Abstract
Adsorption of Cu2+ and Co2+ from aqueous solution using zeolite synthesized from coal gangue was examined at room temperature. Batch experiments were carried out to investigate the effects of contact time, initial ion concentration, and pH on the adsorption process. Under comparable conditions, the adsorption greatly depended on initial ion concentration and pH. Different isotherms were applied to describe equilibrium data, and the results turned out that adsorption data can be better fitted with Langmuir, Temkin, and Dubinin–Radushkevich models, indicating monolayer coverage of adsorption sites on the surface of zeolite NaX and a physical process for Co2+ and Cu2+ adsorption. Adsorption capacity calculated from Langmuir was 45.05 mg/g for Cu2+ and 44.53 mg/g for Co2+. The adsorption kinetic could be better described with the pseudo-second-order model, and the removal mechanism of Co2+ and Cu2+ by the synthesized zeolite NaX was governed by surface adsorption, film diffusion, and intra-particle diffusion. The work suggests that coal gangue-derived zeolite NaX can be used as an alternative economical adsorbent for metal ions removal.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Metal ions, non-biodegradable toxic inorganic pollutants (Bradl 2004), tend to accumulate in living organisms, causing various diseases and disorders (Bailey et al. 1999). Metal ions emerge from the course of mineral resources exploiting, metal smelting, and processing practices. As a kind of industrial resultant, metal ions have produced tremendous detriments to the ecological environment due to their non-biodegradation and long-term circulation within food chains. Gradually, chronic damage involved physiological and metabolic activities appears then health problems seem to be inevitable (Srividya and Mohanty 2009). Considering their ever-increasing negative impacts, skyrocketing attentions have been focused on finding out an efficient and green way to solve this environmental issue.
In recent years, removals of metal ions from water have been widely conducted (Dabrowski et al. 2004; Erdem et al. 2004). Many separation methods have been used for removal of metal ions from aqueous solution, including chemical precipitation, coagulation, solvent extraction, membrane filtration, ion exchange, reverse osmosis, chemical reduction, ultrafiltration, ionization, adsorption, biological treatment, and chelating resin (Hui et al. 2005; Baker et al. 2009; Padervand and Gholami 2013; El-Bahy and El-Bahy 2016; Petrovič and Simonič 2016; Iannazzo et al. 2017). However, most of these methods have significant disadvantages, such as the incomplete removal, high-energy requirements, and the production of toxic sludge (Petrovič and Simonič 2016; Iannazzo et al. 2017). Among those methods, adsorption has been proved to be one of the most feasible, simple, easily implemented, and highly efficient method for the removal of metal ions from polluted water (Kumar and Chawla 2014; Rahimi and Mohaghegh 2016; Iannazzo et al. 2017). Some widely used adsorbents for metal ions include activated carbon, clay minerals, industrial solid wastes, orange peel, nanosized metal oxides (such as iron oxides, aluminum oxides, titanium oxides, and manganese oxides), and zeolites (Kumar and Chawla 2014; Rahimi and Mohaghegh 2016; Iannazzo et al. 2017). A green way of absorbing metal ions could be achieved by exploiting industrial derived and reproducible adsorbents, and it thus can contribute to further industrial applications.
Coal exploitation generally creates a large quantity of coal gangue as solid by-product. The accumulation of considerable coal gangue over the past years has resulted in serious environmental problems, e.g., land occupation and soil and groundwater pollution. Several alternatives, such as building materials, heat-insulating materials, and aluminum salt products, have been proposed and implemented to deal with it. In general, an adsorbent can be regarded as green if it needs little processing, abundant in nature, or a by-product even waste material from another industry (Bailey et al. 1999). Conversion of coal gangue to zeolite is one of the potential alternatives for handling the coal gangue as it has high content of silica and alumina, which are the basic components for zeolite formation. In general, there are two main methods (one-step and two-step methods) to synthesize zeolite with solid wastes as silica–alumina source (Qian and Li 2015). As for the application of removing metal ions from aqueous solution, zeolites were reported to exhibit high sorption capacity, sorption affinity, and cation exchange capacity for divalent sorbates (Apiratikul and Pavasant 2008; Rahimi and Mohaghegh 2016). According to our knowledge, however, scarce attempt has been made to convert coal gangue to zeolites (Zhang et al. 2008; Lei et al. 2009; Qian and Li 2015).
