Abstract
Microelectromechanical system (MEMS)-based microneedles are an innovative way of drug delivery that increases the permeability of the skin. It generates microscopic pores inside the skin that leads to the passive diffusion of drugs for dermal microcirculation to take place. This phenomenon helps toward efficient drug penetration. MEMS microneedles are small-sized needles usually in the micron to millimeter range, normally having a length to width of about 150–550 µm and 50–300 µm. respectively. Their tip diameter varies from 1 to 80 µm that can pierce through the epidermis layer directly to dermal tissues devoid of any pain. In this paper, a broad overview of solid microneedles for biomedical applications has been presented. The objective of this review is to collect the state of art main features related to solid microneedles. Particularly, the challenges related to solid microneedles, such as materials and methods used in the fabrication of microneedles, design and their performance, testing, safety concerns, commercialization issues, and applications, have been discussed. Microneedles can be characterized conferring to their fabrication procedure, structure, materials, general shape and size, the shape of the tip, microneedle array thickness, and applications. This comprehensive review on solid microneedles may provide significant useful information for scientists or researchers working on the design and development of solid microneedles for biomedical applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microneedles are considered useful drug delivery devices because they provide painless and efficient drug delivery [1]. Microneedles can be used either way as a stand-alone system or as integrated with other devices forming the complicated microfluidic systems [2,3,4]. Even though the idea of microneedles was suggested in the 1970s, it wasn’t established experimentally till the 1990s once the microelectronics industry delivered the required tools for microfabrication. The first studies of transdermal drug delivery were reported in 1998 [5]. After that, the development and use of microneedles for drug delivery and other pharmaceutical applications have increased rapidly.
Different materials like stainless steel, silicon, titanium, and polymers have been used so far to fabricate microneedles [6, 7]. Some of the microneedles are made with drugs, which means they have a needle shape but are delivered directly into the body and penetrate the skin easily. The microneedles are made in different sizes, shapes, and functions, but all are used as a substitute to former delivery methods like the conventional hypodermal needle or additional vaccination apparatuses [8,9,10]. Microneedle arrays or microneedles can be used as a separate device along with a measure of drug extraction, biological detection, or delivery system. Microneedles, due to their efficiency, can be integrated with biosensors, micropumps, microfluidic chips, and microelectronic devices. Microneedles have many advantages as they have improved the comfort of patients. Microneedles are harmless to be used in humans. Lately, microneedle technology has been proposed for increasing skin penetrability and particularly increase the transdermal delivery for macromolecules.
Correspondingly, several researchers have presented microneedles suitable for many biomedical applications, and they have become a broadly studied technique in this era for biomedicine [10,11,12]. But it still needs to have a brief overview of the latest apprises on the development of different microneedles for biomedical applications as these needles have limited obtainability for marketable use and remain at the study level. Few earlier reviews have been reported on microneedles. These different reviews provide brief information on microneedles and their applications. Donnelly et al. [13] presented a review article that provides facts about various MN types, fabrication approaches, and, prominently, inquiries of the medical safety of MN. Mahmood et al. [14] presented a broad review that focused on current and prospect progresses for microneedle technology containing the up-to-date microneedle design, tests, and schemes in microneedle development besides possible safety features collected from complete literature review relating to microneedle studies toward a date. Ahmed et al. [15] presented a brief review that summarizes the current substantiation for the custom of microneedle arrays as biosensors aimed at constant monitoring of glucose content of the interstitial fluid, concentrating on insertion mechanics, characteristics of microchannels, and protection profile. Florina et al. [16] reported a very substantial review in which delivery device, the central delivery approaches using the microneedles array, materials that are commonly used for fabrication procedure, geometrical, and profile considerations in addition to the present preclinical and clinical requests of the microneedle array. Quinn et al. [17] reported a fundamental and preliminary review that explains how microneedle arrays can upsurge the sum of compounds agreeable to transdermal delivery through piercing the skin’s defensive barrier, the vein corneum, as well as constructing a path for drug infusion to the dermal nerve below. Kevin Ita [10] presented a review on hydrogel-forming microneedles, and special consideration is compensated to hydrogel-forming microneedles as they are new microneedles that do not comprise drugs however absorb interstitial fluid to custom constant conduits among dermal microcirculation as well as an involved patch-form reservoir. Numerous microneedles accepted through regulatory experts for experimental use are also studied. Chen et al. [18] presented a review on the perspective, as well as the experiments of applying microneedles, to provide nucleic acids aimed at gene therapy. It was also suggested that a grouping of microneedles and additional gene delivery methods may provide a path for the improved delivery method for gene rehabilitation. Marwah et al. [19] presented an introductory review that explains that new vigorous drug transport equipment is involved in increasing the transdermal infusion via the actual drug delivery method. Thakur et al. [20] presented a short overview of a variety of tests that are frequently encountered to attain well-organized optical drug levels inside fixed tissues of the eye. It also defines the difficulties met by means of conservative hypodermal needle-based optical vaccinations for frontal and subsequent section drug delivery. It argues research approved in the field of microneedles, up to date. Guojun Ma and Chengwei Wu et al. [21] presented a comprehensive review in which studies were introduced on the mechanical problems with respect to microneedles. All the above-discussed reviews present the facts and information about microneedles only and do not discuss any of its types solely. Here, the authors have given a complete review on solid microneedles that covers any recent development of solid microneedles in the field of medicine or drug delivery. Thus, it is the most comprehensive and simplified review that covers the up-to-date information of solid microneedles concerning the design and development, structure, parameters used for its performance, fabrication methods, materials used for fabrication, safety issues, and challenges, as well as applications.
Solid microneedles have been so far the most common and simplest method of microneedle devices because they are used for the maximum of the early work of microneedle delivery of drugs or vaccines [22]. Solid microneedles have no distribution or flow passages by themselves for drugs and are thus used for pretreatment of skin to generate transitory channels of micron sizes through stratum corneum over mechanically distorting epidermis preceding to drug supervision [23]. Then, drugs were inserted or applied in the form of square patches directly to the skin area penetrated by microneedles [24]. Wang et al. [25] reported a method in which a nanometer-scoped zinc-oxide pyramidal rod array was constructed to form a device in which tip, as well as a base diameter of 50 × 50-µm-long rods, were 60 nm and 150 nm, correspondingly [26]. Li et al. [26] reported a super short microneedle and studied how they can act as a safe and effective substitute in transdermal drug delivery of hydrophilic molecules. It was also studied that after these super short solid microneedles have been injected into a patient, no infection, e.g., Staphylococcus aureus, occurs, and the patient remains safe. Aoyagi et al. [27] proposed a new fabrication method for solid microneedle arrays using numerous tip angles prepared of a biodegradable polymer such as PLA. Their experimentation was confirmed by the simulation of finite element analysis. Coulman et al. [28] reported solid microneedles having a pyramidal shape and pointed/frustum tip to apprehend the performance of nanoparticle preparations inside the biological surroundings and their interface with the skin layers resulting in the disruption of the skin through an innovative delivery device, e.g., solid microneedle arrays. Oh et al. [29] proposed the study which recommends that a biocompatible PC solid microneedle might be an appropriate device for transdermal drug delivery scheme of hydrophilic molecules through the potential applications toward macromolecules, e.g., proteins as well as peptides. Ding et al. [30] fabricated solid microneedle arrays having custom transitory conduits and improve the transportation of vaccine molecules transversely through the skin barrier and without any kind of pain. It was also examined in mice how the immune reactions are after applying TCI by means of two typical antigens, diphtheria-toxoid and respiratory tract infection vaccines. Han et al. [31] presented a new method for fabrication aimed at groove-inserted solid microneedle arrays of a biocompatible polymer as well as the immunization features to ovalbumin transported into mice through the solid microneedle arrays via the skin, and the results proved that it was a compatible way of drug delivery by keeping in mind the dimensions and shapes of microneedle arrays. Chandra Sekhar Kolli and Ajay K. Banga [22] demonstrated maltose solid microneedles characterized and exposed to construct microchannels in the skin that were also categorized and presented to increase the transdermal drug delivery of NH. Jin et al. [32] proposed a unique fabrication approach for an economical microneedle patch prepared by biocompatible polymer, besides efficient confirmations for fabricated solid microneedle patches over animal models, and no side effects were found such as infection or allergic reactions on the application of solid microneedle patches. Kim et al. [33] proposed the investigational factors and mechanical pathways through which deactivated infection vaccine can lose action, besides develop as well as measures upgraded solid microneedle coating preparations that defend the antigen from inactivity. Afterwards, coating microneedles have used a typical vaccine preparation, the constancy of respiratory tract infection vaccine was condensed to just 2%, as calculated by hemagglutination activity. Park et al. [34] reported polymer solid microneedle rollers having a conical shape and discussed that compared to injecting microneedles on a smooth patch, the consecutive inclusion of solid microneedles on a roller row by row require less inclusion strength in the full thickness of swinish skin. Generally, polymer solid microneedle rollers, made from simulated polymer films, provide an unpretentious way to upsurge skin penetrability for drug delivery purposes. Gomaa et al. [35] investigated the issue that by compelling transepidermal water loss depths of dermatome human skin models succeeding the inclusion of solid polymer microneedles. Inclusions triggered an initial severe drop in barricade function tracked by a slow imperfect recovery – an example constant with microneedle generation of microchannels which consequently contract, owing to the skin’s elasticity. Donnelly et al. [36] reported the very first kind of polymeric microneedles that have been engaged for delivering a perfect lipophilic dye, Nile red, into the expunged pigskin. Prominently, the one-step delivery approach is used for the limited delivery of exceedingly hydrophobic agents that overwhelm several disadvantages of existing delivery approaches. Romgens et al. [37] proposed a study that presented that sharp solid