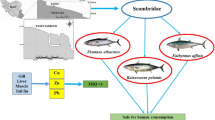
Abstract
Eight heavy metals, namely Cu, Zn, Fe, Mn, Cd, Ni, Pb, and As in the muscles of nine fish species collected from Nansi Lake, China. were determined, and the potential health risks to local residents via consumption of the fishes were estimated. The results of two-way ANOVA that showed the concentrations of heavy metals in the investigated fish samples were influenced significantly by fish species and sampling sites. Correlation analysis indicated that sampling sites had significant effects on the levels of correlation coefficients among different heavy metal concentrations. Interestingly, although none of the hazard quotient (HQ) values of any individual element was greater than 1 for the investigated exposure population through fish consumption, the hazard index (HI) values were more than 1 for local fishermen, suggesting that local fishermen may be experiencing some adverse health effects. Among the investigated nine fish species, Cyprinus carpio had the highest HQ and HI. As, Pb, and Cd were the most concerning heavy metals in the investigated fish samples due to their higher relative contributions to the HI values.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fish is widely consumed by humans in the world due to their high protein supply and omega-3 fatty acids that help reduce the risk of certain types of cancer and cardiovascular diseases (Storelli 2008). However, fish has been reported to accumulate large amounts of some metals and is often at the top of the aquatic food chain (Mansour and Sidky 2002; Tüzen 2003; Bidar et al. 2009). High content of heavy metals leads to severe threat to fish and consequently results the health risks to people associated with fish consumption. On the other hand, fish is often used to monitor heavy metals and the other pollutions of aquatic ecosystems (Dorea 2008; Ahmad and Shuhaimi-Othman 2010; Rahman et al. 2012). The concentrations of heavy metals in the tissues and organs of fishes indicate the concentrations of heavy metals in water and their accumulation in food chains (Pintaeva et al. 2011). So, it is necessary to assess the levels of heavy metals in fishes and to report possible contamination that would represent a health hazard. Lately, numerous investigations and monitoring programs have been carried out on heavy metal concentrations in fishes (Tüzen and Soylak 2007; Uluozlu et al. 2007; Mendil et al. 2005; Reynders et al. 2008; Tüzen 2009; Tüzen et al. 2009; Yi et al. 2011).
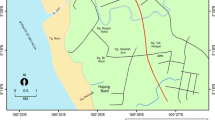
Nansi Lake (34° 27′ N–35° 20′ N, 116° 34′ E–117° 21′ E) is the largest fresh water lake in Shandong Province, China with a surface area of 1266 km2 and an average water depth of 1.46 m. It is composed of four lakes, namely Nanyang Lake, Dushan Lake, Zhaoyang Lake, and Weishan Lake. Nansi area is one of the most economically developed regions in China and has been polluted significantly by heavy metals originating from anthropogenic sources, such as electronics, metalwork, and mining industries, putting thousands of people in this area into tremendous health hazard. Previous studies mostly focused on the sediments in Nansi Lake (An and Li 2009; Li et al. 2009; Liu et al. 2010). However, there is a lack of studies regarding metal levels in edible fishes from Nansi Lake and whether these levels represent a human health risk for local residents. Therefore, the objectives of the present study were to determine the concentrations of Cu, Zn, Fe, Mn, Cd, Ni, Pb, and As in the muscles of nine fish species collected from Nansi Lake and estimate the potential health risk of heavy metals to local residents through fish consumption.
Materials and methods
Sampling
A total of 288 fish samples belonging to ten fish species were collected from four lakes, Nanyang Lake, Dushan Lake, Zhaoyang Lake, and Weishan Lake (Fig. 1) during four seasons, from the spring to the winter of 2012. The species were Ophiocephalus argus, Carassius auratus, Pseudobagrus fulvidraco, Parabramis pekinensis, Atractoscion nobilis, Ctenopharyngodon idellus, Scomberomorus niphonius, Silurus asotus, and Cyprinus carpio. The fish samples collected were washed with clean water at the point of collection, separated by species, placed on ice, brought to the laboratory on the same day, and then frozen at −20 °C until dissection.