This work focused on Co2+ and Cu2+ removal from aqueous solution using zeolite NaX prepared from coal gangue as adsorbent. The adsorption influence factors such as initial ion concentration, pH, and contact time were investigated. Langmuir, Freundlich, Temkin, and Dubinin–Radushkevich isotherm models were used to describe the adsorption equilibrium data. Pseudo-first-order model, pseudo-second-order model, intra-particle model, and film diffusion model were employed to reveal the adsorption mechanism.
Materials and methods
Materials
Coal gangue, used as silicon and aluminum sources in this work, was collected from Baishui coal mine of Weibei area, China. It contains 45.14% SiO2, 26.97% Al2O3, 0.32% MgO, 0.47% CaO, 1.21% Fe2O3, 1.02% K2O, 0.21% Na2O, 0.51% TiO2, and 16.40% loss on ignition. Coal gangue primarily consists of kaolinite, quartz, and hematite. The mineral compositions and chemical components of coal gangue indicate that this kind of mining solid waste could be a promising resource for zeolite synthesis.
Other inorganic chemicals, such as copper nitrate (Cu(NO3)2·3H2O), cobalt nitrate (Co(NO3)2·6H2O), sodium hydroxide (NaOH), nitric acid (HNO3), and hydrochloric acid (HCl) are all analytic-grade reagents. Copper nitrate and cobalt nitrate were purchased from Jinshanting chemical reagent plant of Shanghai, while sodium hydroxide, nitric acid, and hydrochloric acid were bought from Tianjin chemical reagent plant. Stock solutions (1000 mg/L) were prepared by dissolving accurate mass of Co(NO3)2·6H2O and Cu(NO3)2·3H2O into 1000 mL deionized water. Desired concentrations were obtained by diluting the stock solution with deionized water.
Preparation of zeolite
Zeolite NaX was synthesized from coal gangue via hydrothermal treatment after alkali fusion. The milled coal gangue sample was calcinated at 800 °C for 3 h and then mixed with NaOH at an optimal weight ratio of nearly 1.2:1. The resultant was then roasted holding by a nickel crucible at 600 °C for 3 h in a muffle furnace. The alkali-fused product was milled and transferred into a 100-mL-volume beaker, aging for 6 h at a constant liquid/solid ratio at 8 mL/g. The aged product was transferred into a 100-mL-volume polytetrafluoroethylene reactor and crystallized at 110 °C for 12 h in an oven (Chen and Lu 2017). Then, the product was separated from the mixture through vacuum filtration with deionized water to removal excess sodium hydroxide until the pH reaching 9–10 and dried at 105 °C before storing in a desiccator.
Characterization of zeolite
The synthesized products were characterized with X-ray diffraction (XRD) and scanning electron microscope (SEM). XRD pattern was obtained using a Rigaku D/Max2550VB+/PC X-Ray diffractometer with Cu Kα (λ = 0.154 nm) radiation operating at 40 kV, 100 mA, scan rate of 4°/min, and step width of 0.02°. The surface morphology and microstructure of the synthesized zeolite products were characterized by a Hitachi S-3400N scanning electron microscope (SEM) and energy-dispersive X-ray spectrometer (EDXS) combined instrument. The surface area analyzer, JW-BK132F, was applied to determine surface area using BET technique based on adsorption–desorption characteristics of N2 gas on the sample at 77 K. The micropore size distribution was determined by Barrett–Joyner–Halenda (BJH) method using the adsorption branch (Huang et al. 2010). The point of zero charge (pHpzc) was determined with JS94H2 Zeta Meter electrophoresis by measuring the surface charge of zeolite NaX in deionized water at different pH.
Batch sorption experiment
Effect of initial pH
The initial pH of the aqueous solution is a crucial controlling parameter during the adsorption process. To clarify the influence of initial pH of solution, batch sorption experiments were carried out at different initial pH solution (2.0–6.0), adjusted by 0.1 mol/L HNO3 or NaOH solution. In this study, 0.2 g zeolite NaX was, respectively, added into 100 mL Cu2+ solution (100 mg/L) and 100 mL Co2+ solution (100 mg/L), agitated at 300 rpm for 120 min at ambient temperature (27 °C). The equilibrium mixture was filtered with a 0.45-μm filter membrane, and the residual Cu2+ and Co2+ concentrations in the solution were measured by inductively coupled plasma atomic emission spectroscopy (ICP-AES, Spectro Arcos).
