Abstract
The aim of this study was to evaluate the health risk of Cd, Cu, Pb, As, and Hg exposure through consumption of cultured oyster Crassostrea gigas from Sonoran coast. Metals were analyzed by atomic absorption spectrometry, and the risk assessment was estimated according to the hazard quotient (HQ), carcinogenic risk (CR), and hazard index (HI). The mean levels of Cd, Cu, Pb, As, and Hg in oysters were 1.35, 7.69, 0.29, 0.07, and 0.04 mg/kg wet weight, respectively. Results indicate that except for Cd, all mean levels were below than those recommended by the Mexican government, FAO, and European Union. The metal levels were similar to those reported on the Pacific Northwest Coasts. For risk assessment, the HQ, CR, and HI values did not exceed safety levels. Therefore, the oysters cultivated in the studied areas are safe for human consumption.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Contaminants, such as metals and metalloids, can accumulate in the food chain. These contaminants are characterized by their toxicity, persistence, and bioaccumulation (Abdallah 2013; Maanan 2008; Burioli et al. 2017). Food and water are considered the main sources of metals and metalloids for humans (Mok et al. 2015). Due to their physical and chemical characteristics, some metals may accumulate to high levels through the consumption of certain foods, such as oysters. These mollusks are consumed by humans because of their high nutritional value and low cost of production. These organisms are filter feeders; thus, their production is considered to have a low impact on the environment. However, because they filter large amounts of water though their gills and mouth (Cheng and Gobas 2007; Shenai-Tirodkar et al. 2016), oysters may contain higher concentrations of contaminants than those found in the water, independent of environmental pollution (Maanan 2008; Ochoa et al. 2013; Burioli et al. 2017).
A number of regulatory efforts and monitoring programs regarding metals and metalloids have been implemented to allow the commercialization of cultivated oysters (Bendell and Feng 2009; Cheng and Gobas 2007). The majority of producing countries have their own regulations, and other countries have regulations based on international committees, such as the European Union Legislation, Codex Alimentarius Commission, and Joint Expert Committee on Food Additives. In Mexico, the action and guidance levels for Cd, Pb, and Hg in fish and fisheries products were set in 1993 (NOM-127-SSA1-1993) and modified in 2009 (NOM-242-SSA1-2009), in this new guidance was included total As and methyl mercury. However, despite all of these regulations and monitoring programs, the commercialization problems associated with the metal contents in cultivated oysters worldwide have not been solved (Bendell and Feng 2009; Cheng and Gobas 2007; Mok et al. 2015). For this reason, it is important to evaluate the human health risk via ingestion of oysters, a tool that may influence food safety policy.
According to the United States Environmental Protection Agency (USEPA), a human health risk assessment is the process used to estimate the nature and probability of adverse health effects in humans who may be exposed to chemicals in contaminated environmental media now or in the future (USEPA 2009). Risk analysis is a process consisting of three steps: risk assessment, risk management, and risk communication, with the goal of protecting public health. Risk assessment is a key element that ensures the use of scientific knowledge to establish standards, guidelines, and other recommendations related to food safety to provide better protection of the population and facilitate international trade (USEPA 2009). Some countries have established a specific limit of oyster consumption. For example, Canada recommends a maximum intake of three oysters per week (Widmeyer and Bendell-Young 2008). In Mexico, there is no official recommendation for oyster consumption, thus the average consumption reported by Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentaión (SAGARPA) is used.