Sample preparation
Frozen fish samples were thawed at room temperature and dissected using stainless steel scalpels. One gram of accurately weighed epaxial muscle was dissected. One gram of accurately weighed epaxial muscle from each sample was dissected for analysis. They were washed with distilled water, dried in filter paper, weighed, packed in polyethylene bags, and stored at −20 °C until analysis.
Reagents and apparatus
All reagents were of analytical reagent grade unless otherwise stated. HNO3 and H2O2 were of suprapure quality (Merck, Germany). Double deionized water (Milli-Q Millipore; 18.2 M cm−1) was used for all dilutions. The element standard solutions were prepared by dilution of 1000 mg l−1 certified standard solutions (Nssrc, China). XT-9912 model microwave system (Xintuo, China) equipped with advanced composite PTFE vessels was used for digestion of the samples. A Perkin Elmer Analyst 700 (CT, USA) atomic absorption spectrometer equipped with HGA graphite furnace (GF-AAS) was used to determine Cd, Ni, Pb, and As. For graphite furnace measurements, argon was used as inert gas. Pyrolytic-coated graphite tubes (Perkin Elmer part no. B3 001264) with a platform were used. Samples were injected into the graphite furnace using Perkin Elmer AS-800 autosampler. Optima 2100 DV model inductively coupled plasma atomic emission spectroscopy (ICP-AES) (PE, USA) was used for simultaneous multielement detection of Cu, Zn, Fe, and Mn. Detection limit values of elements as milligram per liter were found to be 0.018 for Cu, 0.010 for Zn, 0.016 for Fe, 0.020 for Mn, 0.09 for Cd, 0.35 for Ni, 0.18 for Pb, and 0.17 for As in this study. The operating conditions of ICP-AES and GF-AAS were listed in Table 1.
Microwave digestion
One gram muscle sample was digested with 5 ml of 65 % HNO3 and 2 ml of 30 % H2O2 in microwave digestion system and finally diluted to 10 ml with double deionized water. A blank digest was carried out in the same way. All sample solutions were clear. Digestion conditions for microwave system were applied as 3 min for 400 W, 5 min for 600 W, 8 min for 900 W, 8 min for 1000 W, vent 8 min, respectively.
Quality assurance
All glasswares and equipments were carefully cleaned starting with 2 % HNO3 and ending with repeated rinsing distilled deionized water to prevent contamination. Reagent blank determinations were used to correct the instrument readings. Detection limit is defined as the concentration corresponding to three times the standard deviation of ten blanks. Certified reference material (DORM-2 dogfish muscle) were used for accuracy evaluation, and the recovered values were given in Table 2.
Risk assessment
A potential non-cancer risk for individual heavy metals is expressed as the hazard quotient (HQ) (US EPA 1992, 1999) and can be calculated as follows:
where RfD is the daily intake reference dose (μg kg−1 day−1); ADD is the average daily intake of heavy metals (μg kg−1 day−1); C is the mean concentrations of heavy metals in fish (μg kg−1); IR is the consumption rate of fish (g person−1 day−1); and BW is the average adult body weight (kg). The RfD values were 40, 300, 700, 240, 1, 20, and 0.3 μg kg−1 day−1 for copper, zinc, iron, manganese, cadmium, nickel, and arsenic, respectively (US EPA 2007). Because the RfD for Pb was not available, we used JECFA 3.57 μg kg day−1 (JECFA 2003). If the HQ exceeds 1, there may be concern for potential non-cancer effects. As a rule, the greater the value of HQ above 1, the higher the level of concern.
A hazard index (HI) approach was used to assess the overall potential non-carcinogenic health risk posed by more than one heavy metal. The HI is equal to the sum of the HQs, as described in Eq. (3) (US EPA 1989).
Statistical analysis
Two-way analysis of variance (ANOVA) was employed to test whether varied significantly between the heavy metal concentrations and three factors (species, sites, and seasons). The significance level was set at 0.05. Person correlation analysis was adopted to examine the inter-relationships among the investigated heavy metal concentrations in the fish samples. Data calculation and statistical analysis were performed with EXCEL and SPSS 19.0.