Effect of initial ion concentration
Parallel experiments were conducted to reveal the influence of the initial ion concentration on adsorption. In this study, the initial ion concentration varied from 10 to 200 mg/L, while other experimental conditions such as initial pH (4.0) of solution, zeolite NaX dose (0.2 g), experimental temperature (room temperature, 27 °C), stirring time (120 min), and rotational speed (300 rpm) were fixed through the process. Graph of adsorption capacity versus initial ion concentration was plotted to determine the optimum initial ion concentration.
Adsorption kinetic experiment
Kinetic studies were conducted in 250-mL conical flasks. In total, 0.2 g dried zeolite NaX was thoroughly mixed with 100 mL metal ion solution at five different initial ion concentrations (10, 20, 50, 100 and 200 mg/L), and the pH of the solution was adjusted to 4.0 using 0.1 mol/L HNO3 or 0.1 mol/L NaOH. The suspension was stirred at room temperature (27 °C) under a rate of 300 rpm. Samples of 0.5 mL solution were extracted from the conical flasks at the given time (0, 5, 10, 20, 40, 60, 80, 100, and 120 min). The total extraction volume was no more than 5% of the stock ion solution. The extracted solutions were filtered with 0.45-μm filter membrane. The ion concentration in the solution was determined by ICP-AES.
Metal ions uptake
The removal efficiency (R, %) and the amount of ion adsorbed at time t (q t , mg/g) and at equilibrium (q e , mg/g) were calculated using the following equations, respectively (Srividya and Mohanty 2009; Zhao et al. 2010).
where C 0, C t , and C e (mg/L) represent the ion concentrations at initial, t time, and equilibrium, respectively. V (L) is the volume of ion solution, and M (g) is the mass of zeolite NaX.
Results and discussion
Characterization of zeolite
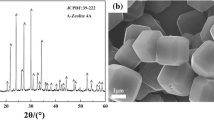
The crystalline phase of the synthesized product was characterized by XRD (Fig. 1). Diffractogram of the synthesized product matches the reference peaks of zeolite NaX (PDF: 38-0237) well. Comparison of the characteristic peak confirmed the conversion of coal gangue into zeolite NaX. Surface morphology was checked by SEM (Fig. 2). Octahedron crystalline structure of the synthesized zeolite NaX particles can be clearly observed (Fig. 2a), which further indicates that the coal gangue was successfully transformed to target product. EDXS analysis result (Fig. 2b) suggests a molar ratio of SiO2/Al2O3 of 2.1. The specific surface area and the micropore total volume of the synthesized zeolite NaX are 676.02 m2/g and 0.263 cm3/g, respectively, indicating that the synthesized zeolite NaX is a potential effective adsorbent. It has a wide range of pore dimension within micropore range (<2 nm), which is possible to separate different metal ions from polluted wastewater.
The point of zero charge (pHPZC) of an adsorbent is crucial to choose the optimum pH for the adsorption study, as well as to understand the adsorption mechanism (Argun 2008; Göde et al. 2010). The pHPZC of the synthesized zeolite NaX is 3.8. The surface charge remains positive when the pH below 3.8 and negatively charged once the pH beyond that point which is favorable for adsorption of positively charged metal ions.
Effect of initial pH
The ionization degree of the adsorbate and the surface property of the sorbent as well as metal ion species were strongly affected by the aqueous solution pH (Lin and Yang 2002; Rivera-Utrilla and Sánchez-Polo 2003). It is necessary to be viewed as an important factor that can greatly affect the removal efficiency in an adsorption system. Hui et al. (2005) pointed out that the ionization of surface hydroxyl groups (Si–OH and Al–OH) can change when zeolite surface exposed to aqueous solution. Covarrubias et al. (2005) stated that positively charged metal ions may react with hydroxyl in aqueous and the zeolite surface may in turn influence the surface charge and finally changed the removal efficiency. Batch adsorption experiments concerning the removal efficiency as the function of initial solution pH (2.0, 3.0, 4.0, 5.0, 6.0) were conducted. pH was adjusted before adding zeolite NaX. Higher initial pH was excluded in the case of precipitation, which could lead to a sharp increase in removal efficiency (Hui et al. 2005). The experiments show that the adsorption amount of Co2+ and Cu2+ onto zeolite NaX increases once the pH rises up from 2.0 to 6.0, especially when pH varied from 2.0 to 4.0. The removal efficiency increases slowly when pH changed from 4.0 to 6.0. This can be elucidated by the balance effect involving the surface charge and solution chemistry as well as the zeolite structure (Moghadam et al. 2013). Zeolite consists of body part, negative charged aluminosilicate frame structure, and balanced by positively charged exchangeable alkali ions (Na+, K+) to keep an electric neutrality state. Exposed to highly acid environment (lower than the isoelectric point of the zeolite NaX) leads to a positively charged zeolite NaX surface due to an exchange reaction between the hydrogen ion and alkali ions. At lower pH, the concentration of H3O+ is much higher than that of metal ions, and hence the former metal ions occupy the binding sites on the zeolite surface, resulting in a lower removal efficiency. On the other hand, Cu2+ and Co2+ can complex with hydroxyl when pH > 4.0 and a steric hindrance effect appears, and then metal ions cannot pass through the pore which lead to a relative slow increase in Cu2+ and Co2+ removal efficiency.