Mexico is the sixth largest producer of oysters after the USA, Japan, Korea, France, and China (FAO 2015–2017). The oyster Crassostrea gigas was cultivated for the first time in Mexico in the 1970s, and it has been marketed since 1980 (SAGARPA 2008). This species has been favored for its excellent adaptation to the conditions of northern Mexico, mainly in the states of Sonora, Baja California, and Baja California Sur. In Sonora, oyster cultivation is one of the alternatives with the greatest potential in aquaculture, presenting with growth of over 50% since 2006, with production of approximately 117 tons in 2012 (SAGARPA 2013). The high demand and market prices make this product an area of opportunity for Sonora producers, as demonstrated by the significant number (32) of farms (SAGARPA 2013). These oysters reach commercial sizes (from 8 to 14 cm) at 12 months, which is faster than oysters cultivated elsewhere (Bendell and Feng 2009; SAGARPA 2008). Because of the global interest in human health and increase of the commercialization of seafood such as cultivate oyster, this study is intended to provide information concerning the presence of metals and metalloids in cultivated oysters and the health effects related to their consumption. The aim of this study was to investigate the human health risk associated with cadmium, copper, lead, arsenic, and mercury intake due to the consumption of oysters from three different locations along the Sonora coast.
Materials and Methods
Study Area
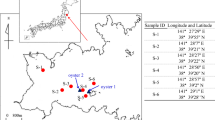
Three important oyster production areas were studied (Morua 31°17′09″N, 113°26′19″W; S. Cruz 28°49′30″N, 115°55′30″W; and S. Barbara 26°69′52″N, 109°65′05″W) along the Sonora Coast, Mexico (Fig. 1). The production of oysters in these areas was for Morua 80.4 Tons, for S. Cruz 647.6 Tons, and for S. Barbara 15.5 Tons, approximately. These areas are recognized by the oyster and shrimp aquaculture, touristic, and shipyard activities. The climate is dry most of the year and the region is characterized by arid to semiarid conditions with rainy summers (< 100 mm). The weather conditions are typical of desert environment, with temperature ranging from 35 to 49 °C during summer and 5 to 8 °C during winter.
Sampling
Oysters (Crassostrea gigas) were sampled at each farm for 1 year during January to December 2010. Since the sampling was done 8 years ago, this study is valuable as it provides a reference for comparative studies and future risk assessment studies. Each month, 10 oysters with similar shell lengths (between 7 and 10 cm) were sampled and packed in polyethylene plastic bags, and transported on ice to the laboratory within 12 h of collection.
The oysters were cleaned with distilled water but were not depurated; thus, the soft tissue included the digestive gland. The oysters were shucked with a knife, and the entire organism was removed from its shell, homogenized in a food processor, and stored frozen until further analysis.
Metal Analysis
Metal concentrations were determined by atomic absorption spectrometry after wet digestion. Briefly, 3.0 g of oyster homogenate was weighed in a microwave digestion vessel and combined with 5 mL of HNO3 (50% v/v) (García-Rico et al. 2001). The samples were digested in a microwave system (MARSX, CEM Corp., Matthews, NC, USA) for 30 min at 200 °C. After microwave digestion, the samples were cooled and diluted with deionized water.
For the Cd, Cu, and Pb quantification, the digested samples were diluted to a final volume of 50 mL. Cu was determined by atomic absorption spectrometry (Varian model SpectrAA-20, Victoria, Australia), whereas Cd and Pb were determined using a graphite furnace (GTA-96, Varian, Victoria, Australia). For the As quantification, 2.0 mL of KI (20% w/v) and 4.0 mL of HCl (50% v/v) were added to the digested samples and diluted with deionized water to a final volume of 50 mL. The As was quantified via a hydride generation technique (VGA-76, Varian, Victoria, Australia) using the following conditions: 0.35% w/v reducing agent NaBH4 in 10% w/v NaOH and 50% v/v HCl solution. For Hg quantification, 0.5 mL of K2Cr2O7 (1% w/v) was added to the digested samples, after which they were diluted with deionized water to a final volume of 50 mL. Hg was determined via a cold vapor technique after reduction with SnCl2 (20% w/v) in HCl (20% v/v). The wavelength and slit were, respectively, set to the following values (nm): Cd (228.8, 0.5), Cu (324.8, 0.5), Pb (283, 0.5), As (197.2, 1.0), and Hg (253.7, 0.5).
