenization that threatens biodiversity (Allan 2004; Dornelas et al. 2009). The fertilizers applied indiscriminately in intensive agriculture activities are one of the most important sources of contamination by nutrient loading in freshwater ecosystems (Grashof-Bokdam and van Langevelde 2005; Strokal et al. 2016). The excess of these nutrients, such as phosphorus and nitrogen, transported to watercourses reduce water quality due to the increased growth of undesirable algae and low oxygen concentrations (Blann et al. 2009; Woodward et al. 2012). This has several negative effects on freshwater ecosystem biodiversity, like replacement and loss of species in their assemblages (Gustafson and Wang 2002; Craft et al. 2007; Chalar et al. 2013; Lange et al. 2014; Laterra et al. 2018). Furthermore, drainage and water management for agriculture has significantly altered the hydrology of wetlands affecting also their biodiversity (Lacoul and Freedman 2006; Blann et al. 2009; Green et al. 2017). Water withdrawals for agriculture have been found to markedly reduce streamflow, causing a loss of connection between the surface and the groundwater systems (Postel 2000) and resulting in a loss of habitat for aquatic biota (McKay and King 2006).
Riverine wetlands (RWs) are recognized as a major component of biodiversity in fluvial ecosystems (Amoros et al. 2000; Tockner et al. 2002). They are areas where streams overflow and connect with the surrounding terrestrial environment (Ringuelet 1962; Brinson and Malvárez 2002) and where macrophyte assemblages are highly developed (Amoros et al. 2000). As it is known, macrophytes are key components of wetlands ecosystems (Rejmánková 2011). They remove a variety of pollutants from the water (Bonanno and Vymazal 2017), and increase the habitat complexity and heterogeneity of these ecosystems (Thomaz and Cunha 2010; Poi et al. 2021). Also, they provide several resources for other organisms, like microhabitats (Dudley 1988; Warfe et al. 2008), shelter (Heck and Crowder 1991; Thomaz and Cunha 2010) and food (Dvořák 1996; Díaz-Valenzuela et al. 2016).
Within the wetland biota, macroinvertebrates play an important role in the overall functioning of these ecosystems as they occupy a central position in the food web and in organic matter cycling and energy flow (Batzer et al. 1999). The reduction in water quality caused by agricultural land use also leads to decreases in macroinvertebrate richness and density, as well as the decrease in the abundance of sensitive groups (Genito et al. 2002; Lange et al. 2014; Malacarne et al. 2016). In these senses, the macroinvertebrate assemblage is widely used as indicator of in-stream biotic integrity of the land use (Bunn et al. 2010), and the comprehensive analysis of their composition can provide an overview of wetland environmental status (Balcombe et al. 2005; Mereta et al. 2013).
The knowledge of biodiversity of RWs and the effects of land use and hydrological periods is necessary for ecosystem management and conservation plans. Our goal was to investigate the effects of intensive agricultural land use on the environmental variables and the macrophyte and macroinvertebrate assemblages of Pampean lowland RWs of Argentina, in time periods characterized by different hydrological conditions. We hypothesized that (i) intensive agricultural land use induces changes in physical and chemical variables and in the structural and functional responses of macrophyte and macroinvertebrate assemblages; (ii) an extraordinary dry period leads to variations in the assemblages and increases differences between land uses.
Materials and Methods
Study Area
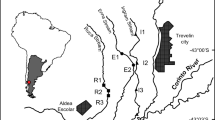
The study was conducted in the Pampean ecoregion of Buenos Aires, Argentina (Fig. 1). This region is a vast grassy plain that covers central Argentina. It has a humid and temperate climate, mean annual precipitation between 1000 and 1200 mm, and a mean annual temperature of 16 °C (Hurtado et al. 2006). Riverine wetlands of this region are threatened by agriculture that affects the water quality and alters the natural habitat (Gómez et al. 2016). In particular, RWs located in the Pampean plain of Argentina represent the low depressions of Pampean stream basins, characterized by abundant and diverse aquatic vegetation. The mentioned streams are characterized by an absence of riparian forest, low current velocities, and high nutrient levels (Rodrigues Capítulo et al. 2001; Feijoó and Lombardo 2007).
Map of the study area and the sampling sites located in Buenos Aires province, Argentina. Land use areas modified from Solis et al. (2019)
We selected two periurban RWs with intensive agricultural land use and two others with extensive livestock located on tributary streams of the freshwater section of the Río de la Plata estuary (Fig. 1, Online Resource: Table S1). The selection of these RWs was based on geohydrological and land use analysis (Rodrigues Capítulo et al. 2020; Gómez et al. 2022). The four of them are located in characteristic Pampean streams and despite their land use, these wetlands have been preserved. Del Gato and the Carnaval RWs are located in the surrounding agricultural area of La Plata city, the capital of the Province of Buenos Aires. This area is one of the main horticultural regions in the country (Baldini et al. 2019) with a high productivity based on intensive use of fertilizers and pesticides along with greenhouse technology (Arias et al. 2020). Downstream, the Carnaval and Del Gato streams run through densely populated areas and their channels have been heavily impacted by anthropic action. Urban areas are increasing their coverage in the region and also threaten the integrity of the water ecosystems (López and Rotger 2020; Paredes del Puerto et al. 2022). The over-extraction for crop irrigation and drinking water supply of the city of La Plata and its surroundings has caused the local deepening of the water tables, contributing to the loss of groundwater supply to the base flow of the streams where these RWs occur (Gómez et al. 2022). The other RWs, Cajaravillas and Chubichaminí, are located in Magdalena, a rural area with extensive livestock grazing on natural grassland (0.7 cows per ha., Gómez et al. 2022). Freshwater systems surrounded by these grasslands are considered the reference for the region (Solis et al. 2018; Arias et al. 2020; Paracampo et al. 2020). These RWs receive groundwater and surface water, with a greater volume being supplied by the former. Both RWs flow downstream through rural areas that do not hinder their natural drainage to the Río de la Plata estuary.
To know the effects of hydrological variation, four sampling campaigns were performed at the RWs during contrasting hydrological scenarios: two samplings were carried out during a period of normal flow, which we call “normal period” (September and October, mean monthly cumulative precipitation of 80 mm and 61 mm respectively), and two samplings in a dry period (February and early March, monthly cumulative precipitation of 2 and 92 mm respectively). Despite the cumulative precipitation of 92 mm recorded in March, only 10 mm were measured before the sampling campaign. The average precipitation recorded in the dry period was lower than the historical mean monthly accumulated precipitation for the last ten years (National Meteorological Service: February 177 mm and March 123 mm). This intense drought coincides with the “La Niña” phase of the ENSO phenomenon (Gómez et al. 2022).
In each RW we selected a section of 50 m in the center of the channel where the physicochemical and biological samplings were carried out. In that section, in each sampling occasion, physical and chemical variables and macrophyte assemblage descriptors were measured and macroinvertebrates were sampled (see below). During the dry period, the RWs studied were characterized by lower width, lower water velocity, and lower flow in the four wetlands studied (Online Resource: Table S1). The Carnaval, one of the agricultural RWs, was completely dry during the February campaign.
Site Characterization
The pH, temperature, dissolved oxygen concentration (DO), conductivity, and total dissolved solids (TDS) were measured in situ in triplicate with a HORIBA Multiparameter U-10. Besides, on each sampling occasion, a water sample was collected for the analysis in the laboratory of nutrient concentrations (Total Nitrogen, Nitrates [N-NO3], Nitrites [N-NO2], Ammonia [N-NH4], Total Phosphorus and Phosphate [P-PO4]) and oxygen demands (biochemical and chemical, BOD and COD, respectively) under standardized protocols (APHA 1998).
Macrophytes
In order to study the total macrophyte coverage, the species composition and their life forms, we chose five evenly-spaced transects perpendicular to the 50 m section studied (wet width length) at each sampling site and sampling campaign. On each transect, the macrophytes species, along with their coverage and life forms, were recorded. For each variable, a single value was calculated from the average of the five transects. The total macrophyte coverage was calculated as the percentage of transect length covered by plants. The relative coverage of each species, as well as each life form, was estimated by measuring the length of the transect covered by each species after Feijoó and Menéndez (2009). For this study we classified the species as emergent, submerged, floating-leaved anchored, and free-floating (Cabrera and Fabris 1948; Lahitte et al. 2004).