Effect of initial metal ion concentration
Metal ions can be adsorbed onto certain amount of zeolite. In that way, attentions should be paid to investigate the influence of initial ion concentration on metal ions removal. The relationship between sorption capacity and the initial concentration (ranged from 10 to 200 mg/L) is shown in Fig. 3. As it can be observed clearly, a rise of initial ion concentration contributes to an increase in metal ion adsorption capacity to a certain point and then reaches a climax, which could be explained by the saturation of adsorption active sites. Additionally, the figure also demonstrates that metal ions were almost removed within the first 40 min though relative longer time was needed to reach equilibrium at higher initial metal concentration when compared with lower ones for both of the cases. Sharp adsorption at the beginning can be ascribed to the greater metal ion concentration difference during the first several minutes, which exhibits stronger adsorption driver force.
Kinetic studies
Further information about the reaction route and mechanism in an adsorption system can be revealed by the adsorption kinetic studies. Moreover, deep insights into the solute adsorption can be obtained through the analysis of the adsorption experiment data using proper sorption kinetic models (Ho and McKay 1999). It can also help to determine the remnant time needs to reach equilibrium at the solid–solution interface. So far, several sorption kinetic models have been proposed to understand the adsorption characteristics. Among them, the most widely used were pseudo-first-order equation, pseudo-second-order equation, intra-particle diffusion model, and liquid film diffusion model.
The pseudo-first-order adsorption kinetic model used to describe adsorption in a liquid–solid system can be expressed as
where q e (mg/g) and q t (mg/g) refer to the amount of adsorbed metal ions on zeolite NaX at equilibrium and time t, respectively. t is the reaction time (min) and K 1 (min−1) represents the pseudo-first-order adsorption rate constant. K 1 and q e can be obtained from the slope and intercept in log (q e −q t ) against t plot, respectively.
The pseudo-second-order kinetic model is expressed as
where q e (mg/g) and q t (mg/g) mean the amount of adsorbed metal ions on zeolite NaX at equilibrium and at time t, respectively. K 2 (g/(mg·min)) is the pseudo-second-order rate constant. K 2 and q e can be known through the graph of t/q t versus t.
To calculate the above rate constants, the pseudo-first-order equation and pseudo-second-order equation were employed to fit sorption data of Co2+ and Cu2+ (under five different concentrations) with a fixed adsorbent dose. The values of the reaction rate constants and correlation coefficients are listed in Table 1. A comparison between the pseudo-first-order and pseudo-second-order kinetic rate constants suggests that Co2+ and Cu2+ adsorption by zeolite NaX closely follows the pseudo-second-order kinetic rather than the other one. This is clearly exhibited in Table 1, since the values of q e obtained from pseudo-second-order kinetic equation was found to be closer to the experimental q e value, whereas pseudo-first-order q e value did not agree with the experimental q e .
The intra-particle diffusion model is represented as
where K id (mg/(g min−1/2)) is the intra-particle diffusion rate constant. q t is the amount of metal ions adsorbed at time t and C (mg/g) is a constant proportional to the thickness of boundary layer (Arivoli and Ravichandran 2013). Applying the intra-particle diffusion model to the experimental data of Co2+ and Cu2+ adsorption, the fitting plot did not give straight lines to pass through the origin (Fig. 4). This may indicate that the mechanism of removal of Co2+ and Cu2+ by the synthesized zeolite NaX is complex, and the surface adsorption as well as intra-particle diffusion contributes to the rate-determining step (Salam 2013; Thamilarasu and Karunakaran 2013).
