Abstract
Background
Interleukin-6 (IL-6) is a potent inflammatory cytokine that appears to play a key role in cancer growth and metastasis. In the present study, the effects of IL-6 receptor (IL-6R) on breast cancer aggressiveness and bone metastases were investigated.
Methods
MDA-MB-231 (MDA-231) cells were treated in the presence or absence of anti-human IL-6 receptor (IL-6R) monoclonal antibody and examined with respect to cell survival. The expressions of signal transducer and activator of transcription 3 (Stat3), vascular endothelial growth factor (VEGF), and receptor activator of NF-κB (RANK) were analyzed by SDS-PAGE and immunoblotting. MDA-231 cells were injected into the left ventricle of mice, and then anti-human IL-6R monoclonal antibody or saline was administered intraperitoneally for 28 days. After 28 days, the incidence of bone metastases was evaluated in the hind limbs by radiography and histology.
Results
Anti-human IL-6R monoclonal antibody reduced bone metastases in an animal model injected with MDA-231 cells on radiological and histomorphometric analyses. The mechanism of bone metastasis inhibition involved inhibited cell proliferation and decreased expressions of phospho-Stat3, VEGF, and RANK in MDA-231 cells.
Conclusions
The results of the present study suggest that inhibition of IL-6 signaling may become a preventive therapeutic option for breast cancer and bone metastases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is a relatively common tumor, with an estimated incidence of 1.2 million new cases worldwide every year [1]. The crude and age-adjusted incidences of breast cancer in women have tended to increase since 1975. The crude incidence of breast cancer including carcinoma in situ in 2010 was 115.7 per 0.1 million population, which was the highest of all cancers [2]. The patients’ outcomes depend mainly on the development of distant metastases [3]. Approximately, 20% of patients with significant bone metastases survive for more than 5 years, whereas those with minor metastases in the bone can survive up to 10 years or more [4].
Bone metastases are most common in patients with primary metastatic or recurrent breast cancers and usually cause disability and morbidity accompanied by pain and neurological disorders [5, 6]. There has been some success with the use of intravenous bisphosphonates and the recent development of denosumab, but treatment with these drugs is mostly palliative [6,7,8], without improving overall survival. Promising new therapeutic approaches are needed for both the primary tumor and distant metastases, as is a greater understanding of bone-tropic tumor cells.
Inflammatory processes can have quite diverse effects on cancer development. TNF-α, IL-1, and IL-6 are among the key inflammatory cytokines implicated in carcinogenesis [9]. High levels of interleukin-6 (IL-6) have been detected in many types of human epithelial cancers [10]. Increased expression of IL-6 is associated with a poor prognosis [11, 12]. IL-6 is a multifunctional cytokine that was originally characterized as acting in immune and inflammatory responses, and it inhibits apoptosis in toxic environments during inflammation [13]. IL-6 can promote tumor cell proliferation in vitro and tumor growth in vivo [14, 15]. According to some studies that evaluated the clinical significance of IL-6 pre-treatment levels, IL-6 concentrations reflected disease status and were commonly associated with metastatic disease [11]. Recent in vitro studies have suggested that IL-6 is pathologically involved in the proliferation and metastasis of breast cancer cells [15, 16]. Clinical studies have suggested that elevated serum IL-6 levels in cancer patients correlate with weight loss [17] and a poor prognosis [12], and that a decrease in serum IL-6 levels induced by medroxyprogesterone acetate (MPA) correlates with a reversion of weight loss in patients with advanced breast cancer [17]. Thus, blocking IL-6-mediated signaling cascades has a potential for treatment of human cancers [18, 19] and metastases. Tocilizumab is an anti-human IL-6 receptor (IL-6R) monoclonal antibody (Mab) that inhibits the binding of IL-6 to IL-6R on the cell surface or the soluble form of IL-6R [20]. Tocilizumab is approved for RA and juvenile idiopathic arthritis (JIA), as well as Castleman’s disease, in Japan. Recent studies have shown that tocilizumab is also effective as an antitumor agent against tumor cells in vitro and in vivo [21, 22].
In this study, whether tocilizumab inhibits bone metastases of a breast cancer cell line (MDA-MB-231) in vitro and in vivo was investigated.