Macroinvertebrates
Macroinvertebrate samples were randomly taken among the vegetation stands at each RW (along the 50 m section) in triplicate at every sampling opportunity, giving an amount of three samples for each site at each sampling date. Samples consisted of all the material collected from the inside of a Plexiglas square (surface of 25 cm x 25 cm) with a sieve of 500-um mesh (Cortelezzi et al. 2013). Each replicate was fixed in situ with 5% (v/v) aqueous formaldehyde. In the laboratory, the invertebrates were separated under a stereomicroscope, counted, and identified to the lowest possible taxonomic level through standard keys (Lopretto and Tell 1995; Merritt et al. 2008; Domínguez and Fernández 2009).
Data Analysis and Statistical Approach
The RWs along the physical and chemical variables were characterized by principal-component analysis (PCA). Before the analysis, the variables were standardized and the average for each sampling date was used for those that were taken in triplicate. The variables that presented little contribution, those that showed low correlation with the first and the second component, were removed to simplify analysis (Kassambara 2017). Differences in physical and chemical variables between land uses and hydrological periods and their interaction were assessed by two-way ANOVA with ‘RW’ nested within ‘land use’. The variables that did not fit with a normal distribution (temperature, conductivity, DO, Total Nitrogen and Total Phosphorus) were log-transformed. Pairwise comparisons on the main fixed factors were performed using Tukey’s post hoc tests. Model residuals were tested for normality using a Shapiro–Wilk test.
The total coverage of macrophytes and the relative coverage of each life form expressed as percentages were calculated to characterize the macrophyte assemblage. Also, the richness was estimated as the number of taxa present, and diversity was estimated by the Shannon–Wiener Index (Shannon and Weaver 1949). In addition, to characterize the macroinvertebrate assemblage, density was expressed as the average number of individuals per square meter, and richness and diversity were estimated. We assigned each taxon to a functional feeding group (FFG) using available references (Cummins et al. 2005; Allan and Castillo 2007; Merritt et al. 2008). The relative abundance values of each FFG were calculated for each sample using macroinvertebrate densities (ind. m-2).
Macrophyte coverage, diversity of both assemblages, as well as density and FFG of macroinvertebrates, were compared between land uses and hydrological periods by two-way ANOVA with ‘RW’ nested within ‘land use’. The interaction between ‘land use’ and ‘hydrological period’ was also assessed. Density of macroinvertebrates data were log-transformed, whereas coverage of macrophytes and FFGs of macroinvertebrates were arcsine transformed to fit with the normal distribution. Pairwise comparisons on the main fixed factors were performed using Tukey’s post hoc tests. Model residuals were tested for normality using a Shapiro–Wilk test. For count data (richness), we used generalized linear models (GLMs) with Poisson error distribution (link: log) for the same factors.
A Permutational Multivariate Analysis of Variance (PERMANOVA) was used to test differences in macroinvertebrate taxonomic composition between ‘land use’ (agriculture and livestock) and ‘hydrological period’ (normal and dry), with ‘RWs’ nested within ‘land use’. The PERMANOVA was applied on a Bray–Curtis dissimilarity matrix calculated from the abundance data of macroinvertebrates. We also used a similarity percentage analysis (SIMPER) to identify taxa separating different land use categories and hydrological periods and to quantify the contribution of individual taxa to each category.
All statistical analysis were performed using the language environment R version 3.6.3 (R Development Core Team 2020) and the RStudio Team (2015). The packages used for the analysis were FactoMineR (Lê et al. 2008), vegan (Oksanen et al. 2019), biodiversityR (Kindt and Coe 2005), and stats (R Development Core Team 2020).
Results
Sites Characterization
The first two PCA axes explained 63.8% of the overall variance (Fig. 2). The first axis (43.2%) illustrated the environmental differences between land uses. Agricultural RWs were characterized by higher nutrient concentration (total phosphorus, total nitrogen, soluble reactive phosphorus, and to a lesser extent, ammonium). By contrast, livestock RWs were characterized by higher values of pH, conductivity, and TDS and lower values of nutrient concentrations (Online Resource: Table S2, correlations between axis and variables). The second axis (20.6% of the total variance) was positively correlated with temperature, nitrate, and nitrite whereas it was negatively correlated with flow and dissolved oxygen. This axis showed the difference between hydrological periods in livestock RWs, where the dry period was characterized by higher temperature, lower flow and DO in comparison with the normal period.
Principal Component Analysis of the analyzed physico-chemical variables in the four riverine wetlands studied in the sampled period. Circles: agricultural land use; squares: livestock land use. Closed symbols: normal period; open symbols: dry period. See Table 1 for abbreviations
Significant differences were found in water physical and chemical characteristics between land uses (Table 1). Agricultural RWs exhibited higher values of total phosphorus (F1,6 = 34.50, p = 0.001), total nitrogen (F1,6 = 116.94, p < 0.001), and P-PO4 (F1,6 = 44.84, p < 0.001). By contrast, livestock RWs had higher values of conductivity (F1,34 = 250.33, p < 0.001), TDS (F1,34 = 147.72, p < 0.001) and pH (F1,34 = 169.51, p < 0.001) in both hydrological periods. Dissolved oxygen concentration was lower in the dry period in comparison with the normal period in RWs of both land uses and also, was lower in agricultural RWs than in livestock RWs in the dry period (F1,34 = 4.480, p = 0.042). In contrast, temperature was higher in the dry period in both land uses (F1,34 = 51.67, p < 0.001). Biochemical oxygen demand showed differences between hydrological periods, with higher values in the dry period, only in agricultural RWs (F1,6 = 16.98, p = 0.006).
Macrophytes
We recorded 15 species during the study, and the total coverage of macrophytes was always greater than 60% in the RWs studied (Table 2). The order Poales was the best represented in both land uses. Typhaceae was the family with higher relative coverage in agricultural RWs and Cyperaceae in livestock ones. No significant differences were found in richness (estimate = 0.18, zvalue = 0.43, p = 0.670; estimate = 0.29, zvalue = 0.65, p = 0.51), diversity (F1,7 = 0.10, p = 0.763; F1,7 = 1.72, p = 0.231) or total coverage (F1,7 = 0.00, p = 0.956; F1,7 = 0.94, p = 0.346) between land uses and hydrological periods, respectively. Coverage of floating-anchored species was higher in agricultural RWs than in livestock RWs (F1,7 = 637.14, p < 0.001), where coverage of emergent macrophytes was higher (F1,7 = 82.41, p < 0.001). Submerged macrophytes were not recorded in agricultural RWs. The emergent macrophyte Typha latifolia, and the floating anchored macrophytes Hydrocotyle ranunculoides, Alternanthera philoxeroides, and Ludwigia peploides were the dominant species in the agricultural RWs, whereas the emergent macrophytes Iris pseudacorus and Schoenoplectus californicus were the dominant in livestock RWs. Unlike the normal period characterized by a higher coverage of emergent macrophytes (F1,7 = 19.70, p = 0.003), the dry period was characterized by a higher coverage of a free-floating species (Lemna gibba, F1,7 = 10.73, p = 0.014, Online Resource: Table S3).
Macroinvertebrates
A total of 63 taxa of macroinvertebrates belonging to 24 orders were collected in the RWs studied (Online Resource: Table S4). During all the study 41 taxa and 20 orders were found in agricultural RWs whereas livestock RWs presented 58 taxa and 23 orders. Taxa richness differed significantly between land uses (Fig. 3, estimate = 0.39, zvalue = 2.68, p = 0.007). Differences in the number of insect families were also found, with a total of 18 families in agricultural RWs and a higher number of 23 families in livestock RWs (Online Resource: Table S4). Mean density also differed between land uses (F1,37 = 31.26, p < 0.001), with agricultural RWs showing half the density of livestock RWs (Fig. 3). In addition, the density found during the dry period for the two land uses was half that recorded for the normal period (F1,37=34.40, p < 0.001, Fig. 3). Diversity only showed significant differences between land uses in the dry period (F1,37 = 5.48, p = 0.025), with higher values in livestock RWs than in agricultural RW (Online Resource: Table S5).
Regarding FFGs, 26 taxa were identified as predators, 23 as collector-gatherers, 7 as scrapers, 5 as collector-filterers, and 2 as shredders (Online Resource: Table S4). Free-living aquatic nematodes were not included in the FFG analysis due to the controversies in the FFG classification (Moens et al. 2006; López van Oosterom et al. 2013). In the comparison between land uses, agricultural RWs showed higher relative abundance of predators (F1, 37 = 5.87, p = 0.020) and scrapers (F1, 37 = 6.15, p = 0.012, Fig. 4). By contrast, livestock RWs exhibited a higher relative abundance of collector-gatherers in both periods (F1, 37 = 6.50, p = 0.015) and a higher relative abundance of collector-filterers in the dry period, coinciding with a reduction of this FFG in the agricultural RWs (significant interaction, F1, 37 = 10.567, p = 0.002; Fig. 4). Differences between hydrological periods were also found: the dry period exhibited higher relative abundance of predators (F1, 37 = 28.63, p < 0.001) than the normal period, which had a significantly higher relative abundance of shredders (F1, 37 = 26.39, p < 0.001, Fig. 4).
