Abstract
Chronic stress causes maladaptive changes in the brain that lead to depressive behavior. In the present study, we investigate whether chronic stress alters gut microbiota compositions that are related to stress-induced maladaptive changes in the brain. Mice treated with daily 2-h restraint for 14 days (CRST) exhibit depressive-like behavior. Sequence readings of 16S rRNA genes prepared from fecal samples taken from CRST-treated mice suggest that chronic stress induces gut microbiota changes that are pronounced in the post-stress period, relative to those that occur in the 14-day stress phase. The genus Lactobacillus is one such microbiota substantially changed following chronic stress. In contrast, intraperitoneal injection of extracellular vesicles (EVs) isolated from culture media of the Gram-positive probiotic Lactobacillus plantarum is sufficient to ameliorate stress-induced depressive-like behavior. Interestingly, EVs from the Gram-positive probiotic Bacillus subtilis and EVs from the Gram-negative probiotic Akkermansia muciniphila also produce anti-depressive-like effects. While chronic stress decreases the expression of MeCP2, Sirt1, and/or neurotrophic factors in the hippocampus, EVs from the three selected probiotics differentially restore stress-induced changes of these factors. These results suggest that chronic stress produces persistent changes in gut microbiota composition, whereas purified EVs of certain probiotics can be used for treatment of stress-induced depressive-like behavior.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic stress is a potent environmental risk factor for depressive behavior. Stress responses proceed with the activation of the hypothalamic–pituitary–adrenal (HPA) axis, resulting in glucocorticoid (GC) release into blood. When stress-induced GC release is excessive or prolonged, GC-dependent maladaptive changes occur in the brain, such as diminished sensitivity to GC in the limbic system and abnormal HPA axis activation [1, 2]. Furthermore, chronic stress and stress-induced excesses of GC induce profound genomic responses that exceed the homeostatic range in the brain. Those changes can cause dendritic and spine atrophy in the limbic system, and produce neural circuit activity changes [3]. These stress-induced maladaptive changes in the limbic system are responsible for behavioral disturbances such as cognitive impairment and mood disorders [3, 4].
Recent studies have reported that the gut microbiota changes are closely associated with the pathophysiology of stress-related mood disorders [5,6,7,8]. Patients with depression have altered gut microbiota composition [9], whereas supplementation with probiotics results in anti-depressant effects in depression patients [10]. Recently, several laboratories have reported that gut microbiota changes are involved in depressive-like behaviors in animal models of depression. Mice exposed to chronic unpredictable mild stress (CUMS) had decreased levels of Lactobacillus, whereas administration of Lactobacillus reuteri during CUMS treatment attenuated despair-like behavior [11]. Transferring fecal microbiota prepared from the mice subjected to 7 weeks of CUMS to healthy recipients produced despair-like behavior, decreased neurogenesis in the hippocampus, and impaired the anti-depressant effects of fluoxetine [12]. Supplementation with Lactobacillus casei also produced anti-depressant-like effects in CUMS-treated rats [13]. These studies support the idea that gut microbiota changes could affect depressive-like behavior, and certain probiotics like Lactobacillus could be beneficial for treatment of stress-induced depressive behavior. However, the mechanisms by which probiotics affect brain functions and produce anti-depressant effects are not clearly understood.
Several mechanisms have been proposed to explain how gut microbiota communicate with the brain [7, 14]. The neural mechanism involving vagus nerve innervation of gut epithelial cells has been suggested, and immune responses stimulated by cytokines and peptide hormones, such as ghrelin, somatostatin, cholecystokinin, gastrin, GLP-1, and peptide YY, released from gut microbiota-stimulated enteroendocrine cells have been implicated. Neuronal effects of bacterial metabolites including dopamine, GABA, tryptophan, or 5-HT precursors have been proposed in other studies. Microbial by-products including short-chain fatty acids, carbohydrates, and bile acids, and/or bacteria-derived extracellular vesicles (EVs) have been proposed as mediators of gut microbiota [7, 15,16,17]. Of those proposed divergent mechanisms, the EV-mediated mechanism has been recently the focus of several studies [17]. Both Gram-negative and Gram-positive bacteria secrete EVs, which contain bacterial genomic DNA, RNA, proteins including various enzymes, and other metabolites. Bacteria are believed to employ EVs to communicate with host organisms or other organisms [17]. For example, EVs from Akkermansia muciniphila contain cargo contents that increase phosphorylation of AMPK and protect against LPS-induced intestinal permeability changes [18], and decrease colitis-induced inflammation [19]. EVs from Lactobacillus plantarum contain cargo materials that increase BDNF expression in cultured hippocampal cells [20]. Thus, EVs derived from specific bacteria could change cellular function in the brain and exert physiological effects on the host body, although the detailed mechanisms by which EVs from different bacteria and associated key components act on the host body remain to be investigated.
In the present study, we investigated the temporal profiles of gut microbiota composition changes during and after chronic stress, and we sought to understand whether administration of EVs derived from Gram-negative and Gram-positive probiotics could affect stress-induced maladaptive changes in the brain and depressive-like behaviors.
Materials and Methods
Animals
Seven-week-old male C57BL6 mice were purchased from Daehan BioLink (Eumsung, Chungbuk, Republic of Korea). Upon arrival, mice were grouped and housed in pairs in standard clear plastic cages in a temperature- (23–24 °C) and humidity (50–60%)-controlled environment under a 12-h light/dark cycle (lights on at 07:00–19:00 h), with ad libitum access to water and food. Animals were handled in accordance with the animal care guidelines of Ewha Womans University. Restraint procedures and EV treatment in this study were approved by the Ewha Womans University Animal Care and Use Committee (IACUC 15–012).
Chronic Restraint Stress and Fecal Collection
Mice were exposed to restraints as described previously [21, 22]. In brief, mice were individually placed in a well-ventilated, 50-ml polypropylene conical tube, with the head of the animal orienting toward the conical side by pushing the backside of the animal with a cut piece of a 15-ml conical tube. Thereafter, animals were restrained in this manner for 2 h daily starting at 10 a.m. After each session of restraint, they were returned to their home cages and housed with their cage mate with free access to food and water. This procedure was repeated each day for 14 days or other indicated time. Control mice housed in pairs were maintained in home cages without disturbance.
To collect fecal samples, mice were placed in an empty autoclaved cage with no bedding. The first one to two fecal pellets per animal were collected in a 1.5-mL microcentrifuge tube using a sterile toothpick, and were immediately stored at − 80 °C until shipping to MD Healthcare Inc. for analysis.