Materials and methods
Reagents
Tocilizumab was provided from Chugai Pharmaceutical Co. (Tokyo, Japan). Rabbit polyclonal antibodies to phospho-Stat3, phospho-ERK, ERK, and gp130 were from Cell Signaling Technology (Danvers, MA, USA). Rabbit polyclonal antibodies to VEGF and goat polyclonal antibody to β-actin were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Mouse monoclonal antibody to RANK was from Abcam (Cambridge, MA, USA).
Cell culture
The human breast cancer cell lines MDA-MB-231 (MDA-231), MCF7, and ZR-75-1 were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). MDA-231 cells were cultured in DMEM supplemented with 10% FBS and grown in a 5% CO2 atmosphere at 37 °C. MCF7 and ZR-75-1 were cultured in RPMI medium 1640 supplemented with 10% FBS.
Animals
Five-week-old, female BALB/c nu/nu mice (SLC, Hamamatsu, Japan) were used for all in vivo experiments. Procedures involving animals and their care were conducted in conformity with national and international laws and policies and approved by our Institutional Review Board.
Reverse transcriptase-polymerase chain reaction
For investigation of the expression of mRNA, total RNA was isolated using ISOGEN and treated with DNase (Wako Pure Chemical Industries, Japan). cDNA was synthesized using PrimeScript Reverse Transcriptase (Takara Bio, Shiga, Japan). PCR amplification was performed using the following specific primers and cycling parameters: human IL-6, forward primer 5-GGGAAGCTTGCTATGAACTCCTCCTCCACA-3, reverse primer, 5-GGGGAATTCATGCTACATTTGCCGAAGAGC-3, 30 s at 94 °C, 30 s at 58 °C, and 30 s at 72 °C for 35 cycles; human IL-6 receptor, forward primer 5-AAGGACCTCCAGCATCACTGTGTCA-3, reverse primer, 5-CCTTCAGAGCCCGCAGCTTCCACGT-3, 30 s at 94 °C, 30 s at 60 °C, and 30 s at 72 °C for 35 cycles; human TNF-α, forward primer 5-GTGGCAGTCTCAAACTGA-3, reverse primer, 5-TATGGAAAGGGGCACTGA-3, 30 s at 94 °C, 30 s at 58 °C, and 30 s at 72 °C for 35 cycles; human TNF-R1, forward primer 5-TCGATTTGCTGTACCAAGT-3, reverse primer 5-GAAAATGACCAGGGGCAACAG-3, 30 s at 94 °C, 30 s at 56 °C, and 30 s at 72 °C for 35 cycles; human TNF-R2, forward primer 5-CAGTGCGTTGGACAGAAG-3, reverse primer 5-GGCTTCATCCCAGCATCA-3, 30 s at 94 °C, 30 s at 60 °C, and 30 s at 72 °C for 35 cycles; and human GAPDH, forward primer 5-CATGGAGAAGGCTGGGGCTC-3, reverse primer 5-CACTGACACGTTGGCAGTGG-3, 30 s at 94 °C, 30 s at 55 °C, and 30 s at 72 °C for 35 cycles. The PCR products were loaded in 2% agarose gel and stained with ethidium bromide. The size of the fragments was confirmed by reference to a 100-bp DNA ladder. Quantification of amplified mRNA was done by densitometry assisted by the image analysis software Scion Image (Scion Corporation, Frederick, MD, USA).
SDS-PAGE and immunoblotting
Western blots were performed as described previously [23]. Briefly, samples (cell lysates) were separated by SDS-PAGE, transferred to nitrocellulose membranes, and immunoblotted with primary antibodies. The primary antibodies were polyclonal rabbit antiserum against phospho-Stat3, phospho-ERK, ERK, gp130, VEGF polyclonal goat antiserum against β-actin, and monoclonal mouse antiserum against RANK. Separated proteins were visualized with HRP-conjugated anti-rabbit antibody, anti-goat antibody, or anti-mouse antibody (Dako Cytomation, Carpinteria, CA, USA) with enhancement by chemiluminescence using ECL+ (Amersham Pharmacia Biotech, Arlington Heights, IL); chemiluminescence detection used the LAS-1000 plus (Fujifilm, Tokyo, Japan), in accordance with the manufacturer’s specifications. Quantification of immunoblots was done by densitometry assisted by the image analysis software Scion Image.
Cell proliferation assay
Cell proliferation of MDA-231 cells treated with tocilizumab (96-well plates) was assayed using the WST-1 assay according to the manufacturer’s instructions. The data values of the y-axis were converted into rates, with 1.0 as control at 0 h (baseline).
Migration assay
Cell migration analyses using Boyden chambers were performed as described previously [23].