Human Health Risk Assessment
A health risk assessment model from the USEPA (Integrated Risk Information System, IRIS) was applied to calculate the metal intake attributed to oysters to estimate the non-cancer risk and carcinogenic exposure for adults (USEPA 2013) as follows:
where CDI is the chronic daily intake (mg of metal/kg/day); C is the average metal concentration (mg/kg) in oyster samples on a fresh weight basis; I is the daily average consumption of oysters (0.39 kg/year = 1.07 g/day) (SAGARPA 2013); ED is the exposure duration (70 years); EF is the exposure frequency (365 days/year); BW is body weight (70 kg); and AT is the exposure duration (in years) multiplied by the average time (in days) (USEPA 2013).
The potential non-carcinogenic (hazard quotient, HQ) and carcinogenic risk (CR) was calculated using the following Eqs. 2 and 3, respectively:
HQ is an estimation of the daily exposure of the human population that is likely to be without an appreciable risk of deleterious effects during a lifetime. An HQ ≤ 1 suggests that adverse health effects are unlikely. In this study, the oral reference dose (RfD) values were as follows (µg/kg day): Cd (1), Cu (40), Pb (3.5), As (0.3), and Hg (0.5) (USEPA 2013). Because of the lack of information for total As, the RfD data of inorganic As were used. CR was estimated only for As. CR is the probability of an individual developing any type of cancer from lifetime exposure to carcinogenic hazards. The slope factor (SF0) was 1.5 mg/kg day. The acceptable or tolerable risk for regulatory purposes is over the range of 1E−06 to 1E−04 (USEPA 2013). The hazard index (HI) was used to assess the overall potential non-carcinogenic health risk posed by more than one metal. For this study, the HI was calculated as the sum of the HQ for each studied metal.
Quality Control
All glassware were treated with a 20% (v/v) Pierce solution (Pierce, IL, USA), rinsed with cold tap water, treated with HNO3 (20% v/v), and finally rinsed with double-distilled water. For quality control purposes, duplicate reagent blanks and samples were analyzed during the procedure. All chemicals were trace metal grade. The standard reference materials SBR-1566b, Oyster Tissue, and DORM-2 from the National Institute of Standards and Technology (NIST, Gaithersburg, MD, USA) were analyzed for quality assurance. Percent recovery means were as follows (%): Cd (96), Cu (100), Pb (99), As (100), and Hg (103). The variation coefficient was below 7%. The detection limits (µg/kg) determined by the blanks method were as follows: Cd (6), Cu (20), Pb (3), As (6), and Hg (2).
Statistical Analysis
Descriptive statistics (mean, standard deviation, and range) were calculated for all the studied elements. Statistical analysis were conducted with NCSS version 6.0 Software (2007, UT, USA) using one-way ANOVA comparisons in oyster metal content among spatial distribution with a significance level of p < 0.05.
Results and Discussion
Metal Concentrations in Oysters
The greatest variations in the metal concentrations were found to be 2.41–20.18 mg/kg wt for Cu, 0.2–4.01 mg/kg wt for Cd, and 0.08–0.68 mg/kg wt for Pb (Table 1). Arsenic and Hg exhibited similar variations (0.01–0.15 mg/kg wt) (Table 1). The Cu levels were higher than those previously reported by García-Rico et al. (2001) in cultivated oysters (Crassostrea gigas) from the Sonora Coast (1.40–7.87 mg/kg wt for Cu; and 0.40–1.43 mg/kg wt for Cd) (Table 1). The Cu enrichment may be explained by the presence of significant natural deposits and their increased extraction over the last decade throughout the state of Sonora. Therefore, the input of contaminated water along the coast of the Gulf of California has increased. Another important contribution that may enhance the Cu concentration in coastal water is its use as an additive in shrimp cultivation. Similar behavior has been observed by Frías-Espericueta et al. (2015) along the Sonora and Sinaloa coasts. These authors have related Cu enrichment to the shrimp diet, which contains 8–12 mg/kg of Cu and discharges 2.6–3 kg Cu/year into lagoons. Other important sources of Cu are agriculture and urban effluents along the coast (Frías-Espericueta et al. 2015; Garcia-Rico et al. 2011). From a toxicological perspective, Cu is an essential enzyme cofactor, but high Cu levels may be related to liver damage in humans (Ochoa et al. 2013).