The assemblage compositions differed significantly between land uses (PERMANOVA: pseudo-F1,37 = 16.46, P = 0.001) and hydrological periods (PERMANOVA: pseudo-F1,37 = 12.39, P = 0.001). Based on SIMPER analysis results, the dissimilarity between land uses in the normal period was 48%, whereas the dissimilarity increased to 67% in the dry period. The taxa that contributed the most to differences between agriculture and livestock were the amphipod, Hyalella curvispina (Shoemaker, 1942) (5.80%), the aquatic worms, Oligochaeta (5.33%), the limpet, Uncancylus concentricus (dOrbigny, 1835) (5.29%), the copepods, Cyclopoida (5.18%), the water fleas, Cladocera (4.97%), the non-biting midges, Chironomidae (4.79%), and the mayfly, Caenis sp. (4.64%) (with higher abundance in livestock than agricultural RWs) and the planarians, Dugesiidae (4.68%) and the springtails, Entomobryidae (3.47%) with higher abundance in agricultural RWs. The differences between hydrological periods were attributed to the decreased abundance of H. curvispina (7.92% in livestock, 7.48% in agriculture), Dugesiidae (5.29% in livestock, 10.42% in agriculture), and Caenis sp. (4.88% in livestock) in dry periods and the increased abundance of particular taxa such as the roundworms, Nematoda (4.00% in livestock, 4.09% in agriculture), Cyclopoida (3.79% in livestock, 5.73% in agriculture), and the biting midge, Ceratopogonidae (4.43% in livestock).
Discussion
Our results suggest that agricultural land use had a strong influence on the physical and chemical parameters, macrophyte structure, and macroinvertebrate metrics of the Pampean lowland RWs studied. The physical and chemical characteristics found for agricultural RWs denote water quality degradation of these wetlands in comparison with livestock RWs according to the first studies of the region (Tarda et al. 2019; Cochero et al. 2020; Gómez et al. 2022).
The high concentration of nutrients in RWs with agriculture in their catchment is typical of freshwater systems in agricultural areas because the high amount of fertilizer used in crops increases the concentration of nutrients in the surrounding freshwater systems (Carpenter and Bennett 2011; Egler et al. 2012; Mugni et al. 2013; Ruiz-Picos et al. 2016). Phosphorus concentration found in livestock RWs was near the base values for basins of the region (Feijoó and Lombardo 2007) and agriculture values were similar to those found on lowland streams with the same land use (Solis et al. 2016, 2018). However, for both land uses the concentration exceeded the local guide values for biota protection (PT < 0.01 mg/l, ACUMAR 2019) being classified as highly eutrophic waters (PT > 0.02 mg/l) by international criteria CCME (2004). In contrast to the agricultural RWs, the higher conductivity, TDS, and pH found in livestock RWs agreed with the characterization performed by Molina et al. (2017) for aquatic environments associated with pastures, herbaceous, and shrub vegetation. In particular, in pasture catchments like those studied, cattle have free access to wetlands for watering, which can increase the total dissolved solids and conductivity due to the constant disturbance and resuspension of the substrate (Gary et al. 1983; Roche et al. 2013). Also, the higher conductivity of the livestock RWs could be produced by the groundwater contribution (Caruso 2002).
In accordance with our results, changes in macrophytes species resulting from agricultural land use were also recorded for USA wetlands (Gustafson and Wang 2002). Submerged macrophytes were absent and floating macrophyte coverage increased in agricultural lands, as documented in the context of increased nutrient load (Rasmussen and Anderson 2005). Besides, other authors addressed a lower coverage of emergent macrophytes in wetlands (Lougheed et al. 2001) and lowland streams (Zgola 2014) rich in nutrients. Species composition could change in relation to agricultural land use as well. In our work, we found T. latifolia and H. ranunculoides as the dominant species in agricultural sites. Typha spp. are considered to increase their dominance in nutrient-enriched wetlands (Cooper et al. 2006; Craft et al. 2007; Rejmánková 2011). These species can benefit from agricultural nutrient pulses by taking up nutrients rapidly and funneling them into growing tissues (Zedler and Kercher 2004). Similarly, H. ranunculoides is known to increase its growth and biomass with increasing nutrient availability in laboratory conditions (Hussner and Lösch 2007). But, not only the surrounding land use can determine the macrophyte composition, also the physical attributes and the hydrologic disturbance affects the relative abundance of macrophyte species (Lacoul and Freedman 2006). The higher concentration of TDS and the shallow and wide characteristic of the livestock RWs allowed the development of a higher coverage of emergent macrophytes in agreement with previous studies (Feijoó et al. 1999; Egertson et al. 2004). Despite the land use, macrophyte coverage was high in the four RWs studied, giving these wetlands a high habitat complexity (Thomaz and Cunha 2010). On the other hand, as it was found in other wetlands (Cooper et al. 2006; Craft et al. 2007; Moges et al. 2017) the order Poales was the best represented. Regarding hydrological conditions, the high coverage of L. gibba found in the dry period correlates with the increase in the coverage of floating macrophytes documented at low flows by other authors (Cao et al. 2018; Fraaije et al. 2018). Lower flow velocities lead to a predominance of more typically lentic plant communities, such as free-floating species (Fraaije et al. 2018).
Although the use of macroinvertebrates as indicators of agricultural wetland land use is still discussed (Batzer 2013; Gleason and Rooney 2017), we found that this assemblage responded to the effect of agriculture on the RWs studied, and can be considered good indicators of land use in this wetlands. In agreement with the results found in other types of wetlands around the world, lower richness and density were observed in wetlands with intensive agricultural land use. That is the case for example for some Mediterranean littoral wetlands (Ortega et al. 2004) and ponds (Della Bella and Mancini 2009), North American floodplain wetlands (Chipps et al. 2006) and Chinese wetlands (Wu et al. 2019) impacted by intensive agricultural activities. Similar values of taxonomic richness were found between our research and those previously mentioned, showing mean values near to 25 in reference wetlands and near to 13 in agricultural ones. Besides, Epele and Miserendino (2015), found a mean taxonomic richness of 20 in Patagonian wetlands with extensive livestock and a decreased number of insect families with the increased concentration of total phosphorus and nitrogen. In line with our results for RWs, Arias et al. (2020) and Solis et al. (2017) also found that mean density was twice as high between streams of the Pampean region with extensive livestock and intensive agriculture in their surroundings. Interestingly, in comparison with Pampean streams (Ocon and Rodrigues Capítulo 2012; Solis et al. 2017, 2018; Arias et al. 2020) the RWs studied, regardless of the land use, presented a higher number of macroinvertebrate orders, highlighting the importance of its presence in the streams.
The reduction in taxonomic richness is usually related to the loss of sensitive taxa in agricultural land use (Gerth et al. 2017). High percentages of agricultural land cover reduced the number of sensitive macroinvertebrate taxa and produced a macroinvertebrate community composition that reflected an altered habitat (Genito et al. 2002). In coincidence with Mugni et al. (2013), Solis et al. (2017, 2018) and Marrochi et al. (2021), who studied streams in the Pampean region, our results in RWs suggest that the deterioration in water quality generated by intensive agriculture could cause a decrease on the abundance of sensitive macroinvertebrate taxa such as H. curvispina and Caenis sp. Cooper et al. (2006) also found less abundance of a Hyallela species (Hyalella azteca) in wetlands adjacent to agricultural land. In addition, we found that tolerant taxa such as Dugesiidae and Entomobryidae characterized agricultural RWs, in coincidence with the results of Solis et al. (2018). We also recorded the presence of some sensitive invertebrates according to Rodrigues Capítulo et al. (2001) that were found only in livestock RWs such as Lestes, Rhionaeschna, and individuals of the Simuliidae family.