Intracardiac experimental metastasis model
Subconfluent cells were fed with fresh medium 24 h before intracardiac injection. Cells (1 × 105) were suspended in 0.1-mL sterile PBS and injected with a 29-gauge needle into the left ventricle of 5-week-old, female nude mice under anesthesia with pentobarbital (0.05 mg/g). Animals were maintained in accordance with the guidelines of the Institutional Animal Care and Use Committee.
Effect of anti-IL-6R antibody on bone metastasis in vivo
Tocilizumab was administered intraperitoneally at a dose of 8 mg/kg on days 1, 8, 15, and 22 after tumor cell inoculation (n = 15). Untreated mice received saline by intraperitoneal injection (n = 16). Mice were killed 4 weeks after inoculation. X-ray and pathology examinations of bilateral hind limbs were conducted.
Radiographic analysis of bone metastases
Development of bone metastases was monitored by X-ray imaging 4 weeks after tumor inoculation, as described previously [23, 24]. All radiographs were analyzed carefully by three orthopedists. The incidence of bone metastases per mouse and the number of metastatic foci in all bone including ribs or vertebrae were determined for each mouse. Osteolytic metastatic foci as small as 0.5 mm in their longer diameter, which were recognized as demarcated radiolucent lesions in the bone, were projected and amplified on the monitor and enumerated. Data are shown as the incidence and the numbers of metastases per mouse.
Histological analysis of bone metastases and histochemical analysis of osteoclasts
Mice were killed 4 weeks after intracardiac inoculation of tumor cells. Bilateral hind limbs were removed and fixed in 4% paraformaldehyde for 1 day. Femurs and tibias were decalcified in 10% EDTA for 2 weeks. After embedding in paraffin, sections were stained with hematoxylin and eosin. Histochemical examination of TRAcP staining was also performed, as described previously [24]. Areas of bone metastases and tumor burden were determined in middle sections under a microscope.
Tumor burden and number
Histomorphometric analyses of tumor burden in the metastatic tumors in the distal femoral and proximal tibial metaphyses of both hind limbs were performed using longitudinal sections stained with hematoxylin and eosin. Tumors were measured in the intraosseous and extraosseous regions of the distal femur and proximal tibia on the central section of the tumor (largest tumor area). The measurement area in each bone was ~ 1.5 mm, beginning 100 µm below the growth plate, as described previously [23, 24]. All measurements were made using the image analysis software Scion Image. Tumor burden is shown as tumor area (%) per femur and tibia. The area of tumor foci associated with metastases was recorded on each section for each hind limb bone (femur and tibia) per mouse.
Osteoclast number
The number of TRAcP-positive multinucleated osteoclasts at the interface between tumor and bone in the distal femur and proximal tibia was counted in 5 fields of each section and expressed per millimeter of this interface distance, as described previously [24].
Statistics
All numerical values are expressed as means ± (standard deviation, SD). Statistical analysis was performed using the Mann–Whitney U test and Fisher’s exact test; p < 0.05 was considered significant.
Results
Analysis of IL-6 and TNF-α expressions
TNF-α mRNA, TNF-R1 mRNA, and IL-6R mRNA were expressed in breast cancer cell lines, but TNF-R2 mRNA was not expressed in all cell lines (data not shown). IL-6 mRNA was produced in MDA-231 cells, but it was not observed in MCF7 and ZR-75-1 cells (Fig. 1). In a previous study, IL-6 mRNA was undetectable by RT-PCR in estrogen receptor (ER)(+) breast cancer cell lines (including MCF-7 and ZR75-1). In contrast, MDA-231 displayed high levels of IL-6 mRNA [16]. This previous study was similar to the present experimental results. This previous study by ELISA showed that MDA-231 cells secreted IL-6 constitutively in culture; MCF-7 cells, in which no detectable IL-6 mRNA was found, did not secrete measurable amounts of IL-6.