Analysis of 16S Ribosomal RNAs and Taxonomic Assignment
DNA Sequencing of Amplified Variable Regions of 16S Ribosomal DNA
Bacterial DNA isolation and emulsion-based PCR (emPCR) were carried out as described previously [23, 24]. Bacterial DNAs were extracted from fecal samples using a PowerWater® DNA Isolation Kit (MO BIO Laboratories, Carlsbad, CA, USA). PCR products were used to construct a single-stranded DNA library with adaptors for each sample using the 454 sequencing library preparation procedure (Roche, Branford, CT, USA). Constructed libraries were quantified using a Quant-iT PicoGreen dsDNA Assay Kit (Thermo Fisher Scientific, Waltham, MA), and were amplified using the 454 GS-FLX system and emulsion-based PCR (emPCR) Kit (Roche). In brief, single-stranded DNA libraries constructed with adaptors were immobilized onto DNA capture beads, which were added to a mixture of amplification mix and oil. They were then vigorously shaken on a TissueLyser II (Qiagen, Valencia, CA, USA) to create “micro-reactors” containing both a DNA capture bead and PCR amplification reagents in water-in-oil emulsions. The emulsion was dispensed into a 96-well plate and PCR amplification was carried out according to the manufacturer’s instructions. Each PCR reaction contained 20 ng of DNA in 50 μl of a PCR reaction volume. The universal primers 27F (5′-GAGTTTGATCMTGGCTCAG-3′) and 518R (5′-WTTACCGCGGCTGCTGG-3′) were used to amplify the variable regions 1 to 3 (V1-V3) of 16S ribosomal RNA (16S rRNA) genes. PCR was carried out using the FastStart High Fidelity PCR System (Roche) under the following conditions: 94 °C for 3 min followed by 35 cycles at 94 °C for 15 s, 55 °C for 45 s, and 72 °C for 1 min, followed by a final elongation step at 72 °C for 8 min. After emPCR, PCR products were purified using an Agencourt AMPure Bead kit (Beckman Coulter Inc., Brea, CA), and DNA concentration and quality was quantified using the Picogreen method (Invitrogen, Carlsbad, CA, USA).
Analysis of Sequencing Reads of 16S rDNA and Taxonomic Assignment
Analysis of sequencing reads of 16S rDNAs and taxonomic assignment were carried out as described previously [23]. DNA sequencing of amplified variable regions of 16S ribosomal DNA was carried out by Macrogen Inc. (Seoul, South Korea) using a Genome Sequencer FLX + System (Roche, Basel, Switzerland). Briefly, the emulsion containing emPCR products was chemically lysed and the beads carrying amplified DNA libraries were recovered and washed by filtration. Biotinylated primer A (complementary to adaptor A) was used to purify streptavidin-coated magnetic beads. The amplified single-stranded DNAs were separated from magnetic beads by melting double-stranded amplification products. The sequencing primer was then annealed to the amplified single-stranded DNA. The beads carrying amplified single-stranded DNA were counted with a Particle Counter (Beckman Coulter). Each sample was loaded on the 75-mm PicoTiter plate (Roche Diagnostics) fitted with an 8-lane gasket.
Sequencing reads with the lengths greater than > 300 bp and average Phred scores > 20 were selected. Operational taxonomy units (OTUs) were assigned using the sequence clustering algorithm UCLUST. Subsequent taxonomy assignment was achieved using QIIME by searching the 16 s ribosomal RNA sequence database of GreenGenes 8.15.13 using the following similarity cut-offs: species, > 97% similarity; genus, > 94% similarity; family, > 90% similarity; order, > 85% similarity; class, > 80% similarity; and phylum, > 75% similarity. The bacterial composition at the genus level was plotted in the heatmap if a genus cluster shows significant difference (> twofold) between two groups.
Preparation of EVs from Lactobacillus plantarum, Bacillus subtilis, and Akkermansia muciniphila
Bacterial culture and EV isolation were carried out as described previously [25,26,27,28,29]. In brief, Lactobacillus plantarum (KCTC 11401BP) was cultured in MRS broth (MB Cell, CA, USA) for 18 h at 37 °C with gentle shaking (150 rpm) as described previously [25, 26]. When the optical density of the cultures at 600 nm reached 1.0, bacteria were pelleted by centrifugation at 10,000 × g for 20 min. The supernatant was passed through a 0.22-μm bottle-top filter (Corning, NY, USA) to remove remaining cells or cell debris. The filtrate was concentrated using a MasterFlex pump system (Cole-Parmer, IL, USA) and a 100-KDa Pellicon 2 Cassette filter membrane (Merck Millipore, MA, USA), and passed through a 0.22-μm bottle-top filter again. EVs were pelleted from the resulting filtrate by ultracentrifugation at 150,000 × g for 3 h at 4 °C. Pellets were washed and resuspended in PBS (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4). The relative amount of Lactobacillus plantarum (Lac-EV) was quantified on the basis of protein levels carried by Lac-EV. Protein concentration was measured using a BCA protein assay kit (Thermo Fisher Scientific, MA, USA). Resuspended Lac-EVs were stored at − 80 °C until use.
Bacillus subtilis var. natto. which was isolated from Cheonggukjang, a Korean traditional food made by fermenting soybeans, was grown in brain heart infusion (BHI) media (Becton–Dickinson, Franklin Lakes, NJ) for 12 h at 37 °C as described previously [27]. Microscopic analysis indicated that sporulation had begun at 17 h, but not at 12 h. Bacillus subtilis cells cultured for 12 h, which had not undergone sporulation, were pelleted by centrifugation at 10,000 × g for 20 min and the supernatant was retained. Bacillus subtilis EVs (Bac-EV) were collected from the supernatants as described above, and collected EVs were stored at − 80 °C until use. The relative amount of Bac-EV was quantified on the basis of protein levels contained in Bac-EV. Protein concentration was determined.
Akkermansia muciniphila (ATCC BA-835), obtained from the American Type Culture Collection (Manassas, VA, USA), was cultured under anaerobic conditions (99% N2 at 37 °C) until the optical density at 600 nm reached 1.5, as described previously [28, 29]. Bacterial cultures were pelleted at 10,000 × g for 20 min and the supernatant was collected. EVs were isolated from the supernatant as described above, and isolated Akkermansia muciniphila EVs (Akk-EV) were stored at − 80 °C until use. The relative amount of Akk-EV was quantified on the basis of protein levels contained in Akk-EV.
HT22 Cell Culture and Treatment with Glucocorticoid and Bacterial EVs
HT22 mouse hippocampal cells were cultured as described previously [20]. Briefly, HT22 cells were cultured at 37 °C under 5% CO2 in Dulbecco’s modified Eagle’s medium (DMEM; LM001-05, Welgene, Gyeongan-si, Korea) supplemented with 10% heat-inactivated fetal bovine serum (FBS; FB02-500, Serum Source International, Charlotte, NC, USA), penicillin (20 units/ml), and streptomycin (20 mg/ml) (LS020-02, Welgene). HT22 cells cultured to reach 70–80% confluency were trypsinized and were plated at 1.0 × 105 cells/well in a 6-well plate containing the growth media.