Results and discussion
The concentrations of heavy metals in fish
The concentrations of heavy metals in the studied nine fish species were given in Table 3. All metal concentrations were determined on a wet weight basis. The concentrations of copper, zinc, iron, manganese, cadmium, nickel, lead, and arsenic in fish samples were found to be 1.47–5.67, 11.43–28.23, 8.73–35.81, 1.48–4.62, 0.12–0.35, 0.84–3.12, 0.28–0.65, and 0.05–0.10 μg g−1, respectively. Among all determined heavy metals, iron was found to be the dominant elemental ion followed by zinc and copper.
There was a great variation in zinc concentrations among the investigated fish muscles. Fishes are known to have a high threshold level of zinc. The minimum and maximum lead levels observed were 11.43 μg g−1 in A. nobilis and 28.23 μg g−1 in C. auratus. Zinc is known to be involved in most metabolic pathways in humans, and zinc deficiency can lead to loss of appetite, growth retardation, skin changes, and immunological abnormalities. The concentrations of zinc in all the fish samples were found to be lower than the standard of 30 μg g−1 (FAO 1983) and 50 μg g−1 (MAFF 1995).
Iron was found to be the dominant element as compared with other heavy metals in fish samples. The iron contents in the investigated fish samples ranged from 8.73 to 35.81 μg g−1. C. auratus had the highest iron concentration whereas P. fulvidraco had the lowest. Iron is vital for almost all living organisms, participating in a wide variety of metabolic processes, such as oxygen transport, DNA synthesis, and electron transport. The RDA of iron for 7–12 months infants and 51–70 years adults is 11 and 8 mg day−1, respectively (Ikem and Egiebor 2005).
Copper is an essential part of several enzymes and is necessary for the synthesis of hemoglobin (Sivaperumal et al. 2007). However, high intake of Cu has been recognized to cause adverse health problem (Gorell et al. 1997). The lowest and highest Cu levels in fish species were found as 1.47 μg g−1 in O. argus and 5.67 μg g−1 in A. nobilis. The maximum copper level permitted is 30 μg g−1 (wet weight) for FAO (1983) and 20 μg g−1 for WHO (1996). The Australian Food Standard Code established the maximum concentration for Cu at 10 μg g−1 (wet weight) (Alam et al. 2002). The copper levels in analyzed fish samples were found to be lower than these limits.
Manganese, one of the essential trace elements, is a structure component of some enzymes and activates the actions of some enzymes. The deficiency of manganese can produce severe skeletal and reproductive abnormalities in mammals. High doses of manganese produce adverse effects primarily on the lungs and brain. In this study, the lowest iron content was 1.48 μg g−1, for C. auratus, whereas the highest was 4.62 μg g−1, for C. carpio. The US National Academy of Science recommends 2.5–5 mg manganese per day (National Academy of Sciences 1980), and the World Health Organization recommended 2–9 mg per day for an adult (WHO 1994).
In the present investigation, the lowest concentration of nickel was found in C. auratus (0.82 μg g−1) and the highest in C. idellus (3.12 μg g−1). Nickel normally occurs at very low levels in the environment, and it can cause variety of pulmonary adverse health effects, such as lung inflammation, fibrosis, emphysema, and tumors (Forti et al. 2011). The WHO recommends 100–300 μg nickel for daily intake (WHO 1994).
Cadmium is not a part of natural biochemical processes and is extremely hazardous. The cadmium concentrations were found in the range of 0.12–0.35 μg g−1. O. argus had the lowest lead concentration whereas C. carpio had the highest. The concentrations of cadmium in all fish samples were far below the Western Australian authorities’ proposed level of 5.5 μg g−1 (Plaskett and Potter 1979). But, the cadmium levels in some fish samples were higher than the standard of 2.0 μg g−1 (FAO 1983) and 50 μg g−1 (MAFF 1995).
The minimum and maximum lead levels observed were 0.28 μg g−1 in S. asotus and 0.65 μg g−1 in O. argus. Lead is similar to cadmium that has no beneficial role in human metabolism, producing progressive toxicity. Lead creates health disorders such as sleeplessness, tiredness, and hear and weight loss. The maximum permitted lead level is 2.0 μg g−1 set by MAFF (1995) and WHO (1996). The maximum permitted concentration of Pb proposed by Australian National Health and Medical Research Council is 2.0 μg g−1 (wet weight) (Plaskett and Potter 1979). The lead levels in the investigated fish samples were found to be lower than the standard.