The Cd levels (0.2–4.01 mg/kg wt) (Table 1) detected in this study were also higher than those previously reported by García-Rico et al. (2001) within the same oyster species (0.40–1.43 mg/kg wt). Significant Cd levels in the surface water column of the Gulf of California have been reported (Garcia-Rico et al. 2011; Osuna-Martínez et al. 2011). Previous studies reported important concentrations of Cd, Cu, and Pb in oxide fraction of the sediments at the Gulf of California coasts (García-Rico et al. 2005; Ochoa-Valenzuela et al. 2009). It is well documented that metals bound to this fraction form stable complexes but may accumulate in estuarine sediments. Therefore, in reducing conditions, metals may be mobilized and taken up by oysters. Also, it is important to note that in the Gulf of California, high Cd concentrations in phytoplankton and sediment due natural phenomenons as the hydrothermal systems are reported (García-Rico et al. 2003).
Mineralization and recycling of Cd in the upper water column and sediment has been related to the enrichment of Cd in oysters (Osuna-Martínez et al. 2011). Cd is a toxic metal with multiple effects on aquatic organisms, including effects on the respiration and osmoregulation mechanisms (Hajeb et al. 2014). In aquatic foods, such as oysters, Cd, at high concentrations, may be bound to metallothioneins through cysteine residues; however, the Cd-metallothionein complex has been reported to be unstable and readily degrades to inorganic Cd (Hajeb et al. 2014). Moreover, according to Francesconi (2007), when Cd-metallothionein is ingested through oyster consumption, the acidic conditions of the human stomach allow for the release of inorganic Cd. Therefore, because Cd may induce severe dysfunction in humans, such as kidney dysfunction, osteomalacia, and reproductive deficiencies (Francesconi 2007), it is important to perform studies of Cd bioavailability in cultivated oysters to elucidate how it is metabolized. The concentrations of the remaining metals studied (As, Pb, and Hg) were similar to those previously reported (García-Rico et al. 2001) in Sonoran cultivated oysters (Crassostrea gigas) (0.05 mg/kg wt for As and 0.03 mg/kg wt for Hg), and they exhibited low levels of enrichment.
The spatial distribution of metals may reflect anthropogenic impact by the industrial activities developed near the study areas, as well as natural flows. In previous works have been found important levels of Cd, Cu, and Pb in sediments and water from Sonora coastal (García-Rico et al. 2003; Ochoa-Valenzuela et al. 2009), in which the tourist resorts, agricultural, and mining activities were reported as the main sources of metals. The spatial distributions of the studied metals are shown in Table 1. Cd presented with the highest mean concentration (1.51 mg/kg wt) in oysters purchased in Morua, an area with important tourist resorts, whereas Cu (10.34 mg/kg wt) and Hg (0.09 mg/kg wt) presented with the highest mean levels in oysters from S. Barbara, which is located close to an important modern agricultural zone in which large amounts of agrochemicals, such as lead arsenate and cupric oxide, are used. Significant variations (p < 0.05) were detected for Cd between Morua and S. Cruz and for Cu and Hg between Morua and S. Barbara.
