Abstract
Type 2 diabetes mellitus (T2DM) is considered one of the most common disorders worldwide. Although several treatment modalities have been developed, the existing interventions have not yielded the desired results. Therefore, researchers have focused on finding treatment choices with low toxicity and few adverse effects that could control T2DM efficiently. Various types of research on the role of gut microbiota in developing T2DM and its related complications have led to the growing interest in probiotic supplementation. Several properties make these organisms unique in terms of human health, including their low cost, high reliability, and good safety profile. Emerging evidence has demonstrated that three of the most important signaling pathways, including nuclear factor kappa B (NF-κB), phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt), and nuclear factor erythroid 2–related factor 2 (Nrf2), which involved in the pathogenesis of T2DM, play key functions in the effects of probiotics on this disease. Hence, we will focus on the clinical applications of probiotics in the management of T2DM. Then, we will also discuss the roles of the involvement of various probiotics in the regulation of the most important signaling pathways (NF-κB, PI3K/Akt, and Nrf2) involved in the pathogenesis of T2DM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM) is generally known as a chronic metabolic disorder triggered by genetic and environmental factors and has become one of the major global health problems, affecting all main sectors of societies and placing a great burden on the global economy [1]. According to a recent report, 463 million people are suffering from DM worldwide; if the trend continues uncontrolled, the numbers are anticipated to elevate to 578 million and 700 million by 2030 and 2045, respectively [2]. DM can be subdivided into three main classes: type 1 (T1DM), type 2 (T2DM), and gestational (GDM); among these, T2DM includes the massive majority (approximately 90%) of cases around the world [3]. T2DM is mainly characterized by hyperglycemia, low-grade inflammation, insulin resistance (IR), oxidative stress, dysfunction of pancreatic β-cells, and gut dysbiosis. These impairments can result in macrovascular (coronary heart disease, cardiomyopathy) and microvascular (retinopathy, nephropathy, neuropathy) complications [4]. Nowadays, there are still only two common approaches to the medical treatment of diabetes including oral anti-diabetic and insulin injection with their different adverse effects [5]. As a result, researchers’ attention has been paid to finding treatment choices with low toxicity and few adverse effects that could control T2DM efficiently.
In more recent years, accumulating evidence has demonstrated that gut microbiota plays a critical role in human health [6, 7]. Many studies have indicated that dysbiosis of the intestinal flora can result in intestinal problems and metabolic diseases, particularly T2DM. Besides, it has been elucidated that the resident microbiota associated with chronic inflammation contributes to the development of T2DM [8]. Moreover, the gut microbiota is changed in the progression of T2DM, and its comorbidities [9]. As a result, alteration of microbiota may be helpful for understanding and treatment of T2DM. In this line, it was reported that intestinal microbiota is also strongly conducive to increased adiposity, β-cell dysfunction, metabolic endotoxemia, inflammation, and oxidative stress [10]. Thus, by modifying the host’s gene expression and metabolic processes, reverse intestinal microbiota dysbiosis may affect the metabolism of diabetes-related organs and organs impacted by metabolic diseases.
A worldwide epidemic has developed over the past few years regarding T2DM management. A variety of therapeutic techniques have been developed, and many drugs have been recommended to promote glycemic control by improving insulin production and intake, reducing glucose production and absorption, inhibiting glucose reabsorption, and enforcing urinary glucose excretion [11]. Several different drugs are used to achieve these goals, such as thiazolidinediones (TZDs), α-glucosidase inhibitors, sulfonylureas, and biguanides, which are used in the treatment of hyperglycemia. The different anti-diabetic medications are known to cause some unpleasant side effects, including gastrointestinal problems, liver problems, and lactic acidosis [12]. The investigation of alternative methods focused on the gut microbiota indicated promising approaches for managing T2DM in the future [13]. Many studies have been conducted in the last two decades regarding the beneficial effects of gut microbiota in metabolic diseases such as T2DM [14].
The beneficial modulation of the intestinal microbiota can be achieved by administering probiotics [15]. Probiotics, which are live microorganisms capable of colonizing the human intestinal tract, have recently attracted much attention. Several properties make these organisms unique in terms of human health, including their low cost, high reliability, and good safety profile [16]. Nevertheless, the precise mechanisms by which probiotics are involved in improving various diseases, especially T2DM, are yet to be thoroughly investigated. Emerging data demonstrated that probiotics possess beneficial effects for relieving T2DM in the animal model and clinical experiments, including reduction of blood glucose levels, improvement of IR, modulation of the intestinal microbiota [17], amelioration of diabetes-related symptoms, and most importantly, regulation of pathways involved in the pathogenesis of T2DM, like nuclear factor kappa B (NF-κB), phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt), and nuclear factor (erythroid-derived 2)-like 2 (Nrf2) pathways [18].
NF-κB plays a crucial role in the early pathobiology of T2DM, as several cytokines, chemokines, and inflammatory molecules are activated when hyperglycemia is present in the body. There are certain DNA-binding proteins that are members of the NF-κB family that cause pro-inflammatory cytokines to be produced. Hence, several studies have proposed that NF-κB can be a candidate target for T2DM treatment [19]. Another important pathway involved in the pathogenesis of T2DM is the PI3K/AKT signaling pathway which mediates growth factor signals that are pivotal to organismal growth and various cellular events, such as glucose metabolism, lipid metabolism, and protein synthesis; thus, manipulation of the PI3K/AKT pathway and its downstream mediators is a favorable target for the management of T2DM [20]. In addition, Nrf2, with its negative regulator, Kelch-like ECH-associated protein 1 (Keap1), regulates genes in response to oxidative stress via the antioxidant response element (ARE). Multiple enzymes catalyze antioxidant reactions which include glutathione peroxidase (GPx), superoxide dismutase (SOD), catalase (CAT), heme oxygenase-1 (HO-1), NADPH-quinone oxidoreductase-1 (NQO-1), and glutamate cysteine ligase (GCL); these enzymes are found to be the downstream targets of Nrf2 [21]. By increasing phase II detoxification potential via modulating the Nrf2 pathway, the antioxidant defenses of the cells are enhanced, providing a novel therapeutic strategy for protection against insults like inflammation and oxidative stress, both as main mediators of T2DM [22]. Taken together, we will focus on the clinical applications of probiotics in managing of T2DM. Then, we will also discuss the roles of the involvement of various probiotics in regulating of the most important signaling pathways that contributed to the pathogenesis of T2DM.
Historical Background of Microbiota
According to its etymology, the word “probiotic” is derived from the Greek term “probios,” which means “for life.” The history of probiotics started over a century ago when Henry Tessler (1899) discovered Bifidobacterium in the intestines of breastfed infants and discovered that it could prevent diarrheal episodes in these infants [23]. The hypothesis that microorganisms can be used for health purposes by replacing gut flora with beneficial ones was first introduced by Elie Metchnikoff (1907) [24]. Shirota reported in the early 1930s that intestinal bacteria can survive in the gut passage and therefore developed the fermented milk containing Lactobacillus casei strain shirota, still widely referred to today as Yakult [25]. The term “probiotic” was first used by Lilly and Stillwell (1965) to describe a substance that stimulates the growth of other microorganisms [26]. In addition to supporting evidence, a new definition of probiotics was proposed by Parker (1974) as a group of microorganisms and substances that maintain intestinal microbial homeostasis [27]. In the following decade, many scientists have widened the definition of probiotics to include their host’s health benefits. Following this, both the Food and Agriculture Organization of the United Nations/World Health Organization (FAO/WHO) described probiotics as “living microorganisms that confer a beneficial effect on their host when administered in adequate quantities” [28]. These recommendations and guidelines have been widely embraced and have proved helpful to researchers. Over the past few decades, studies in the field of probiotics have grown exponentially, and research has focused on understanding the role of a variety of probiotics in dealing with chronic diseases.
An Overview of Probiotics: Focus on Therapeutic Applications in T2DM
It has been shown that probiotics can maintain glucose homeostasis in multiple ways. For instance, altering the intestinal microbiota leads to the suppression of inflammatory processes [29]. As a result of altered gut microbiota, gut hormones are also released in disorganized manners. In maintaining glucose homeostasis, gut hormones are important as they control the growth and survival of β-cells. Probiotics can enhance the antioxidant system in the β-cells, which will consequently improve glucose homeostasis by decreasing IR as well as strengthening the antioxidant system [30]. Numerous animal studies have found that supplementing gliclazide drugs with probiotics results in increased bioavailability of the drugs, which leads to blood glucose homeostasis [31]. In addition, maintaining insulin sensitivity may also be another effect of probiotics in maintaining glucose homeostasis [32]. In addition, gut microbiota can alter glucose metabolism by converting polysaccharides, indigestible to human enzymes, into glucose readily absorbed in the gastrointestinal system [33].