The liquid film diffusion is another kinetic model that assumes the flow of the adsorbate molecules through a liquid film surrounding the solid adsorbent is the slowest step in an adsorption process and it thus determines kinetic of the process (Salam 2013). The liquid film diffusion model is given by the following equation (Salam 2013)
where F = q t /q e , the proportion of equilibrium achievement. K fd (min−1) is the film diffusion rate coefficient. A linear plot of −ln(1−F) versus t with zero intercept suggests that the kinetic of the adsorption process is controlled by diffusion process when passing through the liquid film. The liquid film diffusion model fitting plot did not cover all the experimental data points well and did not provide a straight line through the origin (Fig. 5). It indicates that the liquid film diffusion model was not the only determining step (Salam 2013).
Adsorption isotherm
The adsorption isotherm is the relationship between equilibrium concentration of the solute in the solution and quantity of the adsorbate adsorbed by sorbent at constant temperature (Yuan et al. 2013). In the study, the experimental adsorption data were fitted with the most widely used isotherm models, that is, Langmuir, Freundlich, Temkin, and Dubinin–Radushkevich isotherm models (Erdem et al. 2004; Srividya and Mohanty 2009; Göde et al. 2010; Abdelnaeim et al. 2016; Chaudhry et al. 2017a, b).
The Langmuir model is based on the assumption that the uptake of metal ions occurs on a homogeneous surface by monolayer adsorption without any interaction between adsorbed ions (Srividya and Mohanty 2009; Abdelnaeim et al. 2016). The model further hypothesizes that the energy is same for all the active adsorption sites. The Langmuir isotherm model represents the equilibrium distribution of metal ions between the solid and liquid phases. The linear form of Langmuir equation is given as (Zhao et al. 2010; Arivoli and Ravichandran 2013)
where C e (mg/L) is the equilibrium concentration of metal ion in solution. q e (mg/g) is the amount of metal ion adsorbed at equilibrium. q m (mg/g) is the maximum metal ion uptake per unit mass of the adsorbent, which relates to adsorption capacity. b is the Langmuir constant (L/mg) which relates to the adsorption rate. The q m and b can be determined from C e /q e versus the C e plot which gives a straight line of slope 1/q m and intercept 1/(bq m ). According to the Langmuir model, the maximum adsorption capacity of zeolite NaX is 44.53 mg/g for Co2+ and 45.05 mg/g for Cu2+ (Table 2).
The essential characteristics of Langmuir adsorption isotherm can be explained in terms of dimensionless constant called separation factor or equilibrium parameter, R L (R L = 1/(1 + bC 0)), where b is the Langmuir constant (L/mg) and C 0 is the initial concentration of metal ions. Four adsorption types were categorized according to the value of separation factor R L (Arivoli and Ravichandran 2013): (1) favorable adsorption, 0 < R L < 1; (2) unfavorable adsorption, R L > 1; (3) linear adsorption, R L = 1 and (4) irreversible adsorption, R L = 0. R L is a positive number whose magnitude determines the feasibility of the adsorption process (Srividya and Mohanty 2009). The values of R L for Co2+and Cu2+ adsorption on the synthesized zeolite NaX are all between 0 and 1, indicating that the synthesized zeolite NaX is an appropriate adsorbent for Co2+ and Cu2+.
The Freundlich isotherm is the most frequently used to describe the adsorption of inorganic and organic components in solution (Hui et al. 2005). This fairly satisfactory empirical isotherm can be applied to a non-ideal sorption that involves heterogeneous sorption, and it is expressed as
where C e (mg/L) is the equilibrium concentration. K F (mg/g(L/mg)1/n) is roughly an indicator of the adsorption capacity and 1/n is the adsorption intensity of metal ion on the synthesized zeolite NaX. The magnitude of the exponent 1/n gives an indication of the favorability of adsorption. By plotting log q e versus log C e , values of K F and 1/n can be calculated from the slope and intercept of the plot. Table 2 shows that the 1/n values for Co2+ and Cu2+ are between 0 and 1, implying a favorable adsorption (Abdelnaeim et al. 2016).