Feeding strategies of macroinvertebrates could also reflect the adaptation of species to stressors and form part of a unified measure across communities differing in taxonomic composition (Tomanova et al. 2006). The effect of agricultural land use could be reflected in a greater relative abundance of scrapers and predators in RWs. The increase in the frequency of scraper feeding habits is expected to occur in nutrient-enriched environments (Dolédec et al. 2006; Statzner and Bêche 2010). On the other hand, predator abundance was associated with the higher density of Dugesiidae and Helobdella sp., considered tolerant taxa by other authors (Rodrigues Capítulo et al. 2001; Solis et al. 2019). The lowest proportion of collector-filterers in agricultural RWs in the dry period was in accordance with Gebrehiwot et al. (2017), who found a decrease in this FFG at organic polluted sites of Ethiopian wetlands in a dry period. Also, the relative abundance of this FFG is considered to be a useful metric for characterizing the ecological condition of river-associated wetlands in Eastern Africa (Mereta et al. 2013).
The presence of different life forms of macrophytes can also drive the composition of invertebrate FFG by determining the availability and type of food resources (Cremona et al. 2008; de Souza Rezende et al. 2019). In this sense, submerged macrophytes are usually related to a greater abundance of collectors-gatherers (Cremona et al. 2008; Peiró et al. 2015; de Souza Rezende et al. 2019), because its architecture allow the retention of more detritus (Sand-Jensen 1998). In our study, this life form was absent in agricultural RWs in concordance with the lower proportion of this FFG. On the other hand, the highest proportion of predators in agricultural RWs and in the dry period could be related to the higher coverage of floating-leaved macrophytes. This life form provides little habitat in the vertical dimension that promotes a higher exposure to visual predators compared to the other macrophyte life forms (Gosselain et al. 2005). In this sense, the land use and hydrological period effects observed in the macrophyte assemblages could also contribute to the differences in the proportion of FFGs.
Much of the current knowledge about the ecological response of macroinvertebrates to droughts is related to the effects of largely predictable seasonal droughts rather than supra-seasonal events (Lake 2003). Species inhabiting temporal ecosystems that suffer predictable droughts have different resistance mechanisms, including tolerance to the deterioration of water quality conditions and the presence of desiccation-resistant life history stages (Bogan et al. 2017). Also, resilience mechanisms such as dispersal to rewetted habitats from refugia are common among taxa adapted to temporary-wetland habitats (Boulton and Lake 2008). On the other hand, faunal recovery from supra-seasonal droughts varies from one case to another (Lake 2003). Generally, species are vulnerable to these events as they are not adapted and cannot escape the disturbance events in time (Boulton 2003). The unusual absence of precipitation, the high evapotranspiration in summer, and the loss of connection with the groundwater in agricultural areas (Rodrigues Capítulo et al. 2020) led the Carnaval RW to be dry in the February campaign. The lower oxygen concentration in RWs surrounded by agricultural land indicated a higher effect of the dry period on the water characteristics of these RWs. According to Robinson et al. (2004), reduced flow commonly leads to decreases in dissolved-oxygen content, which is critical to the survival of many aquatic species and can also affect their distribution and abundance. This condition was associated with the lower macroinvertebrate diversity, the wide difference in taxa richness, and the higher dissimilarity in macroinvertebrate composition found in agricultural RWs in comparison with livestock RWs during the dry period.
In line with our findings on the RWs studied, it is known that natural low flows cause decreases in invertebrate densities (Wood and Armitage 2004). Some authors suggested that this decrease occurs in response to changes in competition and predation because habitat area decreases and food quality and quantity are altered by flow reduction (Cowx et al. 1984; Wood et al. 2000). Furthermore, invertebrate community composition often changes in response to low or reduced flow in streams (Gore et al. 2001; Suren et al. 2003) and wetlands (Sim et al. 2013). The increase in the density of particular taxa associated with the dry period in macroinvertebrate assemblages was similar to that reported by other authors. Larned et al. (2007) also found Nematoda and Copepoda to be resistant to desiccation. Desiccation-resistant stages are well-known in copepods (Dahms 1995), and aquatic nematodes are believed to survive extended dry periods in a state of anhydrobiosis (Drummond et al. 2015). Moreover, Ceratopogonidae larvae appear almost immediately when surface flows resume, suggesting that they use the hyporheic zone as a refuge from surface drying (Stanley et al. 1994; Stubbington 2012). Conversely, H. curvispina and Caenis sp. decrease their abundance in the dry period. There are no previous reports on changes in the abundances of these taxa under drought conditions. However, other related species have been studied. Ladle and Bass (1981) and Wood and Armitage (2004) recorded similar responses of the amphipod Gammarus pulex (L.) to dry conditions. Regarding Caenis sp., the reduced flow and decreased water velocity were documented to be detrimental to other Ephemeroptera taxa (Calapez et al. 2017). With regards to FFGs, in agreement with our results, the effects of a flow reduction in streams and wetlands were associated with an increase in the prevalence of predators and a decrease in shredders (Bêche et al. 2006; Statzner and Bêche 2010; Gebrehiwot et al. 2017). Reduced velocities could favor invertebrate predators by removing velocity-mediated predation refugia (Dewson et al. 2007) and affect shredder abundance by modifying the quality of resources (Statzner and Bêche 2010).
The results showed the combined effects of nutrient loading and hydrological disturbances in the RWs studied. Similar results were mentioned by Green et al. (2017) for the Doñana wetlands in Spain, a more complex system of wetlands than the studied and recently for headwater streams of the region on fish assemblages (Paredes del Puerto et al. 2022). As Green et al. 2017) mentioned, decisions from informed policy makers can promote ecosystem resilience to global threats through local measures. The correct management of agricultural activities in the land surrounding a river, as part of integrated watershed management, is of paramount importance to the conservation of associated wetland water quality (Wang 2001). Therefore, unsustainable agricultural practices could be replaced with environmentally-friendly, ecological agriculture to preserve wetland ecosystems (Zou et al. 2018).
Conclusions
The findings provide evidence of the importance that the surrounding landscape has in the environmental characteristics and macrophyte and macroinvertebrate assemblages of the lowland RWs studied. The differences between land uses were greater during a drought period, which could indicate a higher sensitivity to different hydrological conditions in those wetlands surrounded by intensive agricultural systems in comparison with those surrounded by extensive livestock considered as reference for the region.
Despite the land use, the great macrophyte coverage and the high number of macroinvertebrate orders in the RWs studied indicates the importance of these systems in the basin and the value of their conservation. The differences found in macroinvertebrate FFGs could also be influenced by the composition of macrophyte assemblage, indicating the role and importance of aquatic plants in lowland RWs. In this sense, the combined use of macrophytes and macroinvertebrate assemblages were a powerful tool for describing and assessing the riverine wetlands studied. The metrics evaluated were useful for studying the land use of the catchment and the hydrological conditions.
Our study was constrained by the low number of RW still preserve in the region and thus low replication of riverine wetlands at each land use. However, further studies in the region and in the study sites will allow for more robust and generalized assessments of patterns we observed in this study. Despite this observation, considering the limited knowledge about this type of wetlands, we provide valuable information for future projects of conservation and management of these environments.
Data Availability
We affirm that the data supports the results. The datasets used and analyzed during the current study are available in the supplementary material and from the corresponding author on reasonable request.
Code Availability
Not applicable.