The metal concentrations observed in this study were compared to values obtained elsewhere (Table 2). Investigations regarding metals in oysters (Crassostrea gigas) have focused on Cd because this element frequently exceeds regulations, thereby making commercialization difficult. The Cd levels measured in the present study were similar to those reported on the Pacific Northwest Coast, which is an important region for the production of Crassostrea gigas oysters (Bendell 2009; Kruzynski 2004). With regard to the remaining elements highlighted in this study, the Cu levels were similar to those reported by Osuna-Martínez et al. (2011) in the Gulf of California and by Ochoa et al. (2013) in Spain. Also, the Pb levels were similar to those reported in the Pacific Northwest (Widmeyer and Bendell-Young, 2008), whereas the As and Hg levels were higher than those reported in cultivated oysters from Spain (Ochoa et al. 2013).
As a safeguard to human health, the maximum permissible concentrations of metals and metalloids in oysters have been set to limit the intake of these contaminants. When the studied mean metal concentrations were compared to the levels set by Mexican regulation (NOM-242-SSA1-2009), all of the metal concentrations, except those of Cd, were found to be below the regulatory limit. Moreover, only 14% of the analyzed samples exceeded the Cd limit allowed by Mexican, Hong Kong, and Australian regulations (2 mg/kg wt) and 33% of the analyzed samples exceeded EU regulations (1 mg/kg wt). Therefore, to ensure that 100% of the oyster production meets the Cd requirements, it is important to continue and improve good manufacturing practices.
Risk Assessment
Chronic Daily Intake (CDI)
The CDI values for metals from cultivated oysters are shown in Table 3. The daily intake (mg/kg day) ranged from 3.1E−06 to 6.1E−05 for Cd, 3.7E−05 to 3.1E−04 for Cu, 1.2 E−06 to 1.0E−05 for Pb, 1.5E−07 to 2.3E−06 for As, and 1.5E−04 to 2.0E−06 for Hg. These daily intakes for each studied metal were less than the RfD safe intake levels (USEPA 2009), which implies that an intake of 0.39 kg/year of oysters does not represent a health risk. However, Cd was the only element with a CDI value that was more than 1% (1.4–2.3%) of its PTWI (1 µg/kg day).
The average daily intake values of Cd and Pb were lower than those reported by Abdallah (2013) in mollusca species consumed in Egypt (2E−05 to 1.1E−03 mg/kg day for Cd, 7.8E−04 to 4.2E−03 mg/kg day for Pb) as well as by Widmeyer and Bendell-Young (2008) and Cheng and Gobas (2007) in oysters (Crassostrea gigas) from Canada (Cd values of 1E−03 to 1.05E−03 mg/kg day and 8.4E−04 to 1.1E−03 mg/kg day, respectively).
Hazard Quotient (HQ)
A risk assessment was conducted based on ingested oysters. The metal and metalloid HQs for non-carcinogenic effects are shown in Table 3, and they exhibited the following order: Cd > As > Cu > Hg ≈ Pb. All HQ values did not exceed the safe level of HQ = 1, thus the intake of these metals via consumption of oyster may not induce adverse health effects. For risk assessment, the pattern of fish and fisheries products consumption is important. In Mexico, consumption of fish and fisheries products is low, but its intake is higher in coastal cities, even more in isolated coastal communities dedicated to the capture and cultivated sea products (Delgado-Álvarez et al. 2015). The HI is a useful parameter for estimating the risk of mixing metals present in food and is obtained by adding the HQ values. In this study, the HI values ranged from 5.1E−03 to 8.3E−02, corresponding to 0.5–8.4% of the safe level of HI = 1. Notably, Cd was the contaminant that contributed most to the mean calculated value HI (70%), followed by As (12%), Cu (9.7%), Pb (4.3%), and Hg (4%).
