Abstract
Designation of essential fish habitat requires a detailed understanding of how species-specific vital rates vary across habitats and biogeographical regions. This is especially true for species like the economically important brown shrimp (Farfantepenaeus aztecus) which occurs in multiple habitat types across a wide geographic range (southeastern US Atlantic and Gulf of Mexico (GoM) coasts) and exhibits variation in vital rates to small-scale variability in habitat conditions. As juveniles, brown shrimp occupy a suite of interconnected habitats within the estuarine mosaic before migrating offshore as adults. In the southeastern US, intertidal creeks make up a substantial proportion of available habitat within the estuarine mosaic, yet habitat-specific vital rates, including growth, are currently unavailable. We therefore sought to (1) estimate growth rates of juvenile brown shrimp in intertidal creek habitat within a high salinity, southeastern US estuary, the North Inlet estuary in South Carolina, and (2) compare our estimated rates with those from salt marsh habitats in northern GoM estuaries, the only other estuaries where field-derived estimates for juvenile brown shrimp are available. Juvenile brown shrimp collected over a 10-week period (May–July 2021) ranged from 25 to 95 mm TL and appeared to emigrate from the intertidal creek to deeper waters beginning at ~ 65 mm TL. Daily growth rates ranged from 0.45 to 2.30 mm day−1, with the highest rates estimated early in the study period. Despite differences in estimation method, salt marsh habitat type, and region, estimated growth rates from the North Inlet estuary were nearly identical to those from northern GoM estuaries. Collectively, our results suggest that despite differences in habitat geomorphology, spatial extent, and temporal availability, intertidal creeks may provide juvenile brown shrimp with similar nursery function to other habitats within the estuarine seascape.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Estuaries provide critical ecosystem services to humans, including serving as nursery habitat for populations of fishes and crustaceans that support important fisheries worldwide (Beck et al. 2001; Barbier et al. 2011; Baker et al. 2020). The Sustainable Fisheries Act of 1996 defines essential fish habitat (EFH) as “waters and substrate necessary to fish for spawning, breeding, feeding, and/or growth to maturity,” and EFH must be identified for all federally managed stocks in the USA (Rosenberg et al. 2000). However, in practice, nearly all habitat types that are utilized over the life history of an animal are often considered “essential,” such that EFH can become overly broad and the designation less meaningful. For example, in the southeastern USA, EFH for penaeid shrimp includes all inshore estuaries and offshore spawning grounds, amounting to tens of thousands of square kilometers (SAFMC 1998). Levin and Stunz (2005) narrowed the definition of EFH to include only habitat with significant impact on demographic rates of sensitive life history stages, and they demonstrated that restoration of two specific habitat types (salt marsh and seagrass) could reverse a population decline for a key sport fish. Many fishery species use estuaries during their early life stages, and the availability of multiple habitat types within the estuarine mosaic can support high growth and survival rates and, ultimately, successful recruitment to adult populations (Minello et al. 2003). However, vital rates may vary among habitat types; thus, habitat-specific vital rates should be quantified to most effectively utilize the EFH framework within an ecosystem approach to fishery management.
Two species of penaeid shrimp, white shrimp (Litopenaeus setiferus) and brown shrimp (Farfantepenaeus aztecus), make up the bulk of the commercial shrimp landings along the southeastern US Atlantic coast. Penaeid shrimp life history occurs on an annual scale; adults spawn offshore and post-larvae are transported by currents into estuaries and then grow rapidly as juveniles during their period of estuarine residency before emigrating offshore to adult habitats. Generally, shrimp populations exhibit high interannual variability that is likely driven by their sensitivity to factors such as habitat availability and access (Webb and Kneib 2002; Shervette and Gelwick 2008; Minello et al. 2012) and environmental conditions, including temperature and salinity (Mace and Rozas 2017; Fowler et al. 2018), though the preference for specific environmental conditions may differ across species (Doerr et al. 2016).