microneedles are important to insert microneedles in a well-organized manner to a preferred depth. Zhang et al. [38] studied the permeation as well as the delivery of poly(lactic-co-glycolic acid) (PLGA) nanoparticles in human skin cured with solid microneedles. Fluorescent PLGA nanoparticles were arranged to show the transdermal transference procedure of nanoparticles. The studied results proposed that solid microneedles could increase the intradermal distribution of the PLGA nanoparticles. Julia et al. [39] studied the effect of solid microneedles on the transdermal delivery of particular antiepileptic drugs, and it was tested on pigskin to demonstrate the consequence of solid microneedle rollers. Hoang et al. [40] studied the effect of solid microneedles on transdermal delivery of amantadine hydrochloride as well as pramipexole di-hydrochloride through a pig ear skin in vitro and statistical analysis was also conceded. Mj Uddin et al. [41] proposed an innovative inkjet printing technology for coating solid microneedle arrays of metal material using three anticancer agents including 5-fluorouracil, cisplatin, as well as curcumin, for transdermal delivery. Witting et al. [42] highlighted precisely the protein balance throughout storage and also demonstrated that discriminatory intraepidermal installation of proteins or peptides via solid microneedles is a practical approach. The solid microneedle array pierces the strong barrier of the stratum corneum, causing the active molecules to diffuse through the arranged channels. When the microneedles array is removed, the drug formulation is customarily applied onto the pretreated area of the skin. The pores are formed in the skin which helps the variety of molecules to transport through gaps of closely damaged skin as well as reach the epidermis and dermis [43]. Different researchers have been working on solid microneedles using different materials for their fabrication and making them useful for human skin or other treatments. Solid microneedles made of metals are an attractive setup for having painless drug delivery. Though, fabrication procedures for metal microneedles are complex and require a large setup. An accessible building of metallic microneedle arrays by means of thermoplastic representation of metallic glasses has been reported. Microneedles with tuneable lengths as well as tips are created through monitoring the rheology and splintering of metallic glasses. And when these solid metallic glass microneedles are taken for in vitro insertion tests, they give an excellent piercing of porcine skin [44]. The first-time usage of TA.XTplus Texture Analyser toward characterization of spurt force in pigskin for drug delivery tests has been reported. With this force, the pigskin can easily be ruptured as demonstrated in their experiments and results [40]. It is reported that the sharper-tipped solid silicon microneedles having a higher aspect ratio have been fabricated. Tetramethylammonium hydroxide etching techniques have been improved and used for the fabrication of stretched and pointed microneedles. The fabricated solid microneedles are considered to be appropriate for applications of drug delivery [45]. The safety measures of solid polymer microneedles for humans offered at different applications have been proposed. Also it was taken into account the diverse application locations of vaccination, cosmetology, and insulin delivery tangled through microneedles method, reasons persuading user receiving of microneedles comprising the length, density, and the proportions of microneedle patches by putting on diverse microneedle patches on the forearm, forehead, and abdomen skins of human participants [46]. It is reported that patch pretreatment of solid polymer microneedle improves the penetration of drug particles in the skin [46]. The solid microneedles are also made up of biodegradable polymers and biocompatible polymers for transdermal drug delivery. Polymer microneedles have the benefits of being easily fabricated, cost-efficient, and can be mass-produced, besides precise drug release by means of the water solubility and deprivation properties of polymers. Polymer microneedles, by using various physical and chemical properties of polymers, offer a promising method for drug delivery [47]. Solid microneedles are used for skin pretreatment as they have increased skin permeability. Solid microneedles are used in cosmetic surgery and other skin treatments due to their good skin permeability [48]. An innovative method for manufacturing solid polymer microneedles utilizing laser-ablated molds in a shot molding procedure has been reported. Research has been successfully conducted to create cone-shaped microholes through low tip areas in a device steel pattern, utilizing a femtosecond laser through a cross-hatching approach. Lastly, the achieved mold was made to be used in an injection molding procedure for replicating the polypropylene microneedles [49]. Verbaan et al. [50] described the enhanced piercing method of microneedle patches in dermatome human skin by an influence enclosure method. A detailed review of solid microneedles is presented in Table 1 below.(see Table 2)
Classification of Microneedles
There are four types of microneedles, i.e., solid microneedles, coated microneedles, hollow microneedles, and dissolving microneedles. (Fig. 1) Each type has pros and cons depending on its delivery methods and their functionalities [51]. Each type is explained below concerning its design, fabrication techniques, materials used for its fabrication, and applications. Solid microneedles deliver drugs to the body in two parts; firstly, the microneedle array is applied to the patient’s skin to make microscopic bores, which are much deeper, to easily penetrate the skin’s outermost layer, and secondly, the transdermal patch is used for the application of the drug. Solid microneedles are now used by dermatologists in skin treatment or collagen stimulation therapy [52]. Hollow microneedles are comparable to solid microneedles in terms of materials used. They have reservoirs through which drug is delivered directly into the body. Meanwhile, the drug delivery is reliant on the flow rate of the microneedle; therefore, this microneedle array has a possibility of becoming clogged through extreme swelling or faulty design. Hollow microneedles have the disadvantage of increasing the possibility of clipping under the pressure of, and consequently inadequate to deliver drugs []. Coated microneedles are commonly made from metals or polymers just like solid microneedles. Instead of being applied from any applicators or patches, the drug is transferred directly to the human body through microneedles or microneedle arrays; therefore, they have been named coated microneedles [53]. To make sure that the required dose of a drug is properly delivered to the body, these microneedles are often enclosed in other thickening agents or surfactants. Most of the chemicals used on coated microneedles are named irritants. When these microneedle arrays are applied, and there is a risk of local inflammation to the body where applied, then these arrays can be removed directly without any harm to the patient [54]. In the latest adaptation of microneedle designs, dissolvable microneedles have drugs encapsulated in a harmless polymer that formerly dissolves inside the skin [2].
Drug Administration on Insertion of Microneedles
The drug is inserted into the skin via different types of microneedles in the form of a patch that is placed on the body of a human, and then the transportation of the drug takes place as described below in Fig. 2. Also, when a drug is delivered properly, the microneedles are removed without giving any pain to the patient, and it is a very effective or easy process.
Design and Geometry of Solid Microneedles
To decide the performance and mechanical behavior of microneedles, it is very important to understand the design and geometry, which includes the shape of microneedles, their tip radius, length, aspect ratio, base width, and diameter, etc. So, it is very important to wisely select a correct material as well as an accurate methodology for the fabrication of ideal microneedles that are biocompatible, involuntarily strong to penetrate skin layers, proficient in loading miscellaneous active drug materials, and measured or continued drug delivery [7]. Wang et al. [63] carried an exceptional review on the characteristic of diverse polymer materials and approaches engaged in fabricating and getting ideal microneedles. The most important feature is that the geometry of microneedles simplifies the smooth insertion of needles into the skin. This is essential as human skin is robust and flexible in nature, and thus it tends to prevent the insertion of microneedles or may lead to the breaking of microneedles. This is usually seen with microneedles that are fabricated with weak materials having rounded tips. For that reason, the geometry of microneedles is subtle and critical for effective drug delivery.
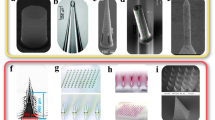
Different shapes of microneedles that were established include rectangular with a sharp edge, conical, and pointed, etc., with variable widths and lengths. Figure 3 shows the design and geometry of solid microneedles. For example, Lee et al. reported solid polymer microneedles having pyramidal shapes without good mechanical strength vis-a-vis the conical molded microneedles by way of the pyramidal ones are related with an advanced cross-sectional area at a similar base width [60]. Moreover, Chen et al. reported that greater insertion depths were attained using chitosan microneedles having a 5-µm-tip radius [64]. Likewise, the microneedles having a sharp tip are vivacious for insertion because they have a higher potential to pierce with a small force, and vice versa is correct for microneedles with large diameter tips [65, 66].
Forces Used During Penetration
In solid microneedles, pharmaceutical materials are coated to deliver the drugs into the patient’s body. In the course of penetration, several forces, e.g., bending, lateral, buckling, axial, and resistive are usually experienced by microneedles. Therefore, it is very important to have perfectly designed microneedles that tolerate all these forces without any deflection because these forces can cause breakage of microneedles at the time of penetration. The force, which at the time of insertion becomes more dominant on the microneedle’s tip, is axial [67]. The axial force is compressive, plus it points toward the buckling of microneedles. On the insertion of microneedles into the skin, another force called resistive force is applied by the skin. Hereafter, to penetrate the microneedle, the applied force must be larger than the resistive force. Also, uneven skin surface, as well as human inaccuracy in the course of needle penetration, may cause bending. As a result, it is essential to understand the geometry of microneedles and the material’s mechanical properties concerning getting the perfect design of microneedles as well as the estimation of failure caused by microneedles [65, 68, 69]. The buckling force acted on microneedles in skin penetration is given as follows:
[70, 71]where E is Young’s modulus, I is the moment of inertia, and L is the microneedle’s length.
The bending force that the microneedle can bear without breakage is given as follows:
[70]where c is the distance from the vertical axis to the outer edge of the skin.
The axial force that the microneedle can bear without breakage is given as follows:
[11, 70]where \({\sigma }_{y}\) represents yield strength of a material or tip area.
Also, the resistive force which the skin applied when penetration takes place is given as follows:
[70]where \({P}_{\mathrm{pierce}}\) is the pressure needed to penetrate the solid microneedle in to the skin [72,73,74,75,76,77]. Afterward, the skin is penetrated by microneedles; the only force acting on microneedles is the frictional force that resulted from the tissues’ and needles’ interaction [74, 78].
Materials Used in the Fabrication of Solid Microneedles and Their Issues
For the design and fabrication of microneedles, it is very important to select appropriate materials for any particular application. Materials used for the fabrication of microneedles can be classified into two groups:
-
Degradable
-
Nondegradable
Degradable materials include materials like metals, glass, silicon, and ceramics, whereas degradable materials consist of polysaccharides as well as biodegradable polymers.
