Abstract
We obtained an approximate solution of the Schrodinger equation for the modified Kratzer potential plus screened Coulomb potential model, within the framework of Nikiforov–Uvarov method. The bound state energy eigenvalues for N2, CO, NO, and CH diatomic molecules were computed for various vibrational and rotational quantum numbers. Special cases were considered when the potential parameters were altered, resulting into modified Kratzer potential, screened Coulomb potential, and standard Coulomb potential, respectively. Their energy eigenvalues expressions and numerical computations agreed with the already existing literatures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Researchers have devoted their interest over the years, towards investigating the bound state solutions of nonrelativistic wave equations for different potentials [1, 2]. A few of these potentials have been solved exactly [3], while others can only be solved approximately [4,5,6,7,8,9,10], with the use of different approximation schemes [11,12,13]. Also, different methods have been employed to obtain the solutions of the nonrelativistic wave equations with a chosen potential model. These include the factorization method [14], functional analysis approach [15,16,17], supersymmetry quantum mechanics (SUSYQM) [18,19,20,21], asymptotic iteration method (AIM) [22, 23], algebraic approach [24], exact and proper quantization rules [25, 26], Laplace transformation [27], Nikiforov–Uvarov method (NU) [28,29,30,31,32,33,34], and others.
The Kratzer potential [35] is mostly applied in atomic physics, molecular physics, and quantum chemistry [36]. It is used to describe the interactions of molecular structure in quantum mechanics. The Kratzer potential is made up of a long-range attraction and a repulsive part. The integration of these parts makes this potential reliable in terms of its vibrational and rotational energy eigenvalues [37, 38]. The Kratzer potential is known to approach infinity when the internuclear distance approaches zero, due to the repulsion that exist between the molecules of the potential. As the internuclear molecular distance approaches infinity, the potential decomposes to zero [39, 40].
The screened Coulomb potential, which is also known as the Yukawa potential, is greatly important with applications cutting across nuclear physics and condensed matter physics [41]. The screened Coulomb potential is used mostly in short-range interactions [42,43,44]. The screened Coulomb potential is known to be the potential of a charged particle in a weakly non-ideal plasma. It also describes the charged particle effects in a sea of conduction electrons in solid-state physics [45].
Recently, Bayrak et al. [38] have presented an exact analytical solution of the radial Schrodinger equation for the Kratzer potential using the asymptotic iteration method (AIM). The exact bound state energy eigenvalues (Enl) and corresponding eigenfunctions (Rnl) were calculated for various values of n and l quantum numbers for selected diatomic molecules. In another development, a noncentral modified Kratzer potential was considered and the solutions of the Schrodinger equation obtained using the factorization method [36]. An approximate solution of the Schrodinger equation interacting with an inversely quadratic Yukawa potential was obtained using SUSYQM [46], where the screened Coulomb potential was obtained as a special case by varying the potential strength. Also, an approximate analytical solution of the radial Schrodinger equation for the screened Coulomb potential was obtained, with energy eigenvalues and its corresponding eigenfunctions computed in closed forms [47]. With the above-mentioned studies on these different potentials and their lofty importance, we seek to investigate the bound state solutions of the Schrodinger equation with the combined modified Kratzer and screened Coulomb potential of the form:
where De is the dissociation energy, re is the equilibrium internuclear separation, A is the depth of the potential, and α is the range of the potential. It can be deduced that when De = 0, the above combined potential reduces to the screened Coulomb potential. When De = 0 and \( \alpha \to 0 \), the potential of Eq. 1 reduces to the Coulomb potential. Also, when A = 0, Eq. 1 reduces to the modified Kratzer potential. Using the conventional NU method, we derive the \( \ell - \) wave bound state solutions and their eigenfunctions of the Schrodinger equation for the modified Kratzer potential plus screened Coulomb potential, analytically and numerically. Special cases are also considered, and our results are compared with existing literatures for confirmation sake.