Early life stage penaeid shrimps occur in estuaries across a broad range of habitat types and generally have wide tolerances to varying environmental conditions such as water temperature and salinity (Zein-Eldin and Renaud 1986; Zink et al. 2017). However, studies examining brown shrimp growth in salt marsh habitats have primarily focused on two research avenues: (1) laboratory experiments investigating the impacts of water temperature, salinity, or their interaction and (2) field studies conducted in microtidal salt marshes of varying salinities in northern Gulf of Mexico estuaries. Laboratory experiments focusing on the impact of salinity have shown that the growth rates of juvenile brown shrimp were higher at higher salinities (8 and 12 vs 2 and 4, Saoud and Davis 2003; 38 vs 33, Perez-Castaneda et al. 2012) and that juvenile brown shrimp preferred higher salinity conditions, between 17 and 35 (Doerr et al. 2016). Similarly, field enclosure experiments conducted in salt marshes adjacent to the Gulf of Mexico revealed that growth rates of brown shrimp were depressed in low salinity (< 2) environments, while higher growth rates were documented for enclosed and free-ranging shrimp occupying higher salinity salt marshes (15–30; St. Amant et al. 1966; Rozas and Minello 2009, 2011, 2015; Leo et al. 2018). As a result, while shrimp can occupy a diverse set of habitat types and survive within a range of environmental conditions, key demographic rates, such as growth, can vary as a function of physical factors, such as salinity, which are highly dynamic both spatially and temporally in estuaries.
In addition to environmental conditions within tidal creeks, biogeographic patterns in tidal flooding can be an important predictor of estuarine nekton nursery utilization (Minello 2017). Prior studies examining growth of brown shrimp in salt marsh habitats have all been conducted in estuaries in the northern Gulf of Mexico, where tide ranges are generally < 1 m and flooding durations are irregular (Minello et al. 2012). This contrasts with southeastern US Atlantic coast estuaries which exhibit regular tidal ranges > 1 m (and up to 3 m). These tides lead to predictable flooding frequencies and substantial expanses of intertidal habitat, with the greatest extent in South Carolina and Georgia (Dame et al. 2000; Minello et al. 2012). Because the value of intertidal habitats to penaeid shrimps (e.g., for trophic support) is largely determined by flooding frequencies and durations, which vary greatly across the entire southeastern USA from Texas to North Carolina (Minello et al. 2012; Baker et al. 2013), examination of habitat-specific growth rates throughout this geographic range is needed.
Along the southeastern US Atlantic coast, post-larval brown shrimp generally begin to arrive in estuaries from late winter (February) to early spring (April) at sizes ranging from 9 to 12 mm total length (TL; Bearden 1961; Wenner and Beatty 1993; DeLancey et al. 1994). The specific timing of peak recruitment (as measured by density of post-larvae) displays substantial variability both within and among years and is likely influenced by factors such as water temperature, lunar cycles, bathymetry, and tidal circulation (Bearden 1961; Williams 1969; DeLancey et al. 1994). Following settlement in the estuary, post-larvae grow into juveniles that can be found in tidal creeks and marsh surface habitats from late spring (May) through mid-summer (July), generally exhibiting maximum abundance in May and June in shallow intertidal creeks (Hackney and Burbank 1976; Hunter and Feller 1987; Allen et al. 2007, 2017; Mace et al. 2019; Kimball et al. 2023). Departure from shallow estuarine habitats generally occurs when individuals reach between 65 and 100 mm TL; at this sub-adult stage, they begin to emigrate to deeper, open water habitats prior to moving into offshore waters when they reach 110–140 mm TL (Knudsen et al. 1985; Wicker et al. 1988; Fry et al. 2003; Kimball et al. 2023).