So far, scientists have fabricated the following:
Numerous researchers have used silicon for the fabrication of microneedles but Si is a brittle material and is dangerous to health [26, 101,102,103,104,105,106,107,108,109,110,111,112]. Metal microneedles have the adequate mechanical strength to pierce the skin; however, they also have the disadvantage of generating probable biological wastes [2, 9] [113]. Many polymers have a history of biocompatibility. They display tremendous mechanical and chemical properties which are appropriate for the fabrication of microneedles. Different polymers have been reported for the fabrication of microneedles which include polyglycolide (PGA), poly(l-lactic acid) (PLLA), polydimethylsiloxane (PDMS), polycarbonate (PC), poly(methyl methacrylate) (PMMA), etc. The fabrication of microneedles using polymeric materials has been reported by numerous researchers. However, many polymers are so soft that during the insertion process, they cause buckling failure. Several other materials for the fabrication of microneedles have also been reported, e.g., glass, alloy, metal, silver, copper, and gold. Glass microneedles having hollow oval tips have been fabricated for intradermal delivery utilizing the micropipette pulling method, but they can also break easily inside the skin on the application of the drug. [70, 80, 93, 114115,116,117].
Fabrication Techniques: Their Advantages and Disadvantages
Different fabrication techniques have been used to fabricate microneedles which include the following:
Photolithography [118]
This process is commonly used for the fabrication of polymer microneedles, and this process, unlike other techniques, involves simple steps and less time. The tips of microneedles fabricated by photolithography are blunt and sharper as compared to others. However, it also has some limits, i.e., expensive masks, polymers must be photosensitive, and harsh processing conditions, therefore cannot be used in biological models.
Chemical Etching [119]
This fabrication method is highly selected by researchers as they are inexpensive and damage-free due to their pure chemical nature. But, it also has some disadvantages, i.e., reduced process control, temperature sensitivity, reduced particle control, and high costs for biochemical disposal.
Deep Reactive Ion Etching [120]
This method is used for the high-volume fabrication of microneedles and is commonly known as the Bosch process, but it has the disadvantage that it takes a long time because each phase lasts for several seconds.
Hot Embossing [121]
The hot embossing technique might assist in the development of greatly accurate and extremely effective fabrication of microneedles. It has the disadvantage of difficulty in demolding, substantial enduring thermal stress owing to varying coefficients of thermal expansion among the mold and polymer materials.
Laser Ablation [122]
In this technique, light pulses are used for giving the prominence of the desired shape of microneedles on a metal plate, thus creating solid-metal microneedle arrays. However, owing to their high-intensity pulses of laser, the creation of plasma ions, as well as electrons, is not appropriate for the fabrication of required designs or structures.
Surface Micromachining [123]
The surface micromachining technique offers a benefit that can be used on different materials as compared to substrates of a single crystal. This technique is extensively used by the researcher as it provides less loss of materials, better mechanical properties, and good dimensional control. However, it is expensive because higher steps of fabrication are involved, repetitive processes, and difficulty in implementing it for larger structures.
Atomized Spraying Method [124]
This method disables the problems related to limited volume for the mass production of dissolving microneedles through the required geometry as well as physical characteristics. Moreover, the difficulties related to liquid surface tension effects, as well as viscosity once filling the microneedle molds, can be reduced. However, it has issues of higher air emissions and the use of highly compressed air.
Micro-Molding [125]
In this method, microneedles are made by using molds and filled with liquids or chemicals. It has the advantages of being comparatively simple and is a cost-effective microneedle production.
Every fabrication method has its benefits and limits as described briefly. For silicon microneedle fabrication, lithography and deep reactive ion etching methods are commonly used. The key phenomenon in the growth of microneedles is etching and deposition [126,127,128]. Laser ablation and hot embossing methods are favorable fabrication methods for polymeric microneedles. However, electrochemical etching is the most effective and is an economic way for fabricating microneedles of silver, gold, copper, other metals, etc. [129,130,131,132].
Testing and Evaluation
After the successful fabrication and designing of microneedles till 2005, the researchers switched their interests and started exploring the methods for testing fabricated microneedles.
For microneedle testing, different skins from animals and vegetables besides humans have been used until now:
Also, microneedles are coated using a solid-state influenza vaccine to increase the efficiency of the vaccine and then tested on the skin of mice [143]. A short closely pointed microinjection array has been established to understand the result of stress rate on the accuracy of permeation into animal skin [144]. Reference [145] has examined that influenza disease-like elements coated on microneedle arrays can produce stimulatory consequences on Langerhans cells in the skin of a human. The extremely short microneedle has been fabricated by Si wet-etching technology as well as tested for transdermal drug delivery into human skin [26]. The detachable-tipped microneedles have been presented and verified for simple delivery of drugs and vaccines into human remains skin [146]. A nominally intrusive structure has been established employing a microneedle conductor array to transport macromolecular medicines to the subterranean skin nerves and tested on tonsured mouse skin [138]. Solid-silicon microneedle patches have been consumed using altered lengths and geometry to pierce the human epidermis [147, 148]. Microneedle array rollers have been established and tried on human and pigskin to rise skin penetrability and for cosmetic surgery [149]. Microneedles have also been used for delivering PLGA nanoparticles in the skin of humans [38]. Solid-based polymer microneedles have been established to examine the transepidermal water loss depths of dermatome human skin [35]. The effectiveness of transdermal delivery of insulin has been examined by means of microneedle rollers on rats which are diabetic [150]. The influenza vaccine having viral particles has been studied and tested by means of microneedle patches on bone marrow cells and lungs of mice [151]. Thus, many researchers have tested their fabricated needles to make sure their quality is good and they are safe to use for biomedical applications.
Safety Concerns
Solid microneedles are being used for harmless and effective drug delivery and for vaccines through generating flexible microchannels in the skin [152].
Microneedles are insignificantly invasive drug delivery structures that have been considered or deliberated for safety. Safety concerns are primarily associated with any kind of infection risk. Other factors to study include user suitability as well as environmental issues.
-
Pain
-
Bleeding
-
Skin irritation
-
Remaining vaccine left in structure and on the surface of the skin
On experience using hypodermic needles, the contaminated needles can cause the greatest risk of infection. Therefore, single-use adequately clean microneedles that are sterilized would cause a little risk of infection. Though, the skin is continually in connection with environmental entities and becomes voluntarily colonized through definite microbial species. Any hole in the skin can give entry to microorganisms that might cause confined or total infection. Microneedle arrays comprising hundreds as well as thousands of microneedles might consequently be problematic. Though, the threat of infection is linked to a large number of factors including.
-
the number of breaches as well as their sizes,
-
the penetration of breaches,
-
the number of microbes entering the skin as well as their nature, and
-
the discrete vulnerability of the patient.
In clinical practice, it looks improbable that any trivial injuries caused by the usage of microneedles would result in substantial safety concerns. Certainly, the skin barrier is regularly breached in the course of communal experiences of slight abrasion, e.g., shaving; nonetheless, infection hardly occurs [153, 154].
Even though pain is not a safety concern per capita; it, however, has an emotional impact on patient reception and safety perception. Initial studies about sharp-tipped microneedles revealed that they are considered normal as painless in human subjects [51, 155].
Also, the epidermis is lacking vasculature, and the utmost shallow capillary bed is situated in the upper dermis near the junction of the dermal-epidermal. As a consequence, microneedles piercing the skin deeper than nearly 100 µm might rupture capillaries. Regardless of this expectation, many animal and human studies have not witnessed bleeding after treatment with microneedles [156, 157].
Also, no skin irritation occurs in any of the studies when insertion of drug or vaccination is done by using microneedles. However, some more sensitive skins can have some redness or tenderness; otherwise, there is no such proof found [24, 158].
The insinuations of having vaccine residual left on microneedles or on the skin surface rest on the particulars of the vaccines and their preparation. In any situation, minimalizing the residuals could be useful from a safety and conservational standpoint [154].
Jiang et al. [159] studied the existence of microchannels or infections after the inclusion of microneedles into the rabbit’s cornea through a standard lamp. They identified that microchannels vanished in 3 h, and there was no sign of a provocative response. Thus, it proposes that microneedles can be considered appropriate for drug delivery to the eyes for the cure of ocular diseases. Damme et al. administered the influenza injection intradermally through microneedles as well as intramuscularly via conventional hypodermic needles. They considered that pain linked with the insertion of a needle was considerably less through microneedles. After the insertion of the microneedle, ephemeral reactions happening on the skin at the site where microneedles were applied were found to be endurable. They determined that efficient vaccination could be accomplished utilizing microneedles as compared to the conservative intramuscular distribution of influenza injection. Laurent et al. [160] proved a study in which protection, as well as the effectiveness of rabies vaccination, were administered via microneedles. They also found acceptability with effective vaccination via microneedles as compared to conservative vaccination. Gill et al. [161] deliberated the consequence of microneedle length as well as their number on pain. They described that pain increases with the increase in length of microneedles, while pain increases slightly when the number of microneedles increases. Thus, it was concluded that skin permeation should have an increase in the number of microneedles as compared to microneedle length to consider less pain. Bal et al. [162] and Kaushik et al. [51] considered the protection of microneedles and stated that drugs could be transported deprived of any adversarial reactions and pain via microneedles. Generally, it is concluded that microneedles are secure; nonetheless, few trials remain to be confronted for their commercialization and improvement as an effective transdermal drug delivery technology.
Applications of Microneedles
Today there are numerous and diverse applications of microneedles. In genetic engineering, cell biology, and molecular engineering, it is preferred to change a method to host peptides, oligonucleotides, proteins, DNA, as well as other inquiries into the cells to modify their functions. Therefore, solid microneedles can be applied to the cells for the distribution of molecules over impervious membranes. It has been verified that microfabricated needle patches could be used to transport DNA into plants and mammalian cells, persuading cell alteration [12, 163, 164].
