Abstract
We present an implantable, microfabricated device for the treatment of hydrocephalus. Hydrocephalus is a medical condition, in which an abnormal accumulation of cerebrospinal fluid (CSF, a water-like fluid that circulates around and protects the brain and spinal cord.) occurs in the brain. The novel microdevice presented here mimics the function of natural one-way valves, arachnoid villi, found in the human brain. Hence, we name it microfabricated arachnoid villi (MAV). The MAV consists of an array of one-way microvalves and hollow microneedles. The one-way microvalves control flow based on pressure differential. A Parylene microvalve array with a dome petal geometry was designed and fabricated. Initial flow tests demonstrated the desired low cracking pressure of the valve and a sufficient mechanical stability. The hollow microneedle array was designed to pierce the dura mater membrane (A tough fibrous membrane covering the brain and the spinal cord and lining the inner surface of the skull.) and provide a conduit for CSF. An SU-8 microneedle array was designed and successfully microfabricated. The innovative MAV may open a new era in the treatment of hydrocephalus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Cerebrospinal fluid (CSF) is produced in the brain at a rate of 0.3–0.5 ml/min; it circulates around and protects the brain and spinal cord (Fig. 1a). The average volume of intracranial CSF is 125 ml in an adult. The total volume of CSF is turned over 4–5 times in a 24 h period. It is believed that CSF is absorbed into the superior sagittal sinus through biological one-way valves called arachnoid villi (AV), which are located in dura mater tissue. The production and absorption of CSF is therefore in a dynamic equilibrium.
Hydrocephalus is an abnormal accumulation of CSF within the subarachnoid space surrounding the brain due to impaired CSF absorption (Kent and Van De 1984). Hydrocephalus is one of the most frequently encountered problems in Neurosurgery. 8,305 newly diagnosed cases of hydrocephalus were treated in the United States in the year 2000 (Patwardhan et al. 2005). Hydrocephalus can be classified as either non-communicating or communicating. The communicating form is a defect at the level of the AV while the non-communicating type is caused by an obstruction to outflow within the interior of the brain. The majority of hydrocephalus cases are of the communicating form. The CSF pressure in the subarachnoid area varies according the age group. In infants, it is estimated to be 40–50 mmH2O and in children from 40–100 mmH2O. In older age groups it remains constant at about 150 mmH2O which is usually about 40–50 mmH2O above the intracranial venous pressure. Pressures above 200 mmH2O are considered abnormal. The pathological conditions of hydrocephalus can be life-threatening and results in brain injury if left untreated.
Currently, hydrocephalus is treated by a surgical procedure, performed by a neurosurgeon, in which a shunt device is placed into the patient’s body (Fig. 1b). The shunt channels the flow of fluid away from the brain into another part of the body where it will be safely absorbed. The shunt device consists of a proximal catheter within the ventricular space, a distal draining catheter located within an absorptive surface, and an intervening one-way valve device that is placed subcutaneously outside the skull. Most valves allow flow at a pre-set pressure. The most common absorptive surface for the draining catheter is the peritoneum of the abdominal cavity. This is referred to as a ventriculo-peritoneal (VP) shunt. Although VP shunts have prevented death and disability from hydrocephalus for the past few decades, they still have persistent shortcomings.
The first major shortcoming of the current VP shunt is the high failure rate. Drake has reported failure rates of 40% after 1 year and 50% after 2 years (Drake et al. 1998). It is estimated that 50% of mechanical shunt failure is due to shunt blockage. The failure rate is usually highest in the immediate post operative period. The proximal catheter can become occluded if brain debris or parts of choroids plexus become attached to the pores of the proximal catheter resulting in diminished flow. The valve may similarly become occluded by debris, blood clot or infection. Other causes of failure include tubing breakage, kinking or shortening due to patient growth or movement. Another problem with the current VP shunts is the imprecise flow of CSF which results in over or under shunting. Under-shunting occurs when CSF is not evacuated from the brain fast enough to maintain equilibrium with production. The limitations of valve design as well as some partial occlusion can produce this under-shunting. Over-shunting is also a limitation of the current shunt design. In this case too much flow is allowed through the valve and a lower than normal pressure exists around the brain. This is usually exacerbated by changes in patient posture from a recumbent to an erect position.