After 24 h, HT22 cells were treated with glucocorticoid (GC; corticosterone, 400 ng/ml) or GC plus bacterial EVs (20 ug/ml) in DMEM containing 1% FBS and no antibiotics and cultured for an additional 24 h. The doses of GC and EVs used in the present study were based on the previous study [20]. HT22 cells were harvested and were used for further analysis.
Administration of EVs to Mice
EVs were administered to mice via the intraperitoneal route as described previously [20]. Lac-EV, Bac-EV, or Akk-EV were administered, each with 6 μg in 100 μl of injection volume, for the indicated days.
Quantitative Real-time PCR
Quantitative real-time PCR (qPCR) was carried out as described previously [20, 30]. Briefly, HT22 cells or hippocampal tissues were homogenized using pellet pestles (Z359971, Sigma-Aldrich) in TRI-zol reagent (15,596–018, Invitrogen), and total RNA was isolated from the homogenates. Two micrograms of total RNA was converted to cDNA using a reverse transcriptase system (Promega, Madison, WI, USA).
qPCR reaction contained 4 μl of 1/8 diluted cDNA, 10 μl of 2 × iQTM SYBR Green Supermix (Bio-Rad Laboratories, Foster City, CA, USA), and 1 μl each of 5 pmol/μl forward and reverse primers in 20 μl of volume. The qPCR reaction was carried out using the CFX 96 Real-Time PCR System Detector (Bio-Rad Laboratories). Transcript levels were normalized relative to Gapdh and L32 levels.
The primers used in this study were tBdnf (total form), forward 5′-TGGCTGACACTTTTGAGCAC-3′ and reverse 5′-GTTTGCGGCATCCAGGTAAT-3′; Bdnf1, forward 5′-CCTGCATCTGTTGGGGAGAC-3′ and reverse 5′-GCCTTGTCCGTGGACGTTTA-3′; Bdnf4, forward 5′-CAGAGCAGCTGCCTTGATGTT-3′ and reverse 5′-GCCTTGTCCGTGGACGTTTA-3′; Nt3, forward 5′-TACTACGGCAACAGAGACG-3′ and reverse 5′-GTTGCCCACATAATCCTCC-3′; Nt4/5, forward 5′-AGCGTTGCCTAGGAATACAGC-3′ and reverse 5′-GGTCATGTTGGATGGGAGGTATC-3′; Ngf, forward 5′-AGCATTCCCTTGACACAG-3′ and reverse 5′-GGTCTACAGTGATGTTGC-3′; Hdac2, forward 5′-GGGACAGGCTTGGTTGTTTC-3′ and reverse 5′-GAGCATCAGCAATGGCAAGT-3′; MeCP2, forward 5′-ACAGCGGCGCTCCATTATC-3′ and reverse 5′-CCCAGTTACCGTGAAGTCAAAA-3′; Sirt1, forward 5′-GATCCTTCAGTGTCATGGTTC-3′ and reverse 5′-ATGGCAAGTGGCTCATCA-3′; Gapdh, forward 5′-AGAAGGTGGTGAAGCAGGCATC-3′ and reverse 5′-CGAAGGTGGAAGA GTGGGAGTTG-3′; and L32, forward 5′-GCTGCCATCTGTTTTACGG-3′ and reverse 5′-TGACTGGTGCCTGATGAACT-3′.
Behavioral Tests
The behavioral tests were performed as described previously [21, 22]. Mice were allowed to adapt to the behavior testing room for a minimum of 30 min prior to the start of the test. All behavioral tests were monitored with a video tracking system (SMART; Panlab, Barcelona, Spain) and/or a webcam recording system (HD Webcam C210, Logitech, Newark, CA, USA).
Sociability Test
The sociability test was carried out as described previously [21, 22]. Briefly, an open field (40 × 40 cm2) was partitioned by a wall (20-cm wide and 20-cm high) at the center point to prepare a U-shaped two-choice field. Circular grid cages (12 cm in diameter × 33 cm height) were placed on each side of the U-shaped two-choice field. A subject mouse was allowed to freely explore this space placed with an empty circular grid cage on each side for 5 min and was then returned to the home cage. After 10 min, a social target was loaded into a circular grid cage on one side of the field and the habituated subject mouse was placed in the center of the U-shaped two-choice field. The subject mouse was allowed to explore both fields for 10 min while the trajectory of the mouse’s movements and time spent in each field was recorded by a video tracking system. Social targets were the same age and sex as the subject mice. The position of the field placed with the circular grid cage containing a social target and the position of the field containing an empty grid cage were defined as the target field and non-target field, respectively.
Tail Suspension Test
The tail suspension test (TST) was carried out as described previously [21, 22]. Mice were suspended individually by fixing their tails with adhesive tape to the ceiling of a shelf 50 cm above a bottom floor, and were recorded with a webcam recording system for 6 min. Cumulative immobility time was measured. Immobility was defined as the time the animal spent suspended with all limbs motionless.
Forced Swim Test
The forced swim test (FST) was performed as described previously [21, 22]. Mice were placed in a Plexiglas cylinder (15 cm in diameter × 27 cm height) containing water at a temperature of 24 °C and a depth of 15 cm. Mice were placed in the cylinder for 6 min and the cumulative immobility time was measured for the last 5 min. Immobility was defined as time of the animal spent floating with all limbs motionless. The performance during the test was recorded using a webcam recording system and then analyzed.
Statistical Analysis
A two-sample comparison was carried out using the Student t-test. Multiple comparisons were performed by one-way ANOVA followed by the Newman-Keuls post hoc test or two-way ANOVA followed by the Bonferroni post hoc test. All data are represented as mean ± SEM, and statistical significance was accepted at the 5% level.
Results
Metagenome Analysis Revealed that Gut Microbiota Composition Was Markedly Changed During and After Chronic Stress
Mice treated with daily 2-h restraint for 14 days, called chronic restraint stress (CRST), exhibit depressive-like behaviors that last longer than 3 months [31, 32]. CRST-induced behavioral deficits are produced by stress-induced maladaptive changes in the hippocampus and neuroendocrine systems [33]. In the present study, we investigated whether CRST produces changes in gut microbiota.
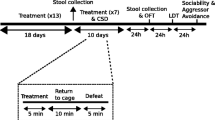
After C57BL6/J mice were purchased from a local vendor and habituated in our animal room facility for 5 days, they were randomly divided into two groups: control and stress groups. Mice assigned to the stress group were treated with daily 2-h restraint for 14 days and stools were collected at day 1, day 14, and post-stress day 14. Stools were collected from control mice in parallel (Fig. 1A). Bacterial DNA was isolated from fecal samples and used to obtain DNA sequence readings of variable regions of 16S ribosomal RNA (rRNA) genes. This led to the identification of 21,811 and 19,148 operational taxonomic units (OTUs) in the control and CRST groups, respectively. Expected sample taxonomic richness increased with the number of DNA sequence reads in both groups (Fig. 1B). The microbiota identified in CRST and control groups consisted of 22 OTUs at the phylum level, 43 OTUs at the class level, 79 OTUs at the order level, 176 OTUs at the family level, and 376 OTUs at the genus level. Analysis of the relative occupancy of the most abundant 15 phyla with an occupancy ≥ 0.1%, which counted for > 95% of identified OTUs, indicated that the CRST group exhibited significant changes in the composition of the top 15 phyla relative to the control group over the test period, with a particularly dramatic change at post-stress day 14 (Fig. 1C).