Another significant application of microneedle is in nominally invasive drug delivery. Due to the slight cross-sectional area of normally several-hundred square micrometers, any risk of damaging effects can be easily reduced. Also, the drug delivery can be restricted to a precise and confined tissue or area in the human body. Also, to study the neural events through some degree of trauma to the tissues, solid microneedles have been used. The short channel of solid microneedles offers another benefit in drug delivery. Microfabrication technology permits the needles to have channel lengths easily controllable at a microscopic scale. The needles can be premeditated to pierce just below the outer layer of skin which is called stratum corneum, and it has precise permeability. As the endings of nerves occur at the penetration of ~ 100 μm, therefore delivery at this position will ease pain, contamination, or wound. Furthermore, since there is a large number of capillaries present inside the skin layer, the drug will be freely immersed in the body, thus allowing quick treatment [165].
Microneedles can likewise be used to extract samples, hence finding substantial applications in the health monitoring field as well as performing biochemical analysis. For example, in patients having diabetes, microneedles are the finest way to check their glucose level and to manage multiple dosages of insulin. The usage of microneedles can make it practically a painless and considerably more pleasant experience for patients.
Microneedles also have applications in the electronics field as well as sensors. They have been used in scanning tunneling microscopes, Millipede data storage techniques, and atomic force microscopes, as probes for superficial modification and reporting. Microneedles have been functional in microdialysis where the microneedles are made penetrable only to trivial molecular weight combinations. This guards the sensors against advanced molecular weight composites, e.g., proteins assisting to maintain the operative viability of the medicinal monitors. Further applications comprise heads of printer and electrospray emitter control valve [66, 166,167,168,169,170,171].
Products Made with Different Types of Microneedles
Prospective of microneedles to change the worldwide transdermal market is emphasized in terms of the accomplishment rate of microneedle technologies in clinical trials getting to the worldwide market. Thus, the arrival of commercial microneedle products in the market is extremely estimated as they have the potential of representing the incredible impact on clinical medication in the nearby future. The summary of designed products using different types of microneedles is given in Table 3.
Commercialization Issues
It has been observed that there are numerous applications of solid microneedles, but only a small number of products have been marketed until now. For the delivery of drugs at both large and small scale, it is necessary to consider the efficiency and safety of microneedles while developing them. When metallic microneedles are inserted into the skin, there is a chance of some metallic traces to be retained under the skin which afterward causes irritation, swelling, erythema, discoloration, or further side effects. Repeated insertion of microneedles at the same spot may result in the above-mentioned problems. Application of microneedles at altered sites each time or deviation in skin depth in persons may result in deviation in bioavailability that needs to be measured while developing the microneedles. Nowadays, research is more fixated on the expansion of new technologies for the supervision of current molecules that are previously confirmed as safe, therefore reducing growth time and promising an increased success rate. That is why staff in the pharmaceutical industry struggle for the successful expansion of microneedles as transdermal drug delivery systems [185, 186].
Additionally, biohazardous piercing waste may be left behind after practice which needs to destruct carefully. Dissolving microneedles on the other hand are usually made of polysaccharides, which on insertion completely dissolve in the skin, leaving behind no harmful waste. Whole dissolution, correct insertion into the skin as well as filling of drugs comprehensively at the tip merely are the top challenges to be confronted during the development of dissolving microneedles. The usage of hollow microneedles is an alternative approach that gains the interest of researchers owing to its capacity to control a larger range of molecules. However, hollow microneedles do not hold enough strength, and this is their main issue that must be focused on [187, 188].
Other Challenges
There are several challenges related to microfluidic devices in the biomedical field which include fabrication issues, designing level issues, and packing level issues when considering these devices to be used in different practical applications. The more common and severe issues related to microneedles at fabrication and design levels are evading of clogging consequence, appropriate length, toughness, strength, piercing tip to evade pain, drug confrontation, less cost of fabrication, dependability, and biocompatibility. It is needed to adopt appropriate group fabrication techniques to decrease the budget for devices. The main consideration is the packaging of these microfluidic devices. Packaging should be vigorous and resilient to avoid any damage or infection caused by these microfluidic devices [10, 189]. Concurrently, the accidental drug discharge in storage from the reservoir should be stopped. A protective covering may be mandatory to protect such kinds of small-sized devices, e.g., sharp-tipped microneedles. Mostly, the microneedles stated in the literature have been suggested as stand-alone devices. The integration of microneedles is a prodigious challenge that confines the usage of these microfluidic devices for biomedical applications. The final price of these delivery devices should be reasonable for the patients [190,191,192]. Nowadays, the trend is changing toward the usage of polymer materials (e.g., PDMS, PGA, PMMA) for the fabrication of microneedles to overawe most of the above-referred issues as these stated materials are biocompatible, inexpensive, and they show exceptional mechanical as well as chemical properties [193,194,195].
Discussion
Solid microneedles are therefore considered to be crucial components for biomedical systems. Material selection is an important concern in biomedical devices. Silicon has been extensively used as a material in microfluidic devices; however, polymer materials (e.g., PGA, PMMA, PDMS, PLLA, PC, and PLA) are exchanging Si owing to biocompatibility, ease of fabrication, low cost, as well as exceptional structural properties [196,197,198].
Solid microneedles having sharp tips are considered to be more useful for drug delivery/transport. The efficiency of drug delivery in recent years has also been explained through various researchers by microneedle testing on mice, pigs, chickens, and humans. Many researchers have described the structure, as well as breakage studies of microneedles, through applying force and stress to foresee the failure and twisting of microneedles [199]. The graphic illustration of force and stress comparison for microneedles is given below in Fig. 4.
The material selection is very important for the fabrication of microneedles, and how much strength they endure when applied to human skin or any animal body is also important. The strength of materials concerning their ultimate tensile strength and Young’s modulus is given in the graph below:
The length and diameter of microneedles are also important parameters to be studied while developing microneedles and how they are useful in the insertion or application of microneedles. The graph for length and diameter comparison is given in Fig. 5 below.
Conclusion
Microneedles give many different benefits compared to the conventional needle/syringe as well as other delivery techniques that might also be harmless and effective. For instance, the patch-based setup of numerous microneedle designs would assist simple vaccine supervision and probably self-administration by patients. The minor size of microneedle structures should also ease storing and prompt distribution to dominant locations. Solid microneedles are considered to be a striking platform for drug delivery as they have a low cost of manufacturing, and they can play a significant role in the medicinal response to a virus pandemic. Solid microneedles can be easily fabricated and are stronger as compared to hollow and dissolving microneedles. Solid microneedles using diverse materials, such as glass, metals, silicon, and polymers, have been stated for biomedical applications; however, silicon has been typically used as a substrate among other materials in the fabrication of microfluidic devices. Silicon is brittle and each time involves some risks for health care. Biocompatibility is very essential for well-being, and the reason trend is shifting toward polymer materials. Most polymers, such as PDMS, PGA, PLA, and PMMA, are precisely appropriate for biomedical devices owing to their upright biocompatibility, low budget, easiness of fabrication, and exceptional chemical as well as mechanical properties. According to the given literature review, the authors determine that microneedles, their commercialization, and biomedical applications continue to be growing day by day and still need improvements and advancements.
References
Nandagopal MG, Antony R, Rangabhashiyam S, Sreekumar N, Selvaraju N. Overview of microneedle system: a third generation transdermal drug delivery approach. Microsyst Technol. 2014;20(7):1249–72.
Park J-H, Allen MG, Prausnitz MR. Biodegradable polymer microneedles: fabrication, mechanics and transdermal drug delivery. J Control Release. 2005;104(1):51–66.
Bariya SH, Gohel MC, Mehta TA, Sharma OP. Microneedles: an emerging transdermal drug delivery system. J Pharm Pharmacol. 2012;64(1):11–29.
van der Maaden K, Jiskoot W, Bouwstra J. Microneedle technologies for (trans) dermal drug and vaccine delivery. J Control Release. 2012;161(2):645–55.
Henry S, McAllister DV, Allen MG, Prausnitz MR. Microfabricated microneedles: a novel approach to transdermal drug delivery. J Pharm Sci. 1998;87(8):922–5.
Kim Y-C, Park J-H, Prausnitz MR. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev. 2012;64(14):1547–68.
Indermun S, Luttge R, Choonara YE, Kumar P, Du Toit LC, Modi G, et al. Current advances in the fabrication of microneedles for transdermal delivery. J Control Release. 2014;185:130–8.
Moon SJ, Lee SS, Lee H, Kwon T. Fabrication of microneedle array using LIGA and hot embossing process. Microsyst Technol. 2005;11(4–5):311–8.
Cheung K, Das DB. Microneedles for drug delivery: trends and progress. Drug Delivery. 2016;23(7):2338–54.
Ita K. Transdermal delivery of drugs with microneedles—potential and challenges. Pharmaceutics. 2015;7(3):90–105.
Escobar-Chávez JJ, Bonilla-Martínez D, Angélica M, Molina-Trinidad E, Casas-Alancaster N, Revilla-Vázquez AL. Microneedles: a valuable physical enhancer to increase transdermal drug delivery. J Clin Pharmacol. 2011;51(7):964–77.
Sivamani RK, Liepmann D, Maibach HI. Microneedles and transdermal applications. Expert Opin Drug Deliv. 2007;4(1):19–25.
Donnelly RF, Singh TRR, Woolfson AD. Microneedle-based drug delivery systems: microfabrication, drug delivery, and safety. Drug Delivery. 2010;17(4):187–207.
Tuan-Mahmood T-M, McCrudden MT, Torrisi BM, McAlister E, Garland MJ, Singh TRR, et al. Microneedles for intradermal and transdermal drug delivery. Eur J Pharm Sci. 2013;50(5):623–37.