References
ACUMAR (Autoridad de Cuenca Matanza Riachuelo) (2019) Resolución 283/2019. https://www.argentina.gob.ar/normativa/nacional/resoluci%C3%B3n-283-2019-334281/texto. Accessed 14 Dec2021
Allan JD (2004) Landscapes and riverscapes: the influence of land use on stream ecosystems. Annu Rev Ecol Evol Syst 35:257–284. https://doi.org/10.1146/annurev.ecolsys.35.120202.110122
Allan JD, Castillo MM (2007) Stream ecology: structure and function of running waters, 2nd edn. Springer Science & Business Media, Dordrecht, The Netherlands
Amoros C, Bornette G, Henry CP (2000) A vegetation-based method for ecological diagnosis of riverine wetlands. Environ Manag 25:211–227. https://doi.org/10.1007/s002679910017
APHA (American Public Health Association) (1998) Standard Methods for the Examination of Water and Waste Water, 20th edn. APHA, Washington
Arias M, Scalise A, Solis M, Paracampo A, Indaco M, Fanelli S, Mugni H, Bonetto C (2020) Horticulture affects macroinvertebrate assemblages in adjacent streams (Buenos Aires, Argentina). Knowl Manag Aquat Ecosyst 421:5. https://doi.org/10.1051/kmae/2019048
Balcombe CK, Anderson JT, Fortney RH, Kordek WS (2005) Aquatic macroinvertebrate assemblages in mitigated and natural wetlands. Hydrobiologia 541:175–188. https://doi.org/10.1007/s10750-004-5706-1
Baldini C, Marasas ME, Drozd AA (2019) Entre la expansión urbana y la producción de alimentos. Revista de la Facultad de Agronomía; Universidad Nacional de La Plata 118:031–031. https://doi.org/10.24215/16699513e031
Batzer DP, Rader RB, Wissinger SA (1999) Invertebrates in freshwater wetlands of North America: ecology and management. Wiley, New York
Batzer DP (2013) The seemingly intractable ecological responses of invertebrates in North American Wetlands: A review. Wetlands 33:1–15. https://doi.org/10.1007/s13157-012-0360-2
Bêche LA, Mcelravy EP, Resh VH (2006) Long-term seasonal variation in the biological traits of benthic‐macroinvertebrates in two Mediterranean‐climate streams in California, USA. Freshw Biol 51:56–75. https://doi.org/10.1111/j.1365-2427.2005.01473.x
Blann KL, Anderson JL, Sands GR, James LA, Vondracek B (2009) Effects of agricultural drainage on aquatic ecosystems: A Review. Crit Rev Environ Sci Technol 39:909–1001. https://doi.org/10.1080/10643380801977966
Bogan MT, Hwan JL, Cervantes-Yoshida K, Ponce J, Carlson SM (2017) Aquatic invertebrate communities exhibit both resistance and resilience to seasonal drying in an intermittent coastal stream. Hydrobiologia 799:123–133. https://doi.org/10.1007/s10750-017-3205-4
Bonanno G, Vymazal J (2017) Compartmentalization of potentially hazardous elements in macrophytes: insights into capacity and efficiency of accumulation. J Geochem Explor 181:22–30. https://doi.org/10.1016/j.gexplo.2017.06.018
Boulton AJ (2003) Parallels and contrasts in the effects of drought on stream macroinvertebrate assemblages. Freshw Biol 48:1173–1185. https://doi.org/10.1046/j.1365-2427.2003.01084.x
Boulton AJ, Lake PS (2008) Effects of drought on stream insects and its ecological consequences. In: Lancaster J, Briers RA (eds) Aquatic Insects: Challenges to Populations. CAB International, Wallingford, UK, pp 81–102
Brinson MM, Malvárez AI (2002) Temperate freshwater wetlands: Types, status, and threats. Environ Conserv 29:115–133. https://doi.org/10.1017/S0376892902000085
Bunn SE, Abal EG, Smith MJ, Choy SC, Fellows CS, Harch BD, Kennard MJ, Sheldon F (2010) Integration of science and monitoring of river ecosystem health to guide investments in catchment protection and rehabilitation. Freshw Biol 55:223–240. https://doi.org/10.1111/j.1365-2427.2009.02375.x
Cabrera AI, Fabris H (1948) Plantas acuáticas de la Provincia de Buenos Aires. Publicación técnica Dirección Agropecuaria, La Plata, Argentina
Calapez AR, Branco P, Santos JM, Ferreira T, Hein T, Brito AG, Feio MJ (2017) Macroinvertebrate short-term responses to flow variation and oxygen depletion: a mesocosm approach. Sci Total Environ 599:1202–1212. https://doi.org/10.1016/j.scitotenv.2017.05.056
Cao HX, Fourounjian P, Wang W (2018) The importance and potential of duckweeds as a model and crop plant for biomass-based applications and beyond. In: Hussain C (ed) Handbook of environmental materials management. Springer, Cham, pp 1–16. https://doi.org/10.1007/978-3-319-58538-3_67-1
Carpenter SR, Bennett EM (2011) Reconsideration of the planetary boundary for phosphorus. Environ Res Lett 6:14009
Caruso BS (2002) Temporal and spatial patterns of extreme low flows and effects on stream ecosystems in Otago, New Zealand. J Hydrol 257:115–133. https://doi.org/10.1016/S0022-1694(01)00546-7
CCME (Canadian Council of Ministers of the Environment) (2004) Canadian water quality guidelines for the protection of aquatic life: Phosphorus: Canadian Guidance Framework for the Management of Freshwater Systems. Canadian environmental quality guidelines. Canadian Council of Ministers of the Environment, Winnipeg
Chalar G, Delbene L, González-Bergonzoni I, Arocena R (2013) Fish assemblage changes along a trophic gradient induced by agricultural activities (Santa Lucía, Uruguay). Ecol Ind 24:582–588. https://doi.org/10.1016/j.ecolinD2012.08.010
Chipps SR, Hubbard DE, Werlin KB, Haugerud NJ, Powell KA, Thompson J, Johnson T (2006) Association between wetland disturbance and biological attributes in floodplain wetlands. Wetlands 26:497–508. https://doi.org/10.1672/0277-5212(2006)26[497:ABWDAB]2.0.CO;2
Cochero J, Di Giorgi H, Donadelli J, Suárez J, Simonetti R, Finkler NR, Cunha DGF (2020) El rol de los bañados de desborde fluvial en la retención de nutrientes y su actividad metabólica. Biologia Acuatica 35:013–013. https://doi.org/10.24215/16684869e013
Cooper MJ, Uzarski DG, Burton TM, Rediske RR (2006) Macroinvertebrate community composition relative to chemical/physical variables, land use and cover, and vegetation types within a Lake Michigan drowned river mouth wetland. Aquatic Ecosystem Health and Management 9:463–479. https://doi.org/10.1080/14634980600892655
Cortelezzi A, Sierra MV, Gómez N, Marinelli C, Rodrigues Capítulo AR (2013) Macrophytes, epipelic biofilm, and invertebrates as biotic indicators of physical habitat degradation of lowland streams (Argentina). Environ Monit Assess 185:5801–5815. https://doi.org/10.1007/s10661-012-2985-2
Cowx IG, Young WO, Hellawell JM (1984) The influence of drought on the fish and invertebrate populations of an upland stream in Wales. Freshw Biol 14:165–177. https://doi.org/10.1111/j.1365-2427.1984.tb00030.x
Craft C, Krull K, Graham S (2007) Ecological indicator of nutrient enrichment, freshwater wetlands, Midwestern United States (US). Ecol Ind 7:733–750. https://doi.org/10.1016/j.ecolinD2006.08.004
Cremona F, Planas D, Lucotte M (2008) Biomass and composition of macroinvertebrate communities associated with different types of macrophyte architectures and habitats in a large fluvial lake. Fundam Appl Limnol 171:119–130
Cummins KW, Merritt RW, Andrade PC (2005) The use of invertebrate functional groups to characterize ecosystem attributes in selected streams and rivers in south Brazil. Studies on Neotropical Fauna and Environment 40:69–89. https://doi.org/10.1080/01650520400025720
Dahms HU (1995) Dormancy in the Copepoda – an overview. Hydrobiologia 306:199–211. https://doi.org/10.1007/BF00017691
Daneshvar F, Nejadhashemi AP, Adhikari U et al (2017) Evaluating the significance of wetland restoration scenarios on phosphorus removal. J Environ Manage 192:184–196. https://doi.org/10.1016/j.jenvman.2017.01.059
Davidson NC (2014) How much wetland has the world lost? Long-term and recent trends in global wetland area. Mar Freshw Res 65:934–941. https://doi.org/10.1071/MF14173
de Souza Rezende R, Monção FS, Gonçalves Junior JF, dos Santos AM (2019) Macroinvertebrate associated with macrophyte beds in a Cerrado stream. Limnetica 38:639–652. https://doi.org/10.23818/limn.38.37