In an attempt to solve problems of imprecise shunting, programmable valves (electro-magnetically adjustable) have been developed. Neurosurgeons can pre-select one of multiple pressure settings at the time of implantation. After implantation, the valve can be adjusted non-invasively using a magnetic device to individualize the flow rate to a particular patient’s situation. However, since the actual intracranial pressure cannot be monitored after implantation, finding an optimum pressure setting still remains very difficult. Also, it was reported that household magnets could change the programmable shunt valves (Liu et al. 2005). In addition, these valves do not adapt to changing patient positions once set. Anti-siphon devices have also been employed to correct problems of over shunting due to changes in patient position. But this too has not alleviated the problems associated with imprecise flow (Drake and Saint-Rose 1995). Despite its promise, the programmable valve has not been proven to be a great advance.
Recently, an active shunt system has been proposed (Yoon et al. 2004). The system consists of a micro telemetry pressure sensor, a micropump, and a controller. The controller controls the micropump according to the intracranial pressure measured with a telemetry pressure sensor. They have demonstrated the pressure sensor and micropump prototypes, and tested them in vitro. This is a novel approach that could provide an active form of moving CSF in relation to instantly measured pressure gradients. The telemetry of the system would also be useful monitoring of the intracranial pressure after implantation and hence diagnosis. However, there are many obstacles to overcome with this proposed system such as power supply, complex circuitry, multiple moving parts as well as other problems of the current system such as breakage, clogging, infection and a single outlet.
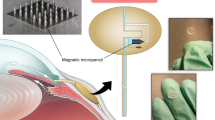
Here we report an innovative approach for the treatment of hydrocephalus. We are attempting to replace the deficient AV that produces the pathologic condition of communicating hydrocephalus with a microfabricated device that mimics normal AV. The “Microfabricated arachnoid villi (MAV)” is to be implanted against the dura mata (Fig. 2). The MAV consist of an array of microneedles and microvalves. The hollow microneedle array is to pierce the dura mater and provide a conduit for CSF. The one-way microvalves attached to the bottom of the microneedles control flow. The outflow of CSF is controlled passively by the pressure differential between the venous channel and the subarachnoid space analogous to normally functioning AV. Therefore, the MAV closely mimics the normal CSF physiologic mechanism. Failure of the current shunt systems due to occlusion by the choroid plexus (a frond-like structure that occupies the ventricular spaces of the brain) can be eliminated since the proposed MAV does not reside in the ventricular system but rather in the subarachnoid space that communicates with the ventricular space. Shunt failure as the result of infection would also be drastically reduced because the MAV does not have long tubes that serve as a nidus of infection along its path. Shunt failure as the result of tubing disconnection, cracking, kinking and shortening as the result of patient growth is similarly eliminated with the elimination of the long tube. The MAV is compact and completely contained within the confines of the skull. The potential for damage is minimal as it is afforded the protection of the skull. The potential for dislodgement is minimized by proper placement and anchoring. Over-shunting due to the change in the patient’s posture is also be minimized since the source and drain areas are adjacent to each other unlike current VP shunt systems. Under-shunting due to the preset pressure existing in the current system can also be addressed by employing thin-film microvalves with very low cracking pressure. The innovative MAV may open a new era in the treatment of hydrocephalus. This paper describes the initial progress in the design and fabrication of the two major components of the MAV: one-way microvalve and hollow microneedle.
The design of an implantable MAV that mimics the natural AV. The device consists of arrays of microneedle and microvalve. The hollow microneedle array is to pierce the dura mater and provide a conduit for CSF. The one-way microvalves attached to the bottom of the microneedles control flow based on the pressure differential between subarachnoid area and sagittal sinus
2 Design and simulation
The microvalve of the MAV must be capable of diverting the CSF at low pressure differences (<50 mmH2O) to keep the subarachnoid area within the normal pressure range. The valve should also be one-way valve such that it opens for the CSF to flow out but closes to prevent backflow. Also, the device must be able to hold the CSF pressure (150–200 mmH2O) when implanted in the brain. In order to minimize back flow (from sinus to the intracranial area), a normally closed valve design is preferred. The size of the device is also critical because it will be surgically implanted adjacent to the brain in the subarachnoid space. The dura mater has many AV to drain CSF. The MAV must have similar redundancy built into the device to lower the failure rate. Other things to be considered in the design of MAV include biocompatibility, surgical implantation capability, and cost.