Animal Study in Probiotics and T2DM
Numerous animal studies have also confirmed the association between gut microbiota and T2DM [34, 35]. The biological effects of probiotics in diabetic animals have been extensively investigated, including the effects of Bifidobacterium and Lactobacillus on glucose tolerance and IR [36, 37]. In an experiment done on mice model of T2DM induced by high-fat diets (HFDs) and streptozotocin (STZ), Lactobacillus plantarum CCFM0236 was given, and it was found that this supplement ameliorated IR, pancreatic beta-cell dysfunction, and systemic inflammation [38]. The supplementing of Lactobacillus plantarum Ln4 to mice fed HFD resulted in weight loss and alleviation of IR; this was measured by improving the insulin tolerance test (ITT), oral glucose tolerance test (OGTT), and IR measure (HOMA-IR) indices [39]. In HFD- and STZ-induced diabetic mice, Bifidobacterium longum DD98 and Bifidobacterium longum DD98 enriched with selenium reduced fasting blood glucose (FBG) levels and hemoglobin A1c (HbA1c) levels and improved glucose tolerance [40]. In another study, a composite probiotic containing Lactobacillus strains and saccharomycetes was found to alleviate the signs and symptoms of T2D in db/db mice by improving FBG, OGTT, and HbA1c levels while increasing glucagon-like peptide-1 (GLP-1) secretion [41]. It has been shown that nano selenium-enriched Bifidobacterium longum inhibits the progression of STZ-induced diabetes and ameliorates the renal function damage caused by high glucose levels [42]. The treatment of HFD-induced diabetic mice with Lactobacillus fermentum MTCC 5689 improved IR and prevented the development of diabetes [43]. Lactobacillus paracasei TD062 was found to improve glucose homeostasis and activate insulin signaling pathways, preventing the development of T2DM [44]. The effectiveness of multiple probiotic formulae, which include Bacillus subtilis, Lactobacillus crispatus, and Lactobacillus reuteri, has been investigated in STZ-induced diabetic rats, revealing the consumption of a probiotics formula daily may alleviate glucose intolerance and impaired insulin secretion [45]. HFD-induced mice treated with Liposilactobacillus fermentum MG4295 showed improvement in insulin, glucose, and GLP-1 levels [46]. In T2DM induced by HFD, Pediococcus acidilactici pA1c was protected from body weight gain and IR and improved intestinal histology [47]. A study reported that Lactobacillus plantarum SHY130 reduced hyperglycemia in HFD-/STZ-induced diabetic mice via the regulation of the enteroinsular axis [48]. It is interesting to note that several bacterial taxa were associated with diabetic patients and animal models, including Akkermansia muciniphila [49], Lactobacillus [50], and Bacteroides [51]. As a result, it is very relevant to investigate the molecular mechanisms through which these bacterial taxa participate in the development of diabetes since their function does not seem to be well known [52].
Human Study in Probiotics and T2DM
In the following, we reviewed studies on intestinal microbiota’s effects on glycemic control in humans with T2DM. Probiotics may have varying strain-specific effects on glycemic control, as revealed by two meta-analyses [53, 54]. Several published studies indicated that genes from Bifidobacterium, Akkermannsia, Bacteroides, Roseburia, and Faecalibacterium negatively correlate with T2DM. In contrast, genes from Fusobacterium, Blautia, and Ruminococcus have been observed to be positively correlated with T2DM [14, 54]. Although Lactobacillus is still the most frequently identified and reported genus, there has not been a consistent correlation between Lactobacillus and its effects on T2DM [55]. Bifidobacterium has not been used alone as probiotics for T2DM. However, nearly all animal studies investigating several species of Bifidobacterium indicated amelioration of glucose tolerance [56, 57]. Therefore, research on animals supports the notion that Bifidobacterium probiotics have a preventive effect on T2DM [57, 58]. It has been revealed that Bacteroides have negative correlations with T2DM, whereas few studies that included some medications indicated positive associations [59, 60]. This apparent dispute can be explained by metformin’s previously known antibiotic effect and/or putative feedback mechanisms on gut microbiota brought on by improved human physiology [61]. Roseburia, Faecalibacterium, and Akkermansia were illustrated to be negatively associated with T2D in human studies. About half of T2D microbiome studies reported a decrease in at least one of these five phylogenetically distant species in patients, suggesting that they may have a role other than as a biomarker. The bulk of these microorganisms have been studied as probiotics for animal metabolic disorders, but less frequently in humans, which is crucial to highlight [62,63,64,65]. Ruminococcus, Fusobacterium, and Blautia have been positively associated with T2DM in fewer investigations. Studies that looked at these bacteria’s species levels found contradicting information. For instance, although one study revealed that Ruminococcus sp. SR1/5 was enriched by the use of metformin [66], another study discovered that Ruminococcus bromii and Ruminococcus torques reduced following bariatric surgery and the remission of diabetes [67]. The inconsistent results of these studies could be caused largely by the various sorts of therapies.
Here are details on recent probiotic clinical trials conducted on T2DM in Supplementary Table 1. There is no agreement on the exact mechanism by which probiotics achieve their advantages. However, many of their favorable effects can be explained by hypothesized processes. Various mechanisms contribute to probiotics’ effect on T2DM, including modulating inflammation, lipid metabolism, gut permeability, and interacting with dietary components [68, 69]. It is believed that short-chain fatty acids (SCFAs) are the major anions in the colon, which are produced largely by the probiotic bacteria in the colon from indigestible polysaccharides. By increasing GLP-1 levels and improving intestinal barrier function, SCFAs improve intestinal health [70]. Insulin production from the β-cells is stimulated by GLP-1, while glucagon production is inhibited by GLP-1. GLP-1 is a gut incretin hormone that contributes to glucose homeostasis [71]. By producing vitamins and hormones, probiotics improve gut physiology and promote epithelial cell growth [72, 73]. Probiotic supplementation has been found to reduce glucose levels in diabetics, improve oxidative stress markers, lipid profiles, blood pressure, and body mass index, and reduce other metabolic abnormalities associated with T2DM [74]. Additionally, some studies have shown that probiotics have positive effects on mental health. Studies have not reported any hepatotoxic or nephrotoxic effects compared to other synthetic drugs [75, 76].
Extracellular Vesicles from Microbiota
Extracellular vesicles (EVs) derived from microbiota carry a wide variety of compounds that can affect various pathways and affect the host. In biology, EVs refer to structures containing lipid bilayers that range in size from 30 to 400 nm and include diverse groups of proteins, lipids, and deoxyribonucleic acid (DNA) [77]. It has been shown that EVs derived from gut microbiota and probiotic bacteria can encapsulate an extensive range of bioactive molecules which can travel long and short distances to modulate specific biological functions affecting the host [78]. In Gram-negative and Gram-positive bacteria, EVs are typically outer membrane vesicles (OMVs) or membrane vesicles (MVs), respectively. It is highly likely that OMVs and MVs play roles as both bacterial survival factors and as hosts about bacterial interactions, including intra- and inter-kingdom communications without direct contact between cells [79]. Some recent studies have reported that some EVs may possess therapeutic potential in treating T2DM. The mechanism of action of the EV treatment consists of (a) increased insulin receptor substrate 1 (IRS-1) phosphorylation and (b) enhanced glucose transporter 4 (GLUT4) translocation in muscle, and (c) increased glycogen storage in the liver to sustain glucose homeostasis [80]. Based on a study by Chelakkot et al. EVs of Akkermansia muciniphila are directly associated with improving gut barrier integrity and metabolic profile in mice induced with HFD. By treating mice with Akkermansia muciniphila EVs orally, the permeability of the gut barrier was decreased, body weight gain was reduced, and glucose tolerance was improved [81]. However, Choi et al. have demonstrated that gut microbiota-derived EVs potentially play a role in the progression of diet-induced metabolic disorders in the case of dysbiosis of the gut microbiota. According to the authors, they observed that stool EVs isolated from HFD-fed mice lead to a blunting of glucose metabolism in both skeletal muscle and adipose tissue as a consequence of promoting IR. It was shown that Pseudomonas panacis Lipopolysaccharides (LPS)-containing EVs were significantly higher in HFD-fed mice. These EVs also contributed to the negative effects of HFD on glucose metabolism [82]. The limited data available makes it difficult to evaluate the potential of EVs in the treatment of T2DM and its complications, which needs further investigation.
Most Important Signaling Pathways Involved in the Pathogenesis of T2DM
According to generally accepted beliefs, the IR condition in T2DM is generally attributed to defects at one or several levels of the insulin-signaling pathway in the liver, the adipose tissue, and the skeletal muscles. Therefore, investigating the biochemical events involved in the intracellular action of insulin has quickly been followed up by studies seeking to determine which molecular defects are responsible for the onset of the state of IR. Among the most important signaling pathways, we review NF-κB, PI3K/Akt, and Nrf2, which are the crucial pathways responsible for IR and T2DM.
The Critical Role of NF-κB in the Pathogenesis of T2DM
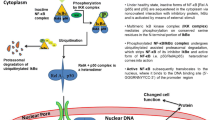
Through its regulation of many genes involved in cellular functions, NF-κB plays a critical role in a various functions related to human health. These functions include the development and maintenance of both the innate and adaptive immune systems, as well as several other functions [83]. It is now widely accepted that the role of NF-κB in the development and progression of diabetes and its complications is pivotal [84]. There is evidence that NF-κB has both pro-inflammatory and anti-inflammatory effects. However, it seems to be more pro-apoptotic in β-cells. When cells are healthy β-cells, NF-κB is suppressed, but when they are stressed or inflamed, translocation of NF-κB to the nucleus occurs upon activation [85]. NF-κB regulates various genes that participate in the β-cells dysfunction and death. NF-κB and its target genes have been well documented in their ability to contribute to the development of T2DM and IR [86]. Studies based on selective transgenic expression and a liver IKKβ (IκB kinase β) knockout provide sufficient evidence to support the critical role of NF-κB in IR [87, 88]. In experimental models of diabetes, it has been reported that HFD and obesity-induced IR are mimicked by the overexpression of IKKβ, leading to the activation of NF-κB in mice [89]. One of the most well-known inducers of NF-κB is tumor necrosis factor-α (TNF-α), a cytokine that promotes inflammation and induces IR by phosphorylating IRS1 [90]. TNF family members activate NF-κB leading to rapid gene transcription linked to cell proliferation, differentiation, and inflammation [91]. As well as various evidence for high levels of TNF-α in obese human and animal adipose tissues, it has been discovered that neutralization of TNF-α can reverse IR, an indication that NF-κB may contribute to IR [92, 93]. According to a study by Romzova, the NF-κB polymorphism has been implicated in the pathogenesis of T2DM. This is based on the finding that the AA genotype of IκBα gene shows an increase in people with T2DM [94]. It was shown that two common variants of NF-κB1 (− 94 insertion/deletion (indel) polymorphism in the promoter, and rs7667496, intronic) were independent risk factors for developing T2DM in Caucasian elderly subjects [84]. Even though most studies have demonstrated that NF-κB causes apoptosis in pancreatic β-cells, other reports have shown that NF-κB possesses both protective and destructive properties, which depend on the pathophysiological condition and the type of tissue involved [95]. When the NF-κB gene is blocked or knocked out, the genes associated with insulin secretion are reduced, and the pancreatic endocrine cells decrease [96]. Moreover, another study found that A20 is an anti-apoptotic gene NF-κB dependent that prevents the occurrence of apoptosis in β-cells induced by TNF-α [97]. By affecting the expression of glucose transporter 2 (GLUT2), NF-κB plays a role in insulin secretion [98]. Numerous studies have indicated that inhibition of GLUT2 transcription factor in pancreatic β-cells, liver, and kidney can affect IR and the development of T2DM [99, 100]. Although NF-κB plays an imperative role in regulating insulin levels, the mechanism by which it contributes to the pathogenesis of T2DM in humans is not well understood at present (Fig. 1).