Della Bella V, Mancini L (2009) Freshwater diatom and macroinvertebrate diversity of coastal permanent ponds along a gradient of human impact in a Mediterranean eco-region. In: Oertli B, Céréghino R, Biggs J, Declerck S, Hull A, Miracle MR (eds) Pond Conservation in Europe. Developments in Hydrobiology. Springer, Dordrecht, pp 181–197. https://doi.org/10.1007/978-90-481-9088-1_16
Dewson ZS, James ABW, Death RG (2007) A review of the consequences of decreased flow for instream habitat and macroinvertebrates. J N Am Benthol Soc 26:401–415. https://doi.org/10.1899/06-110.1
Díaz-Valenzuela J, Barva-Alvarez R, Merlo-Galiazzi A, Zambrano L (2016) Macrophytes and metaphyton as habitats for insects in temporary and permanent tropical aquatic ecosystems. Neotropical Biodiversity 2:171–180. https://doi.org/10.1080/23766808.2016.1248709
Dixon AB, Wood AP (2003) Wetland cultivation and hydrological management in eastern Africa: Matching community and hydrological needs through sustainable wetland use. Nat Res Forum 27:117–129. https://doi.org/10.1111/1477-8947.00047
Dolédec S, Phillips N, Scarsbrook M, Riley RH, Townsend CR (2006) Comparison of structural and functional approaches to determining landuse effects on grassland stream invertebrate communities. J N Am Benthol Soc 25:44–60. https://doi.org/10.1899/0887-3593%282006%2925%5B44%3ACOSAFA%5D2.0.CO%3B2
Domínguez E, Fernández HR (2009) Macroinvertebrados bentónicos sudamericanos: sistemática y biología Fundación Miguel Lillo. Tucumán, Argentina
Dornelas M, Moonen AC, Magurran AE, Bàrberi P (2009) Species abundance distributions reveal environmental heterogeneity in modified landscapes. J Appl Ecol 46:666–672. https://doi.org/10.1111/j.1365-2664.2009.01640.x
Drummond LR, Mcintosh AR, Larned ST (2015) Invertebrate community dynamics and insect emergence in response to pool drying in a temporary river. Freshw Biol 60:1596–1612. https://doi.org/10.1111/fwB12591
Dudley TL (1988) The roles of plant complexity and epiphyton in colonization of macrophytes by stream insects. Verh Int Ver Theor Angew Limnol 23:1153–1158. https://doi.org/10.1080/03680770.1987.11899786
Dvořák J (1996) An example of relationships between macrophytes, macroinvertebrates and their food resources in a shallow eutrophic lake. Hydrobiologia 339:27–36. https://doi.org/10.1007/BF00008910
Egertson CJ, Kopaska JA, Downing JA (2004) A century of change in macrophyte abundance and composition in response to agricultural eutrophication. Hydrobiologia 524:145–156. https://doi.org/10.1023/B:HYDR.0000036129.40386.ce
Egler M, Buss DF, Moreira JC, Baptista DF (2012) Influence of agricultural land-use and pesticides on benthic macroinvertebrate assemblages in an agricultural river basin in southeast Brazil. Braz J Biol 72:437–443. https://doi.org/10.1590/S1519-69842012000300004
Epele LB, Miserendino ML (2015) Environmental quality and aquatic invertebrate metrics relationships at Patagonian wetlands subjected to livestock grazing pressures. PLoS One 10:e0137873. https://doi.org/10.1371/journal.pone.0137873
Everard M, Wood A (2017) Agricultural management and wetlands: an overview. In: Finlayson CM, Everard M, Irvine K, McInnes R, Middleton B, van Dam A, Davidson NC (eds) The Wetland book I: structure and function, management, and methods. Springer, Beijing, pp 1009–1019. https://doi.org/10.1007/978-90-481-9659-3_194
Feijoó CS, Giorgi A, García ME, Momo F (1999) Temporal and spatial variability in streams of a pampean basin. Hydrobiologia 394:41–52
Feijoó CS, Lombardo RJ (2007) Baseline water quality and macrophyte assemblages in Pampean streams: a regional approach. Water Res 41:1399–1410. https://doi.org/10.1016/j.watres.2006.08.026
Feijoó C, Menéndez M (2009) La biota de los ríos: los macrófitas. In: Elosegi A, Sabater S (eds) Conceptos y Técnicas en Ecología Fluvial. Fundación BBVa, Bilbao, pp 243–251
Fraaije RG, Poupin C, Verhoeven JT, Soons MB (2018) Functional responses of aquatic and riparian vegetation to hydrogeomorphic restoration of channelized lowland streams and their valleys. J Appl Ecol 56:1007–1018. https://doi.org/10.1111/1365-2664.13326
Gary HL, Johnson SR, Ponce SL (1983) Cattle grazing impact on surface water quality in a Colorado front range stream. J Soil Water Conserv 38:124–128
Gebrehiwot M, Awoke A, Beyene A, Kifle D, Triest L (2017) Macroinvertebrate community structure and feeding interactions along a pollution gradient in Gilgel Gibe watershed, Ethiopia: Implications for biomonitoring. Limnologica 62:68–76. https://doi.org/10.1016/j.limno.2016.11.003
Genito D, Gburek WJ, Sharpley AN (2002) Response of stream macroinvertebrates to agricultural land cover in a small watershed. J Freshw Ecol 17:109–119. https://doi.org/10.1080/02705060.2002.9663874
Gerth WJ, Li J, Giannico GR (2017) Agricultural land use and macroinvertebrate assemblages in lowland temporary streams of the Willamette Valley, Oregon, USA. Agric Ecosyst Environ 236:154–165. https://doi.org/10.1016/j.agee.2016.11.010
Gleason JE, Rooney RC (2017) Aquatic macroinvertebrates are poor indicators of agricultural activity in northern prairie pothole wetlands. Ecol Ind 81:333–339. https://doi.org/10.1016/j.ecolinD2017.06.013
Gómez N, Rodriguez Capítulo A, Collautti D et al (2016) La puesta en valor de los servicios ecosistémicos que ofrecen los arroyos de llanura pampeana como una medida de mitigación de las inundaciones: el caso de arroyo del gato en el partido de la plata. In: Volpedo A, de Cabo L, Arreghini S, Fernández Cirelli A et al (eds) Ecología y manejo de ecosistemas acuáticos pampeanos. Buenos Aires, Argentina, pp 39–52
Gómez N, Siri A, Capítulo LR et al (2022) Effects of urban demand for food and water on physicochemicals and biotic structure of riverine wetlands in the Pampean plain. Ecohydrology and Hydrobiology 22:355–369. https://doi.org/10.1016/j.ecohyD2021.08.006
Gore JA, Layzer JB, Mead J (2001) Macroinvertebrate instream flow studies after 20 years: a role in stream management and restoration. Regul Rivers: Res Manage 17:527–542. https://doi.org/10.1002/rrr.650
Gosselain V, Hudon C, Cattaneo A, Gagnon P, Planas D, Rochefort D (2005) Physical variables driving epiphytic algal biomass in a dense macrophyte bed of the St-Lawrence River (Quebec, Canada). Hydrobiologia 534:11–22. https://doi.org/10.1007/s10750-004-1318-z
Grashof-Bokdam CJ, van Langevelde F (2005) Green veining: landscape determinants of biodiversity in European agricultural landscapes. Landsc Ecol 20:417–439. https://doi.org/10.1007/s10980-004-5646-1
Green AJ, Alcorlo P, Peeters ET et al (2017) Creating a safe operating space for wetlands in a changing climate. Front Ecol Environ 15:99–107. https://doi.org/10.1002/fee.1459
Gupta G, Khan J, Upadhyay AK, Singh NK (2020) Wetland as a sustainable reservoir of ecosystem services: prospects of threat and conservation. In: Upadhyay AK, Singh R, Singh DP (eds) Restoration of wetland ecosystem: A trajectory towards a sustainable environment. Springer, Singapore, pp 31–43. https://doi.org/10.1007/978-981-13-7665-8_3
Gustafson S, Wang D (2002) Effects of agricultural runoff on vegetation composition of a priority conservation wetland, Vermont, USA. J Environ Qual 31:350–357. https://doi.org/10.2134/jeq2002.3500
Heck KL, Crowder LB (1991) Habitat structure and predator—prey interactions in vegetated aquatic systems. In: Bell SS, McCoy ED, MushinsCraftky HR (eds) Habitat Structure. Population and Community Biology Series. Springer, Dordrecht, pp 281–299. https://doi.org/10.1007/978-94-011-3076-9_14
Hurtado MA, Giménez JE, Cabral MG (2006) Análisis ambiental del partido de La Plata aportes del ordenamiento territorial, 1st edn. Consejo Federal de Inversiones, Buenos Aires
Hussner A, Lösch R (2007) Growth and photosynthesis of Hydrocotyle ranunculoides L. fil. in Central Europe. Flora:Morphology, Distribution, Functional Ecology of Plants202:653–660. https://doi.org/10.1016/j.florA2007.05.006
Kassambara A (2017) Practical guide to principal component methods in R: PCA, M (CA), FAMD, MFA, HCPC, factoextra. STHDA Marsella