Taxonomic richness and percent composition of most abundant top 15 phyla of gut microbiota in mice exposed to chronic stress. (A) Experimental design for treatment with 2-h restraint for 14 days (CRST) and time points for fecal collection. (B) Rarefaction curves with mean OTUs for each group over the number of DNA sequence reads of 16S ribosomal RNA genes in the control (blue) and CRST (red) groups (n = 12 for control and 14 for CRST group). Data points are mean occupancy (%) + / − SEM. (C) Clustered stacked column bar graphs depicting the relative occupancy of the most abundant phyla of gut microbiota in the control and CRST groups. Resolved OTUs with occupancies ≥ 0.1% are presented. Each column represents resolved OTUs from 4 to 5 independent fecal samples (CON, n = 4 fecal samples for all time points; CRST, n = 4 fecal samples for day 1; n = 5 fecal samples for day 14, and n = 5 fecal samples for post-stress day 14). (D) The relative occupancy of most abundant top 15 phyla in the control and CSRT groups at the indicated time points and the difference (z-score) in the mean occupancy between control and CSRT groups (CON vs. CRST). SD, standard deviation. Data are presented as mean occupancy (%) + / − SD (CON, n = 4 for all time points; CRST, n = 4–5). The z-score is X − µ/σ, where µ = mean, X = individual score, and σ = standard deviation. < < denotes increase in the mean occupancy over 100
The relative occupancy of the top 15 phyla in CRST and control groups was calculated and converted to % composition of the mean value and the mean difference between the control group and CRST group, expressed as a standard deviation unit (z score) at each time point. On post-stress day 14, the occupancy of p_Bacteroidetes decreased from 36.90 to 15.33% (− 1.95 × z-score), the occupancy of p_Tenericutes decreased from 0.17 to 0.00% (− 3.38 × z), and the occupancy of an unassigned phylum decreased from 7.95 to 3.32% (− 5.28 × z) (Fig. 1D). In contrast, the occupancy of p_Actinobacteria increased from 4.11 to 12.76% (6.14 × z), the occupancy of p_TM7 increased from 0.07 to 1.50% (56.63 × z), and the occupancy of p_Cyanobacteria increased from 0.11 to 1.09% (18.6 × z) (Fig. 1D).
Chronic Stress Caused Dramatic Changes in Gut Microbiota Composition at the Genus Level
We next analyzed stress-induced changes in gut microbiota at the genus level. The identified OTUs contained 104 genus members with a relative occupancy of ≥ 0.1% in control or CRST groups at any of the three time points. The most abundant top 10, 20, and 30 genus members in the control group comprised total 88.7, 96.8, and 98.6%, respectively, of those identified OTUs at stress day 1; 91.8, 96.7, and 98.5%, respectively, at stress day 14; and 91.7, 97.2, and 98.7%, respectively, at post-stress day 14 (Fig. 2; Supplemental Table 1). In contrast, those top 10, 20, and 30 genus members in the CRST group comprised 80.8, 92.8, and 96.1%, respectively, of those identified OTUs at stress day 1; 88.0, 92.8, and 96.0%, respectively, at stress day 14; and 22.4, 39.4, and 41.1%, respectively, at post-stress day 14 (Fig. 2; Supplemental Table 1). Thus, the relative abundance of the most abundant top 10–30 genus members in the control group was not changed within a month of the test period, whereas the relative abundance of the same top 10–30 genus members in the CRST group were reduced slightly during the stress period and severely in the post-stress period.
Temporal changes in summed occupancy of the most abundant top 10, 20, and 30 genus members in control and CRST groups during and after stress phases. The summed occupancy (%) of the most abundant top 10, 20, and 30 genus members among the identified OTUs in the control and CRST (CON(10), CON(20), CON(30), CRST(10), CRST(20), and CRST(30), respectively) groups during and after CRST. The experimental design of Fig. 1A for treatment with 2-h restraint for 14 days (CRST) and time points for fecal collection is applicable here. The shaded box (light blue) represents the stress phase
Analysis of the relative occupancy of identified OTUs indicated that 34 genus members had a relative occupancy of ≥ 0.1% in control or CRST groups at any of the three time points. Of those genus members, 6 members were significantly upregulated and 6 members were downregulated in the CRST group over time (time factor, two-way ANOVA), whereas the remaining 22 members were insignificantly changed or statistically unchanged over the test period (Fig. 3; Supplemental Table 1).
The relative occupancy of the most abundant top 34 individual genus members changed by chronic stress. (A–C) The percent occupancy of 34 individual genus members in the control and CRST groups at stress day 1, stress day 14, and post-stress day 14. The 34 genus members were selected as those having a relative occupancy ≥ 0.1% in the control or CRST groups at any of the three time points. The percent changes of high occupancy (≥ 1% at stress day 1, control) members (A): f__S24-7;g__, g__Lactobacillus, g__Staphylococcus, an unassigned member of bacterium, an unclassified member of o_Clostridiales;f_;g_, g__Corynebacterium, f__Lachnospiraceae;g_, f__Aerococcaceae;g_, g__Jeotgalicoccus, g__Adlercreutzia, f__F16;g_, and g__Desulfovibrio in controls and CRST groups at stress day 1, stress day 14, and post-stress day 14. The percent changes of middle occupancy (≥ 0.1% at stress day 1, control) members (B): f__Planococcaceae;g_, f__Comamonadaceae;g_, f__Ruminococcaceae;g_, f__Rikenellaceae;g_, o__Bacteroidales;f_;g_, g__Bacteroides, f__Enterobacteriaceae;g_, g__Akkermansia, g__Lactococcus, g__Prevotella, g__Oscillospira, g__Rhodococcus, g__Ruminococcus, g__[Ruminococcus], g__Pseudomonas, g__Faecalibacterium, g__Acinetobacter, g__Adlercreutzia, o__Streptophyta;f__;g__, g__Parabacteroides, g__Propionibacterium, and g__Blautia in controls and CRST groups at stress day 1, stress day 14, and post-stress day 14. Data are presented as mean occupancy (%) + / − SEM. * denotes the difference between control and CRST groups (main effect of stress) at post-stress day 14 at p < 0.05 (two-way ANOVA, followed by Bonferroni post hoc test)
Of those altered genus members, the relative occupancy of an unclassified member of f__S24-7 (f__S24-7;g__), an unassigned bacterium, an unclassified member of o_Clostridiales;f_;g_, g__Adlercreutzia, and g__Desulfovibrio decreased in the CRST group at post-stress day 14, and the relative occupancy of g__Lactobacillus decreased in the CRST group at all three time points examined. In contrast, the relative occupancy of g__Bacteroides, f__Enterobacteriaceae;g__, f__Comamonadaceae;g__, g__Rhodococcus, g__Pseudomonas, and g__Enhydrobacter increased in the CRST group at post-stress day 14 (Fig. 3; Supplemental Table 1). The relative occupancy of g__Akkermansia, g__Lactococcus, f__Aerococcaceae;g_, g__Ruminococcus, and other unclassified member of g__Ruminococcus, g__Faecalibacterium, g__Acinetobacter, o__Streptophyta;f__;g__, g__Parabacteroides, g__Propionibacterium, and g__Blautia appeared to be changed in the CRST group at post-stress day 14, although these differences were not statistically significant (Fig. 3; Supplemental Table 1).