Temkin isotherm is based on the assumption that the heat of adsorption of all molecules in the layer decrease linearly with the coverage of adsorbent surface. The isotherm is applicable for adsorption on heterogeneous solid adsorbent surface and liquid adsorbate. The linearized mathematical form of Temkin isotherm is (Abdelnaeim et al. 2016; Chaudhry et al. 2017a, b)
where C e (mg/L) is the equilibrium concentration; A T (L/mg) and b T (kJ/mol) are Temkin constants related to equilibrium binding constant (L/mol) which is related to the maximum binding energy and heat of adsorption, respectively; R is the gas constant; T (K) is the temperature. The values of A T and b T for Co2+ and Cu2+ were calculated from the slope and intercept of the plots q e versus lnC e plots, and the results are given in Table 2. The regression coefficient values for Co2+ (0.998) and Cu2+ (0.996) indicate that the adsorption data were well fitted to Temkin isotherm model. Table 2 shows that the Temkin constant b T related to heat of adsorption for Co2+ and Cu2+ is 0.261 and 0.264 kJ/mol, respectively. It has been reported that the typical range of bonding energy for ion-exchange mechanism is 8–16 kJ/mol (Chaudhry et al. 2017b), and the low values in this study indicate a weak interaction between metal ions (Co2+ and Cu2+) and the synthesized zeolite NaX (Chaudhry et al. 2017b). These results complemented Langmuir and Freundlich isotherm results.
Dubinin–Radushkevich isotherm model is applied to determine the nature of sorption process and the mean energy of sorption (Abdelnaeim et al. 2016). The linear form of Dubinin–Radushkevich isotherm is represented as (Abdelnaeim et al. 2016; Chaudhry et al. 2017a, b)
where k ad is the Dubinin–Radushkevich constant related to mean free energy of adsorption (mol2/kJ2); q s is theoretical Dubinin–Radushkevich monolayer saturation adsorption capacity (mg/g), and ε is Polanyi potential which is equal to RTln(1 + 1/C e ). R and T are gas constant and temperature in Kelvin scale, respectively. The slope and intercept of the straight line plot of lnq e versus ε2 gave k ad and q s . The mean free energy of sorption can be calculated using the following equation
If the value of E is between 8 and 16 kJ/mol, the adsorption process proceeds by ion exchange or chemisorption, and if the value of E less than 8 kJ/mol, the adsorption process is of a physical nature (Abdelnaeim et al. 2016). The values of E for Co2+ and Cu2+ are <8 kJ/mol, implying the adsorption of Co2+ and Cu2+ on the synthesized zeolite NaX is a physical process.
The correlation coefficients demonstrate that Langmuir, Temkin, and Dubinin–Radushkevich models adequately fitted the data for Co2+ and Cu2+ adsorption (Table 2). Such an observation gives a suggestion of monolayer adsorption of metal ions on the surface which means monolayer coverage of adsorption sites distribution (Kumar and Chawla 2014). Furthermore, adsorption capacity of Cu2+ and Co2+ calculated from Langmuir model were compared with the similar adsorbents (Erdem et al. 2004; Hui et al. 2005; Apiratikul and Pavasant 2008) in Table 3, from which we can know that the synthesized zeolite NaX has the relative strong adsorption capacity in removing heavy metal ions. On the other hand, the adsorption capacity order determined from Fig. 3 is Co2+ < Cu2+.
Several factors, such as zeolite framework and hydrated radius sizes of the metal ions may lead to such a contradiction. From the point of the structure, coal gangue-derived zeolite NaX has a wide range distribution of effective pore diameter (6.6 Å, 7.4 Å and 8.4 Å)(Chen and Lu 2017). A specific structure zeolite can play as a retainer for the separation of metal ions that have different hydrated radius. If the size of the metal hydrated radius is greater, compared with the adsorbent, it cannot pass through it. In fact, the hydrated radius of Co2+ (4.23 Å) is higher than Cu2+ (4.04 Å)(Tansel et al. 2006), which can elucidate the adsorption sequence in our experiment, namely, Co2+ < Cu2+. According to the study (Xu et al. 2010), the hydrogen may replace the exchangeable alkali ions (Na+) that exist within the structure, leading to a raise of solution pH. The calculated beginning precipitation pH concerns Co2+ and Cu2+ is 7.94 and 5.74 under the adsorption condition, respectively. Turan et al. (2005) argued that the abated metal ion concentration equals to adsorption plus precipitation in a zeolite adsorption system. Quicker precipitation happens to Cu2+ rather than Co2+, which contributes to faster precipitation of Cu2+ and higher adsorption capacity under experimental condition. Furthermore, we propose that adsorption is a process depends on the variety of adsorbents as well as experiment conditions.