NF-κB signaling pathways, canonical and non-canonical. IL-1R, TLRs, and TNFRs trigger the canonical pathway. In the non-canonical pathway, the activation of the NF-B2 (p100)/RelB complex is dependent on the activation of RANK, CD40, and BAFFR. By targeting the expression of certain genes, such as cytokines, chemokines, and other molecules, the activation of NF-SB signaling regulates various cellular processes
The Critical Role of PI3K/Akt in the Pathogenesis of T2DM
PI3K/Akt signaling plays a crucial role in the regulation of cellular functions during growth and development by mediating growth factor signals to different parts of the organism and regulating critical cellular processes, including glucose homeostasis, protein synthesis, proliferation, and survival of cells and lipid metabolism [101]. Activated Akt contributes to insulin metabolic function in at least three specific ways: (a) GLUTs’ translocation is responsible for the transportation of certain molecules, particularly GLUT4, the main transporter in the lipocyte and the skeletal muscles. (b) It is known that glycogen synthase kinase 3 (GSK3) inhibits glycogen synthase (GS) activity by phosphorylation, a central step in hepatic glucose metabolism [102]. Its two isomers, GSK3α and GSK3β, also contain Akt phosphorylation sites, making GSK3 an important substrate of Akt/PKB. An increase in the expression of GSK3β was found in mice with IR and obesity, which shows that GSK3 and Akt/PKB work reciprocally [103]. (c) The lipid-induced IR is accompanied by a decrease in the peroxisome proliferator-activated receptor coactivator (PGC-1α) pathway in individuals with (pre)diabetes [104]. As a result, any undesirable interference in any part of this pathway may have a detrimental effect on insulin transduction and, consequently, could negatively affect glucose uptake. During this condition, IR may be characterized as a problem with the transduction of the insulin signal [105]. Insulin and insulin-like growth factor 1 are believed to exert their metabolic and mitogenic actions through the PI3K/AKT/mTOR pathway [106]. Insulin-induced glucose and lipid metabolism could be improved by activating the PI3K/AKT/mTOR pathway. Glucose uptake, glycogen synthesis, glucose transporter type 4 translocation, and insulin-induced mitogenesis are all part of this process [107, 108]. DM and hyperglycemia may result from either PI3K/AKT/mTOR pathway node blockage in pancreatic β-cells and peripheral tissues [109]. A serine/threonine kinase downstream of AKT known as PKB has been identified as a key mediator of insulin action [107]. In T2DM skeletal muscle, IR is related to both upstream and downstream defects of Akt/PKB with reduced activity of the PI3 kinase pathway and dephosphorylation of the Akt/PKB substrate AS160 in the studies [110]. Moreover, mice with the Akt2/PKBβ isoform knocked down or depleted suffer from IR and diabetic-like symptoms, with rodents with the Akt2/PKBβ isoform knocked out exhibiting hepatic IR as well [111]. Besides IR, decreased insulin secretion and diminished β-cell mass are crucial factors contributing to the development of T2DM. Studies involving autopsies have revealed a 50–65% reduction in β-cell mass in individuals with impaired fasting glucose. This indicates that the reduction of β-cell mass in T2DM subjects occurs early [112]. PI3K/AKT pathway is involved in the β-cell function and insulin secretion in pancreatic cells, which insulin regulates. Previously, there was no thought insulin affected pancreatic cell synthesis, differentiation, and secretion. There has been consistent evidence that insulin plays an important role in pancreatic cells, mainly through the PI3K/AKT signaling pathway [113]. Insulin secretion from pancreatic β-cells is promoted by activation of the PI3K/AKT pathway [114]. AKT activation constitutive and overexpression in pancreatic β-cells leads to an increase in the number of cells, their growth rate, and their size, mediated by signal transduction intermediates downstream of AKT, such as FoxO1, GSK3, and mTOR1. These experimental results provide further evidence for AKT’s role in pancreatic cells [115]. On the other hand, overexpressing a kinase-dead mutant in β-cells had an 80% reduction in AKT activity, resulting in no insulin secretion. As a result of IR, the number of β-cells is increased, causing additional insulin to be released to maintain a normal glucose tolerance, leading to hyperinsulinemia [116]. T2DM results from impaired glucose tolerance due to β-cell dysfunction in IR (Fig. 2).
An illustration of the PI3K/AKT signaling pathway. Receptor tyrosine kinase (RTK) recruits phosphatidylinositol 3 kinase (PI3K) following activation and phosphorylation and phosphorylates phosphatidylinositol 4,5-bisphosphate (PIP2) to phosphatidylinositol (3,4,5)-trisphosphate (PIP3) which activates AKT (protein kinase B) by recruiting pyruvate dehydrogenase kinase 1 (PDK1) to the PH domain of AKT thereby activating the entire pathway and regulating different mechanisms
The Critical Role of Nrf2/Keap1 in the Pathogenesis of T2DM
NRF2 gene encodes for a transcription factor that modulates a population of several antioxidant responses involved in the control of inflammation, environmental stress, metabolic enzymes, injury, and detoxification enzymes by producing a variety of free radicals. Kelch-like ECH protein 1, also known as Keap1, is located in the cytoplasm and prevents the translocation of Nrf2 into the nucleus [117]. A major challenge of T2DM can be found in the associated macrovascular and microvascular issues resulting from its resistance to insulin. According to studies by Uruno et al. using both genetic knockdown of Keap1 and pharmacological induction of Nrf2 in murine models, the activation of Nrf2 could improve insulin sensitivity in diabetes, as well as obesity and diabetes, which are abrogated in mice [118]. Additionally, increasing Nrf2 signaling may reduce IR, a phenomenon that could also prevent oxidative stress from occurring in the hypothalamus, which could affect the regulation of the body’s metabolism more generally [119]. In Chinese populations, Nrf2 molecule distribution was linked closely with complications of T2DM, including nephropathy, neuropathy, and retinopathy [120]. The absence of Nrf2-induced antioxidants and cytoprotection contributes to IR, which is thought to be aggravated in Nrf2KO mice. Liu et al. showed that Nrf2 deficiency-induced hepatic IR in mice fed HFD by activating NF-κB. Furthermore, malondialdehyde is increased in Nrf2KO mice, which indicates oxidative stress, and glutathione levels are decreased [121]. In the development of T2DM, as well as its complications, in some studies, the Keap1-NRF2 pathway has been found to play an important protective role. Multiple aspects and mechanisms appear to protect pancreatic β-cells via the Keap1-Nrf2 pathway [122, 123]. Further studies have shown that genetically modified upregulation of Nrf2 via the Keap1 knockout preserves β-cell mass and function in diabetic mice [118]. It appears that the Nrf2/Keap1 system protects pancreatic β-cells by scavenging free radicals and reducing inflammation via the NF-κB pathway [124]. Furthermore, the Nrf2/Keap1 pathway is also controversial about its role in insulin secretion. The pancreatic islets of Nrf2 knockout mice have decreased insulin content and secretion, and Nrf2 upregulation seems to improve the insulin-releasing ability of β-cells [125, 126]. At the same time, the Nrf2 knockout mouse models have also shown decreased fat and body weight, reduced blood glucose, and increased insulin signaling [127,128,129,130]. MIN6 β-cells and the islets of mice with stable knockdown of Nrf2 and mice with conditional knockouts of Nrf2 in β-cells significantly decreased antioxidant enzymes in response to diverse stimuli [131, 132]. Nrf2 regulates autophagy in pancreatic β-cells in response to reactive oxygen species (ROS) stimulation, and pancreatic β-cells are suppressed from the inflammatory response [133]. In addition, there is also evidence that the Keap1-Nrf2 pathway exhibits beneficial effects. Among the many functions of Nrf2, one of the most important is to speed up wound healing by inhibiting oxidative DNA damage, matrix metalloproteinase 9 (MMP9), and transforming growth factor-β1 (TGF-β1) [134]. In diabetic mice, Nrf2 has been shown to possess altered macrophage phenotypes and promote autophagy, in addition to promoting the protective effects of atheroprotection on oxidative stress [135]. By reducing oxidative stress, apoptosis, inflammation, and fibrosis in kidney cells and improving their proliferation, the Keap1-Nrf2 pathway showed significant benefits against diabetic kidney disease [136] (Fig. 3).
The signaling pathway involving Nrf2-Keap1-ARE is shown in a schematic diagram. Kelch-like ECH-associated protein1 (Keap1) ubiquitinates nuclear erythroid-2 like factor-2 (Nrf2) constantly and degrades it in the proteasome under normal circumstances. In the presence of oxidative stress (ROS), Keap1 inactivates, and Nrf2 phosphorylates. Heme oxygenase-1 (HO-1) and NAD(P)H quinone dehydrogenase 1 (NQO1) are activated by phosphorylated Nrf2 (p-Nrf2), which accumulates in the nucleus and binds to ARE sites
Crosstalk Between Signaling Pathways and Probiotics in T2DM
Three signaling pathways (NF-κB, PI3K/Akt, and Nrf2) are the crucial pathways that contribute to the pathogenesis of T2DM. Evidence from multiple investigations reveals that several probiotics can improve T2DM via modulation or regulation of these three pathways. Targeting different components of these pathways may intensify their effects directly or indirectly. Supplementary Table 2 represents the interplay between different probiotics with NF-κB, PI3K/Akt, and Nrf2 signaling pathways involved in T2DM pathogenesis.
