Abstract
Copper ions were first adsorbed by zeolite 4A synthesized from bauxite tailings, the desorption of Cu(II) using Na2EDTA solutions was performed, and the recycling of zeolite 4A in adsorption and desorption was systematically investigated. It was observed that the Cu(II) removal efficiency was directly dependent on the initial pH value. The maximum removal efficiency of Cu(II) was 96.2% with zeolite 4A when the initial pH value was 5.0. Cu(II) was completely absorbed in the first 30 min. It was also observed that the desorption efficiency and zeolite recovery were highly dependent on the initial pH and concentration of Na2EDTA in the solution. The desorption efficiency and percent of zeolite recovered were 73.6 and 85.9%, respectively, when the Na2EDTA solution concentration was 0.05 mol L−1 and the pH value was 8. The recovered zeolites were pure single phase and highly crystalline. After 3 cycles, the removal efficiency of Cu(II) was as high as 78.9%, and the zeolite recovery was 46.9%, indicating that the recovered zeolites have good adsorption capacity and can repeatedly absorb Cu(II).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The effluents of wastewater in certain industries, such as paper board mills, fertilizer industries, metal cleaning and plating baths, and wood pulp production, contain many kinds of toxic metals, including copper ions, and a very small amount of Cu(II) is highly poisonous to water-dwelling creatures (Lazaridis and Keenan 2010; Uriu-Adams and Keen 2005). Hence, the removal of Cu(II) from waste effluents is environmentally important. At present, many methods have been used to treat wastewater containing copper, including biological (Kaduková and Virčíková 2005), ion exchange (Dabrowski et al. 2004), and chemical precipitation methods (Chang et al. 2010; Matlock et al. 2002).
Recently, low-cost sorbents have been demonstrated to be easy and practical in the removal of Cu(II) from wastewater. Due to their strong adsorption and ion-exchange capacities, zeolites are regarded as promising materials (Erdem et al. 2004; Wang and Peng 2010).
Zeolites are crystalline, microporous aluminosilicates with a basic crystalline framework composed of SiO4 and AlO4 tetrahedrons connected by shared oxygen atoms, forming characteristic structures and that result in excellent performance in multiple applications (Liu et al. 2013), such as ion exchange (Baldansuren et al. 2009; Sherry and Walton 1967), detergent building (Upadek et al. 1991), gas separation (Ackley et al. 2003; Vareltzis et al. 2003; Du et al. 2014), and catalysis (Purna Chandra Rao et al. 2006). Additionally, zeolite 4A has a high selectivity for metal ion removal, and the exchanged sodium ions are nontoxic. For these purposes, this zeolite has been widely used in both laboratories and industry (Hui and Chao 2006). Zeolite 4A has been synthesized successfully and used to remove Cr3+ in our previous work (Lei et al. 2016).
Hui et al. absorbed Cu(II) using zeolite 4A, and the absorption efficiency was greater than 90% (Hui et al. 2005). However, to reduce the operational cost and adsorbent material loss, heavy metal adsorbents need to be recycled multiple times. Unfortunately, there has been little research on the desorption of the absorbed Cu(II) zeolite. It was reported that the formation constant for Cu(EDTA)2− is as large as 6.3 × 1018, (Skoog et al. 2013) indicating that it is a very stable complex. Mohsen-sia et al. removed Cu(II) with Na2EDTA with an efficiency as great as 99.5% (Mohsen-Nia et al. 2007). Therefore, it is feasible to desorb Cu(II) using Na2EDTA.
In this study, bauxite tailings were used to synthesize zeolite 4A, which was subsequently used to remove Cu(II) in the solution. The desorption of Cu(II) by Na2EDTA aqueous solutions was subsequently undertaken, and finally, the recycling of adsorption and desorption was studied. A simple and economic Cu(II) adsorption and desorption process was explored in this work.
Experimental
Prepared zeolite 4A
Bauxite tailings, produced by Henan Province of China, were used to supply aluminum and a portion of the silicon for the synthesis of zeolite 4A powders. Sodium metasilicate nonahydrate (Na2SiO3·9H2O, SCRC, AR) was used as a supplementary silicon and sodium source. Calcium carbonate (CaCO3, SCRC, AR) and sodium hydroxide (NaOH pellets, SCRC, AR) were used as the fused salt. The synthesis of zeolite 4A from bauxite tailings was conducted as reported in the literature (Lei et al. 2016).