Interleukin-6 and TNF-α expressions in breast cancer cell lines. TNF-α mRNA, TNF-R1 mRNA, and IL-6R mRNA are expressed in breast cancer cell lines on RT-PCR, but IL-6 mRNA is expressed only in MDA-MB-231 cells. Quantification of amplified mRNA was done as described in “Materials and methods” and indicated as fold induction to GAPDH
Analysis of IL-6 signaling
Thus, whether IL-6 signaling can be suppressed by tocilizumab was investigated using MDA-231 cells. IL-6 with gp130 is a well-known regulator of phosphorylated Stat3. Phospho-ERK is a MAPK activated by a variety of environmental stresses. In a recent report, IL-6 was reported to regulate ERK phosphorylation [25]. In the present study, gp130 showed no change, and pStat3 dropped with 10 µl/ml, while pERK decreased at 100 µl/ml (Fig. 2a, b). Treatment with 100 µg/ml of tocilizumab inhibited RANK protein expression (Fig. 2c). These results suggest that tocilizumab suppresses IL-6 signaling in MDA-231 cells.
Effect of anti-interleukin-6 (IL-6) receptor antibody on IL-6 signaling, gp130 expression, phospho-Stat3 expression, and VEGF expression after 48 h of treatment (a), phospho-ERK expression after 60 min of treatment (b), and RANK after 48 h of treatment (c) in MDA-MB-231 cells. Quantification of amplified immune blots was done as described in “Materials and methods” and indicated as fold induction to 0 (μg/ml)/actin
Effect of anti-IL-6R antibody on VEGF expression
Angiogenesis, the process that leads to tumor vascularization by new blood vessel formation, is essential for tumor growth and metastasis. VEGF is the most extensively studied angiogenic factor [26, 27]. Tocilizumab suppressed VEGF expression in MDA-231 cells in a dose-dependent manner (Fig. 2c).
Effect of anti-IL-6R antibody on RANK expression
Many tumors express RANK, the cognate receptor of RANKL, and a high concordance between RANK expression by the primary cancer and its skeletal secondaries have been reported [28]. It was demonstrated that MDA-231 cells express RANK, and tocilizumab suppressed RANK expression in MDA-231 cells (Fig. 2d).
Cell growth and migration
Tocilizumab effectively inhibited the growth of MDA-231 cells. Tocilizumab-treated cells showed a dose-dependent reduction in cell proliferation (Fig. 3); 100 μg/mL tocilizumab suppressed cell proliferation significantly more than 10 and 30 μg/mL tocilizumab. Next, the effect of tocilizumab on the motility of MDA-231 cells was examined. In our previous report, treatment with a TNF inhibitor suppressed cell migration [23]. Thus, whether an IL-6R inhibitor could affect cell migration ability was tested. Specifically, the number of cells that had passed through a membrane was counted. Tocilizumab (≤ 100 μg/mL) had no effect on cell motility (supplemental data). This result suggested that tocilizumab to 100 µg/ml did not cause cell growth inhibition due to toxicity.
Cell proliferation assay in vitro. Tocilizumab (TCZ) at more than 10 μg/mL suppresses cell proliferation (*p < 0.005, **p < 0.001, ***p < 0.0001: control vs TCZ). Tocilizumab-treated cells show a dose-dependent reduction in cell proliferation (#p < 0.05, ##p < 0.001: TCZ 10 and 30 μg/mL vs. 100 μg/mL). Columns, mean; bars, SD. BL: 0 h (baseline)
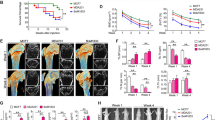
Radiographic analysis of the effect of anti-IL-6R antibody on the development of metastases in nude mice
To test whether tocilizumab directly affects the ability of MDA-231 cells to metastasize, MDA-231 cells were injected into the left ventricles of mice. On X-ray analysis 4 weeks after tumor inoculation, the number of osteolytic lesions was counted. Osteolytic bone metastases appeared around the knee joints (Fig. 4a) in the mice without tocilizumab treatment. While 93.8% (15/16 mice) of untreated mice developed metastases, 60% (9/15) of treated mice developed metastases (Fig. 4b). The incidence of metastases per mouse was decreased significantly by tocilizumab (p < 0.05). The mean number of metastatic foci in bone per mouse was 2.88 (± 1.50) and 1.20 (± 1.27) in untreated and treated mice, respectively. The metastatic number per mouse was significantly decreased (p < 0.005) by treatment.