NF-κB and Probiotics
It has been reported that NF-κB activation may activate either pro-inflammatory or anti-inflammatory cascades [137], but in β-cells, this activity appears to be mostly pro-apoptotic [138]. On the other hand, NF-κB activity is inhibited in healthy β-cells; however, it becomes active in response to inflammation and oxidative stress. Emerging data proposes that probiotic strains play a vital role in modulating the immune and redox system by affecting the NF-κB pathway in the T2DM host [139]. Additionally, experimental in vivo models have demonstrated the effectiveness of probiotics in impotent T2DM. It was reported that oral administration of Lactobacillus paracasei HII01 led to reduce the expression levels of NF-κB in the T2DM mice induced by HFD and STZ [140]. Moreover, SCFAs have also been reported to be produced by probiotics in the large intestine, indicating the probiotics’ effect. In addition to their ability to inhibit histone deacetylase, SCFAs affect various genes that are either directly or indirectly involved in glucose metabolism and T2DM pathogenesis [141]. In line with this concept, SCFA generated by probiotics suppresses NF-κB activity by repressing cullin-1 neddylation, a vital step in the ubiquitination system [142]. Recently, Liu and colleagues found that treatment with Lactobacillus Plantarum Y15 improved the lipid profiles, decreased pro-inflammatory cytokines (IL-6, IL-8, and TNF-a), and increased IL-4, as an anti-inflammatory cytokine. In addition, this probiotic reshaped the structure of gut microbiota and reduced the abundance of LPS-producing and elevated SCFA-producing bacteria, which consequently declined the levels of LPS and pro-inflammatory cytokines. Besides, L. Plantarum Y15 led to upregulation of IκBα, whereas the mRNA expression of TLR4, IKKβ, and NF-κB was significantly downregulated, and it was concluded that L. plantarum Y15 was involved in the amelioration T2DM by regulating NF-κB pathway [143]. A study has suggested that transplanting SCFA (butyrate)-producing probiotic Faecalibacterium prausnitzii can alleviate symptoms of T2DM via NF-κB signaling [144]. Ample evidence has indicated that various pathogens activate NF-κB through their interaction with toll-like receptors (TLRs), especially TLR4. Mechanistically, signal transduction processes triggered by activated TLR4 include phosphorylation of IKKαβ and IκBα, which lead to the activation of NF-κB and subsequent nuclear translocation [145]. Taken together, accumulating lines of evidence reveal that probiotics exert their positive effect by producing SCFAs and modulating TLR4/NF-κB pathway in T2DM. Probiotics are recommended as add-on therapies for T2DM, due to the strong preclinical evidence, alongside the clinical evidence, that they improve the condition without compromising their tolerability. To achieve optimum results, further research must determine the exact strain, therapeutic dose, and study duration.
PI3K/AKT and Probiotics
In T2DM, insulin regulates several pathways associated with lipid and glucose metabolism; of these, PI3K/AKT pathway has been considered the vital pathway of insulin. This key signaling pathway is needed for the body’s normal metabolism due to its functions and the impairment caused to the development of T2DM. Emerging evidence has demonstrated that targeting PI3K/AKT signaling and its downstream mediators can be a good candidate for treating T2DM [146]. Regarding probiotics’ unique features and functions, compelling evidence indicates that these microorganisms could reverse the dysfunction in the PI3K/AKT pathway in T2DM. For instance, the potential therapeutic effects of Lactobacillus plantarum HAC01 on hyperglycemia and T2DM and their potential mechanisms using mice with HFD- and STZ-induced diabetes were investigated. The results showed that L. plantarum HAC01 remarkably reduced blood glucose levels and HbA1c and improved glucose tolerance and HOMA-IR. Meanwhile, this probiotic elevated the phosphorylation of AMPK and Akt [147]. It has been revealed that administration of L. casei CCFM419 regulated blood glucose balance and protected islets in the T2DM mice, accompanied by improved lipid metabolism. The homeostasis model of IR, insulin level and insulin tolerance test, and mRNA expression of PI3K/Akt signaling pathway indexes demonstrated that L. casei CCFM419 positively affected IR [148]. In T2DM diabetic mice, Lactobacillus paracasei TD062 improves IR and glucose homeostasis by lowering GSK-3β and enhancing IRS-2, PI3K, and Akt, thereby preventing T2DM [44]. Moreover, Zhang et al. revealed that two strains of Lactobacillus paracasei 1F-20 and Lactobacillus fermentum F40-4 enhanced the glucose uptake of oleic acid-treated HepG2 cells and elevated the phosphorylation of AKT and the expression of PI3K protein [149]. Similarly, the administration of Lactiplantibacillus plantarum MG4296 (MG4296) and Lacticaseibacillus paracasei MG5012 (MG5012) to palmitic acid-induced HepG2 cells and HFD-induced mice led to the downregulation of p-IRS-1 and upregulation of p-PI3k and p-Akt, thereby, preventing HFD-induced glucose tolerance and hyperglycemia by reversing the IR [150]. As a result of hyperglycemia, the PI3K/AKT pathway is impaired, resulting in apoptosis or cell death, accompanied by cytochrome C release from mitochondria and caspase-3 activity being augmented [151]. It was reported that by reducing caspase-3 levels, Clostridium butyricum had been shown to lessen apoptosis in diabetic type 2 cerebral ischemia/reperfusion injury via activation of the PI3K/Akt signaling pathway [152]. Ample evidence has shown that supplementation with Bifidobacterium species could improve IR and treat T2DM [153]. Oral administration of Bifidobacterium animalis 01 attenuated T2DM symptoms by modulation of IRS-2/PI3K/AKT. So, upon induction of diabetes, the expression of IRS-2, PI3K, and Akt was decreased, whereas their expression was remarkably elevated following treatment with Bifidobacterium animalis 01 [154]. Another study assessed the anti-diabetic effects of isolated 14 probiotics from fermented camel milk. The authors found that protein expression of p-PI3K/t-p-PI3K and p-AKT/t-AKT in the T2DM mice group was lower than in non-diabetic mice. At the same time, these 14 probiotics increased protein levels of p-PI3K/t-p-PI3K and p-AKT/t-AKT, thus improving β-cell function [155]. LE and co-workers indicated that oral administration of Bifidobacterium spp. increased IR-β, IRS-1, and Akt protein levels in diabetic mice, thus improving glucose uptake and symptoms of T2DM [156]. According to recent in vivo results, probiotics protect the pancreas from β-cell apoptosis via activation of the PI3K/Akt/mTOR pathway [157]. Huang et al. conducted an in vitro study, which indicated a negative correlation between the Akt/mTOR pathway and surface components of probiotics during diabetes onset. They proposed that by upregulating Akt-2, AMPK, and GLUT-4 expression, EPS of Lactobacillus plantarum H31 exerts anti-diabetic properties and has a key role in glucose metabolism affecting the pancreatic α-amylase activity [158]. Overall, the PI3K/Akt signaling pathway is one of the most vital signal transduction pathways with multiple physiological functions. The aberrant activation and/or dysregulation in the main mediators of the PI3K/Akt signaling are detectable in various disorders, especially T2DM. Recently, the modulatory function of probiotics on the PI3K/Akt pathway and their promising effects on managing T2DM have been considered. We summarized several in vitro and in vivo studies that used various probiotic strains to summarize their mechanisms of action on the PI3K/Akt network. Despite the efforts of numerous researchers, practically, all of the investigations have been carried out in vitro or using animal models. As a result, definitive proof of their positive effects on human disease is still absent, and more research on human subjects and clinical samples is required. Such studies, particularly those concentrating on probiotic strains with documented effects on components of the PI3K/Akt pathway, would be likely to provide more conclusive outcomes and support further exploration for new therapeutic candidates for the treatment of various highly prevalent disorders. Such investigations, particularly those focusing on probiotic strains with established effects on components of the PI3K/Akt pathway, are likely to yield more definitive results, paving the way for further research into potential therapeutic candidates for T2DM.
Nrf2/Keap1 and Probiotics
The onset of T2DM is associated with cellular distress, and Nrf2 plays a critical role in enhancing cytoprotective responses. In recent years, probiotic bacteria have been shown to protect against oxidative stress by regulating the Nrf2/Keap1 signaling pathway in vivo and in vitro studies. Hence, by activating Nrf2, probiotics can involve in the amelioration of T2DM. In this road map, Zhang et al. explored the effect of oral administration of B. animalis 01 on T2DM and the associated metabolic syndrome using a T2DM rat model. Their findings elucidated that, as compared to normal rats, the T2DM rats expressed a significantly higher level of Keap1, whereas it was remarkably reduced after administration of B.animalis 01. Additionally, after B. animalis 01 administration, Nrf2 expression was significantly higher in diabetic rats than non-diabetic. Therefore, they concluded that B. animalis 01 is implicated in lowering hepatic oxidative stress via activating the Keap1/Nrf2 pathway, thereby attenuating T2DM-related symptoms [154]. It has been revealed that treatment of hyperlipidemic and normal mice with Lactobacillus plantarum CAI6 and Lactobacillus plantarum SC4 increased the levels of Nrf2 in the liver and kidneys [159]. In accordance with this study, Gao and colleagues observed that Lactobacillus Plantarum FC225 increased the radical scavenging activities of superoxide anion radicals. Meanwhile, this probiotic substantially elevated the expression and translocation of Nrf2 in the hepatocytes of mice and prevented the inhibition of antioxidant enzymes by HFD [160]. Moreover, the probiotic Bacillus amyloliquefaciens SC06 reduced ROS levels and enhanced Nrf2 expression in intestinal porcine epithelial cell 1–1) -1 cells undergoing oxidative stress induced by H2O2 [161]. Furthermore, Maherian et al. assessed whether the combination of 4 weeks of aerobic exercise training with probiotic supplementation affects expression levels of Nrf-2 and caspase-3 in T2DM rats. They reported that aerobic exercise training in combination with probiotic treatment had a lowering effect on the expression of caspase-3, and an increasing impact on the levels of the Nrf-2 gene, ameliorating the antioxidant defense and protecting risk factors of diabetic cardiomyocytes [18]. Putting these data together, although there are a few studies focused on the effect of different probiotics on the Nrf2/Keap1 signaling pathway in T2DM, further in vitro, in vivo, and clinical trial investigations are required to understand the mechanism exactly.