During their period of estuarine residency, juvenile brown shrimp can be observed in multiple habitats within the estuarine mosaic, including marsh pools, intertidal creek pools, subtidal creeks, intertidal creeks, marsh edges, and flooded marsh surfaces (Allen et al. 2007, 2017; Mace et al. 2019; Kimball et al. 2023). Examinations of length frequencies and diets suggest that intertidal creeks likely serve as important nursery habitats supporting juvenile brown shrimp (Hunter and Feller 1987; Kimball et al. 2023). However, habitat-specific growth estimates are not available for brown shrimp in these systems despite observations of higher brown shrimp use of shallow salt marsh habitats and intertidal creeks compared with other estuarine habitat types (Fry et al. 2003; Allen et al. 2007). In addition, juvenile penaeid shrimp exhibit limited movement, on the scale of 10s to 100s of meters (Webb and Kneib 2004). The lack of information regarding nursery value for intertidal creeks could result in underestimating this habitat type for supporting production of brown shrimp. Because the abundance of the juvenile stage has been suggested as the critical component in determining year-class strength for brown shrimp populations, this potential miscalculation may undermine efforts to improve forecasts of adult shrimp populations (Haas et al. 2001).
Given their reliance on multiple interconnected estuarine habitats across critical early life stages and because they are a short-lived, fast-growing species which is heavily influenced by small-scale variability in habitat conditions, we examined the growth of juvenile brown shrimp utilizing intertidal creeks in a euhaline estuary. We hypothesized that population-level brown shrimp growth rates would decrease over the course of their estuarine residency period as the initial cohort of recruits emigrates from shallow intertidal creeks when they reach their maximum size for these habitats. This movement leaves behind a mix of smaller, more recent recruits and slower-growing individuals not yet ready to emigrate to deeper, open waters. We then compared our estimated growth rates with those previously determined for juvenile brown shrimp utilizing salt marsh edge, surface, and pond habitats and subtidal creeks in northern Gulf of Mexico estuaries (St. Amant et al. 1966; Parrack 1979; Rozas and Minello 2009, 2011, 2015; Leo et al. 2018). We hypothesized that our growth rate estimates within a temperate estuary would be faster than those from more sub-tropical locations due to the shorter window during which environmental conditions are hospitable for juvenile penaeid shrimp. Habitat-specific growth estimates for species utilizing salt marsh intertidal creeks will clarify our understanding of the nursery role of these habitats for economically and ecologically important penaeid shrimp and other transient nekton species. In addition, as the distribution of penaeid shrimps expands north (e.g., Tuckey et al. 2021), understanding juvenile shrimp growth rates across latitudinal gradients will allow for improved assessments of shrimp fishery stocks.
Materials and Methods
Study Area
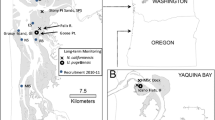
All field collections were conducted in an approximately 100 m section of intertidal creek within the Oyster Landing intertidal creek basin (33° 21′ 01.88 N, 79° 11′ 27.25 W) located within the North Inlet estuary in Georgetown County, South Carolina, USA (Fig. S1). The North Inlet estuary is a well-mixed, high salinity, system in which Spartina alterniflora occupies approximately 75% of the total area (Allen et al. 2014). Oyster Landing creek, the main intertidal tributary (~ 1,300 m in length) servicing the focal 5.1-ha marsh basin, is located near the forest border, about 3.5 km from the inlet, and is subject to periodic influence from rainwater runoff from a 55-ha, undeveloped, forested watershed (Gardner and Bohn 1980; Miller and Gardner 1981).
Field Collections
Juvenile brown shrimp were collected using kick nets (114 cm wide × 114 cm high with a 3-mm mesh and 114 cm wide × 114 cm high with a 1.5-mm mesh) and cast nets (1.8 m radius with 6-mm mesh). Collections occurred during low tide twice a week for 10 weeks (from mid-May through mid-July, 2021). Long-term seine sampling of juvenile nekton in this intertidal creek basin indicated that May to June is the period of peak juvenile brown shrimp abundance (Kimball et al. 2023). To collect all sizes of brown shrimp present in the creek at each sampling event, each net was used multiple times and at multiple locations within the ~ 100 m section of creek, including sub-habitats such as along Spartina-lined creek edges, around oyster reefs, and in creek pools. Up to 60 individuals were collected during each sampling event, and all samples were immediately placed in an ice slurry and returned to the laboratory for processing. After sample collection, all individual brown shrimp were measured (according to Ditty 2011) for total length (TL, in mm).