Post-stress Treatment with EVs from the Three Probiotics to CRST Mice Induced Expression of Neurotrophic Factors in the Hippocampus
Among the taxonomic members of microbiota whose relative abundance was changed by CRST, the stress-dependent decrease of Lactobacillus was particularly remarkable (Fig. 3; Supplemental Table 1). Recently, we reported that EVs derived from Lactobacillus plantarum (Lac-EV) increased neurotrophic factor expression via Sirt1 epigenetic factor in HT22 cells and in the hippocampus of CRST-treated mice [20]. Consistent with the previous study [20], Lac-EV treatment in HT22 cells reversed glucocorticoid (GC)-induced reduced expression of Bdnf, Nt4/5, and Sirt1 expression, and partially increased GC-induced reduced expression of Mecp2, but Lac-EV produced no effect on Hdac2 (Fig. 4A and B). Sirt1, MeCP2, and HDAC2 are epigenetic factors that regulate Bdnf and Nt4/5 expression [20, 30, 34]. Next, we investigated whether EVs from Bacillus subtilis and Akkermansia muciniphila, which are taxonomically remote from Lactobacillus plantarum, could have any effects on the expression of Bdnf, Nt4/5, Mecp2, Hdac2, and Sirt1. Bacillus EV (Bac-EV) treatment in HT22 cells markedly increased GC-induced reduced expression of Bdnf, Mecp2, Hdac2, and Sirt1 expression (Fig. 4A and B). Akkermansia EV (Akk-EV) treatment in HT22 cells reversed GC-induced reduced expression of Bdnf, Nt4/5, and Hdac2, whereas Akk-EV produced no effect on Mecp2 and Sirt1 (Fig. 4A and B). These results indicated that Lac-EV, Bac-EV, and Akk-EV all have an ability to reverse GC-induced reduced expression of Bdnf or Nt4/5, whereas their effects on GC-induced reduced expression of Mecp2, Hdac2, and Sirt1 expression are partially overlapped, but not identical.
Lac-EV, Bac-EV, and Akk-EV treatment in HT22 cells reversed GC-induced reduced expression of Bdnf, Nt4/5, Mecp2, Hdac2, and Sirt1. (A, B) Transcript levels of total Bdnf (tBdnf), Nt4/5 (A), Mecp2, Hdac2, and Sirt1 (B) in HT22 cells treated with glucocorticoid (GC, corticosterone; 400 ng/ml), GC plus Lac-EV (20 μg/ml), GC plus Bac-EV (20 μg/ml), and GC plus Akk-EV (20 μg/ml) for 24 h (n = 4 per group). Data are presented as mean ± SEM. *,**, difference between indicated group. *p < 0.05; **p < 0.01 (one-way ANOVA followed by Newman-Keuls post hoc test)
Next, we investigated whether Lac-EV, Bac-EV, and Akk-EV induce similar genomic responses in the brain of mice as those in HT22 cells and produce behavioral effects. Lac-EV, Bac-EV, and Akk-EV were administered via the intraperitoneal route, which allows EVs to enter the systemic circulation [35]. Post-stress treatment with Lac-EV to CRST-treated mice reversed stress-induced reduced expression of Bdnf, Nt3, and Nt4/5 in the hippocampus. Lac-EV also increased stress-induced reduced expression of Mecp2 and also tended to increase Sirt1 (Fig. 5A–C). Post-stress treatment with Bac-EV to CRST-treated mice restored stress-induced reduced expression of Bdnf and Nt3, and also tended to reverse stress-induced reduced expression of Sirt1, although its effect on Sirt1 was not statistically significant, and Bac-EV produced no effect on Mecp2 and Hdac2 expression (Fig. 5D and E). Post-stress treatment with Akk-EV reversed stress-induced reduction of Bdnf, and Nt3 expression, whereas it produced a subtle effect on Mecp2, Hdac2, and Sirt1 (Fig. 5F and G). These results indicated that Lac-EV, Bac-EV, and Akk-EV similarly reverse stress-induced reduced expression of Bdnf, Nt3, and/or Nt4/5 in the hippocampus of CRST mice, whereas their effects on Mecp2, Hdac2, and Sirt1 expression are only partially overlapped.
Post-stress treatment with Lac-EV, Bac-EV, and Akk-EV reversed stress-induced reduced expression of neurotrophic factors, Mecp2, and Sirt1 in the hippocampus of CRST mice. (A) Experimental design for treatment of mice with 2-h restraint for 14 days (CRST) followed by treatment with EVs from Lactobacillus plantarum (Lac-EV), Bacillus subtilis (Bac-EV), and Akkermansia muciniphila (Akk-EV). A purple arrow at post-stress day 17, time point for tissue prep. EVs, 2 μg/100 μl/mouse/day (i.p.). The experimental design is applicable to this figure and Fig. 6. Control mice injected with saline (CON + veh) or EVs (CON + Lac-EV, CON + Bac-EV, and CON + Akk-EV); CRST-treated mice injected with saline (CRST + veh) or EVs (CRST + Lac-EV, CRST + Bac-EV, and CRST + Akk-EV). (B, C) Expression levels of total Bdnf (tBdnf), Bdnf1, Bdnf4, Nt3, Nt4/5, Ngf (B), Mecp2, Hdac2, and Sirt1 (C) in the hippocampus of CRST mice treated with Lac-EV. Veh, vehicle (n = 6–12 qPCR repeats). (D, E) Expression levels of total Bdnf (tBdnf), Bdnf1, Bdnf4, Nt3, Nt4/5, Ngf (D), Mecp2, Hdac2, and Sirt1 (E) in the hippocampus of mice treated with Bac-EV. Veh, vehicle (n = 6–10 qPCR repeats). (F, G) Expression levels of total Bdnf (tBdnf), Bdnf1, Bdnf4, Nt3, Nt4/5, Ngf (F), Mecp2, Hdac2, and Sirt1 (G) in the hippocampus of mice treated with Akk-EV. Veh, vehicle (n = 6–10 qPCR repeats). Data are presented as mean ± SEM. *p < 0.05; **p < 0.01 (two-way ANOVA followed by Bonferroni post hoc test)
Post-stress Treatment with EVs from the Three Probiotics Produced Anti-depressive-Like Effects
We tested whether administration of Lac-EV, Bac-EV, and Akk-EV could produce behavioral changes in CRST mice. Mice exposed to CRST exhibited increased immobility in the TST and FST (Fig. 6A and B). In contrast, post-stress treatment with Lac-EV to CRST-treated mice for 14 days reversed the stress-induced increased immobility in the TST and FST (Fig. 6A and B).