Kindt R, Coe R (2005) Tree diversity analysis. A manual and software for common statistical methods for ecological and biodiversity studies. World Agroforestry Centre (ICRAF), Nairobi, Kenya
Lacoul P, Freedman B (2006) Environmental influences on aquatic plants in freshwater ecosystems. Environ Rev 14:89–136. https://doi.org/10.1139/a06-001
Ladle M, Bass JA (1981) The ecology of a small chalk stream and its responses to drying during drought conditions. Fundam Appl Limnol 90:448–466
Lahitte HB, Hurrell JA, Mehltreter K et al (2004) Biota Rioplatense I. Plantas de la costa: Nativas y exóticas. LOLA, Buenos Aires
Lake PS (2003) Ecological effects of perturbation by drought in flowing waters. Freshw Biol 48:1161–1172. https://doi.org/10.1046/j.1365-2427.2003.01086.x
Lange K, Townsend CR, Matthaei CD (2014) Can biological traits of stream invertebrates help disentangle the effects of multiple stressors in an agricultural catchment? Freshw Biol 59:2431–2446. https://doi.org/10.1111/fwB12437
Larned ST, Datry T, Robinson CT (2007) Invertebrate and microbial responses to inundation in an ephemeral river reach in New Zealand: effects of preceding dry periods. Aquat Sci 69:554–567. https://doi.org/10.1007/s00027-007-0930-1
Laterra P, Booman GC, Picone L, Videla C, Orúe ME (2018) Indicators of nutrient removal efficiency for riverine wetlands in agricultural landscapes of Argentine Pampas. J Environ Manage 222:148–154. https://doi.org/10.1016/j.jenvman.2018.05.070
Lê S, Josse J, Husson F (2008) FactoMineR: an R package for multivariate analysis. J Stat Softw 25:1–18
López I, Rotger DV (2020) Expansión urbana, humedales y evolución en los usos del suelo en el Gran La Plata. Biologia Acuatica 35:017. https://doi.org/10.24215/16684869e017
López van Oosterom MV, Ocón CS, Brancolini F, Maroñas ME, Sendra ED, Rodrigues Capítulo A (2013) Trophic relationships between macroinvertebrates and fish in a pampean lowland stream (Argentina). Iheringia - Serie Zoologia 103:57–65. https://doi.org/10.1590/S0073-47212013000100009
Lopretto EC, Tell G (1995) Ecosistemas de aguas continentales. Ediciones Sur, La Plata, Argentina
Lougheed VL, Crosbie B, Chow-Fraser P (2001) Primary determinants of macrophyte community structure in 62 marshes across the Great Lakes basin: latitude, land use, and water quality effects. Can J Fish Aquat Sci 58:1603–1612. https://doi.org/10.1139/cjfas-58-8-1603
Malacarne TJ, Baumgartner MT, Moretto Y, Gubiani ÉA (2016) Effects of land use on the composition and structure of aquatic invertebrate community and leaf breakdown process in Neotropical streams. River Res Appl 32:1958–1967. https://doi.org/10.1002/rrA3031
Marrochi MN, Hunt L, Solis M, Scalise AM, Fanelli SL, Bonetto C, Mugni H (2021) Land-use impacts on benthic macroinvertebrate assemblages in pampean streams (Argentina). J Environ Manage 279:111608. https://doi.org/10.1016/j.jenvman.2020.111608
McKay SF, King AJ (2006) Potential ecological effects of water extraction in small, unregulated streams. River Res Appl 22:1023–1037. https://doi.org/10.1002/rrA958
Mereta ST, Boets P, De Meester L, Goethals PL (2013) Development of a multimetric index based on benthic macroinvertebrates for the assessment of natural wetlands in Southwest Ethiopia. Ecol Ind 29:510–521. https://doi.org/10.1016/j.ecolinD2013.01.026
Merritt RW, Allan KW, Berg MB (2008) An Introduction to the Aquatic Insects of North America, 4th edn. Kendall/Hunt Publishing Company, Dubuque, IaKendall-Hunt, Dubuque, United States
Mitsch WJ, Gosselink JG (2000) The value of wetlands: importance of scale and landscape setting. Ecol Econ 35:25–33. https://doi.org/10.1016/S0921-8009(00)00165-8
Moens T, Traunspurger W, Bergtold M (2006) Feeding ecology of free-living benthic nematodes. Freshwater Nematodes. Ecology and Taxonomy. CAB International Publishing, pp 105–131
Moges A, Beyene A, Ambelu A, Mereta ST, Triest L, Kelbessa E (2017) Plant species composition and diversity in wetlands under forest, agriculture and urban land uses. Aquat Bot 138:9–15. https://doi.org/10.1016/j.aquabot.2016.12.001
Molina MC, Roa-Fuentes CA, Zeni JO, Casatti L (2017) The effects of land use at different spatial scales on instream features in agricultural streams. Limnologica 65:14–21. https://doi.org/10.1016/j.limno.2017.06.001
Mugni H, Paracampo A, Bonetto C (2013) Nutrient concentrations in a pampasic first order stream with different land uses in the surrounding plots (Buenos Aires, Argentina). Bull Environ Contam Toxicol 91:391–395. https://doi.org/10.1007/s00128-013-1079-3
Ocón C, Rodrigues Capítulo A (2012) Assessment of water quality in temperate-plain streams (Argentina, South America) using a multiple approach. Ecol Austral 22:81–91
Oksanen J, Blanchet FG, Friendly M et al (2019) vegan: Community Ecology Package. R package version 2.5-6. https://CRAN.R-project.org/package=vegan
Ortega M, Velasco J, Millán A, Guerrero C (2004) An ecological integrity index for littoral wetlands in agricultural catchments of semiarid mediterranean regions. Environ Manage 33:412–430
Paracampo A, Marrochi N, García I et al (2020) Fish Assemblages in Pampean Streams (Buenos Aires, Argentina): Relationship to Abiotic and Anthropic Variables. Anais. Academia Brasileira de Ciencias 92:e20190476. https://doi.org/10.1590/0001-3765202020190476
Paredes del Puerto JM, García ID, Maiztegui T et al (2022) Impacts of land use and hydrological alterations on water quality and fish assemblage structure in headwater Pampean streams (Argentina). Aquat Sci 84:6. https://doi.org/10.1007/s00027-021-00836-1
Peiró DF, do Amaral GF, Saulino HHL (2015) Structure community of aquatic insects associated with different macrophytes in ornamental lakes in a Savanna region, Southeastern Brazil. Pan-American Journal of Aquatic Sciences 10:273–282
Poi AS, Gallardo LI, Casco SL, Sabater LM, Úbeda B (2021) Influence of macrophyte complexity and environmental variables on macroinvertebrate assemblages across a subtropical wetland system. Wetlands 41:1–13. https://doi.org/10.1007/s13157-021-01508-4
Postel SL (2000) Entering an era of water scarcity: the challenges ahead.Ecological Applications10:941–948. https://doi.org/10.1890/1051-0761(2000)010[0941:EAEOWS]2.0.CO;2
R Development Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
RStudio Team (2015) RStudio: Integrated Development for R. RStudio, Inc., Boston
Rasmussen P, Anderson NJ (2005) Natural and anthropogenic forcing of aquatic macrophyte development in a shallow Danish lake during the last 7000 years. J Biogeogr 32:1993–2005. https://doi.org/10.1111/j.1365-2699.2005.01352.x
Rejmánková E (2011) The role of macrophytes in wetland ecosystems. Journal of Ecology and Environmental 34:333–345. https://doi.org/10.5141/JEFB2011.044
Ringuelet RA (1962) Ecología acuática continental. Manual de EUDEBA, Buenos Aires
Robinson CT, Uehlinger U, Monaghan MT (2004) Stream ecosystem response to multiple experimental floods from a reservoir. River Res Appl 20:359e377. https://doi.org/10.1002/rrA743
Roche LM, Kromschroeder L, Atwill ER, Dahlgren RA, Tate KW (2013) Water quality conditions associated with cattle grazing and recreation on national forest lands. PLoS One 8:e68127. https://doi.org/10.1371/journal.pone.0068127
Rodrigues Capítulo A, Tangorra M, Ocon C (2001) Use of benthic macroinvertebrates to assess the biological status of Pampean streams in Argentina. Aquat Ecol 35:109–119. https://doi.org/10.1023/A:1011456916792
Rodrigues Capítulo L, Kruse E, Gómez N (2020) Los bañados de desborde fluvial: una mirada desde la geohidrología. Biologia Acuatica 35:011. https://doi.org/10.24215/16684869e011
Ruiz-Picos RA, Sedeño-Díaz JE, López-lópez E (2016) Ensambles de macroinvertebrados acuáticos relacionados con diversos usos del suelo en los ríos Apatlaco y Chalma-Tembembe (cuenca del Río Balsas), México. Hidrobiológica 26:443–458
Sand-Jensen K (1998) Influence of submerged macrophytes on sediment composition and near-bed flow in lowland streams. Freshw Biol 39:663–679. https://doi.org/10.1046/j.1365-2427.1998.00316.x