Environmental conditions in the intertidal creek basin were continuously monitored during the study period. Specifically, water temperature and salinity were recorded every 15 min with a datasonde (YSI, Inc.) at the nearby Oyster Landing monitoring station (~ 300 m downstream from the sampling site; 33° 20′ 57.70 N, 79° 11′ 20.11 W) maintained by the North Inlet-Winyah Bay National Estuarine Research Reserve’s (NI-WB NERR) System-Wide Monitoring Program (Fig. S1). These data were downloaded from the NERRS Centralized Data Management Office (http://cdmo.baruch.sc.edu/).
Length and Size Structure
To evaluate population-level brown shrimp growth rates, we first explored patterns in the size structure of organisms collected over our 10-week sampling period. We analyzed size structure based on TL measured at the weekly scale (n = 2 sampling events per week; maximum weekly n = 120 individual brown shrimp) by constructing length-frequency histograms for each week (n = 10 weeks total). Descriptive statistics, including the mean, minimum, and maximum lengths, as well as the proportion of sampled individuals less than 40 mm TL (smaller juveniles) and larger than 70 mm TL (large juveniles—sub-adults), were calculated on a weekly basis. Next, we examined the change in the size structure of the sampled brown shrimp population over time using a generalized additive model (GAM). GAMs are an extension of generalized linear models (GLM) that allow for the use of smooth functions (e.g., thin plate regression splines) to model non-linear response variables (Wood 2017), such as organismal growth. We fit a GAM using brown shrimp TL as the response variable and Day of Year as a continuous smooth predictor, assuming normally distributed error and using the identity link. The Day of Year variable was standardized so that day 1 was the start of week 1 (May 16, 2021). To further examine the directionality and magnitude of size structure changes, we calculated the first derivative of the fitted GAM model using the derivative function in the R package gratia, which determines the derivatives of fitted smooth functions using finite differences (Simpson 2021). Predicted values and simultaneous 95% confidence intervals were determined for both the fitted GAM and the estimated first derivative. GAM fits were conducted in R and RStudio using the mgcv package, and predicted values and simultaneous 95% confidence intervals were determined for the fitted GAM and derivative using gratia (Wood 2017; R Core Team 2021, RStudio Team 2021).
Cohort Analysis and Growth Estimation
The presence of multiple cohorts (common for species exhibiting pulsed recruitment and relatively fast growth rates) can confound growth rate estimation using length-frequency data. Initial results from both the descriptive and analytical analyses of brown shrimp size structure (above) indicated the potential for multiple cohorts, specifically, the presence of multiple modal lengths in several weeks, and a general plateauing of average length. Therefore, prior to calculating explicit growth rates, we sought to (1) identify the presence of multiple cohorts and (2) restrict our estimation procedures to periods during which a distinct cohort(s) increased in length through time.
We tested for multiple cohorts by fitting a series of finite mixture models using the R package mixR, which performs maximum likelihood estimation of finite mixture models using the EM (expectation–maximization) algorithm (Yu 2022). Finite mixture models are commonly applied tools in cohort identification and analysis (e.g., Mace et al. 2015). We fit a suite of 5 candidate mixture models to each weekly length-frequency distribution (n = 10) and used model selection procedures to identify the most likely mixture model. The candidate models varied in number of modeled cohorts (1, 2, or 3) and variance structure among cohorts (equal or unequal) (Table S1). All models assumed a Gaussian distribution. We deemed the best fit model to be the finite mixture with the lowest BIC (Bayesian Information Criterion).