Inorganic arsenic is considered to be the most toxic to human health. Arsenic at low doses over a long period of time is known to cause skin changes that may lead to skin cancer. The lowest and highest arsenic content in fish species were found as 0.05 μg g−1 in S. niphonius and 0.10 μg g−1 in P. fulvidraco. The maximum arsenic level permitted for fishes is 1.0 μg g−1 according to Australia standard (Australia New Zealand Food Authority 1998). US Environmental Protection Agency (EPA) has set arsenic tissue residues of 1.3 μg g−1 in freshwater fish (wet weight) as the criterion for human health protection (Burger and Gochfeld 2005). The arsenic concentrations in the studied fish samples were far below these standards.
Two-way ANOVA analysis
The results of two-way ANOVA presented in Table 3 indicating the concentrations of each heavy metal in the investigated fish samples changed significantly among different fish species. This might be a result of different ecological needs, metabolism, and feeding patterns for different species. The results clearly showed that sites had significant effects on the levels of Zn, Cd, Ni, Pb, and As in fish samples. Season variation, however, did not bring out significant content changes of heavy metals except Cu and Cd. The effects of the interaction between species and sites suggested that the significant variations were only found in Fe and Pb concentrations. It is also observed that only Cu level was influenced significantly by interaction of species × seasons. On the whole, the concentrations of heavy metals in the investigated fish samples were mainly influenced by fish species and sites. The fishes collected from Nanyang Lake had the highest concentrations of Cu, Fe, Mn, Cd, Pb, and As, while fishes from Weishan Lake had the lowest concentrations of Zn, Fe, Mn, Ni, Cd, Pb, and As. The metal contents in fishes of other two lakes were found in between. This might suggest that heavy metal pollution in Nanyang Lake was more serious than the other three lakes. The previous study showed that the concentrations of heavy metals in fishes were seriously affected by river input to the lake (Yu et al. 2012). In fact, the Nanyang Lake receives a large amount of wastewater containing heavy metals as several main river branches flow across the important industrial cities such as Jining, Yunzhou, and Hezhe, resulting the increase of heavy metal concentrations in the water and sediment environment of Nanyang Lake. Consequently, the metal concentrations in fishes living in this lake area increase.
Correlation analysis
Inter-element relationships can provide important information on the sources and pathways of the heavy metals. The correlation coefficients between different heavy metal concentrations in the investigated fish samples were given in Table 4. The results clearly showed that correlation coefficients were influenced significantly by the sampling sites. For Nanyang Lake, there were good correlations between Cu and Zn, Fe and Cd, Fe and Pb, and Ni and As for the investigated fish species. For Dushan Lake, significant correlations were found between Fe and Cd, Mn and Ni, As and Ni, and As and Pb. For Zhaoyang Lake, between Zn and Mn, Fe and Mn, Fe and Cd, Fe and Pb, Mn and Cd, Mn and Pb, and Cd and Pb were found to have significant correlations. For Weishan Lake, Cu–Pb, Mn–Pb, Cd–Pb, and Ni–As were significantly correlated. These significant correlations might indicate that the distributions of these pairs of metals were regulated by common local inputs and similar dispersion processes in the study area.
Comparison of metal levels in fish from Nansi Lake with reported values
In order to determine the degree of contamination in the studied area, the concentrations of heavy metals in the investigated fish samples collected from Nansi Lake were compared with the reported values in other lakes (Table 5). Reported results in the literatures showed that metal concentrations in the fish muscles varied widely depending on where and which species were caught. Considering the conversion factor 4.8 from wet weight to dry weight, the heavy metal levels in fish from Nansi Lake were mostly in the high levels compared with the other lakes. For all collected fresh water fish species considered collectively, Zn, Mn, and Cd concentrations were around the 90th percentile (n = 15 for Zn, n = 9 for Mn, n = 14 for Cd) and Pb, Cu, and As around the 75th percentile of the collected worldwide results (n = 15 for Pb, n = 14 for Cu, n = 4 for As). Fe and Ni concentrations in fishes from Nansi Lake were found to be higher than the reported values in other lakes. The above results may indicate that Nansi Lake has been suffering more severe pollution.