Post-stress treatment with Lac-EV, Bac-EV, and Akk-EV improved stress-induced depressive-like behavior in CRST mice. (A, B) Immobility time in the TST (A) and FST (B) of the indicated groups. Mice were exposed to CRST followed by treatment with Lac-EV, Bac-EV, and Akk-EV. Behavior tests were performed on post-stress days 15–16 (p15 ~ p16). Control mice injected with saline (CON + veh) or EVs (CON + Lac-EV, CON + Bac-EV, and CON + Akk-EV); CRST-treated mice injected with saline (CRST + veh) or EVs (CRST + Lac-EV, CRST + Bac-EV, and CRST + Akk-EV). The experimental design of Fig. 5A is applicable here. EVs, 2 μg/100 μl/mouse/day (i.p.). n = 6–12 animals. (C) A summary diagram depicting stress-dependent changes in the relative occupancy of the most abundant phyla of gut microbiota and EV-induced upregulation of MeCP2, Sirt1, and Bdnf expression and anti-depressive-like effects. Data are presented as mean ± SEM. *p < 0.05; **p < 0.01 (one-way ANOVA followed by Newman-Keuls post hoc test among the EV-treated control groups or EV-treated CRST groups; two-way ANOVA followed by Bonferroni post hoc test among CON + veh, CON + EVs, CRST + veh, and CRST + EVs groups)
Post-stress treatment with Bac-EV or Akk-EV to CRST-treated mice for 14 days also reversed stress-induced increased immobility in the FST, which were comparable to those induced by Lac-EVs (Fig. 6A and B). However, post-stress treatment with Bac-EV or Akk-EV to CRST-treated mice partially reversed stress-induced increased immobility in the TST, and their effects were statistically insignificantly (Fig. 6A).
These results suggest that Lac-EV, Bac-EV, and Akk-EV all confer anti-depressive-like effects in CRST-treated mice, although the behavioral effects of Bac-EV and Akk-EV are relatively weak.
Discussion
Chronic Restraint Changed Gut Microbiota Composition Prominently in the Post-stress Period
In the present study, we demonstrated that chronic stress evoked by daily 2-h restraint for 14 days (CRST) changed gut microbiota composition at multiple levels, from phylum to genus. Interestingly, stress-induced changes were more dramatic in the post-stress period than those observed in the stress phase. This finding raises several important interrelated issues. First, repeated restraint stress treated with daily 2-h restraint for 14 days produced gut microflora changes in mice that were housed in a relatively constant physical environment and with a regular food supply. Furthermore, these changes occurred in a period of a month. Although underlying mechanisms need to be studied further, our results suggest that repeated stress imposed by our experimental regimen not only produce maladaptive changes in the brain and depressive-like behaviors, but also significantly impact the relative composition of gut microbiota. Our results are partly consistent with previous reports; 5 weeks of chronic unpredictable mild stress (CUMS) caused changes in gut microbiota composition [11]. In an independent study, 9 weeks of CUMS also changed gut microbiota composition, whereas transferring gut microbiota prepared from stools of CUMS-treated mice to normal recipient mice decreased hippocampal neurogenesis and induced depressive-like behaviors in the recipients [12]. These results support that chronic stress causes maladaptive changes in the brain and also gut microbiota composition in different stress-induced models of depression. Second, the relative abundance of gut microbiota was changed more drastically in the post-stress period compared to the stress phase. It remains to be determined whether these changes represent a state of imbalance in gut microbiota that might occur following the removal of stressors, or if stress-induced changes in gut microbiota are advanced in a protracted manner. Third, concerning the detailed mechanisms by which repeated stress produces gut microbiota changes, it is possible that stress-induced adaptive changes in the brain produce not only HPA axis dysregulation, but also gut microbiota composition. Neuronal dysfunction that involves the autonomic nervous system was associated with altered cellular function of gut epithelial cells [36]. This possibility supports the notion that brain dysfunction is associated with gut microbiota changes via the neuroendocrine system, but more detailed studies will be required to understand the underlying mechanisms.
Post-stress Treatment with EVs from Three Types of Probiotics Improved Stress-Induced Depressive-Like Behavior
We demonstrated that parenterally injected EVs of Lactobacillus plantarum, Bacillus subtilis, and Akkermansia muciniphila all produced anti-depressive-like effects in CRST-treated mice (Fig. 6), although the three bacterial families are taxonomically unrelated. Lactobacillus plantarum is a catalase-negative, facultatively heterofermentative, non-spore-forming, rod-shaped, Gram-positive bacterium belonging to the phylum Firmicutes, class Bacilli, order Lactobacillales, family Lactobacillaceae, and genus Lactobacillus [37]. Bacillus subtilis is a catalase-positive, spore-forming, rod-shaped, Gram-positive bacterium belonging to the phylum Firmicutes, class Bacilli, order Bacillales, family Bacillaceae, and genus Bacillus [38]. Akkermansia muciniphila is a strictly anaerobic, non-spore-forming, oval-shaped, Gram-negative, mucin-degrading bacterium belonging to the phylum Verrucomicrobia, class Verrucomicrobiae, order Verrucomicrobiales, family Akkermansiaceae, and genus Akkermansia [39]. Thus, Lactobacillus and Bacillus are Gram-positive bacteria that share some distinctive prokaryotic features, whereas Akkermansia is a Gram-negative bacterium that is far remote from Lactobacillus and Bacillus at the taxonomic level. Nonetheless, Lac-EV, Bac-EV, and Akk-EV similarly reversed stress-induced reduced expression of Bdnf, Nt3, and/or Nt4/5 in HT22 cells and also in the hippocampus (Figs. 4 and 5) and produced anti-depressive-like effects (Fig. 6). However, the detailed genomic responses of Mecp2, Hdac2, and Sirt1 expression induced by Lac-EV, Bac-EV, and Akk-EV were slightly different, although their effects were partially overlapped (Figs. 4 and 5). Sirt1, MeCP2, and HDAC2 regulate Bdnf, Nt3, and Nt4/5 expression [20, 30, 34]. These results suggest that EVs from the three probiotics might contain the cargo components that directly act on neuronal cells, increase the transcription of neurotrophic factors, and produce anti-depressant-like effects, and that those genomic and behavioral effects are mediated by slightly different, but overlapped, pathways which involve MeCP2, HDAC2, and Sirt1. Whether each type of EVs contains specific contents that induce those genomic and behavioral responses remains to be characterized in the future. EVs used in the present study were prepared from bacterial culture media by filtration through a 0.22-μm bottle-top filter and ultracentrifugation at 150,000 × g for 3 h, as described in the “Materials and Methods” section. Although it is less likely that isolated EVs contain cell debris- or culture-related simple particles, the present study did not test if purified EVs contain EV-like microvesicles derived from culture media itself. Therefore, it will be necessary to test in the future study if EV media control contains any components that induce any genomic responses in cultured cells or produce any behavioral effects.