2 Bound state solution
The radial Schrodinger equation is given as [48]:
where \( \mu \) is the reduced mass, \( E_{nl} \) is the rotational–vibrational energy spectra of the diatomic molecules, \( \hbar \) is the reduced Planck’s constant, and n and ℓ are the radial and orbital angular momentum quantum numbers, respectively (or vibration–rotation quantum numbers in quantum chemistry) [49]. Substituting Eq. (1) into Eq. (2) gives:
We employ the approximation scheme to get rid of the centrifugal barrier as [50]
Sustituting Eqs. (4) and (5) into Eq. (3), we have
Equation (6) can be simplified into the form
where
By using the coordinate transformation
we obtain the differential equation of the form
Comparing Eqs. (10) and (41) (see the appendix section), we have the following parameters
Substituting these polynomials into Eq. (48), we obtain \( \pi (s) \) to be
where
To find the constant k, the discriminant of the expression under the square root of Eq. (12) must be equal to zero. As such, we have that
Substituting Eq. (14) into Eq. (12) yields
From the knowledge of NU method, we choose the expression \( \pi (s)_{ - } \) in which the function \( \tau (s) \) has a negative derivative. This is given by
with \( \tau (s) \) being obtained as
Referring to Eq. (49), we define the constant \( \lambda \) as
Substituting Eq. (18) into Eq. (50) and carrying out simple algebra, where
and
We have
where
Substituting Eqs. (8) and (22) into Eq. (21) yields the energy eigenvalue equation of the modified Kratzer potential plus screened Coulomb potential in the form
The corresponding wave functions can be evaluated by substituting \( \pi (s)_{ - } \,\,{\text{and}}\,\,\sigma (s) \) from Eqs. (15) and (11), respectively, into Eq. (44) and solving the first-order differential equation. This gives
The weight function \( \rho (s) \) from Eq. (46) can be obtained as
From the Rodrigues relation of Eq. (45), we obtain
where \( P_{n}^{{\left( {\theta ,\vartheta } \right)}} \) is the Jacobi polynomial.
Substituting \( \varPhi (s)\,\,{\text{and}}\,\,y_{n} (s) \) from Eqs. (24) and (27), respectively, into Eq. (42), we obtain
where
From the definition of the Jacobi polynomials [51],
In terms of hypergeometric polynomials, Eq. (28) can be written as
3 Special cases
In this section, we make some adjustments of constants in Eq. (1) to have the following cases:
3.1 Screened Coulomb potential
If De = 0 in Eq. (1), we can obtain the screened Coulomb potential of the form
From Eq. (23), the energy eigenvalue equation for the screened Coulomb potential reduces to
Equation (33) is in full agreement with the results in Ref. [46, 47].
3.2 Standard Coulomb potential
We can rewrite Eq. (23) to have
As \( \alpha \to 0\,\,{\text{and}}\,\,D_{\text{e}} = 0, \) Eq. (1) reduces to the standard Coulomb potential of the form
Its energy eigenvalue equation can be deduced from Eq. (34) as
The result of Eq. (36) is very consistent with the result obtained in Eq. (101) of Ref. [52]. Also, comparing our work with the result obtained in Eq. 33 of Ref. [46], it is worthy to note here that the authors in Ref. [46] failed to set the screening parameter (i.e. \( \delta \) in Eq. (1) of Ref. [46]) equal to zero. If that is done, then there would be a clear consistency in the energy eigenvalue equation obtained in Eq. (36) of our computation and Eq. (33) of Ref. [46].
3.3 Modified Kratzer potential
When the parameter A is set to zero, Eq. (1) reduces the potential to the modified Kratzer potential of the form
And its energy eigenvalue equation can also be deduced from Eq. (23) as
Rewriting Eq. (38), we have
As \( \alpha \to 0, \) we obtain the energy eigenvalue for the modified Kratzer potential to be
The result in Eq. (40) is very consistent with result of Eq. (14) in Ref. [53].
4 Results and discussion
In our study, the energy eigenvalues of the modified Kratzer potential plus the screened Coulomb potential are computed for N2, CO, NO, and CH diatomic molecules using Eq. (23), with the aid of the spectroscopic parameters given in Table 1. The explicit values of these energies for different vibrational and rotational quantum numbers are presented in Table 2. For validity purposes, we have also computed the energy eigenvalues of the modified Kratzer potential for the selected diatomic molecules, using the reduced energy equation given in Eq. (40) as a special case. Our results shown in Tables 3 and 4 are in good agreement with the results given in Ref. [53].
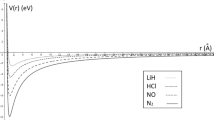
We have plotted the shape of the modified Kratzer potential plus screened Coulomb potential for the different diatomic molecules considered, as shown in Fig. 1. This figure gives an insight into the behaviour of the combined potential when r = re. Also, the variation in the energy eigenvalues with different parameters of the combined potential such as \( D_{\text{e}} ,\,\,r_{\text{e}} ,\,\,\alpha ,\,\,{\text{and}}\,\,A \) is shown in Figs. 2–5, respectively, for various values of n and l quantum numbers. In these figures, there is an increase in energy eigenvalues as the various parameters increase. In Figs. 2 and 3, there exists an asymptotic convergence at zero energy. In Fig. 4, the increase in energy tends to spread out from zero position for different vibrational quantum numbers. We also observe a uniform increase in energy as the parameter “A” increases for the different vibrational quantum numbers.