Cd is present in all foods and, as indicated above, has been found in high levels in oysters reported elsewhere (Bendell and Feng 2009; Cheng and Gobas 2007). Cd is associated with early renal injury and mild tubular dysfunction, especially in populations exposed to chronic low doses (Cheng and Gobas 2007). Therefore, it is important to communicate to vulnerable groups, such as smokers, women with mineral deficiencies, people with kidney dysfunction, and hypertensive patients, the need to limit their oyster consumption. Therefore, it is necessary to consider the Cd bioavailability in oysters and in the rest of the human diet to integrate this value into a risk assessment. Additionally, Cd accumulation in oysters has been identified in dissolved form and/or as a suspended particulate matter in an undissolved form (Garcia-Rico et al. 2011; Osuna-Martínez et al. 2011). Therefore, water and sediments have been implicated in the transport and accumulation of this metal in oysters (Osuna-Martínez et al. 2011; Widmeyer and Bendell-Young 2008). More studies regarding stable carbon and nitrogen isotopes are necessary to determine sources of Cd in cultivated oyster (Serrano-Grijalva et al. 2011; Widmeyer and Bendell-Young 2008).
For the study metals, only inorganic As has been proved as human carcinogen. In this study, the inorganic As CR ranged from 2.3E−07 to 3.4E−06. The USEPA considers that CR lower than E−06 is negligible, values between E−06 and E−04 are considered an acceptable range, whereas values above E−04 are sufficiently large such that remediation is desirable. The CR values calculated in this study were within the acceptable range of E−06 to E−04, suggesting that intake of As detected in this study via consumption of oyster may not induce carcinogenic effects. However, it is important to consider that As CR may increase through consumption of other fishery foods, rice, and red meat (Hajeb et al. 2014; Gundert-Remy et al. 2015; Zhu et al. 2015).
Conclusions
The concentrations of the metals in the studied oysters, except for those of Cu and Cd, were similar to those previously reported in the same studied areas, and they indicated low levels of enrichment. Also, the metal levels were similar to those reported on the Pacific Northwest Coasts. Results indicate that except for Cd, all mean levels were below than those recommended by the Mexican government, FAO, and European Union. The estimated HQ, CR, and HI values for the metals did not exceed the safe levels, suggesting that the intake of metals associated with the consumption of 0.39 kg/year of oysters is not hazardous to consumers. Also, it is important to communicate the need to continue and improve farming practices to oyster producers.
References
Abdallah MAM (2013) Bioaccumulation of heavy metals in mollusca species and assessment of potential risks to human health. Bull Environ Contam Toxicol 90:552–557
Bendell LI (2009) Survey of levels of cadmium in oysters, mussels, clams and scallops from the Pacific Northwest coast of Canada. Food Addit Contam Part B 2(2):131–139
Bendell LI, Feng C (2009) Spatial and temporal variations in cadmium concentrations and burdens in the Pacific oyster (Crassostrea gigas) sampled from the Pacific north-west. Mar Pollut Bull 58(8):1137–1143
Bergés-Tiznado ME, Páez-Osuna F, Notti A, Regoli F (2013) Arsenic and arsenic species in cultured oyster (Crassostrea gigas and C. corteziensis) from coastal lagoons of the SE Gulf of California, Mexico. Biol Trace Elem Res 151:43–49
Burioli EAV, Squadrone S, Stella C, Foglini C, Abete MC, Prearo M (2017) Trace element occurrence in the Pacific oyster Crassostrea gigas from coastal marine ecosystems in Italy. Chemosphere 187:248–260