Recently, it was reported that exposure to 7 weeks of CUMS in rats changed intestinal microbiota composition and produced depressive-like behaviors. In contrast, administration of Lactobacillus casei from 4 weeks to the end of the 7 weeks of the CUMS regimen in rats improved depression-like behavior and reversed stress-induced reduction of BDNF expression and TrkB signaling in the frontal cortex [13], although the mechanism whereby Lactobacillus casei increased BDNF expression was not explored in this study. Administration of Lactobacillus reuteri from 4 to 7 weeks in mice similarly exposed to 7 weeks of the CUMS regimen improved depression-like behavior in the FST by inhibiting the expression of indoleamine-pyrrole 2,3-dioxygenase, a key enzyme in the kynurenine pathway in the intestine, and lowering circulating kynurenine levels [11]. The tryptophan-kynurenine pathway is functional not only in intestinal cells, but also in the liver [40]. A close relationship between the tryptophan-kynurenine pathway and BDNF-TrkB signaling in the limbic system has been proposed [41]. According to these studies, Lactobacillus casei and Lactobacillus reuteri can induce BDNF-TrkB signaling in the limbic system via the tryptophan-kynurenine pathway. These results are similar to those changed by Lac-EV, Bac-EV, and Akk-EV in the hippocampus of CRST-treated mice (Fig. 5). Considering the results of the present study, it might be important to investigate whether anti-depressive-like effects of Lactobacillus casei and Lactobacillus reuteri observed in those studies are produced by the mechanism that depends on EVs, or if they act through other independent mechanisms.
In conclusion, chronic stress induces gut microbiota composition changes that are dramatic particularly in the post-stress period. Post-stress treatment with EVs derived from selective probiotics restores stress-induced decreased expression of MeCP2, Sirt1, and/or neurotrophic factors in the hippocampus, and produces anti-depressive-like effects.
Data Availability
Data and materials will be made available on reasonable request.
Code Availability
Not applicable.
Abbreviations
- Bdnf:
-
Brain-derived neurotrophic factor
- CREB:
-
cAMP response element binding protein
- CRST:
-
Chronic restraint stress
- EVs:
-
Extracellular vesicles
- GC:
-
Glucocorticoid
- HDAC2:
-
Histone Deacetylase 2
- IMI:
-
Imipramine
- MeCP2:
-
Methyl CpG binding protein 2
- Ngf:
-
Nerve growth factor
- NT3:
-
Neurotrophin 3
- NT4/5:
-
Neurotrophin 4/5
- OTUs :
-
Operational taxonomic units
- rRNA :
-
Ribosomal RNA
- Sirt1:
-
Sirtuin 1
- TrkB:
-
Tropomyosin receptor kinase B
References
Radley J, Morilak D, Viau V, Campeau S (2015) Chronic stress and brain plasticity: mechanisms underlying adaptive and maladaptive changes and implications for stress-related CNS disorders. Neurosci Biobehav Rev 58:79–91
de Kloet ER, Joels M, Holsboer F (2005) Stress and the brain: from adaptation to disease. Nature Rev Neurosci 6:463–475
McEwen B, Nasca C, Gray JD (2016) Stress effects on neuronal structure: hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology 41:3–23
Lee EH, Han PL (2019) Reciprocal interactions across and within multiple levels of monoamine and cortico-limbic systems in stress-induced depression: a systematic review. Neurosci Biobehav Rev 101:13–31
Foster JA, Rinaman L, Cryan JF (2017) Stress & the gut-brain axis: regulation by the microbiome. Neurobiol Stress 7;124e136.
Karl JP, Hatch AM, Arcidiacono SM, Pearce SC, Pantoja-Feliciano IG, Doherty LA, Soares JW (2018) Effects of psychological, environmental and physical stressors on the gut microbiota. Front Microbiol 9:2013
Rea K, Dinan T, Cryan JF (2020) Gut microbiota: a perspective for psychiatrists. Neuropsychobiology 79:50–62
Barandouzi ZA, Starkweather AR, Henderson WA, Gyamfi A, Cong XS (2020) Altered composition of gut microbiota in depression: a systematic review. Front Psychiatry 11:541
Jiang H, Ling Z, Zhang Y, Mao H, Ma Z, Yin Y, Wang W, Tang W, Tan Z, Shi J, Li L, Ruan B (2015) Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun 48:186–194
Wallace CJK, Milev R (2017) The effects of probiotics on depressive symptoms in humans: a systematic review. Ann Gen Psychiatry 16:14
Marin IA, Goertz JE, Ren T, Rich SS, Onengut-Gumuscu S, Farber E, Wu M, Overall CC, Kipnis J, Gaultier A (2017) Microbiota alteration is associated with the development of stress-induced despair behavior. Sci Rep 7:43859
Siopi E, Chevalier G, Katsimpardi L, Saha S, Bigot M, Moigneu C, Eberl G, Lledo PM (2020) Changes in gut microbiota by chronic stress impair the efficacy of fluoxetine. Cell Rep 30:3682–3690
Gu F, We Y, Liu Y, Dou M, Jiang Y, Liang H (2020) Lactobacillus casei improves depression-like behavior in chronic unpredictable mild stress-induced rats by the BDNF-TrkB signal pathway and the intestinal microbiota. Food Funct 11(7):6148–6157
Cryan JF, O’Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, Codagnone MG, Cussotto S, Fulling C, Golubeva AV, Guzzetta KE, Jaggar M, Long-Smith CM, Lyte JM, Martin JA, Molinero-Perez A, Moloney G, Morelli E, Morillas E, O’Connor R, Cruz-Pereira JS, Peterson VL, Rea K, Ritz NL, Sherwin E, Spichak S, Teichman EM, van de Wouw M, Ventura-Silva AP, Wallace-Fitzsimons SE, Hyland N, Clarke G, Dinan TG (2019) The microbiota-gut-brain axis. Physiol Rev 99(4):1877–2013
Collins SM, Surette M, Bercik P (2012) The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol 10(11):735–742
Kim OY, Park HT, Dinh NTH, Choi SJ, Lee J, Kim JH, Lee SW, Gho YS (2017) Bacterial outer membrane vesicles suppress tumor by interferon-γ-mediated antitumor response. Nat Commun 8(1):626
Gill S, Catchpole R, Forterre P (2019) Extracellular membrane vesicles in the three domains of life and beyond. FEMS Microbiol Rev 43(3):273–303
Chelakkot C, Choi Y, Kim DK, Park HT, Ghim J, Kwon Y, Jeon J, Kim MS, Jee YK, Gho YS, Park HS, Kim YK, Ryu SH (2018) Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp Mol Med. 50(2):e450