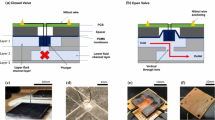
Parylene (poly paraxylylene) was selected as the microvalve material because it is a biocompatible thin polymer film with a low rigidity that can open and close at low pressure differences. A dome petal design was chosen to obtain low cracking pressure and flow resistance (Fig. 2). The design also resembles the natural AV that has multilayer petal geometry. The outflow of CSF is controlled passively by the pressure differential between the sagittal sinus and the subarachnoid space just like normally functioning AV. Arrays of 4 × 4 and 10 × 10 microvalves were designed an area of 5 × 5 mm2 for the assembly with microneedle part. Each microvalve consists of a thin (2 μm) dome membrane and a thick (20 μm) orifice membrane. The dome membrane works as the valve while the orifice membrane provides mechanical support. Each microvalve has a dimension of 200 × 200 × 50 μm3. The thin profile of the device would still fit against the sinus in the subarachnoid space.
The valve performance was investigated using numerical simulation for design optimization. Comsol Multiphysics/MEMS module (Comsol, Burlington, MA, USA) was used to calculate the membrane displacement, stress distribution, pressure distribution, and flow rate as a function of design parameters such as membrane thickness and diameter. Structural and fluidic analyses were coupled and moving mesh mode was used for the simulation of a simplified 2D model. The pressure difference across the valve was varied between 10–100 mmH2O (approximately 100–1,000 Pa). Assuming that the final device will contain an array of 10 × 10 valves, each valve must be able to divert of al least 4 μl/min of CSF (the CSF production rate is 0.4 ml/min). 2 μm thick valve membrane produced an opening of 17 μm after deflection under a pressure differential of 50 mmH2O (Fig. 3). This will provide a volumetric flow rate of 5.5 μl/min through each valve. Stress distribution was also obtained in the simulation and the maximum stress (∼15 MPa) at the hinge region calculated based on von-Mises criteria was much less than the yield strength of Parylene (3.2 GPa).
Design optimization work is predominantly focused on the microvalve design because the microneedle design would not affect the CSF flow significantly. However, the microneedle design is important for the perforation of dura mater. The microneedle was designed to have an off-centered inner hollow channel (Fig. 2) similar to the previously reported silicon microneedles (Wilke et al. 2005; Griss and Stemme 2003). In this way, we can still take advantage of the sharpness of the needle at the time of surgical perforation. Also, it is recommended to align the needle with the direction of blood flow in the sagittal sinus in a way that the holes of the needle may be screened by the tip of the needle. An array of 10 × 10 microneedles were designed in an area of 5 × 5 mm2. Each hollow needle has been designed to have a space on the bottom where the microvalve would be placed.
3 Fabrication
Parylene surface micromachining (Wang et al. 1999; Noh et al. 2004) was employed in the microvalve fabrication. Figure 4a shows the fabrication process flow. A 20 μm thick layer of Parylene C (Specialty Coating Systems, Indianapolis, IN, USA) was deposited on a glass substrate using a Labcoater (PDS 2010, Specialty Coating Systems) to serve as the orifice membrane. A thin (∼0.2 μm) Aluminum layer was then patterned on the Parylene layer via a lift-off process that consists of photolithography, metal deposition, and photoresist (PR) dissolution. Shipley 1827 photoresist was used in the photolithography step and the thin Al film was deposited using electron beam evaporation. The patterned Al layer was then used as a mask for oxygen reactive ion etching (RIE) of Parylene. Parylene was etched with a mixture of O2 and CF4 in a PlasmaTherm RIE system with a pressure of 200 mTorr and a power of 250 W. The approximate etch rate was 0.5 μm/min. Another photolithography step was performed on the patterned Parylene membrane with a thick photoresist. The substrate is then heated on hot plate (90–100°C) to induce the reflow of PR. A 2 μm thick Parylene layer was then deposited on the PR pattern to serve as a valve membrane. An SU-8 rim pattern was then created around the valve for the assembly with the microneedle. An excimer laser (KrF, 248 nm) was used to cut the Parylene valve membrane open. Finally, the Parylene microvalve was released from the glass substrate and the sacrificial PR was removed in acetone. The overall process requires 3 photomasks.