Cheng WWL, Gobas FAPC (2007) Assessment of human health risks of consumption of cadmium contaminated cultured oysters. Hum Ecol Risk Assess 13:370–382
Delgado-Álvarez CG, Ruelas-Inzunza J, Osuna-López JI, Voltorina D, Frías-Espericueta MG (2015) Total mercury content in cultured oysters from NW Mexico: health risk assessment. Bull Environ Contam Toxicol 94:209–213
EU (European Community) (2006) Regulation 1881/2006 of 19th December 2006. Setting maximum levels for certain contaminants in foodstuffs. Off J Eur Union L 364/5e24. https://www.fsai.ie/uploadedFiles/Consol_Reg1881_2006.pdf
EU (European Union) (2014) Regulation 488/2014 of 12th May 2014, Amending Regulation (EC)1881/2006 as regards maximum levels of cadmium in foodstuffs. Off J Eur Union L 138/75e79. https://www.fsai.ie/uploadedFiles/Reg488_2014.pdf
EU (European Union) (2015) Regulation 1005/2015 of 25th June 2015. Amending regulation 1881/2006 as regards maximum levels of lead in certain foodstuffs. Off J Eur Union L 161/9e16. https://www.fsai.ie/uploadedFiles/Reg2015_1005.pdf
FAO (Food and Agriculture Organization of the United Nations) (1989) Report of the workshop and study tour on mollusk sanitation and marketing, Regional Sea Farming Development and Demonstration Project RAS/86/024 15–28 October. http://www.fao.org/docrep/field/003/AB710E/AB710E24.htm
FAO (Food and Agriculture Organization of the United Nations) (2015–2017) Cultured Aquatic Species Information Programme. Crassostrea gigas. In: Helm, MM (ed) FAO Fisheries and Aquaculture Department, Rome. http://www.fao.org/fishery/culturedspecies/Crassostrea_gigas/en
Francesconi KA (2007) Toxic metal species and food regulations—making a healthy choice. Analyst 132:17–20
Frías-Espericueta MG, Osuna-López JI, Delgado-Alvarez CG, Muy-Rangel MD, López-López G, Izaguirre-Fierro G, Jaimes-Bustamante F, Zazueta-Padilla HM, Aguilar-Juárez M, Rubio-Carrasco W, Voltolina D (2015) Changes in metal contents in shrimp cultured in NW Mexico (2000–2010). Environ Monit Assess 187:269. https://doi.org/10.1007/s10661-015-4494-6
Garcia-Rico L, Tejeda-Valenzuela L, Jara-Marini ME, Gomez-Alvarez A (2011) Dissolved and particulate metals in water from Sonora Coast: a pristine zone of Gulf of California. Environ Monit Assess 176:109–123
García-Rico L, Ramos RE, Vivian J (2001) Determination of total metals in cultivated oysters from the northwest coast of Mexico determined by microwave digestion and atomic absorption spectrometry. J AOAC Int 84:1909–1913
García-Rico L, Wilson-Cruz S, Frasquillo-Félix MC, Jara-Marini ME (2003) Total metals in intertidal surface sediment of oyster culture areas in Sonora, Mexico. Bull Environ Contam Toxicol 70:1235–1241
García-Rico L, Soto-Cruz MS, Jara-Marini ME, Gómez- Álvarez A (2005) Fracciones geoquímicas de Cd, Cu y Pb en sedimentos marinos superficiales de zonas ostrícolas del estado de Sonora, México. Rev Int Contam Ambient 3(20):159–167
Gundert-Remy U, Damm G, Foth H, Freyberger A, Gebel T, Golka K, Röhl C, Schupp T, Wollin KM, Jan Hengstler JG (2015) High exposure to inorganic arsenic by food: the need for risk reduction. Arch Toxicol 89:2219–2227
Hajeb P, Sloth JJ, Shakibazadeh Sh, Mahyudin NA, Afsah-Hejri L (2014) Toxic elements in food: occurrence, binding, and reduction approaches. Compr Rev Food Sci Food Saf 13:457–472
Kruzynski GM (2004) Cadmium in oysters and scallops: the BC experience. Toxicol Lett 148:159–169
Maanan M (2008) Heavy metal concentrations in marine molluscs from the Moroccan coastal region. Environ Pollut 153:176–183