Effects of tocilizumab on bone metastases of MDA-MB-231 human breast cancer cells. Representative radiographs of bone metastases treated without (a, metastatic sites with arrow) or with tocilizumab (8 mg/kg/week, data not shown). Representative histological views of bone metastases treated without (b) or with tocilizumab (8 mg/kg/week) (c) are shown with hematoxylin–eosin staining
Histomorphometric analysis of the effect of anti-IL-6R antibody on development of osteolytic lesions
The effects of tocilizumab on bone metastases of MDA-231 cells in nude mice were then examined histologically. Histological examination of femurs and tibias with MDA-231 cells showed wide tumor burden with trabecular bone destruction (Fig. 4c). Histomorphometric analysis demonstrated that tocilizumab decreased the area of osteolytic lesions. The area of metastatic foci per hind limb bone was 22.7% (± 6.1%) and 8.3% (± 4.5%) in untreated and treated mice, respectively (p < 0.05). Histomorphometric analysis also showed that the area of metastatic foci in tocilizumab-treated mice was decreased significantly compared with untreated mice (Fig. 4c, d). Histomorphometric analysis of sections histochemically stained for the osteoclast marker TRAcP showed a significant decrease in osteoclast surface/bone surface in mice treated with tocilizumab compared with untreated mice (Fig. 5a, b, and supplemental data).
Discussion
Bone metastases with severe pain, fractures, and spinal cord compression should be treated immediately. These symptoms may reduce the patient’s performance status and worsen quality of life.
Animal models of bone metastasis using cancer cell lines derived from human carcinomas have been studied to understand the intrinsic pathological event. The most common tumor to cause osteolytic lesions is breast carcinoma; MDA-231 cells of human breast carcinoma selectively colonize the tibias and femurs of nude mice, including osteolytic metastases [24]. In this report, it was confirmed that the breast cancer cell line MDA-231 expressed IL-6 and its receptor, IL-6R. IL-6 signals via a receptor complex composed of IL-6R and gp130 [20]. Activation of this complex causes gp130 to phosphorylate and thereby activate JAK 1 and 2 [29]. IL-6-induced JAK family members activate three major pathways, JAK–Stat3, Ras–MAPK, and PI3K–Akt [30]. The JAK–STAT3 signal pathway was found to be the key in tumor progression [13]. Scherzad et al. demonstrated that human mesenchymal stem cells enhance cancer cell proliferation via IL-6 secretion and activation of ERK1/2 [31], and Shi et al. showed that IL-6 stimulated tumor growth by activation of Ras, Raf, MEK, and ERK1/2 [32]. In the present study, tocilizumab decreased gp130 and phosphorylated Stat3 expressions in MDA231 cells, and it suppressed phosphorylated ERK expression. The humanized monoclonal antibody to the IL-6 receptor, tocilizumab (Actemra®), inhibits the binding of IL-6 to IL-6R on the cell surface or the soluble form of IL-6R [20]. Tocilizumab was approved in Japan and by the European Medicines Agency (EMEA) in 2008, and it was approved by the United States Food and Drug Administration (FDA) in 2010. Tocilizumab has also been shown to be effective in blocking cartilage and bone destruction in IL-6-mediated autoimmune diseases such as synovitis and rheumatoid arthritis (RA). Thus, tocilizumab inhibited IL-6 signaling in MDA231 cells, followed by inhibition of tumor cell proliferation and suppression of bone metastases in the animal model.
Previous work has demonstrated that, in bone metastases, tumor-derived IL-6 can stimulate osteoclast differentiation and osteoclast-mediated bone resorption [33]. The interplay of RANKL-RANK-IL-6 in a previous study further consolidates the role of a direct interaction between osteoblasts and cancer cells within the bone microenvironment. IL-6 expressed by tumor cells induces, via an autocrine mechanism, expression of RANK on the surface of the tumor cells, thus sensitizing these cells to the action of osteoblast-derived RANKL [34]. Further, many tumors express RANK, the cognate receptor of RANKL, and a high concordance between RANK expression by the primary cancer and its skeletal secondaries has been reported [28]. In the present study, it was demonstrated that tocilizumab suppressed RANK expression in MDA-231 cells in a dose-dependent manner.
As another mechanism of inhibition of bone metastasis, tocilizumab might inhibit angiogenesis. Angiogenesis is a central step in tumor progression because it promotes tumor invasion and metastatic spread [35]. VEGF is a potent angiogenic agent that acts as a specific mitogen for vascular endothelial cells through specific cell surface receptors. IL-6 also promotes angiogenesis through induction of VEGF expression [36]. The results of the present study demonstrated that tocilizumab suppressed VEGF expression in MDA231 cells.
IL-6 promoted cell migration of MDA-231 cells [37]. The previous study reported that MDA-231 cells secreted IL-6 constitutively in culture [15, 16]. However, tocilizumab did not affect cell mobility. One of the reasons was no stimulation by IL-6. On the other hand, Ahmad et al. reported IL-6 caused a slight increase in MDA-231 migration in comparison to unstimulated cells, but no statistical significance was observed [38]. IL-6 may not contribute to cell mobility greatly.