Potential health risk to local residents via consumption of fish
Fish from Nansi Lake is an important daily food resource for local residents. Therefore, it is necessary to assess the potential health risks caused by the consumption of local fishes. A total of 1450 adult volunteers who have been told the objectives of this study before the face-to-face survey were randomly recruited from local villages in 2012. The results of our questionnaires showed that the average body weight is 62.8 kg, and the average consumption rates of fresh fish were 38.4 and 98.5 g per day for general population and fishermen, respectively.
The HQ values of individual metal through fish consumption were calculated and listed in Table 6. For different exposure population, HQs of individual metal were all below 1, which means that the daily intake of individual metal through the consumption of fish would be unlikely to cause adverse health effects for local residents. However, for different exposure groups, HQ values exhibited different potential risks. The HQ of every metal for fishermen was higher than for general population, which is a coincidence with those found in previous report (Yu et al. 2012). There was a big discrepancy of HQ among different metals. HQs of As and Cr were the biggest, ranging from 0.102 to 0.471 and 0.073 to 0.549. HQ of Cr was the lowest, having concentrations between 0.004 and 0.030. On the whole, A. nobilis had the highest HQs whereas S. niphonius had the lowest.
It is very interesting that there is no single metal exposure that exceeds its RfD, but the potential risk due to the combined effects of all heavy metals through fish consumption is significant for fishermen other than general population. As shown in Table 6, the HI values were observed in the range of 0.480–0.679 for general population, suggesting that general population were not likely to be hurt by heavy metals through fish consumption. However, the HI values ranged from 1.165 to 1.742 for fishermen, indicating that local fishermen may experience some adverse health effects. Generally, C. carpio consumption had the highest health risk for the investigated exposure groups, while P. fulvidraco consumption had the lowest. The results indicated that the consumption of the fishes from Nanyang Lake had the highest health risk for local residents, while the consumption of the fishes from Weishan Lake had the lowest. The data listed in Table 6 and Fig. 2 showed that As, Pb, and Cd were the key elements contributing to the HI values, and the sum of the three components was more than 50 %, with Cu and Ni being secondary and Zn, Mn, and Fe the least important. High potential risk due to As, Pb, and Cd may be related to wastewater released from metallurgy and chemical plants in this area.
It is worth mentioning that the exposure risk estimates in this study were only for fish, without accounting for any other sources. Many studies showed that human beings are significantly exposed to heavy metals through other foods such as rice, wheat, and vegetable (Zheng et al. 2007; Huang et al. 2008; Hang et al. 2009). If potential health risks of heavy metals through these exposure routs were considered, total heavy metals would be likely to pose higher health risks to local residents.
Conclusions
The heavy metal concentrations found in the fish muscles sampled from Nansi Lake decreased in the order of Fe > Zn > Cu > Mn > Ni > Pb > Cd > As. In general, the levels of Zn, Cu, Ni, Pb, and As in the investigated fish samples were found to be lower than the legal limits. However, the cadmium levels in some fish samples were higher than the standards set by FAO and MAFF. The results of two-way ANOVA showed that the concentrations of heavy metals in the investigated fish samples were affected significantly by fish species and sampling sites. The HQ values for any individual heavy metal were below 1, which means that the daily intake of individual metal through fish consumption would be unlikely to cause health risk for local residents. It should be noted, however, that the aggregate effects of eight heavy metals were more than 1 for local fishermen, indicating that the fishermen were more likely to experience adverse health effects. As, Pb, and Cd are the key elements contributing to the potential health risk of non-carcinogenic effects for general population and fishermen. Furthermore, considerable attention should also be paid to the potential health risks of heavy metals through other exposure pathways.
References
Ahmad, A. K., & Shuhaimi-Othman, M. (2010). Heavy metal concentrations in sediments and fishes from Lake Chini, Pahang, Malaysia. Journal of Biological Sciences, 10(2), 93–100.
Alam, M. G. M., Tanaka, A., Allinson, G., Laurenson, L. J. B., Stagnitti, F., & Snow, E. T. (2002). A comparison of trace element concentrations in cultured and wild carp (Cyprinus carpio) of Lake Kasumigaura, Japan. Ecotoxicology and Environmental Safety, 53, 348–354.