The fabrication process flow. a Microvalve: 1 Parylene deposition (20 μm), 2 Aluminum patterning through lift-off process (photolithography, Al deposition, photoresist dissolution), 3 oxygen reactive ion etching (RIE) of Parylene, 4 photolithography and thermal reflow, 5 Parylene deposition (2 μm), 6 SU-8 rim patterning, 7 laser machining for valve opening 8 release, b Microneedle: 1 Chrome pattern on glass, 2 SU-8 patterning for base layer, 3 SU-8 patterning through bottom exposure, 4 release, 5 sharpening through oxygen RIE, 6 laser machining for internal channels, 7 Parylene coating
Figure 5a shows the fabricated Parylene microvalve arrays. 4 × 4 and 10 × 10 arrays have been fabricated on 2 × 2 in.2 glass plates. One of the problems in the fabrication process was the delamination of the orifice membrane from the substrate during the subsequent fabrication process. This was caused by liquid penetration through the etched holes. This problem can be reduced by roughening the substrate using RIE before the Parylene deposition. Figure 5a shows a Parylene valve membrane with no orifice membrane. The valve opening created by laser ablation was much larger (25–30 μm) than the initial design. This large opening was due to the large diameter (30 μm) of the laser beam. An alternative would be to use oxygen RIE to cut through the membrane.
The fabrication flow of a SU-8 microneedle is shown in Fig. 4b. A Cr pattern was first created on a glass substrate through Cr deposition, photolithography, and Cr etching. A layer of SU-8 was then patterned to serve as a base plane. A thicker SU-8 layer was then spin-coated and exposed to UV light from the bottom through the Cr pattern. A tapered geometry was created in this way due to the light scattering effect. The tapered SU-8 patterns were then sharpened through RIE etching (Choi et al. 2007). Through-holes were made via laser machining. Larger holes (300 μm) were made on the bottom while smaller holes (25 μm) were on the top side (Choi et al. 2007). Finally, a thin Parylene layer (1 μm) was deposited on the needle. Figure 5b shows SU-8 microneedles with hollow channels. Sharp microneedles (500 μm high, 300 μm wide at the base, and a tip radius of a few microns) were successfully fabricated and internal channels were machined by laser ablation.
The assembly of the microvalve and microneedle is yet to be accomplished. Our approach for the assembly is to make self-fitting structures on the microvalve and microneedle. The SU-8 rim on the microvalve has been designed to have the same dimension as the large hole on the bottom of the microneedle. Therefore, the completed microvalve array can be inserted into the bottom of the microneedle array for simple assembly. To ensure a leakage-free attachment, Parylene–Parylene thermal bonding technique (Noh et al. 2002, 2004) will be applied in the assembly process.
4 Proof-of-concept testing and discussion
To test the performance of the Parylene microvalve and the future MAV in vitro, a bench-top “CSF simulator” was developed. The CSF simulator mimics the fluid environment of the intracranial compartment. The CSF simulator consists of two fluid chambers separated with a membrane analogous to dura mater where the Parylene microvalve was installed (Fig. 6). The first and second fluid chambers represent the subarachnoid space and sagittal sinus respectively. The known production rate of CSF in the human brain (approximately 0.4 mL/min) and consequent pressure increase was simulated by pumping fluid to chamber 1 using a syringe pump. Fluid flow through the microvalve was quantified precisely by weighing the fluid coming out of chamber 2. The pressure difference between the two fluid chambers was monitored by a manometer connected between the two chambers during the experiment.
Figure 7 shows the results of initial flow tests for the microvalve prototype shown in Fig. 5. Deionized water was used in these experiments. First of all, the cracking pressure was very low. As soon as the inlet flow was applied to the chamber 1, the water manometer indicated a pressure difference of several mmH2O, but soon the outlet flow was measured and the pressure difference was reduced to a very low value. The outlet flow remained same as the inlet flow up to 4 ml/min, which is 10 times larger than average CSF production rate. Therefore, we can safely say that the device will have a normal valve function in the low flow rate conditions. The Parylene microvalves were visually checked before and after the test to see if they have been damaged during the test. Flow tests were run at higher flow rates than the CSF production flow rate in the brain and no significant damage or deformation was observed in the device.