5 Conclusion
In this study, the approximate bound state solutions of the Schrodinger equation with the modified Kratzer molecular potential plus screened Coulomb potential model were obtained, via the Nikiforov–Uvarov method. The energy eigenvalues of the selected diatomic molecules (N2, CO, NO, and CH) were computed, and a special case was considered. Our results are consistent with the results in available literatures. The shape of the combined potential model for the diatomic molecules was plotted, and this gives a better understanding to the behaviour of the selected diatomic molecules when the equilibrium bond length equals the interatomic distance of the molecules. The variation in the combined energy eigenvalue with the potential parameters (De, re, α, and A) was also plotted. It was discovered that the energy eigenvalues increase as the various potential parameters increase. This study can be extended even to the relativistic regime using other methods [54,55,56]. Recently, there has been investigation into areas covering vibrational partition function [57,58,59] and thermochemical properties of diatomic molecules [60,61,62]. Worth mentioning is the current research done on the prediction of enthalpy and entropy of gaseous dimers [63, 64].
References
H Hassanabadi, E Magshoodi and S Zarrinkamar Few-Body Syst.53 271 (2012)
R Sever, C Tezan, O Yesiltas and M Bucurgat Int. J. Theor. Phys.49 2243 (2008)
A N Ikot, L E Akpabio and E B Umoren J. Sci. Res.3 25 (2011)
O Bayrak and I Boztosun Phys. Scri.76 92 (2007)
H Egrifes, D Demirhan and F Buyukkilic Phys. Lett. A.275 229 (2000)
S M Ikhdair and R Sever Ann. Phys.18 189 (2009)
C Onate, K Oyewumi and B Falaye Few-Body Syst.55 61 (2014)
M Hamzavi, K E Thylwe and A Rajabi Commun. Theor. Phys.60 1 (2013)
S M Ikhdair and B J Falaye Chem. Phys.421 84 (2013)
C Y Chen, F L Lu and D S Sun Cent. Eur. J. Phys.6 884 (2008)
C Pekeris Phys. Rev.45 98 (1934)
W C Qiang and S H Dong Phys. Lett. A.363 169 (2007)
C Berkdemir and J Han Chem. Phys. Lett.409 203 (2005)
S H Dong Factorization Method in Quantum Mechanics (Armsterdam: Springer) (2007)
J Y Liu, G D Zhang and C S Jia Phys. Lett. A.377 1444 (2013)
H M Tang, G C Liang, L H Zhang, F Zhao and C S Jia Can. J. Chem.92 341 (2014)
C S Jia and Y Jia Eur. Phys. J. D.71 3 (2017)
A N Ikot, H P Obong, T M Abbey, S Zare, M Ghafourian and H Hassanabadi Few-Body Systems (Berlin: Springer) (2016)
C A Onate, M C Onyeaju, A N Ikot and J O Ojonubah Chin. J. Phys.000 1 (2016)
C A Onate, A N Ikot, M C Onyeaju and M E Udoh Karbala Int. J. Modern Sci.3 1 (2017)
A N Ikot, O A Awoga, A D Antia, H Hassanabadi and E Maghsodi Few-Body Syst.54 2041 (2013)
H Ciftci, R L Hall and N Saad J. Phys. A: Math. Gen.36 11807 (2003)
B J Falaye Cent. Eur. J. Phys.10 960 (2012)
M R Setare and E Karimi Phys. Scri.75 90 (2007)
W C Qiang and S H Dong Eur. Phys. Lett.45 10003 (2010)
S M Ikhdair and R Sever J. Math. Chem.45 1137 (2009)
G Chen Phys. Lett. A.326 55 (2004)
C Berkdemir J. Math. Phys.46 13 (2009)
C Tezcan and R Sever Int. J. Theor. Phys.48 337 (2009)
A N Ikot, I O Akpan, T M Abbey and H Hassanabadi Commun. Theor. Phys.65 569 (2016)
C A Onate and J O A Idiodi Chin. J. Phys.53 7 (2015)
A N Ikot, O A Awoga, H Hassanabadi and E Maghsodi Commun. Theor. Phys.61 457 (2014)
B J Falaye, K J Oyewumi and M Abbas Chin. Phys. B22 110301 (2013)
A D Antia, A N Ikot, H Hassanabadi and E Maghsodi Indian J. Phys.87 1133 (2013)
A Kratzer Z. Phys.3 289 (1920)
J Sadeghi Acta Phys. Pol.112 23 (2007)
R J Le Roy and R B Bernstein J. Chem. Phys.52 3869 (1970)
O Bayrak, I Boztosun and H Cifti Int. J. Quant. Chem.107 540 (2007)
N Saad, R J Hall and H Cifti Cent. Eur. J. Phys.6 717 (2008)
H Hassanabadi, H Rahimov and S Zarrinkamar Adv. High Energy Phys.458087 1 (2011)
S Dong, G H Sun and S H Dong Int. J. Mod. Phys. E22 1350036 (2013)
S M Ikhdair and R Sever J. Mol. Struct: Theochem.809 103 (2007)