There are no public data on the efficacy of tocilizumab in inhibiting the progression of bone metastases. In this report, it was demonstrated that anti-IL-6 therapy using tocilizumab suppressed bone metastases in an animal model, and this therapy inhibited MDA-231 cell proliferation and Stat3, RANK, and VEGF expressions in vitro. The results of the present study suggest that inhibition of IL-6 using an IL-6R inhibitor may become a preventive therapeutic option for breast cancer and bone metastases.
The present study had several limitations. First, other organ metastases such as lung or liver were not examined. Second, bone metastases of other breast cancer cell lines (e.g., ER-positive cells, such as MCF-7 or ZR75-1) were not examined. Clinical studies have demonstrated that hormone-responsive (i.e., estrogen receptor (ER)-positive) tumors have a much stronger metastatic predilection for bone than their ER-negative tumors [39]. MDA-231 cells secreted IL-6, but are ER negative. The ER-positive cell lines MCF-7 and ZR-75-1 had undetectable levels of IL-6 whereas the ER-negative cell line MDA-231 produced IL-6 in previous report and our study. Our results cannot be applied to ER-positive breast cancer. However, Sasser et al. demonstrated that IL-6 can act as a potent growth factor for the ER-positive cell line MCF-7 in vitro and in vivo. IL-6 produced by bone marrow mesenchymal stem cells (a common fibroblastic cell population within bone) potently induces growth in ER-positive breast cancer cells [15]. Of interest, the bone marrow microenvironment in postmenopausal women maintains stable levels of IL-6 protein, regardless of systemic estradiol levels [40]. And we demonstrated that IL-6 receptor mRNA was expressed in both ER-positive and -negative breast cancer cell lines (Fig. 1). In the previous reports and our results, IL-6 produced by bone marrow fibroblasts and/or autocrine tumor cells was biologically linked with either ER-positive or -negative breast cancer cells. This link may induce morbidity and mortality in patients with bone metastases clinically. We plan to study these in the future.
In conclusion, tocilizumab greatly reduced bone metastases in an animal model injected with a metastatic breast cancer cell line, MDA-231. Inhibition of IL-6 signaling using tocilizumab may become a preventive therapeutic option for breast cancer.
Abbreviations
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- DNase:
-
Deoxyribonuclease
- EDTA:
-
Ethylenediamine tetraacetic acid
- ERK:
-
Extracellular signal-regulated kinase
- FBS:
-
Fetal bovine serum
- GAPDH:
-
Glyceraldehyde 3-phosphate dehydrogenase
- gp130:
-
Glycoprotein 130
- HRP:
-
Horseradish peroxidase
- JAK:
-
Janus kinase
- MAPK:
-
Mitogen-activated protein kinase
- NF-kB:
-
Nuclear factor-kappa B
- PBS:
-
Phosphate-buffered saline
- PCR:
-
Polymerase chain reaction
- PI3K:
-
Phosphoinositide 3-kinase
- RPMI:
-
Roswell Park Memorial Institute
- Stat3:
-
Signal transducer and activator of transcription 3
- TNF:
-
Tumor necrosis factor
- VEGF:
-
Vascular endothelial growth factor
- RNA:
-
Ribonucleic acid
References
Henderson BE, Ross RK, Pike MC. Hormonal chemoprevention of cancer in women. Science. 1993;259(5095):633–8.
Taira N, Arai M, Ikeda M, Iwasaki M, Okamura H, Takamatsu K, Nomura T, Yamamoto S, Ito Y, Mukai H. The Japanese Breast Cancer Society clinical practice guidelines for epidemiology and prevention of breast cancer, 2015 edition. Breast Cancer. 2016;23(3):343–56.
Greenberg PA, Hortobagyi GN, Smith TL, Ziegler LD, Frye DK, Buzdar AU. Long-term follow-up of patients with complete remission following combination chemotherapy for metastatic breast cancer. J Clin Oncol. 1996;14(8):2197–205.
Coleman RE, Rubens RD. The clinical course of bone metastases from breast cancer. Br J Cancer. 1987;55(1):61–6.