Conclusion
The present study shows that the coal gangue was successfully converted into zeolite NaX with a specific area of 676.02 m2/g and wide pore size distribution. Batch experiments suggests that the coal gangue prepared zeolite NaX exhibits great affinity to metal ions such as Co2+ and Cu2+. Such a process was strongly depends on the initial metal ion concentration and pH of the aqueous solution. Longer time was necessary to reach equilibrium at higher initial metal ion concentration than lower ones. Kinetic adsorption mechanism studies indicated that the mechanism of removal of Co2+ and Cu2+ by the synthesized zeolite NaX was controlled by surface adsorption, intra-particle diffusion, and film diffusion. Langmuir model demonstrates a higher correlation coefficient compared with Freundlich model for Co2+ and Cu2+, signifying monolayer coverage of active sites on the surface of the zeolite NaX. The adsorption capacity determined from Langmuir model was 44.53 and 45.05 mg/g for Co2+ and Cu2+, respectively. Temkin and Dubinin–Radushkevich models indicate that the adsorption of Co2+ and Cu2+ on the synthesized zeolite NaX is a physical process. Above findings suggests that coal gangue-derived zeolite NaX can be used as an alternative economical adsorbent for metal ions removal.
References
Abdelnaeim MY, El Sherif IY, Attia AA, Fathy NA, El-Shahat MF (2016) Impact of chemical activation on the adsorption performance of common reed towards Cu(II) and Cd(II). Int J Miner Process 157:80–88
Apiratikul R, Pavasant P (2008) Sorption of Cu2+, Cd2+, and Pb2+ using synthesized zeolite from coal fly ash. Chem Eng J 144:245–258
Argun ME (2008) Use of clinoptilolite for the removal of nickel ions from water: kinetics and thermodynamics. J Hazard Mater 150:587–595
Arivoli S, Ravichandran T (2013) Adsorption of Rhodamine-B from aqueous solution by activated calcite powder studies on equilibrium isotherm, kinetics and thermodynamics. Int J Pollut Abat Technol 2:6–12
Bailey SE, Olin TJ, Bricka RM, Adrian DD (1999) A review of potentially low-cost sorbents for heavy metals. Water Res 33:2469–2479
Baker HM, Massadeh AM, Younes HA (2009) Natural Jordanian zeolite: removal of heavy metal ions from water samples using column and batch methods. Environ Monit Assess 157:319–330
Bradl HB (2004) Adsorption of heavy metal ions on soils and soils constituents. J Colloid Interface Sci 277:1–18
Chaudhry SA, Khan TA, Ali I (2017a) Zirconium oxide-coated sand based batch and column adsorptive removal of arsenic from water: isotherm, kinetic and thermodynamic studies. Egypt J Pet 26:553–563
Chaudhry SA, Zaidi Z, Siddiqui SI (2017b) Isotherm, kinetic and thermodynamics of arsenic adsorption onto iron-zirconium binary oxide-coated sand (IZBOCS): modeling and process optimization. J Mol Liq 229:230–240
Chen J, Lu X (2017) Synthesis and characterization of zeolites NaA and NaX from coal gangue. J Mater Cycles Waste Manag. doi:10.1007/s10163-017-0605-5
Covarrubias C, Arriagada R, Yáñez J, García R, Angélica M, Barros SD, Arroyo P, Sousa-Aguiar EF (2005) Removal of chromium (III) from tannery effluents, using a system of packed columns of zeolite and activated carbon. J Chem Technol Biotechnol 80:899–908
Dabrowski A, Hubicki Z, Podkościelny P, Robens E (2004) Selective removal of the heavy metal ions from waters and industrial wastewaters by ion-exchange method. Chemosphere 56:91–106
El-Bahy SM, El-Bahy ZM (2016) Synthesis and characterization of a new iminodiacetate chelating resin for removal of toxic heavy metal ions from aqueous solution by batch and fixed bed column methods. Korean J Chem Eng 33:2492–2501
Erdem E, Karapinar N, Donat R (2004) The removal of heavy metal cations by natural zeolites. J Colloid Interface Sci 280:309–314
Göde F, Öztürk N, Sert Y, Bahçeli S (2010) Adsorption of Cr(VI) from aqueous solutions onto raw and acid-activated Reşadiye and Hançılı clays. Spectrosc Lett Int J Rapid Commun 43:68–78
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Huang Y, Wang K, Dong D, Li D, Hill MR, Hill AJ, Wang H (2010) Synthesis of hierarchical porous zeolite NaY particles with controllable particle sizes. Microporous Mesoporous Mater 127:167–175