El-Laboudi A, Oliver NS, Cass A, Johnston D. Use of microneedle array devices for continuous glucose monitoring: a review. Diabetes Technol Ther. 2013;15(1):101–15.
Iliescu F, Dumitrescu-Ionescu D, Petrescu M, Iliescu C. A review on transdermal drug delivery using microneedles: current research and perspective. Ann Acad Rom Sci Series Sci Technol Inf. 2014;7(1):734.
Quinn HL, Kearney M-C, Courtenay AJ, McCrudden MT, Donnelly RF. The role of microneedles for drug and vaccine delivery. Expert Opin Drug Deliv. 2014;11(11):1769–80.
Chen W, Li H, Shi D, Liu Z, Yuan W. Microneedles as a delivery system for gene therapy. Front Pharmacol. 2016;7:137.
Marwah H, Garg T, Goyal AK, Rath G. Permeation enhancer strategies in transdermal drug delivery. Drug Delivery. 2016;23(2):564–78.
Thakur Singh RR, Tekko I, McAvoy K, McMillan H, Jones D, Donnelly RF. Minimally invasive microneedles for ocular drug delivery. Expert Opin Drug Deliv. 2017;14(4):525–37.
Ma G, Wu C. Microneedle, bio-microneedle, and bio-inspired microneedle: a review. J Control Release. 2017;251:11–23.
Kolli CS, Banga AK. Characterization of solid maltose microneedles and their use for transdermal delivery. Pharm Res. 2008;25(1):104–13.
McAllister DV, Wang PM, Davis SP, Park J-H, Canatella PJ, Allen MG, et al. Microfabricated needles for transdermal delivery of macromolecules and nanoparticles: fabrication methods and transport studies. Proc Natl Acad Sci. 2003;100(24):13755–60.
Lin W, Cormier M, Samiee A, Griffin A, Johnson B, Teng C, et al. Transdermal delivery of antisense oligonucleotides with microprojection patch (Macroflux®) technology. Pharm Res. 2001;18(12):1789–93.
Wang H, et al. "Microneedle array integrated with CNT nanofilters for controlled and selective drug delivery." Journal of Microelectromechanical Systems 23.5 (2014): 1036-1044.
Wei-Ze L, Mei-Rong H, Jian-Ping Z, Yong-Qiang Z, Bao-Hua H, Ting L, et al. Super-short solid silicon microneedles for transdermal drug delivery applications. Int J Pharm. 2010;389(1–2):122–9.
Aoyagi S, Izumi H, and Fukuda M. "Biodegradable polymer needle with various tip angles and consideration on insertion mechanism of mosquito's proboscis." Sensors and Actuators A: Physical 143.1 (2008): 20-28.
Coulman SA, Anstey A, Gateley C, Morrissey A, McLoughlin P, Allender C, et al. Microneedle mediated delivery of nanoparticles into human skin. Int J Pharm. 2009;366(1–2):190–200.
Oh J-H, Park H-H, Do K-Y, Han M, Hyun D-H, Kim C-G, et al. Influence of the delivery systems using a microneedle array on the permeation of a hydrophilic molecule, calcein. Eur J Pharm Biopharm. 2008;69(3):1040–5.
Ding Z, Verbaan FJ, Bivas-Benita M, Bungener L, Huckriede A, van den Berg DJ, et al. Microneedle arrays for the transcutaneous immunization of diphtheria and influenza in BALB/c mice. J Control Release. 2009;136(1):71–8.
Han M, Kim DK, Kang SH, Yoon H-R, Kim B-Y, Lee SS, et al. Improvement in antigen-delivery using fabrication of a grooves-embedded microneedle array. Sens Actuators, B Chem. 2009;137(1):274–80.
Jin CY, Han MH, Lee SS, Choi YH. Mass producible and biocompatible microneedle patch and functional verification of its usefulness for transdermal drug delivery. Biomed Microdevice. 2009;11(6):1195–203.
Kim Y-C, Quan F-S, Compans RW, Kang S-M, Prausnitz MR. Formulation and coating of microneedles with inactivated influenza virus to improve vaccine stability and immunogenicity. J Control Release. 2010;142(2):187–95.
Park J-H, Choi S-O, Seo S, Choy YB, Prausnitz MR. A microneedle roller for transdermal drug delivery. Eur J Pharm Biopharm. 2010;76(2):282–9.
Gomaa YA, Morrow DI, Garland MJ, Donnelly RF, El-Khordagui LK, Meidan VM. Effects of microneedle length, density, insertion time and multiple applications on human skin barrier function: assessments by transepidermal water loss. Toxicol In Vitro. 2010;24(7):1971–8.
Donnelly RF, Morrow DI, Fay F, Scott CJ, Abdelghany S, Singh RRT, et al. Microneedle-mediated intradermal nanoparticle delivery: potential for enhanced local administration of hydrophobic pre-formed photosensitisers. Photodiagn Photodyn Ther. 2010;7(4):222–31.
Römgens A, Bader D, Bouwstra J, Baaijens F, Oomens C. Monitoring the penetration process of single microneedles with varying tip diameters. J Mech Behav Biomed Mater. 2014;40:397–405.
Zhang W, Gao J, Zhu Q, Zhang M, Ding X, Wang X, et al. Penetration and distribution of PLGA nanoparticles in the human skin treated with microneedles. Int J Pharm. 2010;402(1–2):205–12.
Nguyen J, Ita KB, Morra MJ, Popova IE. The influence of solid microneedles on the transdermal delivery of selected antiepileptic drugs. Pharmaceutics. 2016;8(4):33.
Hoang MT, Ita KB, Bair DA. Solid microneedles for transdermal delivery of amantadine hydrochloride and pramipexole dihydrochloride. Pharmaceutics. 2015;7(4):379–96.
Uddin MJ, Scoutaris N, Klepetsanis P, Chowdhry B, Prausnitz MR, Douroumis D. Inkjet printing of transdermal microneedles for the delivery of anticancer agents. Int J Pharm. 2015;494(2):593–602.
Witting M, Obst K, Pietzsch M, Friess W, Hedtrich S. Feasibility study for intraepidermal delivery of proteins using a solid microneedle array. Int J Pharm. 2015;486(1–2):52–8.
Dang N, Liu TY, Prow TW. "Nano-and microtechnology in skin delivery of vaccines." Micro and Nanotechnology in Vaccine Development. William Andrew Publishing, 2017. p. 327-341.
Hu Z, Meduri CS, Ingrole RS, Gill HS, Kumar G. Solid and hollow metallic glass microneedles for transdermal drug-delivery. Appl Phys Lett. 2020;116(20):203703.
Narayanan SP, Raghavan S. Solid silicon microneedles for drug delivery applications. Int J Adv Manuf. 2017;93(1–4):407–22.
Chen BZ, Liu JL, Li QY, Wang ZN, Zhang XP, Shen CB, et al. Safety evaluation of solid polymer microneedles in human volunteers at different application sites. ACS Appl Bio Mater. 2019;2(12):5616–25.
Lee JW, Han M-R, Park J-H. Polymer microneedles for transdermal drug delivery. J Drug Target. 2013;21(3):211–23.
Li QY, Zhang JN, Chen BZ, Wang QL, Guo XD. A solid polymer microneedle patch pretreatment enhances the permeation of drug molecules into the skin. RSC Adv. 2017;7(25):15408–15.
Evens T, Malek O, Castagne S, Seveno D, Van Bael A. A novel method for producing solid polymer microneedles using laser ablated moulds in an injection moulding process. Manuf Lett. 2020;24:29–32.
Verbaan F, Bal S, Van den Berg D, Dijksman J, Van Hecke M, Verpoorten H, et al. Improved piercing of microneedle arrays in dermatomed human skin by an impact insertion method. J Control Release. 2008;128(1):80–8.
Kaushik S, Hord AH, Denson DD, McAllister DV, Smitra S, Allen MG, et al. Lack of pain associated with microfabricated microneedles. Anesth Analg. 2001;92(2):502–4.
Shin JH, et al. "C-di-GMP with influenza vaccine showed enhanced and shifted immune responses in microneedle vaccination in the skin." Drug Deliv Transl Res. 2020;10(3):815–25.
Gill HS, Prausnitz MR. Coating formulations for microneedles. Pharm Res. 2007;24(7):1369–80.
Bilal M, Mehmood S, Raza A, Hayat U, Rasheed T, Iqbal HM. Microneedles in smart drug delivery. Adv Wound Care. 2021;10(4):204–19.
Ma X, Peng W, Su W, Yi Z, Chen G, Chen X, et al. Delicate assembly of ultrathin hydroxyapatite nanobelts with nanoneedles directed by dissolved cellulose. Inorg Chem. 2018;57(8):4516–23.
Li J, Liu B, Zhou Y, Chen Z, Jiang L, Yuan W, et al. Fabrication of a Ti porous microneedle array by metal injection molding for transdermal drug delivery. PLoS One. 2017;12(2):e0172043.
Ullah A, Kim CM, Kim GM. Porous polymer coatings on metal microneedles for enhanced drug delivery. R Soc Open Sci. 2018;5(4):171609.
Li Y, Zhang H, Yang R, Laffitte Y, Schmill U, Hu W, et al. Fabrication of sharp silicon hollow microneedles by deep-reactive ion etching towards minimally invasive diagnostics. Microsyst Nanoeng. 2019;5(1):1–11.
He X, Sun J, Zhuang J, Xu H, Liu Y, Wu D. Microneedle system for transdermal drug and vaccine delivery: devices, safety, and prospects. Dose-Response. 2019;17(4):1559325819878585.
Lee JW, Park J-H, Prausnitz MR. Dissolving microneedles for transdermal drug delivery. Biomaterials. 2008;29(13):2113–24.