An, W. C., & Li, X. M. (2009). Phosphate adsorption characteristics at the sediment–water interface and phosphorus fractions in Nansi Lake, China, and its main inflow rivers. Environmental Monitoring and Assessment, 148, 173–184.
Australia New Zealand Food Authority. (1998). Food standards code. Standard A12, Issue 37.
Bidar, G., Pruvot, C., Garçon, G., Verdin, A., Shirali, P., & Douay, F. (2009). Seasonal and annual variations of metal uptake, bioaccumulation, and toxicity in Trifolium repens and Lolium perenne growing in a heavy metal-contaminated field. Environmental Science and Pollution Research, 16, 42–53.
Burger, J., & Gochfeld, M. (2005). Heavy metals in commercial fish in New Jersey. Environmental Research, 99, 403–412.
Chale, F. M. M. (2002). Trace metal concentrations in water, sediments and fish tissue from Lake Tanganyika. Science of the Total Environment, 299, 115–121.
Dorea, J. G. (2008). Persistent, bioaccumulative and toxic substances in fish: human health consideration. Science of the Total Environment, 400, 93–114.
FAO. (1983). Compilation of legal limits for hazardous substances in fish and fishery products. FAO Fishery Circular No. 464, 5–10. Rome: Food and Agriculture Organization of the United Nations.
Forti, E., Salovaara, S., Cetin, Y., Bulgheroni, A., Pfaller, R. W., & Prieto, P. (2011). In vitro evaluation of the toxicity induced by nickel soluble and particulate forms in human airway epithelial cells. Toxicology in Vitro, 25, 454–461.
Fu, J., Hu, X., Tao, X. C., Yu, H. X., & Zhang, X. W. (2013). Risk and toxicity assessments of heavy metals in sediments and fishes from the Yangtze River and Taihu Lake, China. Chemosphere, 93, 1887–1895.
Gorell, J. M., Johnson, C. C., Rybicki, B. A., Peterson, E. L., & Kortsha, G. X. (1997). Occupational exposures to metals as risk factors for Parkinson’s disease. Neurology, 48, 650–658.
Hang, X. S., Wang, H. Y., Zhou, J. M., Ma, C. L., Du, C. W., & Chen, X. Q. (2009). Risk assessment of potentially toxic element pollution in soils and rice (Oryza sativa) in a typical area of the Yangtze River Delta. Environmental Pollution, 157, 2542–2549.
Huang, M. L., Zhou, S. L., Sun, B., & Zhao, Q. G. (2008). Heavy metals in wheat grain: assessment of potential health risk for inhabitants in Kunshan, China. Science of the Total Environment, 405, 54–61.
Ikem, A., & Egiebor, N. O. (2005). Assessment of trace elements in canned fishes (mackerel, tuna, salmon, sardines and herrings) marketed in Georgia and Alabama (United States of America). Journal of Food Composition and Analysis, 18, 771–787.
Iqbal, J., & Shah, M. H. (2014). Study of seasonal variations and health risk assessment of heavy metals in Cyprinus carpio from Rawal Lake, Pakistan. Environmental Monitoring and Assessment, 186, 2025–2037.
JECFA. (2003). Summary and conclusions of the 61st meeting of the Joint FAO/WHO Expert Committee on Food Additives. JECFA/61/SC. Rome, Italy.
Karadede, H., & Ünlü, E. (2000). Concentrations of some heavy metals in water, sediment and fish species from the Ataturk Dam Lake (Euphrates), Turkey. Chemosphere, 41, 1371–1376.
Li, H. L., Gao, H., Zhu, C., Li, G. G., Yang, F., Gong, Z. Y., & Lian, J. (2009). Spatial and temporal distribution of polycyclic aromatic hydrocarbons (PAHs) in sediments of the Nansi Lake, China. Environmental Monitoring and Assessment, 154, 469–478.
Liu, E. F., Shen, J., Yang, L. Y., Zhang, E. L., Meng, X. H., & Wang, J. J. (2010). Assessment of heavy metal contamination in the sediments of Nansihu Lake Catchment, China. Environmental Monitoring and Assessment, 161, 217–227.
Lwanga, M. S., Kansiime, F., Denny, P., & Scullion, J. (2003). Heavy metals in Lake George, Uganda, with relation to metal concentrations in tissues of common fish specie. Hydrobiologia, 499, 83–93.