Manders K, van de Poll-Franse LV, Creemers GJ, Vreugdenhil G, van der Sangen MJ, Nieuwenhuijzen GA, Roumen RM, Voogd AC. Clinical management of women with metastatic breast cancer: a descriptive study according to age group. BMC Cancer. 2006;6(6):179.
Coleman RE. Adjuvant bisphosphonates in breast cancer: are we witnessing the emergence of a new therapeutic strategy? Eur J Cancer. 2009;45(11):1909–15.
Barton MK. Denosumab an option for patients with bone metastasis from breast cancer. CA Cancer J Clin. 2011;61(3):135–6.
Aihara T, Toyama T, Takahashi M, Yamamoto Y, Hara F, Akabane H, Fujisawa T, Ishikawa T, Nagai S, Nakamura R, Tsurutani J, Ito Y, Mukai H. The Japanese Breast Cancer Society Clinical Practice Guideline for systemic treatment of breast cancer, 2015 edition. Breast Cancer. 2016;23(3):329–42.
Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30(7):1073–81.
Heikkilä K, Ebrahim S, Lawlor DA. Systematic review of the association between circulating interleukin-6 (IL-6) and cancer. Eur J Cancer. 2008;44(7):937–45.
Salgado R, Junius S, Benoy I, Van Dam P, Vermeulen P, Van Marck E, Huget P, Dirix LY. Circulating interleukin-6 predicts survival in patients with metastatic breast cancer. Int J Cancer. 2003;103(5):642–6.
Yokoe T, Iino Y, Morishita Y. Trends of IL-6 and IL-8 levels in patients with recurrent breast cancer: preliminary report. Breast Cancer. 2000;7(3):187–90.
Hodge DR, Hurt EM, Farrar WL. The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer. 2005;41(16):2502–12.
Malinowska K, Neuwirt H, Cavarretta IT, Bektic J, Steiner H, Dietrich H, Moser PL, Fuchs D, Hobisch A, Culig Z. Interleukin-6 stimulation of growth of prostate cancer in vitro and in vivo through activation of the androgen receptor. Endocr Relat Cancer. 2009;16(1):155–69.
Sasser AK, Sullivan NJ, Studebaker AW, Hendey LF, Axel AE, Hall BM. Interleukin-6 is a potent growth factor for ER-alpha-positive human breast cancer. FASEB J. 2007;21(13):3763–70.
Chiu JJ, Sgagias MK, Cowan KH. Interleukin 6 acts as a paracrine growth factor in human mammary carcinoma cell lines. Clin Cancer Res. 1996;2(1):215–21.
Yamashita JI, Ogawa M. Medroxyprogesterone acetate and cancer cachexia: interleukin-6 involvement. Breast Cancer. 2000;7(2):130–5.
Liu S, Ishikawa H, Li FJ, Ma Z, Otsuyama K, Asaoku H, Abroun S, Zheng X, Tsuyama N, Obata M, Kawano MM. Dehydroepiandrosterone can inhibit the proliferation of myeloma cells and the interleukin-6 production of bone marrow mononuclear cells from patients with myeloma. Cancer Res. 2005;65(6):2269–76.
Liu S, Ma Z, Cai H, Li Q, Rong W, Kawano M. Inhibitory effect of baicalein on IL-6-mediated signaling cascades in human myeloma cells. Eur J Haematol. 2010;84(2):137–44.
Kishimoto T. Interleukin-6: discovery of a pleiotropic cytokine. Arthritis Res Ther. 2006;8(Suppl. 2):S2.
Yoshio-Hoshino N, Adachi Y, Aoki C, Pereboev A, Curiel DT, Nishimoto N. Establishment of a new interleukin-6 (IL-6) receptor inhibitor applicable to the gene therapy for IL-6-dependent tumor. Cancer Res. 2007;67(3):871–5.
Oguro T, Ishibashi K, Sugino T, Hashimoto K, Tomita S, Takahashi N, Yanagida T, Haga N, Aikawa K, Suzutani T, Yamaguchi O, Kojima Y. Humanised antihuman IL-6R antibody with interferon inhibits renal cell carcinoma cell growth in vitro and in vivo through suppressed SOCS3 expression. Eur J Cancer. 2013;49(7):1715–24.
Hamaguchi T, Wakabayashi H, Matsumine A, Sudo A, Uchida A. TNF inhibitor suppresses bone metastasis in a breast cancer cell line. Biochem Biophys Res Commun. 2011;407(3):525–30.