5 Summary and future work
An innovative, implantable microdevice was introduced in this paper for the treatment of hydrocephalus, which is one of the most frequently encountered problems in Neurosurgery. The “Microfabricated arachnoid villi (MAV)” consists of an array of microneedles and microvalves. The hollow microneedle array was designed to pierce the dura mater and provides a conduit for CSF. The one-way microvalves attached to the bottom of the microneedles control flow. The MAV can eliminate most of the problems in the current treatment (VP shunt) such as high failure rate and inaccurate drainage. A thin-film, Parylene microvalve with a dome petal design was chosen to obtain low cracking pressure. Design parameters such as membrane thickness and diameter have been optimized considering the normal flow rate of CSF. Parylene microvalve prototypes containing 4 × 4 and 10 × 10 arrays in an area of 5 × 5 mm2 have been fabricated and proof-of-concept testing has been performed using a “CSF simulator” that mimics the fluid environment of the intracranial compartment. The initial flow tests demonstrated the desired, low cracking pressure and mechanical stability of the valve. An SU-8 hollow microneedle array was also designed and successfully fabricated. This paper reported our recent progress in the design, simulation, and fabrication of the two major component of the MAV: one-way microvalve and hollow microneedle. Future work includes the assembly of the microneedle and microvalve, in-vitro testing of the assembled device, long-term reliability tests, in-vivo tests using animal models and biocompatibility studies.
References
Choi Y, McClain MA, LaPlaca MC, Frazier AB, Allen MG (2007) Three dimensional MEMS microfluidic perfusion system for thick brain slice cultures. Biomed Microdev 9:7–13
Drake JM, Saint-Rose C (1995) The shunt book. Blackwell, New York
Drake JM, Kestle J, Milner R (1998) Randomized trial of cerebrospinal fluid shunt valve design in pediatric hydrocephalus. Neurosurgery 43:294–305
Griss P, Stemme G (2003) Side-opened out-of-plane microneedles for microfluidic transdermal liquid transfer. J Microelectromech Syst 12(3):296–301
Kent M, Van De G (1984) Human anatomy. Brown Publishers, Dubuque
Liu S, Greene R, Thomas GA, Madsen JR (2005) Household magnets can change the programmable shunt valve in hydrocephalus patients. In: IEEE 31st annual Northeast bioengineering conference, pp 22–23
Noh H-S, Huang Y, Hesketh PJ (2004) Parylene micromolding, a rapid and low-cost fabrication method of parylene microchannel. Sens Actuators B 102:78–85
Noh H-S, Hesketh PJ, Frye-Mason GC (2002) Parylene gas chromatographic column for rapid thermal cycling. J Microelectromech Syst 11(6):718–725
Noh H-S, Moon K-S, Cannon A, Hesketh PJ, Wong CP (2004) Wafer bonding using microwave heating of parylene intermediate layers. J Micromech Microeng 14:625–631
Patwardhan, Ravish V, Nanda A (2005) Implanted ventricular shunts in the United States: the billion-dollar-a-year cost of hydrocephalus treatment. Neurosurgery 56(1):139–145
Wang X-Q, Lin Q, and Tai Y-C (1999) A Parylene micro check valve. In: The 12th IEEE international conference on MEMS, pp 177–182
Wilke N, Hibert C, O’Brien J, Morrissey A (2005) Silicon microneedle electrode array with temperature monitoring for electroporation. Sens Actuators A 123–124:319–325
Yoon HJ, Jung JM, Jeong JS, Yang SS (2004) Micro devices for a cerebrospinal fluid (CSF) shunt system. Sens Actuators A 110:68–76
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Emam, M., Abashiya, Y., Chareunsack, B. et al. A novel microdevice for the treatment of hydrocephalus: design and fabrication of an array of microvalves and microneedles. Microsyst Technol 14, 371–378 (2008). https://doi.org/10.1007/s00542-007-0446-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00542-007-0446-6