Conclusion Remarks
The dynamic interactions between diet and gut microbiota play a key role in the pathogenesis of T2DM. Several clinical trials and animal studies have demonstrated that probiotics can be helpful in the management of T2DM. Improvement in glucose metabolism and IR may be achieved by modifying gut microbiota with probiotic strains, especially Lactobacillus and Bifidobacterium. According to the review, the positive effects of probiotics on diabetes can be attributed to their ability to influence signal pathways such as NF-κB, PI3K/Akt, and Nrf2, which might contribute to their benefits in the treatment and management of T2DM. It will take more research to determine the actual effects of probiotic intervention for T2DM, as the results of this study have been controversial. Interventions with broad strains of probiotics are more acceptable in clinics, but it must be determined first what strain to use and whether it should be in either single or multiple forms. To pave the way for the use of probiotics in T2DM, we have to conduct multicenter clinical studies in a standard manner to establish a standardized framework. To establish clinical validity, these studies must be based on the use of a certain strain of probiotics or a certain combination of probiotics that have already been shown to be effective in animal studies and can therefore be used in human studies as well. It is important to note that even though experimental and clinical studies have uncovered the significant potential of these probiotic strains to help manage diabetes, further investigations are still needed to clarify the molecular mechanisms that are involved in being able to develop more effective strategies to manage T2DM and its complications.
Availability of Data and Materials
The data used to support the findings of this study are included in the article.
References
Samavarchi Tehrani S, Goodarzi G, Panahi G, Maniati M, Meshkani R (2021) Multiple novel functions of circular RNAs in diabetes mellitus. Arch Physiol Biochem 1–30
Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N et al (2019) Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas. Diabetes Res Clin Pract 157:107843
Babakhanian M, Razavi A, Pordanjani SR, Hassanabadi S, Mohammadi G, Fattah A (2022) High incidence of type 1 diabetes, type 2 diabetes and gestational diabetes in Central Iran: A six years results from Semnan health cohort. Ann Med Surg 82:1047492
Chawla A, Chawla R, Jaggi S (2016) Microvasular and macrovascular complications in diabetes mellitus: distinct or continuum? Indian J Endocrinol Metab 20(4):546
Khalili L, Alipour B, Asghari Jafarabadi M, Hassanalilou T, Mesgari Abbasi M, Faraji I (2019) Probiotic assisted weight management as a main factor for glycemic control in patients with type 2 diabetes: a randomized controlled trial. Diabetol Metab Syndr 11(1):1–9
Kumar J, Rani K, Datt C (2020) Molecular link between dietary fibre, gut microbiota and health. Mol Biol Rep 47(8):6229–6237
Danneskiold-Samsøe NB, Barros HDDFQ, Santos R, Bicas JL, Cazarin CBB, Madsen L et al (2019) Interplay between food and gut microbiota in health and disease. Food Res Int 115:23–31
Moore RJ, Stanley D (2016) Experimental design considerations in microbiota/inflammation studies. Clin Transl Immunol 5(7):e92
Moreno-Indias I, Cardona F, Tinahones FJ, Queipo-Ortuño MI (2014) Impact of the gut microbiota on the development of obesity and type 2 diabetes mellitus. Front Microbiol 5:190
Grigorescu I, Dumitrascu D (2016) Implication of gut microbiota in diabetes mellitus and obesity. Acta Endocrinol (Bucharest) 12(2):206
Wolfs M, Hofker M, Wijmenga C, Van Haeften T (2009) Type 2 diabetes mellitus: new genetic insights will lead to new therapeutics. Curr Genomics 10(2):110–118
Taylor SI, Yazdi ZS, Beitelshees AL (2021) Pharmacological treatment of hyperglycemia in type 2 diabetes. J Clin Investig 131(2)
Gérard C, Vidal H (2019) Impact of gut microbiota on host glycemic control. Front Endocrinol 29
Gurung M, Li Z, You H, Rodrigues R, Jump DB, Morgun A et al (2020) Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine 51:102590
O’Toole PW, Cooney JC (2008) Probiotic bacteria influence the composition and function of the intestinal microbiota. Interdiscip Perspect Infect Dis 2008:1752853
Panghal A, Janghu S, Virkar K, Gat Y, Kumar V, Chhikara N (2018) Potential non-dairy probiotic products—a healthy approach. Food Biosci 21:80–89
da Cruz Rodrigues VC, Duque ALRF, de Carvalho FL, Simabuco FM, Sartoratto A, Cabral L et al (2020) Modulation of the intestinal microbiota and the metabolites produced by the administration of ice cream and a dietary supplement containing the same probiotics. Br J Nutr 124(1):57–68
Maherinia H, Peeri M, Azarbayjani M, Delfan M (2022) Aerobic exercise training combined with probiotic supplement improves antioxidant defence of cardiomyocytes by regulating Nrf2 and caspase3 gene expression in type 2 diabetic rats. Comp Exerc Physiol 1–10
Liang W-J, Yang H-W, Liu H-N, Qian W, Chen X-L (2020) HMGB1 upregulates NF-kB by inhibiting IKB-α and associates with diabetic retinopathy. Life Sci 241:117146
Huang X, Liu G, Guo J, Su Z (2018) The PI3K/AKT pathway in obesity and type 2 diabetes. Int J Biol Sci 14(11):1483
David JA, Rifkin WJ, Rabbani PS, Ceradini DJ (2017) The Nrf2/Keap1/ARE pathway and oxidative stress as a therapeutic target in type II diabetes mellitus. J Diabetes Res 2017:48267244
Subba R, Ahmad MH, Ghosh B, Mondal AC (2022) Targeting NRF2 in type 2 diabetes mellitus and depression: efficacy of natural and synthetic compounds. Eur J Pharmacol 174993
Islam SU (2016) Clinical uses of probiotics. Medicine (Baltimore) 95(5):e2658
Podolsky SH (2012) Metchnikoff and the microbiome. Lancet 380(9856):1810–1811
Vasiljevic T, Shah NP (2005) Probiotics—from Metchnikoff to bioactives. Int Dairy J 18(7):714–f285
Lilly DM, Stillwell RH (1965) Probiotics: growth-promoting factors produced by microorganisms. Science 147(3659):747–748
Parker R (1974) Probiotics, the other half of the antibiotic story. Anim Nutr Health 29:4–8
Hotel ACP, Cordoba A (2001) Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Prevention 5(1):1–10
Delzenne NM, Neyrinck AM, Cani PD (2011) Modulation of the gut microbiota by nutrients with prebiotic properties: consequences for host health in the context of obesity and metabolic syndrome. Microb Cell Fact 10(1):1–11
Yadav H, Jain S, Sinha PR (2008) Oral administration of dahi containing probiotic Lactobacillus acidophilus and Lactobacillus casei delayed the progression of streptozotocin-induced diabetes in rats. J Dairy Res 75(2):189–195
Al-Salami H, Butt G, Fawcett JP, Tucker IG, Golocorbin-Kon S, Mikov M (2008) Probiotic treatment reduces blood glucose levels and increases systemic absorption of gliclazide in diabetic rats. Eur J Drug Metab Pharmacokinet 33(2):101–106
Andersson U, Bränning C, Ahrné S, Molin G, Alenfall J, Önning G et al (2010) Probiotics lower plasma glucose in the high-fat fed C57BL/6J mouse. Beneficial Microbes 1(2):189–196
Zhang H, DiBaise JK, Zuccolo A, Kudrna D, Braidotti M, Yu Y et al (2009) Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci 106(7):2365–2370