Chen X, Prow TW, Crichton ML, Jenkins DW, Roberts MS, Frazer IH, et al. Dry-coated microprojection array patches for targeted delivery of immunotherapeutics to the skin. J Control Release. 2009;139(3):212–20.
Chen J, Qiu Y, Zhang S, Yang G, Gao Y. Controllable coating of microneedles for transdermal drug delivery. Drug Dev Ind Pharm. 2015;41(3):415–22.
Wang PM, Cornwell M, Hill J, Prausnitz MR. Precise microinjection into skin using hollow microneedles. J Investig Dermatol. 2006;126(5):1080–7.
Chen M-C, Ling M-H, Lai K-Y, Pramudityo E. Chitosan microneedle patches for sustained transdermal delivery of macromolecules. Biomacromol. 2012;13(12):4022–31.
Bodhale DW, Nisar A, Afzulpurkar N. Structural and microfluidic analysis of hollow side-open polymeric microneedles for transdermal drug delivery applications. Microfluid Nanofluid. 2010;8(3):373–92.
Teo AL, Shearwood C, Ng KC, Lu J, Moochhala S. Transdermal microneedles for drug delivery applications. Mater Sci Eng, B. 2006;132(1–2):151–4.
Park J-H, Prausnitz MR. Analysis of mechanical failure of polymer microneedles by axial force. J Korean Phys Soc. 2010;56(4):1223.
Raja Rajeswari, N., P. Malliga, and B. K. Gnanavel. "Buckling analysis of hollow microneedle in transdermal drug delivery." ASME International Mechanical Engineering Congress and Exposition. Vol. 50534. American Society of Mechanical Engineers, 2016.
O’Mahony C. Structural characterization and in-vivo reliability evaluation of silicon microneedles. Biomed Microdevice. 2014;16(3):333–43.
Ashraf MW, Tayyaba S, Afzulpurkar N. Micro electromechanical systems (MEMS) based microfluidic devices for biomedical applications. Int J Mol Sci. 2011;12(6):3648–704.
Davis SP, Landis BJ, Adams ZH, Allen MG, Prausnitz MR. Insertion of microneedles into skin: measurement and prediction of insertion force and needle fracture force. J Biomech. 2004;37(8):1155–63.
Kong X, Zhou P, Wu C. Numerical simulation of microneedles’ insertion into skin. Comput Methods Biomech Biomed Engin. 2011;14(9):827–35.
Gupta J, Gill HS, Andrews SN, Prausnitz MR. Kinetics of skin resealing after insertion of microneedles in human subjects. J Control Release. 2011;154(2):148–55.
Leone M, Van Oorschot BH, Nejadnik MR, Bocchino A, Rosato M, Kersten G, et al. Universal applicator for digitally-controlled pressing force and impact velocity insertion of microneedles into skin. Pharmaceutics. 2018;10(4):211.
Olatunji O, Das DB, Garland MJ, Belaid L, Donnelly RF. Influence of array interspacing on the force required for successful microneedle skin penetration: theoretical and practical approaches. J Pharm Sci. 2013;102(4):1209–21.
Gittard SD, Chen B, Xu H, Ovsianikov A, Chichkov BN, Monteiro-Riviere NA, et al. The effects of geometry on skin penetration and failure of polymer microneedles. J Adhes Sci Technol. 2013;27(3):227–43.
Kochhar JS, Soon WJ, Choi J, Zou S, Kang L. Effect of microneedle geometry and supporting substrate on microneedle array penetration into skin. J Pharm Sci. 2013;102(11):4100–8.
Cha KJ, Kim T, Park SJ, Kim DS. Simple and cost-effective fabrication of solid biodegradable polymer microneedle arrays with adjustable aspect ratio for transdermal drug delivery using acupuncture microneedles. J Micromech Microeng. 2014;24(11):115015.
Lin L, Pisano AP. Silicon-processed microneedles. J Microelectromech Syst. 1999;8(1):78–84.
Griss P, Stemme G. Side-opened out-of-plane microneedles for microfluidic transdermal liquid transfer. J Microelectromech Syst. 2003;12(3):296–301.
Wang J, Lu J, Ly SY, Vuki M, Tian B, Adeniyi WK, et al. Lab-on-a-cable for electrochemical monitoring of phenolic contaminants. Anal Chem. 2000;72(11):2659–63.
Martanto W, Moore JS, Couse T, Prausnitz MR. Mechanism of fluid infusion during microneedle insertion and retraction. J Control Release. 2006;112(3):357–61.
Martanto W, Moore JS, Kashlan O, Kamath R, Wang PM, O’Neal JM, et al. Microinfusion using hollow microneedles. Pharm Res. 2006;23(1):104–13.
Ovsianikov A, Chichkov B, Mente P, Monteiro-Riviere N, Doraiswamy A, Narayan R. Two photon polymerization of polymer–ceramic hybrid materials for transdermal drug delivery. Int J Appl Ceram Technol. 2007;4(1):22–9.
Cai B, Xia W, Bredenberg S, Li H, Engqvist H. Bioceramic microneedles with flexible and self-swelling substrate. Eur J Pharm Biopharm. 2015;94:404–10.
Martanto W, Davis SP, Holiday NR, Wang J, Gill HS, Prausnitz MR. Transdermal delivery of insulin using microneedles in vivo. Pharm Res. 2004;21(6):947–52.
Kim K, Lee J-B. High aspect ratio tapered hollow metallic microneedle arrays with microfluidic interconnector. Microsyst Technol. 2007;13(3):231–5.
Verbaan F, Bal S, Van den Berg D, Groenink W, Verpoorten H, Lüttge R, et al. Assembled microneedle arrays enhance the transport of compounds varying over a large range of molecular weight across human dermatomed skin. J Control Release. 2007;117(2):238–45.
Badran M, Kuntsche J, Fahr A. Skin penetration enhancement by a microneedle device (Dermaroller®) in vitro: dependency on needle size and applied formulation. Eur J Pharm Sci. 2009;36(4–5):511–23.
Donnelly RF, Singh TRR, Garland MJ, Migalska K, Majithiya R, McCrudden CM, et al. Hydrogel-forming microneedle arrays for enhanced transdermal drug delivery. Adv Func Mater. 2012;22(23):4879–90.
Donnelly RF, Moffatt K, Alkilani AZ, Vicente-Pérez EM, Barry J, McCrudden MT, et al. Hydrogel-forming microneedle arrays can be effectively inserted in skin by self-application: a pilot study centred on pharmacist intervention and a patient information leaflet. Pharm Res. 2014;31(8):1989–99.
Sammoura F, Kang J, Heo Y-M, Jung T, Lin L. Polymeric microneedle fabrication using a microinjection molding technique. Microsyst Technol. 2007;13(5–6):517–22.
Aoyagi S, Izumi H, Fukuda M. Biodegradable polymer needle with various tip angles and consideration on insertion mechanism of mosquito’s proboscis. Sens Actuators, A. 2008;143(1):20–8.
Yi-Gui L, Chun-Sheng Y, Jing-Quan L, Susumu S. Fabrication of a polymer micro needle array by mask-dragging x-ray lithography and alignment x-ray lithography. Chin Phys Lett. 2011;28(3):038101.
Bediz B, Korkmaz E, Khilwani R, Donahue C, Erdos G, Falo LD, et al. Dissolvable microneedle arrays for intradermal delivery of biologics: fabrication and application. Pharm Res. 2014;31(1):117–35.
Sullivan SP, Koutsonanos DG, del Pilar MM, Lee JW, Zarnitsyn V, Choi S-O, et al. Dissolving polymer microneedle patches for influenza vaccination. Nat Med. 2010;16(8):915.
Allen EA, O’Mahony C, Cronin M, O’Mahony T, Moore AC, Crean AM. Dissolvable microneedle fabrication using piezoelectric dispensing technology. Int J Pharm. 2016;500(1–2):1–10.
Miyano T, Tobinaga Y, Kanno T, Matsuzaki Y, Takeda H, Wakui M, et al. Sugar micro needles as transdermic drug delivery system. Biomed Microdevice. 2005;7(3):185–8.
Donnelly RF, Morrissey A, McCarron PA, Woolfson DA. Microstructured devices for transdermal drug delivery and minimally-invasive patient monitoring. Recent Pat Drug Delivery Formulation. 2007;1(3):195–200.
Lee K, Lee CY, Jung H. Dissolving microneedles for transdermal drug administration prepared by stepwise controlled drawing of maltose. Biomaterials. 2011;32(11):3134–40.
Brazzle JD, Papautsky I, Frazier AB. Hollow metallic micromachined needle arrays. Biomed Microdevice. 2000;2(3):197–205.
Chandrasekaran S, Brazzle JD, Frazier AB. Surface micromachined metallic microneedles. J Microelectromech Syst. 2003;12(3):281–8.
Chandrasekaran S, Frazier AB. Characterization of surface micromachined metallic microneedles. J Microelectromech Syst. 2003;12(3):289–95.
Stoeber B, Liepmann D. "Design, fabrication and testing of a MEMS syringe." Proceedings of Solid-State Sensor and Actuator Workshop. 2002.
Narayan RJ, Doraiswamy A, Chrisey DB, Chichkov BN. Medical prototyping using two photon polymerization. Mater Today. 2010;13(12):42–8.
Wu Y, Qiu Y, Zhang S, Qin G, Gao Y. Microneedle-based drug delivery: studies on delivery parameters and biocompatibility. Biomed Microdevice. 2008;10(5):601–10.
Haq M, Smith E, John DN, Kalavala M, Edwards C, Anstey A, et al. Clinical administration of microneedles: skin puncture, pain and sensation. Biomed Microdevice. 2009;11(1):35–47.