Absorption of Cu(II) with zeolite 4A
A series of tests were performed to investigate whether the Cu(II) could be removed by zeolite 4A, starting with the preparation of different concentrations of Cu(II) solutions. A specified mass of zeolite 4A and 100 mL of Cu(II) solution of a specified concentration as well as pH value were directly added to the 200 mL beakers. The mixture was allowed to react at ambient temperature for 2 h with continuous stirring at 300 r min−1. Afterwards, the mixture was filtered, and the concentration of Cu(II) remaining in the solution was measured. The removal efficiency of Cu(II) was calculated by the following formula
where c 0 is the initial Cu(II) concentration (mg L−1), and c e is the final Cu(II) concentration (mg L−1).
The initial pH value influenced the removal efficiency. The effect of initial pH value on the removal of Cu(II) by zeolite 4A was investigated using the solution with 100 mg L−1 Cu(II) solution. The initial pH values were adjusted to 4.0, 5.0, 6.0, and 7.0, respectively, using 0.1 mol L−1 sulfuric acid and 0.1 mol L−1 sodium hydroxide solutions. Simultaneously, a series of blank experiments were done to investigate the effects of Cu(OH)2 generated as the pH value increased. Sodium hydroxide solution was used instead of zeolite 4A to adjust the initial pH values, and the concentration of residual Cu(II) in the solution was measured to evaluate the mass of the copper precipitation. Finally, the kinetic study was undertaken to investigate the time required to achieve maximum removal efficiency.
Desorption of Cu(II) by Na2EDTA
A study of desorption efficiency and recovery percentage of zeolite 4A by Na2EDTA solution after Cu(II) absorption was performed. The study primarily investigated the effects of the initial concentration of Na2EDTA and pH value on the desorption of Cu(II). The concentrations of Na2EDTA were 0.02, 0.05, 0.1, 0.15, and 0.2 mol L−1, respectively. The initial pH values of the Na2EDTA solutions were adjusted to 6.0, 7.0, 8.0, 9.0, 10.0, and 11.0 respectively, using 0.1 mol L−1 sulfuric acid and 0.1 mol L−1 sodium hydroxide solutions. The mixtures were subsequently allowed to react at ambient temperature for specified times with continuous stirring at 300 r min−1. Finally, the mixtures were filtered, and the concentration of Cu(II) remaining in the solution was measured. The precipitate was dried at 80 °C for 3 h and weighed. The recovered zeolite was again used to remove Cu(II) as described in the “Absorption of Cu(II) with zeolite 4A” section, and this procedure was repeated three times.
The desorption efficiency of Cu(II) by Na2EDTA solution was calculated by the following equation
where m 0 is the amount of Cu absorbed on the zeolite (g), and m c is the amount of Cu remained in the recovered zeolite after desorption (g), herein including precipitated Cu(OH)2 and the unreacted Cu–zeolite.
The recovery percentage of zeolite was calculated by Eq. (3)
where M Z is the initial weight of zeolite 4A used (g), and M e is the weight of zeolite recovered (g).
Characterization
Solid powders were characterized by the X-ray diffraction (XRD, Rigaku Dmax-2500 diffractometer using Cu Kα radiation from Rigaku Company Japan). The chemical compositions of the solid powders were measured by an X-ray fluorescence spectrometer (XRF-1800, Japan). A field emission scanning electron microscope equipped with an X-ray energy-dispersive spectroscope (FESEM, Supra-55, Carl-Zeiss Microscopy, Oberkochen, Germany) was employed to evaluate the morphology; an accelerating voltage of 10 kV and high vacuum of ca. 1.0×10–5 mbar were generally used. The pH value of the solutions was measured with pH meter (PHS-25C). UV–Vis spectrophotometry was used to detect the concentrations of Cu(II) in the solution (Hui et al. 2005; Lao-Luque et al. 2014).
Results and discussion
Phase and microstructure characterization of zeolite 4A
X-ray diffraction (XRD) analysis in Fig. 1a was performed to characterize the phase of zeolite 4A prepared from bauxite tailings. The sample was identified as pure monocrystalline zeolite 4A. Figure 1b shows that the microstructure of zeolite 4A sample was a chamfering cubic shape, and the average diameter of the as-prepared samples was approximately 2 μm.