E Z Liverts, E G Drukarev, R Krivec and V B Mandelzweig Few-Body Syst.44 367 (2008)
E Maghsoodi, H Hassanabadi and O Aydodu Phys. Scri.86 015005 (2012)
J P Edwards, U Gerber, C Schubert, M A Trejo and A Weber Prog. Theor. Exp. Phys.0873A01 1 (2017)
C A Onate and J O Ojonubah J. Theor. Appl. Phys.10 21 (2016)
H Hamzavi, M Movahedi, K E Thylwe and A A Rajabi Chin. Phys. Lett.29 080302 (2012)
A N Ikot, E O Chukwuocha, M C Onyeaju, C N Onate, B I Ita and M E Udoh Pramana J. Phys.90 22 (2018)
L H Zhang, X P Li and C S Jia Int. J. Quant. Chem.111 1870 (2011)
R L Greene and C Aldrich Phys. Rev. A14 2363 (1976)
M Abramowitz and I A Stegun Handbook of Mathematical Functions with Formulas, Graphs and Mathematical Tables (New York: Dover) (1964)
C Birkdemir Application of the Nikiforov–Uvarov Method in Quantum Mechanics Theor. Concept Quant. Mech. (ed.) M R Pahlavani Chapter 11 (2012)
C Berkdemir, A Berkdemir and J Han Chem. Phys. Lett.417 326 (2006)
H Hassanabadi, E Maghsoodi, S Zarrinkamar and H Rahimov Chin. Phys. B12 120302 (2012)
E Maghsoodi, H Hassanabadi, H Rahimov and S Zarrinkamar Chin. Phys. C37 043105 (2013)
H Hassanabadi, E Maghsoodi and S Zarrinkamar Chin. Phys. C37 113104 (2013)
U S Okorie, E E Ibekwe, A N Ikot, M C Onyeaju and E O Chukwuocha J. Kor. Phys. Soc.73 1211 (2018)
U S Okorie, A N Ikot, M C Onyeaju and E O Chukwuocha Rev. Mex. de Fis.64 608 (2018)
U S Okorie, A N Ikot, M C Onyeaju and E O Chukwuocha J. Mol. Mod.24 289 (2018)
X L Peng, R Jiang, C S Jia, L H Zhang and Y L Zhao Chem. Eng. Sci.190 122 (2018)
C S Jia, C W Wang, L H Zhang, X L Peng, H M Tang, J Y Liu, Y Xiong and R Zeng Chem. Phys. Lett.692 57 (2018)
R Jiang, C S Jia, Y Q Wang, X L Peng and L H Zhang Chem. Phys. Lett.715 186 (2019)
C S Jia, C W Wang, L H Zhang, X L Peng, H M Tang and R Zeng Chem. Eng. Sci.183 26 (2018)
C S Jia, R Zeng, X L Peng, L H Zhang and Y L Zhao Chem. Eng. Sci.190 1 (2018)
A F Nikiforov, V B Uvarov Special Functions of Mathematical Physics (ed.) A Jaffe (Germany: Birkhauser Verlag Basel) p 317 (1998)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix: Review of Nikiforov–Uvarov (NU) method
Appendix: Review of Nikiforov–Uvarov (NU) method
The NU method was proposed by Nikiforov and Uvarov [65] to transform Schrodinger-like equations into a second-order differential equation via a coordinate transformation \( s = s(r), \) of the form
where \( \tilde{\sigma }(s),\,\,\sigma (s) \) are polynomials, at most second degree and \( \tilde{\tau }(s) \) is a first-degree polynomial. The exact solution of Eq. (41) can be obtained by using the transformation
This transformation reduces Eq. (41) into a hypergeometric-type equation of the form
The function \( \varPhi (s) \) can be defined as the logarithm derivative [65]
with \( \pi (s) \) being at most a first-degree polynomial. The second part of \( \psi (s) \) being \( y_{n} (s) \) in Eq. (42) is the hypergeometric function with its polynomial solution given by Rodrigues relation
Here, \( B_{n} \) is the normalization constant and \( \rho (s) \) is the weight function which must satisfy the condition
with
It should be noted that the derivative of \( \tau (s) \) with respect to s should be negative. The eigenfunctions and eigenvalues can be obtained using the definition of the following function \( \pi (s) \) and parameter \( \lambda \), respectively:
and
The value of \( k \) can be obtained by setting the discriminant of the square root in Eq. (48) equal to zero. As such, the new eigenvalue equation can be given as
Rights and permissions
About this article
Cite this article
Edet, C.O., Okorie, U.S., Ngiangia, A.T. et al. Bound state solutions of the Schrodinger equation for the modified Kratzer potential plus screened Coulomb potential. Indian J Phys 94, 425–433 (2020). https://doi.org/10.1007/s12648-019-01477-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12648-019-01477-9