Shannon CE, Weaver W (1949) The mathematical theory of communication. University of Illinois Press, Urbana
Sileshi A, Awoke A, Beyene A, Stiers I, Triest L (2020) Water purifying capacity of natural riverine wetlands in relation to their ecological quality. Front Environ Sci 8:39. https://doi.org/10.3389/fenvs.2020.00039
Sim LL, Davis JA, Strehlow K, McGuire M, Trayler KM, Wild S, Papas PJ, O’Connor J (2013) The influence of changing hydroregime on the invertebrate communities of temporary seasonal wetlands. Freshwater Science 32:327–342. https://doi.org/10.1899/12-024.1
Solis M, Mugni H, Hunt L, Marrochi N, Fanelli S, Bonetto C (2016) Land use effect on invertebrate assemblages in Pampasic streams (Buenos Aires, Argentina). Environ Monit Assess 188:539. https://doi.org/10.1007/s10661-016-5545-3
Solis M, Mugni H, Fanelli S, Bonetto C (2017) Effect of agrochemicals on macroinvertebrate assemblages in Pampasic streams, Buenos Aires, Argentina. Environ Earth Sci 76:180. https://doi.org/10.1007/s12665-017-6476-1
Solis M, Bonetto C, Marrochi N, Paracampo A, Mugni H (2018) Aquatic macroinvertebrate assemblages are affected by insecticide applications on the Argentine Pampas. Ecotoxicol Environ Saf 148:11–16. https://doi.org/10.1016/j.ecoenv.2017.10.017
Solis M, Arias M, Fanelli S, Bonetto C, Mugni H (2019) Agrochemicals’ effects on functional feeding groups of macroinvertebrates in Pampas streams. Ecol Ind 101:373–379. https://doi.org/10.1016/j.ecolinD2019.01.036
Stanley EH, Buschman DL, Boulton AJ, Grimm NB, Fisher SG (1994) Invertebrate resistance and resilience to intermittency in a desert stream. Am Midl Nat 131:288–300. https://doi.org/10.2307/2426255
Statzner B, Bêche LA (2010) Can biological invertebrate traits resolve effects of multiple stressors on running water ecosystems? Freshw Biol 55:80–119. https://doi.org/10.1111/j.1365-2427.2009.02369.x
Strokal M, Ma L, Bai Z, Luan S, Kroeze C, Oenema O, Velthof G, Zhang F (2016) Alarming nutrient pollution of Chinese rivers as a result of agricultural transitions. Environ Res Lett 11:024014. https://doi.org/10.1088/1748-9326/11/2/024014
Stubbington R (2012) The hyporheic zone as an invertebrate refuge: a review of variability in space, time, taxa and behaviour. Mar Freshw Res 63:293–311. https://doi.org/10.1071/MF11196
Suren AM, Biggs BJF, Duncan MJ, Bergey L, Lambert P (2003) Benthic community dynamics during summer low-flows in two rivers of contrasting enrichment 2. Invertebrates. New Zealand Journal of Marine and Freshwater Research 37:71–83. https://doi.org/10.1080/00288330.2003.9517147
Tarda AS, Saparrat MCN, Gómez N (2019) Assemblage of dematiaceous and Ingoldian fungi associated with leaf litter of decomposing Typha latifolia L. (Typhaceae) in riverine wetlands of the Pampean plain (Argentina) exposed to different water quality. J Environ Manage 250:109409. https://doi.org/10.1016/j.jenvman.2019.109409
Thomaz SM, Cunha ER (2010) The role of macrophytes in habitat structuring in aquatic ecosystems: methods of measurement, causes and consequences on animal. Acta Limnol Bras 22:218–236. https://doi.org/10.4322/actalB02202011
Tockner K, Ward JV, Edwards PJ, Kollmann J (2002) Riverine landscapes: an introduction. Freshw Biol 47:497–500. https://doi.org/10.1046/j.1365-2427.2002.00913.x
Tomanova S, Goitia E, Helešic J (2006) Trophic levels and functional feeding groups of macroinvertebrates in neotropical streams. Hydrobiologia 556:251–264. https://doi.org/10.1007/s10750-005-1255-5
Verhoeven JT, Arheimer B, Yin C, Hefting MM (2006) Regional and global concerns over wetlands and water quality. Trends Ecol Evol 21:96–103. https://doi.org/10.1016/j.tree.2005.11.015
Wang X (2001) Integrating water-quality management and land-use planning in a watershed context. J Environ Manage 61:25–36. https://doi.org/10.1006/jemA2000.0395
Warfe DM, Barmuta LA, Wotherspoon S (2008) Quantifying habitat structure: surface convolution and living space for species in complex environments. Oikos 117:1764–1773. https://doi.org/10.1111/j.1600-0706.2008.16836.x
Wood PJ, Agnew MD, Petts GE (2000) Flow variations and macroinvertebrate community responses in a small groundwater-dominated stream in south-east England. Hydrological Processes 14:3133–3147. https://doi.org/10.1002/1099-1085(200011/12)14:16/17<3133::AID-HYP138>3.0.CO;2-J
Wood PJ, Armitage PD (2004) The response of the macroinvertebrate community to low-flow variability and supra-seasonal drought within a groundwater dominated stream. Fundam Appl Limnol 161:1–20. https://doi.org/10.1127/0003-9136/2004/0161-0001
Woodward G, Gessner MO, Giller PS et al (2012) Continental-scale effects of nutrient pollution on stream ecosystem functioning. Science 336:1438–1440. https://doi.org/10.1126/science.1219534
Wu H, Lu K, Lyu X, Xue Z (2019) A macroinvertebrate multimetric index for the bioassessment of wetlands adjacent to agriculture fields in the Sanjiang plain, China. Chin Geogr Sci 29:974–984. https://doi.org/10.1007/s11769-019-1083-6
Zedler JB, Kercher S (2004) Causes and consequences of invasive plants Inachiev wetlands: opportunities, opportunists, and outcomes. Crit Rev Plant Sci 23:431–452. https://doi.org/10.1080/07352680490514673
Zedler JB, Kercher S (2005) Wetland resources: status, trends, ecosystem services, and restorability. Annu Rev Environ Resour 30:39–74. https://doi.org/10.1146/annurev.energy.30.050504.144248
Zgola T (2014) The response of aquatic plants to catchment land use for different types of lowland rivers. Appl Ecol Environ Res 2:143–162
Zou Y, Wang L, Xue Z et al (2018) Impacts of agricultural and reclamation practices on wetlands in the amur river basin, Northeastern China. Wetlands 38:383–389. https://doi.org/10.1007/s13157-017-0975-4
Acknowledgements
We would like to express our thanks to J. Donadelli (ILPLA) for the chemical analysis of water samples and to Alejandra Scotti for the English revision. This study was supported by the Consejo Nacional de Investigaciones Científicas y Técnicas [PIP 2013 − 0570]; the Institutional Project [PI UE-22920160100049CO], the Universidad Nacional de La Plata Projects [UNLP -FCNyM: 2014 11/ N 0738: 2018 11/ N 0869]; and the Fondo Nacional de Ciencia y Tecnología [PICT 2014 − 1342].
Funding
This study was supported by the Consejo Nacional de Investigaciones Científicas y Técnicas [PIP 2013 − 0570]; the Institutional Project [PI UE-22920160100049CO], the Universidad Nacional de La Plata Projects [UNLP -FCNyM: 2014 11/ N 0738: 2018 11/ N 0869]; and the Fondo Nacional de Ciencia y Tecnología [PICT 2014 − 1342].
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Sampling campaigns and data collection were performed by Paula Altieri, Carolina Ocon, Roberto Jensen and Alberto Rodrigues Capítulo. Data analysis and the writing of the first draft of the manuscript were carried on by Paula Altieri. Carolina Ocon and Paula Altieri made the final version of the manuscript, and all authors commented on previous versions. Funding acquisition and the supervision was performed by Alberto Rodrigues Capítulo. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of Interest/Competing Interests
This research does not present conflict of interest and has not being considered for publication elsewhere.
Ethics Approval
Not applicable.
Consent to Participate and Consent for publication
All the authors have fully participated in this manuscript and accept responsibility for it.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
ESM 1
(PDF 444 KB)
Rights and permissions
About this article
Cite this article
Altieri, P., Ocon, C., Jensen, R. et al. Effects of Agriculture and Hydrological Changes on Macrophyte and Macroinvertebrate Assemblages: a Case Study in Lowland Riverine Wetlands of Argentina. Wetlands 42, 48 (2022). https://doi.org/10.1007/s13157-022-01561-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13157-022-01561-7