Kieler IN, Osto M, Hugentobler L, Puetz L, Gilbert MTP, Hansen T et al (2019) Diabetic cats have decreased gut microbial diversity and a lack of butyrate producing bacteria. Sci Rep 9(1):1–13
Wang Y, Ouyang M, Gao X, Wang S, Fu C, Zeng J et al (2020) Phocea, Pseudoflavonifractor and Lactobacillus intestinalis: three potential biomarkers of gut microbiota that affect progression and complications of obesity-induced type 2 diabetes mellitus. Diabetes, metabolic syndrome and obesity: targets and therapy 13:835
Lê K-A, Li Y, Xu X, Liu T, Yang W, He F et al (2013) Alterations in fecal Lactobacillus and Bifidobacterium species in type 2 diabetic patients in Southern China population. Front Physiol 3:496
Holowacz S, Guigne C, Chene G, Mouysset S, Guilbot A, Seyrig C et al (2015) A multispecies Lactobacillus-and Bifidobacterium-containing probiotic mixture attenuates body weight gain and insulin resistance after a short-term challenge with a high-fat diet in C57/BL6J mice. PharmaNutrition 3(3):101–107
Li X, Wang N, Yin B, Fang D, Jiang T, Fang S et al (2016) Effects of Lactobacillus plantarum CCFM0236 on hyperglycaemia and insulin resistance in high-fat and streptozotocin-induced type 2 diabetic mice. J Appl Microbiol 121(6):1727–1736
Lee E, Jung S-R, Lee S-Y, Lee N-K, Paik H-D, Lim S-I (2018) Lactobacillus plantarum strain Ln4 attenuates diet-induced obesity, insulin resistance, and changes in hepatic mRNA levels associated with glucose and lipid metabolism. Nutrients 10(5):643
Zhao D, Zhu H, Gao F, Qian Z, Mao W, Yin Y et al (2020) Antidiabetic effects of selenium-enriched Bifidobacterium longum DD98 in type 2 diabetes model of mice. Food Funct 11(7):6528–6541
Manaer T, Yu L, Nabi X-H, Dilidaxi D, Liu L, Sailike J (2021) The beneficial effects of the composite probiotics from camel milk on glucose and lipid metabolism, liver and renal function and gut microbiota in db/db mice. BMC Complement Med Ther 21(1):1–13
Lin Y, Ren Y, Zhang Y, Zhou J, Zhou F, Zhao Q et al (2018) Protective role of nano-selenium-enriched Bifidobacterium longum in delaying the onset of streptozotocin-induced diabetes. R Soc Open Sci 5(12):181156
Balakumar M, Prabhu D, Sathishkumar C, Prabu P, Rokana N, Kumar R et al (2018) Improvement in glucose tolerance and insulin sensitivity by probiotic strains of Indian gut origin in high-fat diet-fed C57BL/6J mice. Eur J Nutr 57(1):279–295
Dang F, Jiang Y, Pan R, Zhou Y, Wu S, Wang R et al (2018) Administration of Lactobacillus paracasei ameliorates type 2 diabetes in mice. Food Funct 9(7):3630–3639
Memarrast F, Ghafouri-Fard S, Kolivand S, Jafary-Nodooshan S, Neyazi N, Sadroddiny E et al (2017) Comparative evaluation of probiotics effects on plasma glucose, lipid, and insulin levels in streptozotocin-induced diabetic rats. Diabetes Metab Res Rev 33(7):e2912
Kim JE, Lee JY, Kang C-H (2022) Limosilactobacillus fermentum MG4295 improves hyperglycemia in high-fat diet-induced mice. Foods 11(2):231
Cabello-Olmo M, Oneca M, Pajares MJ, Jiménez M, Ayo J, Encío IJ et al (2022) Antidiabetic effects of Pediococcus acidilactici pA1c on HFD-induced mice. Nutrients 14(3):692
Wang G, Song J, Huang Y, Li X, Wang H, Zhang Y et al (2022) Lactobacillus plantarum SHY130 isolated from yak yogurt attenuates hyperglycemia in C57BL/6J mice by regulating the enteroinsular axis. Food Funct 13(2):675–687
Zhang J, Ni Y, Qian L, Fang Q, Zheng T, Zhang M et al (2021) Decreased abundance of Akkermansia muciniphila leads to the impairment of insulin secretion and glucose homeostasis in lean type 2 diabetes. Adv Sci 8(16):2100536
Gu C, Yang Y, Xiang H, Li S, Liang L, Sui H et al (2016) Deciphering bacterial community changes in zucker diabetic fatty rats based on 16S rRNA gene sequences analysis. Oncotarget 7(31):48941
Zhang Y, Wu T, Li W, Zhao Y, Long H, Liu R et al (2021) Lactobacillus casei LC89 exerts antidiabetic effects through regulating hepatic glucagon response and gut microbiota in type 2 diabetic mice. Food Funct 12(18):8288–8299
Zhai L, Wu J, Lam YY, Kwan HY, Bian Z-X, Wong HLX (2021) Gut-microbial metabolites, probiotics and their roles in type 2 diabetes. Int J Mol Sci 22(23):12846
Zhang C, Wang C, Li S, Yu L, Tian F, Zhao J et al (2022) Meta-analysis of randomized controlled trials of the effects of probiotics on type 2 diabetes in adults. Clin Nutr 41(2):365–373
Rittiphairoj T, Pongpirul K, Janchot K, Mueller NT, Li T (2021) Probiotics contribute to glycemic control in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Adv Nutr 12(3):722–734
Qu L, Ren J, Huang L, Pang B, Liu X, Liu X et al (2018) Antidiabetic effects of Lactobacillus casei fermented yogurt through reshaping gut microbiota structure in type 2 diabetic rats. J Agric Food Chem 66(48):12696–12705
Gao R, Zhu C, Li H, Yin M, Pan C, Huang L et al (2018) Dysbiosis signatures of gut microbiota along the sequence from healthy, young patients to those with overweight and obesity. Obesity 26(2):351–361
Wu X, Ma C, Han L, Nawaz M, Gao F, Zhang X et al (2010) Molecular characterisation of the faecal microbiota in patients with type II diabetes. Curr Microbiol 61(1):69–78
Moya-Pérez A, Neef A, Sanz Y (2015) Bifidobacterium pseudocatenulatum CECT 7765 reduces obesity-associated inflammation by restoring the lymphocyte-macrophage balance and gut microbiota structure in high-fat diet-fed mice. PLoS ONE 10(7):e0126976
Yamaguchi Y, Adachi K, Sugiyama T, Shimozato A, Ebi M, Ogasawara N et al (2016) Association of intestinal microbiota with metabolic markers and dietary habits in patients with type 2 diabetes. Digestion 94(2):66–72
Sun L, Xie C, Wang G, Wu Y, Wu Q, Wang X et al (2018) Gut microbiota and intestinal FXR mediate the clinical benefits of metformin. Nat Med 24(12):1919–1929
Malik F, Mehdi SF, Ali H, Patel P, Basharat A, Kumar A et al (2018) Is metformin poised for a second career as an antimicrobial? Diabetes Metab Res Rev 34(4):e2975
Kikuchi K, Othman MB, Sakamoto K (2018) Sterilized bifidobacteria suppressed fat accumulation and blood glucose level. Biochem Biophys Res Commun 501(4):1041–1047
Gauffin Cano P, Santacruz A, Moya Á, Sanz Y (2012) Bacteroides uniformis CECT 7771 ameliorates metabolic and immunological dysfunction in mice with high-fat-diet induced obesity 7(7):e41079
Plovier H, Everard A, Druart C, Depommier C, Van Hul M, Geurts L et al (2017) A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med 23(1):107–113
Zhao S, Liu W, Wang J, Shi J, Sun Y, Wang W et al (2017) Akkermansia muciniphila improves metabolic profiles by reducing inflammation in chow diet-fed mice. J Mol Endocrinol 58(1):1–14
Wu H, Esteve E, Tremaroli V, Khan MT, Caesar R, Mannerås-Holm L et al (2017) Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med 23(7):850–858
Murphy R, Tsai P, Jüllig M, Liu A, Plank L, Booth M (2017) Differential changes in gut microbiota after gastric bypass and sleeve gastrectomy bariatric surgery vary according to diabetes remission. Obes Surg 27(4):917–925
Shen Z, Zhu C, Quan Y, Yang J, Yuan W, Yang Z et al (2018) Insights into Roseburia intestinalis which alleviates experimental colitis pathology by inducing anti-inflammatory responses. J Gastroenterol Hepatol 33(10):1751–1760
Payola Padrosa M (2021) Effects of probiotic consumption on the immune system of athletes.