Absorption of Cu(II) by zeolite 4A
Dependence of the removal of Cu(II) by zeolite 4A on the initial pH value
It is well known that the Cu(OH)2 sediment is generated when the pH value of the solution is above 6.0, whereas the zeolite 4A would be dissolved at lower pH values; an initial pH value range of 4.0 to 7.0 was addressed (Lao-Luque et al. 2014). Figure 2 shows the removal efficiency of Cu(II) ions at a specified pH value with a zeolite 4A mass of 100 mg, and the Cu(II) concentration was 100 mg L−1 in the solution. At lower pH values (pH < 5.0), an excess of H3O+ could compete with Cu(II) for bonding sites, decreasing removal efficiency of Cu(II) by zeolite 4A. To the contrary, when the pH value was increased to 7.0, the Cu(II) removal efficiency of zeolite 4A decreased from 69.8 to 32.1%. This finding was ascribed to the fact that a large proportion of Cu(II) could generate Cu(OH)2 precipitate when the solution pH value was relatively high, leading to a decrease in removal efficiency of Cu(II) by zeolite 4A. Correspondingly, from the contrast experimental results, the removal of Cu(II) due to Cu(OH)2 precipitation was shown to be no more than 5% in an initial pH value range between 4.0 and 5.0, which had little effect on the removal efficiency of Cu(II) by zeolite 4A. However, the precipitation markedly increased to 43.8% at an initial pH of 7.0, which seriously reduced the removal efficiency of Cu(II) by zeolite 4A. Hence, the optimal initial pH value for Cu(II) removal by zeolite 4A was 5.0.
Maximum removal efficiency of Cu(II)
To explore whether the concentration of Cu(II) remaining in the solution after adsorption was lower than the national emission standards, excessive zeolite was used to enhance the removal efficiency of Cu(II). A removal efficiency of 5 mg L−1 Cu(II) solution for different zeolite doses is presented in Fig. 3. As shown, the removal efficiency of Cu(II) gradually increased with increasing amount of zeolite 4A and reached a maximum of 96.2% when the dose of zeolite 4A was 0.175 g L−1. The removal efficiency tended to be stable even as the dose of zeolite 4A was further increased. This stability is observed primarily because the available superficial area of zeolite per unit volume in the solution was kept constant when the amount of zeolite 4A was over a certain amount (Lei et al. 2016). It could be seen that there was 0.19 mg L−1 Cu(II) remaining in the solution under the optimal removal conditions, which was considerably less than the discharge standard of Cu(II) in industrial wastewater (< 1 mg L−1) (Xiong et al. 2012), indicating that Cu(II) could be effectively removed by the zeolite 4A prepared from bauxite tailings.
Dependence of removal efficiency of Cu(II) on reaction time
To remove maximum Cu(II) by zeolite 4A in a minimum time, a series of dynamic experiments were performed with 100 mg L−1 Cu(II) solution and an initial pH value of 5.0; the results are shown in Fig. 4. It could be seen that the Cu(II) removal efficiency increased rapidly to 65% in the first half hour and afterwards became essentially constant, indicating that the removal of Cu(II) mainly occurred within the first 30 min.
Phase and microstructure characterization of Cu–zeolite samples
Figure 5a shows the XRD patterns of the solid product obtained after the zeolite 4A absorbed Cu(II). Only one phase, also a type of zeolite A (JCPDS card 01-084-0389) named Cu–zeolite, is presented in Fig. 5a. This product’s chemical formula is Cu8(OH)2.25Al12Si12O48, demonstrating that Na+ was exchanged by Cu(II). Figure 5b shows the morphology of the Cu–zeolite sample. It could be observed that the surface of the Cu–zeolite sample was very rough, and the chamfering cubic shape was nearly absent compared with the green zeolite 4A.
Surface area measurements
The total surface area of synthesized powder was measured using Standard Volumetric Method by nitrogen adsorption at 77 K and application of BET equation by means of Nova 3200 BET instrument, Quantachrome Corporation, USA.
The specific surface area (m2 g−1) of the synthesized solid powder was measured, and the results indicated that the specific surface area of synthetic 4A zeolite blend was 41.26 m2 g−1 and the specific surface area of Cu–zeolite was 33.45 m2 g−1. The decrease of specific surface area can be ascribed to the adsorption of Cu(II) on the 4A zeolite surface.
Desorption of Cu–zeolite by Na2EDTA solution
Dependence of the desorption of Cu–zeolite by Na2EDTA solution on the initial pH value
Given that zeolite 4A was synthesized in the NaOH solution and that H3O+ would react with zeolite 4A, the minimum pH value was limited to 6.0. Figure 6 shows the desorption efficiency of Cu(II) after 3.5 h desorption when the Cu–zeolite mass was 1 g and initial Na2EDTA concentration was 0.05 mol L−1.