Stoeber B, Liepmann D. "Fluid injection through out-of-plane microneedles." 1st Annual International IEEE-EMBS Special Topic Conference on Microtechnologies in Medicine and Biology. Proceedings (Cat. No. 00EX451). IEEE, 2000.
Paik S-J, Byun S, Lim J-M, Park Y, Lee A, Chung S, et al. In-plane single-crystal-silicon microneedles for minimally invasive microfluid systems. Sens Actuators, A. 2004;114(2–3):276–84.
Wilke N, Hibert C, O’Brien J, Morrissey A. Silicon microneedle electrode array with temperature monitoring for electroporation. Sens Actuators, A. 2005;123:319–25.
Choi JW, et al. "Insertion force estimation of various microneedle array-type structures fabricated by a microstereolithography apparatus." 2006 SICE-ICASE International Joint Conference. IEEE, 2006.
Shibata T, et al. "Fabrication and mechanical characterization of microneedle array for cell surgery." TRANSDUCERS 2007-2007 International Solid-State Sensors, Actuators and Microsystems Conference. IEEE, 2007.
Tu J, Reeves N. Feasibility study of microneedle fabrication from a thin nitinol wire using a CW single-mode fiber laser. Open Engineering. 2019;9(1):167–77.
Ambrose CG, Clanton TO. Bioabsorbable implants: review of clinical experience in orthopedic surgery. Ann Biomed Eng. 2004;32(1):171–7.
Jiang J, Moore JS, Edelhauser HF, Prausnitz MR. Intrascleral drug delivery to the eye using hollow microneedles. Pharm Res. 2009;26(2):395–403.
Parker E, Rao M, Turner K, Meinhart C, MacDonald N. Bulk micromachined titanium microneedles. J Microelectromech Syst. 2007;16(2):289–95.
Yoshida K, Lewinsky I, Nielsen M, Hylleberg M. Implantation mechanics of tungsten microneedles into peripheral nerve trunks. Med Biol Eng Compu. 2007;45(4):413–20.
Chiang K, Amal R, Tran T. Photocatalytic degradation of cyanide using titanium dioxide modified with copper oxide. Adv Environ Res. 2002;6(4):471–85.
Katwal R, Kaur H, Sharma G, Naushad M, Pathania D. Electrochemical synthesized copper oxide nanoparticles for enhanced photocatalytic and antimicrobial activity. J Ind Eng Chem. 2015;31:173–84.
Azimi S, Sandoughsaz A, Amirsolaimani B, Naghsh-Nilchi J, Mohajerzadeh S. Three-dimensional etching of silicon substrates using a modified deep reactive ion etching technique. J Micromech Microeng. 2011;21(7):074005.
Datta P, Goettert J. Method for polymer hot embossing process development. Microsyst Technol. 2007;13(3–4):265–70.
Arya J, et al. "Tolerability, usability and acceptability of dissolving microneedle patch administration in human subjects." Biomaterials 2017;128:1–7.
Linder V, Gates BD, Ryan D, Parviz BA, Whitesides GM. Water‐soluble sacrificial layers for surface micromachining. Small. 2005;1(7):730–6.
Zhang P, Dalton C, Jullien GA. Design and fabrication of MEMS-based microneedle arrays for medical applications. Microsyst Technol. 2009;15(7):1073–82.
Wang MW, Arifin F, Huang JY. "Optimization of the micro molding of a biconcave structure." Int J Technol. 2019;269–79.
Ray P, Mac DB. Determination of the optimal load path for tube hydroforming processes using a fuzzy load control algorithm and finite element analysis. Finite Elem Anal Des. 2004;41(2):173–92.
Ali B, Ashraf MW, Tayyaba S. Simulation, fuzzy analysis and development of ZnO nanostructure-based piezoelectric MEMS energy harvester. Energies. 2019;12(5):807.
Afzal MJ, Ashraf MW, Tayyaba S, Hossain MK, Afzulpurkar N. Sinusoidal microchannel with descending curves for varicose veins implantation. Micromachines. 2018;9(2):59.
Zhang Z, Feng Q, Cai M, Huang L, Jiang Y. Research on stress-etching complex microstructure of aluminum alloy in laser electrochemical machining. Int J Adv Manuf Technol. 2015;81(9):2157–65.
Bassu M, Surdo S, Strambini LM, Barillaro G. Electrochemical micromachining as an enabling technology for advanced silicon microstructuring. Adv Func Mater. 2012;22(6):1222–8.
Hurtony T, Bonyár A, Gordon P. Microstructure comparison of soldered joints using electrochemical selective etching. Materials Science Forum: Trans Tech Publ; 2013. p. 367–72.
Kim K-W, Jeong J-S, An K-H, Kim B-J. A study on the microstructural changes and mechanical behaviors of carbon fibers induced by optimized electrochemical etching. Compos B Eng. 2019;165:764–71.
Zhang P, Jullien GA. "Microneedle arrays for drug delivery and fluid extraction." 2005 International Conference on MEMS, NANO and Smart Systems. IEEE, 2005.
Martanto, W, et al. "Transdermal delivery of insulin using microneedles in vivo." Pharm Res. 2004;21(6):947–52.
Xue P, Zhang L, Xu Z, Yan J, Gu Z, Kang Y. Blood sampling using microneedles as a minimally invasive platform for biomedical diagnostics. Appl Mater Today. 2018;13:144–57.
Chen B, Wei J, Iliescu C. Sonophoretic enhanced microneedles array (SEMA)—improving the efficiency of transdermal drug delivery. Sens Actuators, B Chem. 2010;145(1):54–60.
Chen B, Wei J, Tay FE, Wong YT, Iliescu C. Silicon microneedle array with biodegradable tips for transdermal drug delivery. Microsyst Technol. 2008;14(7):1015–9.
Yan K, Todo H, Sugibayashi K. Transdermal drug delivery by in-skin electroporation using a microneedle array. Int J Pharm. 2010;397(1–2):77–83.
Roxhed N, Griss P, Stemme G. Membrane-sealed hollow microneedles and related administration schemes for transdermal drug delivery. Biomed Microdevice. 2008;10(2):271–9.
Matteucci M, Fanetti M, Casella M, Gramatica F, Gavioli L, Tormen M, et al. Poly vinyl alcohol re-usable masters for microneedle replication. Microelectron Eng. 2009;86(4–6):752–6.
Emam M, Abashiya Y, Chareunsack B, Skordos J, Oh J, Choi Y, et al. A novel microdevice for the treatment of hydrocephalus: design and fabrication of an array of microvalves and microneedles. Microsyst Technol. 2008;14(3):371–8.
Hsu CC, et al. "Fabrication of microneedles." 2007 2nd IEEE International Conference on Nano/Micro Engineered and Molecular Systems. IEEE, 2007.
Pearton M, Kang S-M, Song J-M, Kim Y-C, Quan F-S, Anstey A, et al. Influenza virus-like particles coated onto microneedles can elicit stimulatory effects on Langerhans cells in human skin. Vaccine. 2010;28(37):6104–13.
Crichton ML, Ansaldo A, Chen X, Prow TW, Fernando GJ, Kendall MA. The effect of strain rate on the precision of penetration of short densely-packed microprojection array patches coated with vaccine. Biomaterials. 2010;31(16):4562–72.
Kim Y-C, Quan F-S, Compans RW, Kang S-M, Prausnitz MR. Formulation of microneedles coated with influenza virus-like particle vaccine. AAPS PharmSciTech. 2010;11(3):1193–201.
Chu LY, Prausnitz MR. Separable arrowhead microneedles. J Control Release. 2011;149(3):242–9.
Yan G, Warner KS, Zhang J, Sharma S, Gale BK. Evaluation needle length and density of microneedle arrays in the pretreatment of skin for transdermal drug delivery. Int J Pharm. 2010;391(1–2):7–12.
Song J-M, Kim Y-C, Barlow PG, Hossain MJ, Park K-M, Donis RO, et al. Improved protection against avian influenza H5N1 virus by a single vaccination with virus-like particles in skin using microneedles. Antiviral Res. 2010;88(2):244–7.
Zhou C-P, Liu Y-L, Wang H-L, Zhang P-X, Zhang J-L. Transdermal delivery of insulin using microneedle rollers in vivo. Int J Pharm. 2010;392(1–2):127–33.
Ita K. Transdermal delivery of drugs with microneedles: strategies and outcomes. Journal of Drug Delivery Science and Technology. 2015;29:16–23.
Quan F-S, Kim Y-C, Compans RW, Prausnitz MR, Kang S-M. Dose sparing enabled by skin immunization with influenza virus-like particle vaccine using microneedles. J Control Release. 2010;147(3):326–32.
Al-Japairai KAS, Mahmood S, Almurisi SH, Venugopal JR, Hilles AR, Azmana M, et al. Current trends in polymer microneedle for transdermal drug delivery. Int J Pharm. 2020.
Roth RR, James WD. Microbiology of the skin: resident flora, ecology, infection. J Am Acad Dermatol. 1989;20(3):367–90.
Widera G, Johnson J, Kim L, Libiran L, Nyam K, Daddona PE, et al. Effect of delivery parameters on immunization to ovalbumin following intracutaneous administration by a coated microneedle array patch system. Vaccine. 2006;24(10):1653–64.
Mikszta JA, Alarcon JB, Brittingham JM, Sutter DE, Pettis RJ, Harvey NG. Improved genetic immunization via micromechanical disruption of skin-barrier function and targeted epidermal delivery. Nat Med. 2002;8(4):415–9.
Wang PM, Cornwell M, Prausnitz MR. Minimally invasive extraction of dermal interstitial fluid for glucose monitoring using microneedles. Diabetes Technol Ther. 2005;7(1):131–41.
Burris GHDD, Prausnitz M. Effect of microneedle design on pain in human volunteers. Clin J Pain. 2008;24:585–94.