Macfarlane S, Macfarlane GT (2003) Regulation of short-chain fatty acid production. Proc Nutr Soc 62(1):67–72
Lovshin JA, Drucker DJ (2009) Incretin-based therapies for type 2 diabetes mellitus. Nat Rev Endocrinol 5(5):262–269
Mach N, Fuster-Botella D (2017) Endurance exercise and gut microbiota: a review. J Sport Health Sci 6(2):179–197
Indira M, Venkateswarulu T, Abraham Peele K, Bobby N, Krupanidhi S (2019) Bioactive molecules of probiotic bacteria and their mechanism of action: A review. 3 Biotech 9(8):1–11
Tabrizi R, Ostadmohammadi V, Akbari M, Lankarani KB, Vakili S, Peymani P et al (2019) The effects of probiotic supplementation on clinical symptom, weight loss, glycemic control, lipid and hormonal profiles, biomarkers of inflammation, and oxidative stress in women with polycystic ovary syndrome: a systematic review and meta-analysis of randomized controlled trials. Probiotics Antimicrob Proteins 1–14
Mohammadi AA, Jazayeri S, Khosravi-Darani K, Solati Z, Mohammadpour N, Asemi Z et al (2016) The effects of probiotics on mental health and hypothalamic–pituitary–adrenal axis: a randomized, double-blind, placebo-controlled trial in petrochemical workers. Nutr Neurosci 19(9):387–395
Ansari F, Pourjafar H, Tabrizi A, Homayouni A (2020) The effects of probiotics and prebiotics on mental disorders: a review on depression, anxiety, Alzheimer, and autism spectrum disorders. Curr Pharm Biotechnol 21(7):555–565
Fucarino A, Pitruzzella A, Burgio S, Zarcone MC, Modica DM, Cappello F et al (2021) Extracellular vesicles in airway homeostasis and pathophysiology. Appl Sci 11(21):9933
Sultan S, Mottawea W, Yeo J, Hammami R (2021) Gut microbiota extracellular vesicles as signaling molecules mediating host-microbiota communications. Int J Mol Sci 22(23):13166
Choi J, Kwon H, Kim Y-K, Han P-L (2022) Extracellular vesicles from Gram-positive and Gram-negative probiotics remediate stress-induced depressive behavior in mice. Mol Neurobiol 59(5):2715–2728
Hu W, Song X, Yu H, Sun J, Zhao Y (2020) Therapeutic potentials of extracellular vesicles for the treatment of diabetes and diabetic complications. Int J Mol Sci 21(14):5163
Chelakkot C, Choi Y, Kim D-K, Park HT, Ghim J, Kwon Y et al (2018) Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp Mol Med 50(2):e450–e
Choi Y, Kwon Y, Kim D-K, Jeon J, Jang SC, Wang T et al (2015) Gut microbe-derived extracellular vesicles induce insulin resistance, thereby impairing glucose metabolism in skeletal muscle. Sci Rep 5(1):1–11
Tang C, Zhu G (2019) Classic and novel signaling pathways involved in cancer: targeting the NF-κB and Syk signaling pathways. Curr Stem Cell Res Ther 14(3):219–225
Coto E, Díaz-Corte C, Tranche S, Gómez J, Alonso B, Iglesias S et al (2018) Gene variants in the NF-KB pathway (NFKB1, NFKBIA, NFKBIZ) and their association with type 2 diabetes and impaired renal function. Hum Immunol 79(6):494–498
Cardozo AK, Heimberg H, Heremans Y, Leeman R, Kutlu B, Kruhøffer M et al (2001) A comprehensive analysis of cytokine-induced and nuclear factor-κB-dependent genes in primary rat pancreatic β-cells. J Biol Chem 276(52):48879–48886
Farid A, Moussa P, Youssef M, Haytham M, Shamy A, Safwat G (2022) Melatonin relieves diabetic complications and regenerates pancreatic beta cells by the reduction in NF-kB expression in streptozotocin induced diabetic rats. Melatonin: anti-diabetic drug. Saudi J Biol Sci 103313
Arkan MC, Hevener AL, Greten FR, Maeda S, Li Z-W, Long JM et al (2005) IKK-β links inflammation to obesity-induced insulin resistance. Nat Med 11(2):191–198
Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J et al (2005) Local and systemic insulin resistance resulting from hepatic activation of IKK-β and NF-κB. Nat Med 11(2):183–190
Shoelson S, Lee J, Yuan M (2003) Inflammation and the IKKβ/IκB/NF-κB axis in obesity-and diet-induced insulin resistance. Int J Obes 27(3):S49–S52
Akash MSH, Rehman K, Liaqat A (2018) Tumor necrosis factor-alpha: role in development of insulin resistance and pathogenesis of type 2 diabetes mellitus. J Cell Biochem 119(1):105–110
Hayden MS, Ghosh S (2014) Regulation of NF-κB by TNF family cytokines. Semin Immunol 26(3):253–2666
Borst SE (2004) The role of TNF-α in insulin resistance. Endocrine 23(2):177–182
Chen X, Famurewa AC, Tang J, Olatunde OO, Olatunji OJ (2021) Hyperoside attenuates neuroinflammation, cognitive impairment and oxidative stress via suppressing TNF-α/NF-κB/caspase-3 signaling in type 2 diabetes rats. Nutr Neurosci 1–11
Romzova M, Hohenadel D, Kolostova K, Pinterova D, Fojtikova M, Ruzickova S et al (2006) NFκB and its inhibitor IκB in relation to type 2 diabetes and its microvascular and atherosclerotic complications. Hum Immunol 67(9):706–713
Cnop M, Welsh N, Jonas J-C, Jorns A, Lenzen S, Eizirik DL (2005) Mechanisms of pancreatic β-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes 54(suppl_2):S97–S107
Norlin S, Ahlgren U, Edlund H (2005) Nuclear factor-κB activity in β-cells is required for glucose-stimulated insulin secretion. Diabetes 54(1):125–132
Liuwantara D, Elliot M, Smith MW, Yam AO, Walters SN, Marino E et al (2006) Nuclear factor-κB regulates β-cell death: a critical role for A20 in β-cell protection. Diabetes 55(9):2491–2501
Li X, Wu Y, Song Y, Ding N, Lu M, Jia L et al (2020) Activation of NF-κB-inducing kinase in islet β cells causes β cell failure and diabetes. Mol Ther 28(11):2430–2441
de Souza Cordeiro LM, Bainbridge L, Devisetty N, McDougal DH, Peters DJ, Chhabra KH (2022) Loss of function of renal Glut2 reverses hyperglycaemia and normalises body weight in mouse models of diabetes and obesity. Diabetologia 65(6):1032–1047
Saha S (2020) Association between the membrane transporter proteins and type 2 diabetes mellitus. Expert Rev Clin Pharmacol 13(3):287–297
Abeyrathna P, Su Y (2015) The critical role of Akt in cardiovascular function. Vascul Pharmacol 74:38–48
Seo YH, Jung HJ, Shin HT, Kim YM, Yim H, Chung HY et al (2008) Enhanced glycogenesis is involved in cellular senescence via GSK3/GS modulation. Aging Cell 7(6):894–907
Chakraborty A, Koldobskiy MA, Bello NT, Maxwell M, Potter JJ, Juluri KR et al (2010) Inositol pyrophosphates inhibit Akt signaling, thereby regulating insulin sensitivity and weight gain. Cell 143(6):897–910
Guilherme A, Virbasius JV, Puri V, Czech MP (2008) Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol 9(5):367–377
Iqbal J, Jiang H-L, Wu H-X, Li L, Zhou Y-H, Hu N et al (2022) Hereditary severe insulin resistance syndrome: pathogenesis, pathophysiology, and clinical management. Genes Dis. https://doi.org/10.1016/j.gendis.2022.03.016
Asano T, Fujishiro M, Kushiyama A, Nakatsu Y, Yoneda M, Kamata H et al (2007) Role of phosphatidylinositol 3-kinase activation on insulin action and its alteration in diabetic conditions. Biol Pharm Bull 30(9):1610–1616
Whiteman EL, Cho H, Birnbaum MJ (2002) Role of Akt/protein kinase B in metabolism. Trends Endocrinol Metab 13(10):444–451
Chen X-W, Leto D, Xiong T, Yu G, Cheng A, Decker S et al (2011) A Ral GAP complex links PI 3-kinase/Akt signaling to RalA activation in insulin action. Mol Biol Cell 22(1):141–152
Khan KH, Wong M, Rihawi K, Bodla S, Morganstein D, Banerji U et al (2016) Hyperglycemia and phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/AKT/mTOR) inhibitors in phase I trials: incidence, predictive factors, and management. Oncologist 21(7):855–860
Karlsson HK, Zierath JR, Kane S, Krook A, Lienhard GE, Wallberg-Henriksson H (2005) Insulin-stimulated phosphorylation of the Akt substrate AS160 is impaired in skeletal muscle of type 2 diabetic subjects. Diabetes 54(6):1692–1697
Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB III et al (2001) Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKBβ). Science 292(5522):1728–1731
Matveyenko AV, Butler P (2008) Relationship between β-cell mass and diabetes onset. Diabetes Obes Metab 10:23–31
Kubota N, Tobe K, Terauchi Y, Eto K, Yamauchi T, Suzuki R et al (2000) Disruption of insulin receptor substrate 2 causes type 2 diabetes because of liver insulin resistance and lack of compensatory beta-cell hyperplasia. Diabetes 49(11):1880–1889
Georgia S, Bhushan A (2004) β cell replication is the primary mechanism for maintaining postnatal β cell mass. J Clin Investig 114(7):963–968
Bernal-Mizrachi E, Wen W, Stahlhut S, Welling CM, Permutt MA (2001) Islet β cell expression of constitutively active Akt1/PKBα induces striking hypertrophy, hyperplasia, and hyperinsulinemia. J Clin Investig 108(11):1631–1638
Kahn SE, Prigeon RL, McCulloch DK, Boyko EJ, Bergman RN, Schwartz MW et al (1993) Quantification of the relationship between insulin sensitivity and β-cell function in human subjects: evidence for a hyperbolic function. Diabetes 42(11):1663–1672
Liu M, Grigoryev DN, Crow MT, Haas M, Yamamoto M, Reddy SP et al (2009) Transcription factor Nrf2 is protective during ischemic and nephrotoxic acute kidney injury in mice. Kidney Int 76(3):277–285
Uruno A, Furusawa Y, Yagishita Y, Fukutomi T, Muramatsu H, Negishi T et al (2013) The Keap1-Nrf2 system prevents onset of diabetes mellitus. Mol Cell Biol 33(15):2996–3010
Yagishita Y, Uruno A, Fukutomi T, Saito R, Saigusa D, Pi J et al (2017) Nrf2 improves leptin and insulin resistance provoked by hypothalamic oxidative stress. Cell Rep 18(8):2030–2044
Tao T, Lin X, Tang S, Gui W, Zhu W, Li H (2022) Association of genetic variants in the Sirt1 and Nrf2 genes with the risk of metabolic syndrome in a Chinese Han population. BMC Endocr Disord 22(1):1–8