As shown in Fig. 6, the Cu(II) desorption efficiency was directly pH dependent. When the pH value increased from 6.0 to 11.0, the Cu(II) desorption efficiency was decreased from 95.8 to 42.6%. This finding was partly attributable to the presence of Cu8(OH)2.25Al12Si12O48, which would be dissolved at a lower pH value, after which the dissolved Cu(II) could again be replaced by Na+. This approach results in regeneration of zeolite 4A, lower pH value, and higher Cu(II) desorption efficiency. However, the changing pH value had little influence on the recovery percentage. Because Na+ could also be replaced by H3O+ in acid solution when the pH value was lower than 3, it was not suitable for zeolite 4A recovery. Considering the effects on both the desorption efficiency and the recovery, in the following experiments, the initial pH value was fixed at 8.0, at which desorption efficiency and recovery percent were 91.8 and 75.2%, respectively.
Initial concentration of Na2EDTA versus the desorption of Cu(II)
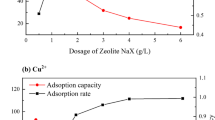
To identify the influence of initial Na2EDTA solution concentration on the desorption efficiency and recovery percentage of zeolite, a series of comparative experiments with different Na2EDTA concentrations were performed at an initial pH of 8.0; the results are presented in Fig. 7.
It could be seen that the zeolite recovery dramatically decreased from 73.6 to 42.4% with increasing initial Na2EDTA concentration (Fig. 7a), indicating that much more zeolite could be recovered at lower initial Na2EDTA concentration. However, 8 h is required to obtain a high recovery, as shown in Fig. 7b. Desorption efficiency was nearly constant at 85% and not influenced by the Na2EDTA concentration. The initial concentration was thus fixed at 0.05 mol L−1. Under this condition, the zeolite recovery was 69.8%, the equilibrium time was shortened to 3.5 h, and the Cu(II) desorption efficiency was 85.9%. Hence, zeolite 4A was successfully regenerated by Na2EDTA solution.
Cu(II) absorption and desorption by recycled zeolite 4A
Effect of recycling times on removal efficiency and recovery of Cu(II)
One hundred milligrams of recovered zeolite 4A after various reaction times was used to remove Cu(II) from 100 mg L−1 solutions at an initial pH = 5 for 2 h. Figure 8 shows that the removal efficiency decreased gradually after multiple recycling cycles. This finding was primarily attributable to a small amount of copper ion remaining on the surface of zeolite that could not be desorbed completely, which thus decreased the removal efficiency. A removal efficiency of Cu(II) of 78.9% could be investigated even after recycling the zeolite 4A three times, indicating that the recycled zeolite still showed good Cu(II) adsorption capacity and can repeatedly remove Cu(II). It was also observed that the recovery percent decreased from 72.8 to 46.9% with the increasing recycle times because the zeolite is dissolved at relatively low pH values. In other words, a partial zeolite 4A dissolved in Na2EDTA solution.
Phase and microstructure characterization of recovered zeolites
XRD and SEM were carried out on the recovered zeolites and compared with the fresh zeolite 4A synthesized from bauxite tailings.
It could be observed that the recovered zeolites were also pure, single-phase, and highly crystalline after recycling three times, as shown in Fig. 9a. However, the surface of the recovered zeolites was rough and defective compared with the fresh zeolite 4A. With increasing recycle time, the defects became more evident, as shown in Fig. 9b. This finding was primarily attributable to a small amount of copper ion remaining on the surface of zeolite and the increasing quantity of Cu(II) ions on the surface of zeolite 4A.
The recovered zeolites were used to absorb Cu(II), and the Cu(II) removal efficiency and recovery percentages of zeolite 4A were 78.9 and 46.9% after three recycles, respectively, indicating that the recovered zeolites have good adsorption capacity and can repeatedly absorb Cu(II).
Conclusions
The present study showed that the Cu(II) was removed by zeolite 4A and desorbed by Na2EDTA. The initial pH value and Cu(II) concentration had a significant effect on the removal efficiency of Cu(II) by zeolite 4A and reached a maximum removal efficiency at pH value of 5 and initial Cu(II) concentration of 5 mg L−1. The concentration of Cu(II) remaining in the solution after the removal reaction was far below the discharge standard of Cu(II) in industrial wastewater. Desorption efficiency of Cu(II) reached 91.4%, and the recovery of zeolite 4A was 72.8% for the first desorption at the pH value of 8 and Na2EDTA concentration of 0.02 mol L−1. However, 8 h is required to reach equilibrium. An initial concentration of 0.05 mol L−1 Na2EDTA solution was recommended, which required 3.5 h to reach equilibrium. Desorption efficiency and recovery of zeolite 4A were 85.9 and 73.6%, respectively. The removal efficiency of Cu(II) reached 78.9%, and the recovery of zeolite was 46.9% after three zeolite 4A recycles, indicating that the recovered zeolites have good adsorption capacity and can repeatedly absorb Cu(II).