Kang CS, Ban M, Choi EJ, Moon HG, Jeon JS, Kim DK, Park SK, Jeon SG, Roh TY, Myung SJ, Gho YS, Kim JG, Kim YK (2013) Extracellular vesicles derived from gut microbiota, especially Akkermansia muciniphila, protect the progression of dextran sulfate sodium-induced colitis. PLoS One. 8(10):e76520
Choi J, Kim YK, Han PL (2019) Extracellular vesicles derived from Lactobacillus plantarum increase BDNF expression in cultured hippocampal neurons and produce antidepressant-like effects in mice. Exp Neurobiol 28(2):158–171
Kim TK, Kim JE, Park JY, Lee JE, Choi J, Kim H, Lee EH, Kim SW, Lee JK, Kang HS, Han PL (2015) Antidepressant effects of exercise are produced via suppression of hypocretin/orexin and melanin-concentrating hormone in the basolateral amygdala. Neurobiol Dis 79:59–69
Choi J, Kim JE, Kim TK, Park JY, Lee JE, Kim H, Lee EH, Han PL (2015) TRH and TRH receptor system in the basolateral amygdala mediate stress-induced depression-like behaviors. Neuropharmacology 97:346–356
Yoo JY, Rho M, You YA, Kwon EJ, Kim MH, Kym S, Jee YK, Kim YK, Kim YJ (2016) 16S rRNA gene-based metagenomic analysis reveals differences in bacteria-derived extracellular vesicles in the urine of pregnant and non-pregnant women. Exp Mol Med 48:e208
Kim MH, Rho M, Choi JP, Choi HI, Park HK, Song WJ, Min TK, Cho SH, Cho YJ, Kim YK, Yang S, Pyun BY (2017) A metagenomic analysis provides a culture-independent pathogen detection for atopic dermatitis. Allergy Asthma Immunol Res 9(5):453–461
Kim MH, Choi SJ, Choi HI, Choi JP, Park HK, Kim EK, Kim MJ, Moon BS, Min TK, Rho M, Cho YJ, Yang S, Kim YK, Kim YY, Pyun BY (2018) Lactobacillus plantarum-derived extracellular vesicles protect atopic dermatitis induced by Staphylococcus aureus-derived extracellular vesicles. Allergy Asthma Immunol Res 10:516–532
Choi JH, Moon CM, Shin TS, Kim EK, McDowell A, Jo MK, Joo YH, Kim SE, Jung HK, Shim KN, Jung SA, Kim YK (2020) Lactobacillus paracasei-derived extracellular vesicles attenuate the intestinal inflammatory response by augmenting the endoplasmic reticulum stress pathway. Exp Mol Med 52:423–437
Kim Y, Edwards N, Fenselau C (2016) Extracellular vesicle proteomes reflect developmental phases of Bacillus subtilis. Clin Proteomics 9(13):6
Kang CS, Ban M, Choi EJ, Moon HG, Jeon JS, Kim DK, Park SK, Jeon SG, Roh TY, Myung SJ, Gho YS, Kim JG, Kim YK (2013) Extracellular vesicles derived from gut microbiota, especially Akkermansia muciniphila, protect the progression of dextran sulfate sodium-induced colitis. PLoS One 8(10):e76520
Chelakkot C, Choi Y, Kim DK, Park HT, Ghim J, Kwon Y, Jeon J, Kim MS, Jee YK, Gho YS, Park HS, Kim YK, Ryu SH (2018) Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp Mol Med 50(2):e450
Choi J, Kwon HJ, Lee JE, Lee Y, Seoh JY, Han PL (2019) Hyperoxygenation revitalizes Alzheimer’s disease pathology through the upregulation of neurotrophic factors. Aging Cell 18(2):e12888
Seo JS, Park JY, Choi J, Kim TK, Shin JH, Lee JK, Han PL (2012) NADPH oxidase mediates depressive behavior induced by chronic stress in mice. J Neurosci 32(28):9690–9699
Kim TK, Lee JE, Kim JE, Park JY, Choi J, Kim H, Lee EH, Han PL (2016) G9a-mediated regulation of OXT and AVP expression in the basolateral amygdala mediates stress-induced lasting behavioral depression and its reversal by exercise. Mol Neurobiol 53(5):2843–2856
Lee JE, Kwon HJ, Choi J, Seo JS, Han PL (2020) Aging increases vulnerability to stress-induced depression via upregulation of NADPH oxidase in mice. Commun Biol 3(1):292
Karpova (2014) Role of BDNF epigenetics in activity-dependent neuronal plasticity. Neuropharmacology 76 Pt C:709–18.
Al Shoyaib A, Archie SR, Karamyan VT (2019) Intraperitoneal route of drug administration: should it be used in experimental animal studies? Pharm Res 37(1):12
Carabotti M, Scirocco A, Maselli MA, Severi C (2015) The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol 28(2):203–209
Salvetti E, Torriani S, Felis GE (2012) The genus Lactobacillus: a taxonomic update. Probiotics Antimicrob Proteins 4(4):217–226
Dijl JM, Hecker M (2013) Bacillus subtilis: from soil bacterium to super-secreting cell factory. Microb Cell Fact 12:3
Payahoo L, Khajebishak Y, Ostadrahimi A (2019) Akkermansia muciniphila bacteria: a new perspective on the management of obesity an updated review. Reviews in Medical Microbiol 30(2):83–89
Davis I, Liu A (2015) What is the tryptophan kynurenine pathway and why is it important to neurotherapy? Expert Rev Neurother 15(7):719–721
Zhang JC, Yao W (2016) Hashimoto K (2016) Brain-derived neurotrophic factor (BDNF)-TrkB signaling in inflammation-related depression and potential therapeutic targets. Curr Neuropharmacol 14(7):721–731
Funding
This research was supported by a grant (2021R1A2B5B02002245) from the Ministry of Science, ICT and Future Planning, Republic of Korea.
Author information
Authors and Affiliations
Contributions
JC and HK carried out the experiments; YKK provided EVs; JC, HK, and PLH designed the experiments, performed the statistical analysis, and wrote the manuscript.
Corresponding author
Ethics declarations
Consent for Publication
All authors consent to the publication of the manuscript in Mol Neurobiol, should the article be accepted by the Editor-in-chief.
Ethics Approval and Consent to Participate
All animals were handled in accordance with the animal care guidelines of Ewha Womans University (IACUC 15–012).
Conflict of Interest
JC, HK, and PLH have no competing financial interests; YKK belongs to MD Healthcare Inc.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Choi, J., Kwon, H., Kim, YK. et al. Extracellular Vesicles from Gram-positive and Gram-negative Probiotics Remediate Stress-Induced Depressive Behavior in Mice. Mol Neurobiol 59, 2715–2728 (2022). https://doi.org/10.1007/s12035-021-02655-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02655-9