Araki K, Wakabayashi H, Shintani K, Morikawa J, Matsumine A, Kusuzaki K, Sudo A, Uchida A. Decorin suppresses bone metastasis in a breast cancer cell line. Oncology. 2009;77(2):92–9.
Ara T, Declerck YA. Interleukin-6 in bone metastasis and cancer progression. Eur J Cancer. 2010;46(7):1223–31.
Maruthanila VL, Elancheran R, Kunnumakkara AB, Kabilan S, Kotoky J. Recent development of targeted approaches for the treatment of breast cancer. Breast Cancer. 2017;24(2):191–219.
Gautam S, Roy S, Ansari MN, Saeedan AS, Saraf SA, Kaithwas G. DuCLOX-2/5 inhibition: a promising target for cancer chemoprevention. Breast Cancer. 2017;24(2):180–90.
Santini D, Perrone G, Roato I, Godio L, Pantano F, Grasso D, Russo A, Vincenzi B, Fratto ME, Sabbatini R, Della Pepa C, Porta C, Del Conte A, Schiavon G, Berruti A, Tomasino RM, Papotti M, Papapietro N, Onetti Muda A, Denaro V, Tonini G. Expression pattern of receptor activator of NFκB (RANK) in a series of primary solid tumors and related bone metastases. J Cell Physiol. 2011;226(3):780–4.
Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117:1175–83.
Levy DE, Darnell JE Jr. Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol. 2002;3(9):651–62.
Scherzad A, Steber M, Gehrke T, Rak K, Froelich K, Schendzielorz P, Hagen R, Kleinsasser N, Hackenberg S. Human mesenchymal stem cells enhance cancer cell proliferation via IL-6 secretion and activation of ERK1/2. Int J Oncol. 2015;47(1):391–7.
Shi Y, Hsu JH, Hu L, Gera J, Lichtenstein A. Signal pathways involved in activation of p70S6K and phosphorylation of 4E-BP1 following exposure of multiple myeloma tumor cells to interleukin-6. J Biol Chem. 2002;277:15712–20.
Hong DS, Angelo LS, Kurzrock R. Interleukin-6 and its receptor in cancer: implications for translational therapeutics. Cancer. 2007;110(9):1911–28.
Zheng Y, Basel D, Chow SO, Fong-Yee C, Kim S, Buttgereit F, Dunstan CR, Zhou H, Seibel MJ. Targeting IL-6 and RANKL signaling inhibits prostate cancer growth in bone. Clin Exp Metastasis. 2014;31(8):921–33.
Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6(4):273–86.
Tzeng HE, Tsai CH, Chang ZL, Su CM, Wang SW, Hwang WL, Tang CH. Interleukin-6 induces vascular endothelial growth factor expression and promotes angiogenesis through apoptosis signal-regulating kinase 1 in human osteosarcoma. Biochem Pharmacol. 2013;85(4):531–40.
Arihiro K, Oda H, Kaneko M, Inai K. Cytokines facilitate chemotactic motility of breast carcinoma cells. Breast Cancer. 2000;7(3):221–30.
Ahmad N, Ammar A, Storr SJ, Green AR, Rakha E, Ellis IO, Martin SG. IL-6 and IL-10 are associated with good prognosis in early stage invasive breast cancer patients. Cancer Immunol Immunother. 2017. https://doi.org/10.1007/s00262-017-2106-8.
James JJ, Evans AJ, Pinder SE, Gutteridge E, Cheung KL, Chan S, Robertson JF. Bone metastases from breast carcinoma: histopathological-radiological correlations and prognostic features. Br J Cancer. 2003;89(4):660–5.
Kassem M, Khosla S, Spelsberg TC, Riggs BL. Cytokine production in the bone marrow microenvironment: failure to demonstrate estrogen regulation in early postmenopausal women. J Clin Endocrinol Metab. 1996;81(2):513–8.
Acknowledgements
We thank Katsura Chiba for excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
12282_2018_853_MOESM1_ESM.pptx
Supplemental data 1. Cell migration analyses. Tocilizumab (≤ 100 μg/mL) has no effect on cell motility. Supplemental data 2. Histological views of TRAcP immunohistochemistry without and with tocilizumab treatment (PPTX 2418 kb)
About this article
Cite this article
Wakabayashi, H., Hamaguchi, T., Nagao, N. et al. Interleukin-6 receptor inhibitor suppresses bone metastases in a breast cancer cell line. Breast Cancer 25, 566–574 (2018). https://doi.org/10.1007/s12282-018-0853-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-018-0853-9