Mok JS, Yoo HD, Kim PH, Yoon HD, Park YC, Lee TS, Kwon JY, Son KT, Lee HJ, Ha KS, Shim KB, Kim JH (2015) Bioaccumulation of heavy metals in oysters from the Southern Coast of Korea: assessment of potential risk to human health. Bull Environ Contam Toxicol 94:749–755
NOM-029-SSA1-1993. Bienes y Servicios. Productos de la pesca. Crustáceos frescos-refrigerados y congelados. Especificaciones Sanitarias. http://www.salud.gob.mx/unidades/cdi/nom/029ssa13.html
NOM-242-SSA1-2009. Productos y servicios. Productos de la pesca frescos, refrigerados, congelados y procesados. Especificaciones sanitarias y métodos de prueba. http://dof.gob.mx/nota_detalle.php?codigo=5177531&fecha=10/02/2011
Ochoa V, Barata C, Riva MC (2013) Heavy metal content in oysters (Crassostrea gigas) cultured in the Ebro Delta in Catalonia, Spain. Environ Monit Assess 185:6783–6792
Ochoa-Valenzuela LE, Gómez-Álvarez A, García-Rico L, Villalba-Atondo AI (2009) Distribution of heavy metals in surface sediments of the Bacochibampo Bay, Sonora, Mexico. Chem Spec Bioavailab 21(4):211–218
Osuna-Martínez CC, Páez-Osuna F, Alonso-Rodrıguez R (2011) Cadmium, copper, lead and zinc in cultured oysters under two contrasting climatic conditions in coastal lagoons from SE Gulf of California, Mexico. Bull Environ Contam Toxicol 87:272–275
Rasmussen RS, Morrissey MT, Cheney D (2007) Effect of age and tissue weight on the cadmium concentration in Pacific oysters (Crassostrea gigas). J Shellfish Res 26(1):173–179
SAGARPA (Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación) (2008) Determinación de agentes causales de alta mortalidad en los cultivos del ostión japonés, Crassostrea gigas, de las costas de Sonora. Gobierno de Sonora—Anexo Técnico 2005. SAGARPA, Mazatlán, Sinaloa, México
SAGARPA (Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación) (2013) Registro y estadística pesquera y acuícola—Consulta específica por especie. SAGARPA, México, DF, México. http://www.conapesca.gob.mx/wb/cona/estadisticas_de_produccion_pesquera
Serrano-Grijalva L, Sánchez-Carrillo S, Angeler DG, Sánchez-Andrés R, Álvarez-Cobelas M (2011) Effects of shrimp-farm effluents on the food web structure in subtropical coastal lagoons. J Exp Mar Biol Ecol 402:65–74
Shenai-Tirodkar PS, Gauns MU, Ansari ZA (2016) Concentrations of heavy metals in commercially important oysters from Goa, Central-West coast of India. Bull Environ Contam Toxicol 97:813–819
USEPA (United States Environmental Protection Agency) (2009) Highlights of the child-specific exposure factors handbook (final report). EPA/600/R-08/135. Washington, DC, USA
USEPA (United States Environmental Protection Agency) (2013) Regional screening levels (RSL) for chemical contaminants at superfund sites. USEPA, Washington, DC
Widmeyer JR, Bendell-Young LI (2008) Heavy metal levels in suspended sediments, Crassostrea gigas and the risk to humans. Arch Environ Contam Toxicol 55:442–450
Zhu F, Qu L, Fan W, Wang A, Hao H, Li X, Yao S (2015) Study on heavy metal levels and its health risk assessment in some edible fishes from Nansi Lake, China. Environ Monit Assess 187:161
Acknowledgements
This research was supported by funds provided by Consejo Nacional de Acuacultura y Pesca (CONAPESCA) 105PI0272. The authors would also like to thank the Instituto de Acuacultura del Estado de Sonora, Comité de Sanidad Acuícola del Estado de Sonora, and the oyster farmers for their logistical support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
García-Rico, L., Tejeda-Valenzuela, L. Metal Concentrations in Oysters Crassostrea gigas Cultured in the Gulf of California and Risk Assessment to Human Health. Expo Health 12, 33–39 (2020). https://doi.org/10.1007/s12403-018-0281-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12403-018-0281-2