MAFF. (1995). Monitoring and surveillance of non-radioactive contaminants in the aquatic environment and activities regulating the disposal of wastes at sea, 1993. Aquatic Environment Monitoring Report No. 44. Direcorate of Fisheries Research, Lowestoft.
Mansour, A. A., & Sidky, M. M. (2002). Ecotoxicological studies: heavy metals contaminating water and fish from Fayum Governorate, Egypt. Food Chemistry, 78, 15–22.
Mendil, D., Uluözlü, Ö. D., Hasdemir, E., Tüzen, M., Sarı, H., & Suiçmez, M. (2005). Determination of trace metal levels in seven fish species in lakes in Tokat, Turkey. Food Chemistry, 90, 175–179.
Mohamed, M., Khidr, A. A., & Dheina, N. (2011). Assessment of heavy metal concentrations in water, plankton, and fish of Lake Manzala, Egypt. Turkish Journal of Zoology, 35, 271–280.
Nakayama, S. M. M., Ikenaka, Y., Muzandu, K., & Choongo, K. (2010). Heavy metal accumulation in lake sediments, fish (Oreochromis niloticus and Serranochromis thumbergi), and crayfish (Cherax quadricarinatus) in Lake Itezhi-tezhi and Lake Kariba, Zambia. Archives of Environmental Contamination and Toxicology, 59, 291–300.
National Academy of Sciences. (1980). Recommended dietary allowances (9th ed.). Washington: National Academic Press.
Petkovšek, S. A. S., Grudnik, Z. M., & Pokorny, B. (2012). Heavy metals and arsenic concentrations in ten fish species from the Šalek lakes (Slovenia): assessment of potential human health risk due to fish consumption. Environmental Monitoring and Assessment, 184(5), 2647–2662.
Pintaeva, E. T., Bazarsadueva, S. V., Radnaeva, L. D., Pertov, E. A., & Smirnova, O. G. (2011). Content and character of metal accumulation in fish of the Kichera River (a tributary of Lake of Baikal). Contemporary Problems of Ecology, 4, 64–68.
Plaskett, D., & Potter, I. C. (1979). Heavy metal concentrations in the muscle tissue of 12 species of teleost from Cockburn Sound, Western Australia. Australian Journal of Marine & Fresh Water Research, 30, 607–616.
Rahman, M. S., Molla, A. H., Saha, N., & Rahman, A. (2012). Study on heavy metals levels and its risk assessment in some edible fishes from Bangshi River, Savar, Dhaka, Bangladesh. Food Chemistry, 134, 1847–1854.
Reynders, H., Bervoets, L., Gelders, M., & De Coen, W. M. (2008). Accumulation and effects of metals in caged carp and resident roach along a metal pollution gradient. Science of the Total Environment, 391, 82–95.
Roach, A. C., Maher, W., & Krikowa, F. (2008). Assessment of metals in fish from Lake Macquarie, New South Wales, Australia. Archives of Environmental Contamination and Toxicology, 54, 292–308.
Schenone, N. F., Avigliano, E., Goessler, W., & Cirelli, A. F. (2014). Toxic metals, trace and major elements determined by ICPMS in tissues of Parapimelodus valenciennis and Prochilodus lineatus from Chascomus Lake, Argentina. Microchemical Journal, 112, 127–131.
Sivaperumal, P., Sankar, T. V., & Nair, P. G. V. (2007). Heavy metal concentrations in fish, shellfish and fish products from internal markets of India vis-à-vis international standards. Food Chemistry, 102, 612–620.
Storelli, M. M. (2008). Potential human health risk from metals (Hg, Cd, and Pb) and polychlorinated biphenyl’s (PCBs) via seafood consumption: estimation of target hazard quotients (THQs) and toxic equivalents (TEQs). Food and Chemical Toxicology, 46, 2782–2788.
Tapia, J., Vargas-Chacoff, L., Bertrán, C., & Peña-Cortés, F. (2012). Heavy metals in the liver and muscle of Micropogonias manni fish from Budi Lake, Araucania Region, Chile: potential risk for humans. Environmental Monitoring and Assessment, 184, 3141–3151.