References
Ackley MW, Rege SU, Saxena H (2003) Application of natural zeolites in the purification and separation of gases. Microporous Mesoporous Mater 61:25–42
Baldansuren A, Eichel RA, Roduner E (2009) Nitrogen oxide reaction with six-atom silver clusters supported on LTA zeolite. Phys Chem Chem Phys 11:6664–6675
Chang JH, Ellis AV, Tung CH, Huang WC (2010) Copper cation transport and scaling of ionic exchange membranes using electrodialysis under electroconvection conditions. J Membr Sci 361:56–62
Dabrowski A, Hubicki Z, Podkościelny P, Robens E (2004) Selective removal of the heavy metal ions from waters and industrial wastewaters by ion-exchange method. Chemosphere 56:91–106
Du T, Liu L, Xiao P, Che S, Wang H (2014) Preparation of zeolite NaA for CO2 capture from nickel laterite residue. Int J Miner Metall Mater 21:820–825
Erdem E, Karapinar N, Donat R (2004) The removal of heavy metal cations by natural zeolites. J Colloid Interf Sci 280:309–314
Hui KS, Chao CYH (2006) Effects of step-change of synthesis temperature on synthesis of zeolite 4A from coal fly ash. Microporous Mesoporous Mater 88:145–151
Hui KS, Chao CYH, Kot SC (2005) Removal of mixed heavy metal ions in wastewater by zeolite 4A and residual products from recycled coal fly ash. J Hazard Mater 127:89–101
Kaduková J, Virčíková E (2005) Comparison of differences between copper bioaccumulation and biosorption. Environ Int 31:227–232
Lao-Luque C, Solé M, Gamisans X, Valderrama C, Dorado AD (2014) Characterization of chromium (III) removal from aqueous solutions by an immature coal (leonardite). Toward a better understanding of the phenomena involved. Clean Techn Environ Polcy 16:127–136
Lazaridis NK, Keenan H (2010) Chitosan heads as barriers to the transport of azo dye in soil column. J Hazard Mater 173:144–150
Lei P-C, Shen X-J, Guo M, Zhang M (2016) An improved implementable process for the synthesis of zeolite 4A from bauxite tailings and its Cr3+. Int J Miner Metall Mater 23:850–857
Liu X, Wang Y, Cui X, He Y, Mao J (2013) Influence of synthesis parameters on NaA zeolite crystals. Powder Technol 243:184–193
Matlock MM, Howerton BS, Atwood DA (2002) Chemical precipitation of heavy metals from acid mine drainage. Water Res 36:4757–4764
Mohsen-Nia M, Montazeri P, Modarress H (2007) Removal of Cu2+ and Ni2+ from wastewater with a chelating agent and reverse osmosis processes. Desalination 217:276–281
Purna Chandra Rao G, Satyaveni S, Ramesh A, Seshaiah K, Murthy KSN (2006) Sorption of cadmium and zinc from aqueous solutions by zeolite 4A, zeolite 13X and bentonite. J Environ Manag 81:265–272
Sherry HS, Walton HF (1967) The ion-exchange properties of zeolites. II. Ion exchange in the synthetic zeolite Linde 4A. J Phys Chem 71:1457–1465
Skoog DA, West DM, Holler FJ, Crouch S (2013) Fundamentals of analytical chemistry. Nelson Education
Upadek H, Smulders E, Poethkow J (1991) Laundry detergent additive containing zeolite, polycarboxylate, and perborate. Zeolites 11:90
Uriu-Adams JY, Keen CL (2005) Copper, oxidative stress, and human health. Mol Asp Med 26:268–298
Vareltzis P, Kikkinides ES, Georgiadis MC (2003) On the optimization of gas separation processes using zeolite membranes. Chem Eng Res Des 81:525–536
Wang S-B, Peng Y-L (2010) Natural zeolites as effective adsorbents in water and wastewater treatment. Chem Eng J 156:11–24
Xiong C, Chen X, Liu X (2012) Synthesis, characterization and application of ethylenediamine functionalized chelating resin for copper preconcentration in tea samples. Chem Eng J 203:115–122
Acknowledgements
The authors would like to thank the National Science Foundation of China (Nos. 51672025, 51572020, 51372019), the National High Technology Research and Development of China (863 program) (No. 2013AA032003), and the Shanxi Collaborative Innovation Center of High Value-added Utilization of Coal-related Wastes for the financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Guilherme L. Dotto
Rights and permissions
About this article
Cite this article
Shen, X., Qiu, G., Yue, C. et al. Multiple copper adsorption and regeneration by zeolite 4A synthesized from bauxite tailings. Environ Sci Pollut Res 24, 21829–21835 (2017). https://doi.org/10.1007/s11356-017-9824-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-9824-5