Based on the output of the above cohort identification process and the results of our initial GAM analysis of population size structure, we calculated brown shrimp growth rates (mm TL day−1) only for weeks 1 through 4, the sole period during which a distinct cohort(s) increased in length through time. First, we estimated absolute growth as \({\text{G}}_{\text{i}}= \text{ } \frac{{\text{TL}}_{\text{i}}-{\text{TL}}_{\text{i-1}}}{7}\), where Gi is the daily growth rate estimate for week i, TLi is the mean TL in week i, TLi-1 is the mean TL of the preceding week, and 7 is the number of days in a week (Shoup and Michaletz 2017). Second, we reapplied the same GAM approach described above to estimate apparent growth, or change in size structure over time (sensu Colombano et al. 2020). Specifically, we refit a GAM with the same model structure as above, but only used length data from weeks 1 to 4. In addition, due to the presence of a third, larger cohort in week 1 (see “Results”), we excluded the largest 7 lengths during that week (all TL > 65 mm), which we assumed to represent the largest cohort, to avoid biasing our estimates. Weekly growth rate estimates were then obtained by taking pointwise estimates of the newly estimated derivative (and upper and lower 95% confidence interval) at each weekly interval.
Growth Rate Comparisons
Finally, we compared our estimated growth rates from juvenile brown shrimp collected in intertidal creek habitat with previous data on shrimp growth in salt marsh habitats in northern Gulf of Mexico estuaries. We restricted our comparison to studies conducted in the field (as opposed to laboratory settings), though we allowed for inclusion of varying growth estimation methods (e.g., length-frequency of free-ranging populations and mark-recapture) and studies in differing salt marsh habitats (e.g., edge, surface, and pond). We focused on studies examining juveniles and small sub-adults (~ 20–100 mm TL) and included only those which provided daily growth rate estimates. Comparisons were also limited to those with similar timing (i.e., May–July).
Results
Environmental Conditions
Average weekly water temperatures at the NI-WB NERR Oyster Landing station ranged from 24.5 to 29.4 ºC, and both daily and weekly means generally trended upward over the 10-week study period, as expected during the late spring to early summer period (Table 1, Fig. 1). Salinity generally remained high during the study period, with mean weekly values exceeding 28, reflecting the ocean-dominated condition of the North Inlet estuary (Table 1). The lowest recorded daily salinities (though still > 20) occurred in June (week 6) following consecutive significant precipitation events, with higher values observed in both May and July (Fig. 1).
Daily mean water temperature (black line) and salinity (gray line) in the Oyster Landing intertidal creek basin during the study period. Daily means calculated from continuous observations recorded at the North Inlet-Winyah Bay NERR Oyster Landing System-Wide Monitoring Program station every 15 min (total daily observations n = 96). Points indicate discrete sampling events (total events n = 19). Vertical lines (and text labels) indicate weekly delineations of the study period (total number of weeks n = 10)
Length and Size Structure
Juvenile brown shrimp collected in the Oyster Landing intertidal creek basin from May to July 2021 ranged in size from 25 to 94 mm TL (Table 1, Fig. 2). Between 90 and 120 individuals were collected every week of the study except the week of July 18th, 2021 (week 10), when only 19 individuals were collected from a single sampling event (Table 1); the second sampling event that week did not collect any brown shrimp and thus was not included in further analyses. The smallest individuals (< 30 mm TL) were primarily collected early in the study (weeks 1–3), though a small juvenile brown shrimp (29 mm TL) was collected as late as week 8 (Table 1, Fig. 3). Maximum lengths were smallest in weeks 1 and 2 (74 and 79 mm TL, respectively), and then, maximum lengths were larger and similar across weeks 3–10 (89–97 mm TL). Likewise, the mean TL of brown shrimp increased steadily from a minimum of 43 mm in week 1 to 66 mm in week 5 (Table 1, Fig. 2), likely reflecting growth of the initial cohort of recruits. Subsequent weeks (weeks 6–10) showed no clear directional trend in mean TL, ranging from 59 to 76 mm TL, with the greatest week-to-week difference occurring between weeks 6 and 7 (nearly 16 mm TL difference in weekly means). The proportion of animals considered to be small juveniles (< 40 mm TL) was greatest in weeks 1 and 2 (0.55 and 0.12, respectively), but never reached above 0.1 in the subsequent weeks (Table 1). In general, the proportion of animals considered to be large juveniles or sub-adults (> 70 mm TL) reflected the opposite pattern, lowest in weeks 1 and 2, and higher (0.2 or greater) in subsequent weeks, reaching a maximum in week 7 (0.79; Table 1).