Matriano JA, Cormier M, Johnson J, Young WA, Buttery M, Nyam K, et al. Macroflux® microprojection array patch technology: a new and efficient approach for intracutaneous immunization. Pharm Res. 2002;19(1):63–70.
Jiang J, Gill HS, Ghate D, McCarey BE, Patel SR, Edelhauser HF, et al. Coated microneedles for drug delivery to the eye. Invest Ophthalmol Vis Sci. 2007;48(9):4038–43.
Laurent PE, Bourhy H, Fantino M, Alchas P, Mikszta JA. Safety and efficacy of novel dermal and epidermal microneedle delivery systems for rabies vaccination in healthy adults. Vaccine. 2010;28(36):5850–6.
Gill HS, Denson DD, Burris BA, Prausnitz MR. Effect of microneedle design on pain in human subjects. Clin J Pain. 2008;24(7):585.
Bal SM, Caussin J, Pavel S, Bouwstra JA. In vivo assessment of safety of microneedle arrays in human skin. Eur J Pharm Sci. 2008;35(3):193–202.
van der Maaden K, Yu H, Sliedregt K, Zwier R, Leboux R, Oguri M, et al. Nanolayered chemical modification of silicon surfaces with ionizable surface groups for pH-triggered protein adsorption and release: application to microneedles. J Mater Chem B. 2013;1(35):4466–77.
Vicente-Perez EM, Larrañeta E, McCrudden MT, Kissenpfennig A, Hegarty S, McCarthy HO, et al. Repeat application of microneedles does not alter skin appearance or barrier function and causes no measurable disturbance of serum biomarkers of infection, inflammation or immunity in mice in vivo. Eur J Pharm Biopharm. 2017;117:400–7.
Xie L, Zeng H, Sun J, Qian W. Engineering microneedles for therapy and diagnosis: a survey. Micromachines. 2020;11(3):271.
Sabri AH, Ogilvie J, Abdulhamid K, Shpadaruk V, McKenna J, Segal J, et al. Expanding the applications of microneedles in dermatology. Eur J Pharm Biopharm. 2019;140:121–40.
Hao Y, Li W, Zhou X, Yang F, Qian Z. Microneedles-based transdermal drug delivery systems: a review. J Biomed Nanotechnol. 2017;13(12):1581–97.
Bhatnagar S, Dave K, Venuganti VVK. Microneedles in the clinic. J Control Release. 2017;260:164–82.
Sachdeva V, K Banga A. Microneedles and their applications. Recent Pat Drug Deliv Formul. 2011;5(2):95–132.
Zhao Z, Chen Y, Shi Y. Microneedles: a potential strategy in transdermal delivery and application in the management of psoriasis. RSC Adv. 2020;10(24):14040–9.
Serrano-Castañeda P, Escobar-Chavez JJ, Rodríguez-Cruz IM, Melgoza LM, Martinez-Hernandez J. Microneedles as enhancer of drug absorption through the skin and applications in medicine and cosmetology. J Pharm Pharm Sci. 2018;21:73–93.
Hirobe S, Azukizawa H, Matsuo K, Zhai Y, Quan Y-S, Kamiyama F, et al. Development and clinical study of a self-dissolving microneedle patch for transcutaneous immunization device. Pharm Res. 2013;30(10):2664–74.
Lhernould MS, Tailler S, Deleers M, Delchambre A. Review of patents for microneedle application devices allowing fluid injections through the skin. Recent Pat Drug Deliv Formul. 2015;9(2):146–57.
Bora P, Kumar L, Bansal AK. Microneedle technology for advanced drug delivery: evolving vistas. Review Article, Department of Pharmaceutical Technology, NIPER, CRIPS. 2008;9(1).
Caffarel-Salvador E, Donnelly RF. Transdermal drug delivery mediated by microneedle arrays: innovations and barriers to success. Curr Pharm Des. 2016;22(9):1105–17.
Halder J, Gupta S, Kumari R, Gupta GD, Rai VK. Microneedle array: applications, recent advances, and clinical pertinence in transdermal drug delivery. J Pharm Innov. 2020:1–8.
Donnelly RF, Singh TRR, Alkilani AZ, McCrudden MT, O’Neill S, O’Mahony C, et al. Hydrogel-forming microneedle arrays exhibit antimicrobial properties: potential for enhanced patient safety. Int J Pharm. 2013;451(1–2):76–91.
Bragazzi NL, Orsi A, Ansaldi F, Gasparini R, Icardi G. Fluzone® intra-dermal (Intanza®/Istivac® Intra-dermal): an updated overview. Hum Vaccin Immunother. 2016;12(10):2616–27.
Levin Y, Kochba E, Kenney R. Clinical evaluation of a novel microneedle device for intradermal delivery of an influenza vaccine: are all delivery methods the same? Vaccine. 2014;32(34):4249–52.
Garland MJ, Migalska K, Mahmood TMT, Singh TRR, Woolfson AD, Donnelly RF. Microneedle arrays as medical devices for enhanced transdermal drug delivery. Expert Rev Med Devices. 2011;8(4):459–82.
Jana BA, Wadhwani AD. Microneedle–future prospect for efficient drug delivery in diabetes management. Indian journal of pharmacology. 2019;51(1):4.
Ali B, ElMahdy N, Elfar NN. Microneedling (Dermapen) and Jessner’s solution peeling in treatment of atrophic acne scars: a comparative randomized clinical study. J Cosmet Laser Ther. 2019;21(6):357–63.
McCrudden MT, McAlister E, Courtenay AJ, González-Vázquez P, Raj Singh TR, Donnelly RF. Microneedle applications in improving skin appearance. Exp Dermatol. 2015;24(8):561–6.
Davis SP, Martanto W, Allen MG, Prausnitz MR. Hollow metal microneedles for insulin delivery to diabetic rats. IEEE Trans Biomed Eng. 2005;52(5):909–15.
Godin B, Touitou E. Transdermal skin delivery: predictions for humans from in vivo, ex vivo and animal models. Adv Drug Deliv Rev. 2007;59(11):1152–61.
Chu LY, Choi S-O, Prausnitz MR. Fabrication of dissolving polymer microneedles for controlled drug encapsulation and delivery: bubble and pedestal microneedle designs. J Pharm Sci. 2010;99(10):4228–38.
Gill HS, Prausnitz MR. Pocketed microneedles for drug delivery to the skin. J Phys Chem Solids. 2008;69(5–6):1537–41.
Yang M, Zahn JD. Microneedle insertion force reduction using vibratory actuation. Biomed Microdevice. 2004;6(3):177–82.
Pettis RJ, Harvey AJ. Microneedle delivery: clinical studies and emerging medical applications. Ther Deliv. 2012;3(3):357–71.
Shin CI, Jeong SD, Rejinold NS, Kim Y-C. Microneedles for vaccine delivery: challenges and future perspectives. Ther Deliv. 2017;8(6):447–60.
Waghule T, Singhvi G, Dubey SK, Pandey MM, Gupta G, Singh M, et al. Microneedles: a smart approach and increasing potential for transdermal drug delivery system. Biomed Pharmacother. 2019;109:1249–58.
Sharma S, Hatware K, Bhadane P, Sindhikar S, Mishra DK. Recent advances in microneedle composites for biomedical applications: advanced drug delivery technologies. Mater Sci Eng C. 2019;103:109717.
Liu G-S, Kong Y, Wang Y, Luo Y, Fan X, Xie X, et al. Microneedles for transdermal diagnostics: recent advances and new horizons. Biomaterials. 2020;232:119740.
Meng F, Hasan A, Babadaei MMN, Kani PH, Talaei AJ, Sharifi M, et al. Polymeric-based microneedle arrays as potential platforms in development of drugs delivery systems. J Adv Res. 2020.
Nagarkar R, Singh M, Nguyen HX, Jonnalagadda S. A review of recent advances in microneedle technology for transdermal drug delivery. J Drug Delivery Sci Technol. 2020:101923.
Rad ZF, Nordon RE, Anthony CJ, Bilston L, Prewett PD, Arns J-Y, et al. High-fidelity replication of thermoplastic microneedles with open microfluidic channels. Microsyst Nanoeng. 2017;3(1):1–11.
Gittard SD, Ovsianikov A, Chichkov BN, Doraiswamy A, Narayan RJ. Two-photon polymerization of microneedles for transdermal drug delivery. Expert Opin Drug Deliv. 2010;7(4):513–33.
Abidin HEZ, Ooi PC, Tiong TY, Marsi N, Ismardi A, Noor MM, et al. Stress and deformation of optimally shaped silicon microneedles for transdermal drug delivery. J Pharm Sci. 2020;109(8):2485–92.
Economidou SN, Pissinato Pere CP, Okereke M, Douroumis D. Optimisation of design and manufacturing parameters of 3D printed solid microneedles for improved strength, sharpness, and drug delivery. Micromachines. 2021;12(2):117.
Joshi M, Pathak S, Sharma S, Patravale V. Design and in vivo pharmacodynamic evaluation of nanostructured lipid carriers for parenteral delivery of artemether: Nanoject. Int J Pharm. 2008;364(1):119–26. https://doi.org/10.1016/j.ijpharm.2008.07.032
Manoj H, et al. Microneedles: Current trends and applications. Microfluidics and Bio-MEMS. Jenny Stanford Publishing, 2020;275-342.
Zhao Z, Chen Y, Shi Y Microneedles: A potential strategy in transdermal delivery and application in the management of psoriasis. Rsc Advances 2020;10(24):14040-14049.
Jeong H-R, et al. "Considerations in the use of microneedles: pain, convenience, anxiety and safety." J Drug Target. 2017;25(1):29–40.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tariq, N., Ashraf, M.W. & Tayyaba, S. A Review on Solid Microneedles for Biomedical Applications. J Pharm Innov 17, 1464–1483 (2022). https://doi.org/10.1007/s12247-021-09586-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-021-09586-x