Liu Z, Dou W, Ni Z, Wen Q, Zhang R, Qin M et al (2016) Deletion of Nrf2 leads to hepatic insulin resistance via the activation of NF-κB in mice fed a high-fat diet. Mol Med Rep 14(2):1323–1331
He J, Zhang X, Lian C, Wu J, Fang Y, Ye X (2019) KEAP1/NRF2 axis regulates H2O2-induced apoptosis of pancreatic β-cells. Gene 691:8–17
Lee S, Hur E-G, Ryoo I-g, Jung K-A, Kwak J, Kwak M-K (2012) Involvement of the Nrf2-proteasome pathway in the endoplasmic reticulum stress response in pancreatic β-cells. Toxicol Appl Pharmacol 264(3):431–438
Song M-Y, Kim E-K, Moon W-S, Park J-W, Kim H-J, So H-S et al (2009) Sulforaphane protects against cytokine-and streptozotocin-induced β-cell damage by suppressing the NF-κB pathway. Toxicol Appl Pharmacol 235(1):57–67
Saha PK, Reddy VT, Konopleva M, Andreeff M, Chan L (2010) The triterpenoid 2-cyano-3, 12-dioxooleana-1, 9-dien-28-oic-acid methyl ester has potent anti-diabetic effects in diet-induced diabetic mice and Leprdb/db mice. J Biol Chem 285(52):40581–40592
Yu Z, Shao W, Chiang Y, Foltz W, Zhang Z, Ling W et al (2010) Oltipraz upregulates the nuclear factor (erythroid-derived 2)-like 2 [corrected](NRF2) antioxidant system and prevents insulin resistance and obesity induced by a high-fat diet in C57BL/6J mice. Diabetologia 54(4):922–934
Zhang Y-KJ, Wu KC, Liu J, Klaassen CD (2012) Nrf2 deficiency improves glucose tolerance in mice fed a high-fat diet. Toxicol Appl Pharmacol 264(3):305–314
Meakin PJ, Chowdhry S, Sharma RS, Ashford FB, Walsh SV, McCrimmon RJ et al (2014) Susceptibility of Nrf2-null mice to steatohepatitis and cirrhosis upon consumption of a high-fat diet is associated with oxidative stress, perturbation of the unfolded protein response, and disturbance in the expression of metabolic enzymes but not with insulin resistance. Mol Cell Biol 34(17):3305–3320
Meher AK, Sharma PR, Lira VA, Yamamoto M, Kensler TW, Yan Z et al (2012) Nrf2 deficiency in myeloid cells is not sufficient to protect mice from high-fat diet-induced adipose tissue inflammation and insulin resistance. Free Radical Biol Med 52(9):1708–1715
Pi J, Leung L, Xue P, Wang W, Hou Y, Liu D et al (2010) Deficiency in the nuclear factor E2-related factor-2 transcription factor results in impaired adipogenesis and protects against diet-induced obesity. J Biol Chem 285(12):9292–9300
Fu J, Zheng H, Wang H, Yang B, Zhao R, Lu C et al (2015) Protective role of nuclear factor E2-related factor 2 against acute oxidative stress-induced pancreatic β-cell damage. Oxid Med Cell Longev
Yagishita Y, Fukutomi T, Sugawara A, Kawamura H, Takahashi T, Pi J et al (2014) Nrf2 protects pancreatic β-cells from oxidative and nitrosative stress in diabetic model mice. Diabetes 63(2):605–618
Zhang L, Li J, Ma J, Chen X, Chen K, Jiang Z et al (2016) The relevance of Nrf2 pathway and autophagy in pancreatic cancer cells upon stimulation of reactive oxygen species. Oxid Med Cell Longev
Long M, Rojo de la Vega M, Wen Q, Bharara M, Jiang T, Zhang R et al (2016) An essential role of NRF2 in diabetic wound healing. Diabetes 65(3):780–793
Lazaro I, Lopez-Sanz L, Bernal S, Oguiza A, Recio C, Melgar A et al (2018) Nrf2 activation provides atheroprotection in diabetic mice through concerted upregulation of antioxidant, anti-inflammatory, and autophagy mechanisms. Front Pharmacol 9:819
Ruiz S, Pergola PE, Zager RA, Vaziri ND (2013) Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney Int 83(6):1029–1041
Arakelyan A, Nersisyan L, Poghosyan D, Khondkaryan L, Hakobyan A, Löffler-Wirth H et al (2017) Autoimmunity and autoinflammation: a systems view on signaling pathway dysregulation profiles. PLoS ONE 12(11):e0187572
Eizirik DL, Mandrup-Poulsen T (2001) A choice of death–the signal-transduction of immune-mediated beta-cell apoptosis. Diabetologia 44(12):2115–2133
Bhardwaj R, Singh BP, Sandhu N, Singh N, Kaur R, Rokana N et al (2020) Probiotic mediated NF-κB regulation for prospective management of type 2 diabetes. Mol Biol Rep 47(3):2301–2313
Toejing P, Khat-Udomkiri N, Intakhad J, Sirilun S, Chaiyasut C, Lailerd N (2020) Putative mechanisms responsible for the antihyperglycemic action of Lactobacillus paracasei HII01 in experimental type 2 diabetic rats. Nutrients 12(10):3015
Khan S, Maremanda KP, Jena G (2017) Butyrate, a short-chain fatty acid and histone deacetylases inhibitor: nutritional, physiological, and pharmacological aspects in diabetes. Handbook of Nutrition, Diet, and Epigenetics 1:15
Kumar A, Wu H, Collier-Hyams LS, Kwon Y-M, Hanson JM, Neish AS (2009) The bacterial fermentation product butyrate influences epithelial signaling via reactive oxygen species-mediated changes in cullin-1 neddylation. J Immunol 182(1):538–546
Liu Y, Zheng S, Cui J, Guo T, Zhang J (2022) Lactiplantibacillus plantarum Y15 alleviate type 2 diabetes in mice via modulating gut microbiota and regulating NF-κB and insulin signaling pathway. Braz J Microbiol 1–11
Ganesan K, Chung SK, Vanamala J, Xu B (2018) Causal relationship between diet-induced gut microbiota changes and diabetes: a novel strategy to transplant Faecalibacterium prausnitzii in preventing diabetes. Int J Mol Sci 19(12):3720
Bayan N, Yazdanpanah N, Rezaei N (2021) Role of toll-like receptor 4 in diabetic retinopathy. Pharmacol Res 105960
Khorami SAH, Movahedi A, Huzwah K, Sokhini A (2015) PI3K/AKT pathway in modulating glucose homeostasis and its alteration in diabetes. Ann Med Biomed Sci 1(2):46–55
Lee Y-S, Lee D, Park G-S, Ko S-H, Park J, Lee Y-K et al (2021) Lactobacillus plantarum HAC01 ameliorates type 2 diabetes in high-fat diet and streptozotocin-induced diabetic mice in association with modulating the gut microbiota. Food Funct 12(14):6363–6373
Li X, Wang E, Yin B, Fang D, Chen P, Wang G et al (2017) Effects of Lactobacillus casei CCFM419 on insulin resistance and gut microbiota in type 2 diabetic mice. Benef Microbes 8(3):421–432
Zhang Z, Liang X, Lv Y, Yi H, Chen Y, Bai L et al (2020) Evaluation of probiotics for improving and regulation metabolism relevant to type 2 diabetes in vitro. J Funct Foods 64:103664
Won G, Choi S-I, Kang C-H, Kim G-H (2021) Lactiplantibacillus plantarum MG4296 and Lacticaseibacillus paracasei MG5012 ameliorates insulin resistance in palmitic acid-induced HepG2 cells and high fat diet-induced mice. Microorganisms 9(6):1139
Meng Y, Wang W, Kang J, Wang X, Sun L (2017) Role of the PI3K/AKT signalling pathway in apoptotic cell death in the cerebral cortex of streptozotocin-induced diabetic rats. Exp Ther Med 13(5):2417–2422
Sun J, Wang F, Ling Z, Yu X, Chen W, Li H et al (2016) Clostridium butyricum attenuates cerebral ischemia/reperfusion injury in diabetic mice via modulation of gut microbiota. Brain Res 1642:180–188
Hashimoto Y, Nakajima H, Hata S, Miyoshi T, Hosomi Y, Majima S et al (2020) Effect of probiotics, Bifidobacterium bifidum G9–1, on gastrointestinal symptoms in patients with type 2 diabetes mellitus: study protocol for open-label, single-arm, exploratory research trial (Big STAR study). J Clin Biochem Nutrition 67(3):223–227
Zhang J, Wang S, Zeng Z, Qin Y, Shen Q, Li P (2020) Anti-diabetic effects of Bifidobacterium animalis 01 through improving hepatic insulin sensitivity in type 2 diabetic rat model. J Funct Foods 67:103843
Wang Y, Dilidaxi D, Wu Y, Sailike J, Sun X, Nabi X-H (2020) Composite probiotics alleviate type 2 diabetes by regulating intestinal microbiota and inducing GLP-1 secretion in db/db mice. Biomed Pharmacother 125:109914
Le TKC, Hosaka T, Nguyen TT, Kassu A, Dang TO, Tran HB et al (2015) Bifidobacterium species lower serum glucose, increase expressions of insulin signaling proteins, and improve adipokine profile in diabetic mice. Biomed Res 36(1):63–70
Mohseni AH, Casolaro V, Bermúdez-Humarán LG, Keyvani H, Taghinezhad-S S (2021) Modulation of the PI3K/Akt/mTOR signaling pathway by probiotics as a fruitful target for orchestrating the immune response. Gut Microbes 13(1):1886844
Huang Z, Lin F, Zhu X, Zhang C, Jiang M, Lu Z (2020) An exopolysaccharide from Lactobacillus plantarum H31 in pickled cabbage inhibits pancreas α-amylase and regulating metabolic markers in HepG2 cells by AMPK/PI3K/Akt pathway. Int J Biol Macromol 143:775–784
Wang L-X, Liu K, Gao D-W, Hao J-K (2013) Protective effects of two Lactobacillus plantarum strains in hyperlipidemic mice. World J Gastroenterol: WJG 19(20):3150
Gao D, Gao Z, Zhu G (2013) Antioxidant effects of Lactobacillus plantarum via activation of transcription factor Nrf2. Food Funct 4(6):982–989
Wang Y, Wu Y, Wang Y, Fu A, Gong L, Li W et al (2017) Bacillus amyloliquefaciens SC06 alleviates the oxidative stress of IPEC-1 via modulating Nrf2/Keap1 signaling pathway and decreasing ROS production. Appl Microbiol Biotechnol 101(7):3015–3026
Author information
Authors and Affiliations
Contributions
FH-L, MT, and SDS designed and implemented the study, FH-L and MT prepared the manuscript, AGh, MH, and SDS critically reviewed the manuscript, FH-L and MT revised the manuscript, and MT and SDS supervised overall project. All authors approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent for Publication
All of the authors consent to publication.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hasanian-Langroudi, F., Ghasemi, A., Hedayati, M. et al. Novel Insight into the Effect of Probiotics in the Regulation of the Most Important Pathways Involved in the Pathogenesis of Type 2 Diabetes Mellitus. Probiotics & Antimicro. Prot. 16, 829–844 (2024). https://doi.org/10.1007/s12602-023-10056-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-023-10056-8