A Weekly length-frequency (mm; total length; 2 mm length bins) and mean length (black dotted line) of juvenile brown shrimp collected in the Oyster Landing intertidal creek basin each week of the 10-week study period. Each week is identified by the date (yyyy-mm-dd) of the starting Sunday. B Box and whisker plots of juvenile brown shrimp total length (mm) by week. Points represent individual shrimp length measurements and are partially transparent to reduce over-plotting; darker points indicate multiple overlapping TL measurements. The middle line of each box represents weekly median length and the lower and upper lines of the 25th and 75th percentiles. Whiskers extend to the most extreme data point no more than 1.5 of the interquartile range
Predicted trends in mean total length (A, upper panel) and apparent growth (change in mean total length, B, lower panel) by week for juvenile brown shrimp. Total length trend estimated from a fitted generalized additive model (GAM) and apparent growth from the first derivate of the fitted GAM. Gray bands show simultaneous 95% confidence intervals. Points in the upper panel are the observed weekly means, but the GAM was fitted to all data points. Effective degrees of freedom (e.d.f.) and approximate significance of the fitted smooth term (p-value) are included in the upper panel
Size structure trends from a fitted GAM showed the same general pattern as the raw length data (Fig. 3). Estimated mean TL increased fastest early in the study period, from mid- to late May, before reaching a plateau of approximately 70 mm TL in June (week 4), then remained at approximately that value throughout the remainder of the sampling period. The fitted GAM yielded an effective degrees of freedom of 3.914, suggesting that the assumption of a non-linear relationship between brown shrimp TL and Day of Year was appropriate (Fig. 3, Table S2). GAM-estimated predictions of mean TL generally aligned with the observed values, though some deviation was apparent in weeks 6 and 7 (Fig. 3).
Cohort Analysis and Growth Rate Estimation
We were able to conduct finite mixture modeling for 9 of the 10 weeks (small sample size in week 10 (n = 19 individuals) prevented a model-based cohort identification approach). There was no evidence of multiple cohorts in 7 of 9 weekly length-frequency distributions (Table S1). Specifically, in each of these cases, model selection procedures suggested that a single cohort provided the best fit to the data. However, three cohorts were apparent in week 1, ranging in mean length from 37 to 68 mm TL (Table 2, Table S3, Fig. S2). The smallest (and likely youngest) cohort comprised the largest proportion of the total number of shrimp measured (0.66; Table S3). Two distinct cohorts were also identified in week 9 (Table S3, Fig. S2).
Based on the cohort analysis, we assumed that the single cohort in weeks 2, 3, and 4 represented a combination of cohorts A and B from week 1. Therefore, we calculated separate absolute growth rates for each cohort during the week 1–week 2 period, then a single rate for each subsequent week. Absolute growth rate measurements during weeks 1 to 4 ranged from 0.45 to 2.30 mm day−1 (Table 2). The generalized additive model (apparent growth) approach yielded similar growth rate estimates for the A + B cohort, ranging from 0.65 to 1.65 mm day−1 (Table 2, Fig. 4, Table S4).
Predicted trends in mean total length (A, upper panel) and apparent growth (change in mean total length, B, lower panel) by week of a single cohort of juvenile brown shrimp sampled across the first 4 weeks of the study. Total length trend estimated from a fitted generalized additive model (GAM) and apparent growth from the first derivate of the fitted GAM. Gray bands show simultaneous 95% confidence intervals. Points in the upper panel are the observed weekly means, but the GAM was fitted to all data points. Effective degrees of freedom (e.d.f.) and approximate significance of the fitted smooth term (p-value) are included in the upper panel
Growth Rate Comparisons
Published estimates fitting our comparison criteria were only available for juvenile brown shrimp from estuaries in the northern Gulf of Mexico. Previous studies estimated growth rates for similar size ranges of juvenile brown shrimp using a diverse set of methods, including caging (collective size range: 27–64 mm TL; Rozas and Minello 2009, 2011, 2015), mark-recapture (minimum size at tagging: 32 mm TL; Leo et al. 2018), and length-frequency of free-ranging shrimp (range of weekly mean sizes for April through May: 23–91 mm TL; St. Amant et al. 1966). Despite these methodological differences, growth rate estimates from our study showed a remarkable similarity to previously published estimates, falling well within the existing range of 0.0 to 2.30 mm TL day−1 (Fig. 5, Table S5).