Tüzen, M. (2003). Determination of heavy metals in fish samples of the Middle Black Sea (Turkey) by graphite furnace atomic absorption spectrometry. Food Chemistry, 80, 119–123.
Tüzen, M. (2009). Toxic and essential trace elemental contents in fish species from the Black Sea, Turkey. Food and Chemical Toxicology, 47, 1785–1790.
Tüzen, M., & Soylak, M. (2007). Determination of trace metals in canned fish marketed in Turkey. Food Chemistry, 101(4), 1395–1399.
Tüzen, M., Karaman, I., Cıtak, D., & Soylak, M. (2009). Mercury(II) and methyl mercury determinations in water and fish samples by using solid phase extraction and cold vapour atomic absorption spectrometry combination. Food and Chemical Toxicology, 47, 1648–1652.
Uluozlu, O. D., Tüzen, M., Mendil, D., & Soylak, M. (2007). Trace metal content in nine species of fish from the Black and Aegean Seas, Turkey. Food Chemistry, 104, 835–840.
US EPA. (1989). Risk assessment guidance for superfund. Human health evaluation manual (part A). Interim final, vol. I. Washington (DC): United States Environmental Protection Agency.
US EPA. (1992). Guidelines for exposure assessment, environmental protection agency, risk assessment forum. Washington DC.
US EPA. (1999). Guidance for performing aggregate exposure and risk assessments, environmental protection agency, office of pesticide programs. Washington DC.
US EPA. (2007). EPA region 3 RBC table. http://www.epa.gov/reg3hwmd/risk/human/rbc/RBCapr07.pdf. Accessed Aug 2008.
Vukosav, P., Mlakar, M., Cukrov, N., Kwokal, Ž., & Pižeta, I. (2010). Heavy metal contents in water, sediment and fish in a karst aquatic ecosystem of the Plitvice Lakes National Park (Croatia). Archives of Environmental Contamination and Toxicology, 59, 291–300.
WHO. (1994). Quality directive of potable water, 2nd ed. (p. 197). WHO.
WHO. (1996). Health criteria other supporting information. In: Guidelines for Drinking Water Quality, vol. 2, 2nd ed. (pp. 31–388). Geneva.
Yang, R. Q., Yao, T. D., Xu, B. Q., Jiang, G. B., & Xin, X. D. (2007). Accumulation features of organochlorine pesticides and heavy metals in fish from high mountain lakes and Lhasa River in the Tibetan Plateau. Environment International, 33, 151–156.
Yi, Y. J., Yang, Z. F., & Zhang, S. H. (2011). Ecological risk assessment of heavy metals in sediment and human health risk assessment of heavy metals in fishes in the middle and lower reaches of the Yangtze River basin. Environmental Pollution, 159, 2575–2585.
Yohannes, Y. B., Ikenaka, Y., Nakayama, S. M. M., Saengtienchai, A., Watanabe, K., & Ishizuka, M. (2013). Organochlorine pesticides and heavy metals in fish from Lake Awassa, Ethiopia: insights from stable isotope analysis. Chemosphere, 91, 857–863.
Yu, T., Zhang, Y., Hu, X. N., & Meng, W. (2012). Distribution and bioaccumulation of heavy metals in aquatic organisms of different trophic levels and potential health risk assessment from Taihu lake, China. Ecotoxicology and Environmental Safety, 81, 55–64.
Zeng, J., Yang, L. Y., Wang, X., Wang, W. X., & Wu, Q. L. (2012). Metal accumulation in fish from different zones of a large, shallow freshwater lake. Ecotoxicology and Environmental Safety, 86, 116–124.
Zheng, N., Wang, Q. C., Zheng, X. W., Zheng, D. M., Zhang, Z. S., & Zhang, S. Q. (2007). Population health risk due to dietary intake of heavy metals in the industrial area of Huludao city, China. Science of the Total Environment, 387, 96–104.
Acknowledgments
The authors would like to thank the key science and technology project of Henan for its financial support [142101310425].
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, F., Qu, L., Fan, W. et al. Study on heavy metal levels and its health risk assessment in some edible fishes from Nansi Lake, China. Environ Monit Assess 187, 161 (2015). https://doi.org/10.1007/s10661-015-4355-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-015-4355-3