Hui KS, Chao CYH, Kot SC (2005) Removal of mixed heavy metal ions in wastewater by zeolite 4A and residual products from recycled coal fly ash. J Hazard Mater 127:89–101
Iannazzo D, Pistone A, Ziccarelli I, Espro C, Galvagno S, Giofre SV, Romeo R, Cicero N, Bua GD, Lanza G, Legnani L, Chiacchio MA (2017) Removal of heavy metal ions from wastewater using dendrimer-functionalized multi-walled carbon nanotubes. Environ Sci Pollut Res 24:14735–14747
Kumar R, Chawla J (2014) Removal of cadmium ion from water/wastewater by nano-metal oxides: a review. Water Qual Expo Health 5:215–226
Lei B, Hu F, Yang X (2009) Synthesis of zeolite A from coal gangue. Deterg Cosmet 9:36–38 (in Chinese)
Lin CY, Yang DH (2002) Removal of pollutants from wastewater by coal bottom ash. J Environ Sci Health Part A 37:1509–1522
Moghadam MR, Nasirizadeh N, Dashti Z, Babanezhad E (2013) Removal of Fe(II) from aqueous solution using pomegranate peel carbon: equilibrium and kinetic studies. Int J Ind Chem 4:1–6
Padervand M, Gholami MR (2013) Removal of toxic heavy metal ions from waste water by functionalized magnetic core–zeolitic shell nanocomposites as adsorbents. Environ Sci Pollut Res 20:3900–3909
Petrovič A, Simonič M (2016) Removal of heavy metal ions from drinking water by alginate-immobilised Chlorella sorokiniana. Int J Environ Sci Technol 13:1761–1780
Qian T, Li J (2015) Synthesis of Na-A zeolite from coal gangue with the in situ crystallization technique. Adv Power Technol 26:98–104
Rahimi E, Mohaghegh N (2016) Removal of toxic metal ions from sungun acid rock drainage using mordenite zeolite, grapheme nanosheets, and a novel metal–organic framework. Mine Water Environ 35:18–28
Rivera-Utrilla J, Sánchez-Polo M (2003) Adsorption of Cr(III) on ozonised activated carbon. Importance of Cπ—cation interactions. Water Res 37:3335–3340
Salam MA (2013) Removal of heavy metal ions from aqueous solutions with multi-walled carbon nanotubes: kinetic and thermodynamic studies. Int J Environ Sci Technol 10:677–688
Srividya K, Mohanty K (2009) Biosorption of hexavalent chromium from aqueous solutions by Catla catla scales: equilibrium and kinetics studies. Chem Eng J 155:666–673
Tansel B, Sager J, Rector T, Garlang J, Strayer RF, Levine LF, Roberts M, Hummeick M, Bauer J (2006) Significance of hydrated radius and hydration shells on ionic permeability during nanofiltration in dead end and cross flow modes. Sep Purif Technol 51:40–47
Thamilarasu P, Karunakaran K (2013) Kinetic, equilibrium and thermodynamic studies on removal of Cr(VI) by activated carbon prepared from Ricinus commuis seed shell. Can J Chem Eng 91:9–18
Turan M, Mart U, Yüksel B, Çelik MS (2005) Lead removal in fixed-bed columns by zeolite and sepiolite. Chemosphere 60:1487–1492
Xu W, Li LY, Grace JR (2010) Zinc removal from acid rock drainage by clinoptilolite in a slurry bubble column. Appl Clay Sci 50:158–163
Yuan Q, Li N, Chi Y, Geng W, Yan W, Zhao Y, Li X, Dong B (2013) Effect of large pore size of multifunctional mesoporous microsphere on removal of heavy metal ions. J Hazard Mater 254–255:157–165
Zhang Z, Wang W, Yang XY, Hu QX, Li YF (2008) Preparation of 4A zeolite from coal gangue through a alkali fusion method. China Surfactant Deterg Cosmet 5:294–297 (in Chinese)
Zhao Y, Zhang B, Zhang X, Wang J, Liu J, Chen R (2010) Preparation of highly ordered cubic NaA zeolite from halloysite mineral for adsorption of ammonium ions. J Hazard Mater 178:658–664
Acknowledgements
The research was supported by the National Natural Science Foundation of China through Grant 41271510, the Research and Development Project of Science and Technology of Shaanxi Province through Grant 2014K15-01-05, and the Fundamental Research Funds for the Central University through Grants GK201601009. Fuqiang Zhao participated in sampling work at the early stage of the project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, X., Shi, D. & Chen, J. Sorption of Cu2+ and Co2+ using zeolite synthesized from coal gangue: isotherm and kinetic studies. Environ Earth Sci 76, 591 (2017). https://doi.org/10.1007/s12665-017-6923-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-017-6923-z