Discussion
The important interactions between habitats and individual organisms motivated the designation of essential fish habitat (EFH), which, in the USA, is broadly defined as the waters and substrate necessary for spawning, feeding, or growth to maturity (Magnuson-Stevens Act, amended 1996). In translating that definition into practice, the US National Marine Fisheries Service identified habitat-specific growth as one of four key criteria to assess EFH (Minello 2017). In our study, juvenile brown shrimp extensively utilized an intertidal creek within a warm-temperate, salt marsh-dominated estuary over a 10-week period from mid-May through mid-July. Size structure and daily growth rate estimates from this period indicate a combination of growth, emigration, and the possible arrival of multiple brown shrimp cohorts into the intertidal creek basin. Substantial growth rates of > 0.8 mm TL day−1 and increasing mean (and median) TL beginning in mid-May and continuing into early June provide evidence that intertidal creeks serve as important nursery habitats by supporting rapid growth of juvenile brown shrimp. Further evidence that our weekly sampling of free-ranging shrimp captured population-level growth of at least the initial cohort was provided by the increase in both minimum and maximum TL during the first several weeks of the study. Despite their potential utility for fishery stock assessment and habitat evaluation purposes, few studies have reported growth rates for juvenile brown shrimp within southeastern US estuaries, and we are unaware of published growth rate estimates from intertidal creek habitats in this region.
The plateau in size (mean TL) and decline in estimated growth rates apparent in the latter half of the study (mid-June to July) suggests a combination of possible processes occurring within the sampled brown shrimp population, including immigration of new cohorts, emigration of larger individuals to other estuarine habitat types, density-dependent growth, competition with other benthic macrofauna, and variation in growth rates across individuals or in response to environmental conditions. The small minimum sizes in weeks 6, 8, and 9 (39, 25, and 25 mm TL, respectively) likely indicate the movement of recently settled juveniles into the creek, or the settlement and subsequent recruitment (to our sampling gear) of post-larval brown shrimp in the period after the initial cohort arrived. The consistency of maximum TL (~ 90 mm) and lack of directional pattern in mean TL in later weeks also suggests that brown shrimp begin to depart intertidal creek habitat within the North Inlet estuary at approximately this size range (65–97 mm TL). Both the timing (mid-June to July) and size align with previous estimates of these movement behaviors in the North Inlet estuary as well as other estuaries of the southeastern USA (Williams 1955; Hunter and Feller 1987; Wenner and Beatty 1993). While these patterns could reflect gear avoidance by larger individuals, we believe the small size of the sampled intertidal creek habitat, shallow water depth, and multiple capture methods (kick net and cast net) mitigate this bias, and thus, the estimated maximum TL more likely reflects size-specific habitat use and movement behavior.
Penaeid shrimp can exhibit reduced condition (mass at length), slower growth rates, and smaller size-at-age in response to increased congeneric abundance (Pérez-Castañeda and Defeo 2005; DeLancey et al. 2008; Blanco-Martinez et al. 2020), evidence that density-dependence can negatively impact growth of these species. Importantly, environmental conditions, particularly salinity, can impact the distribution (and thus density) of penaeid shrimp within estuaries; in particular, rapid fluctuations in salinity are thought to trigger movement both among habitats within the estuary and possibly serve as a cue for movement from coastal areas (Zein-Eldin and Renaud 1986; Wenner and Beatty 1993; Minello 1999; Zink et al. 2017). Thus, the decline in shrimp size and low growth rate estimates between weeks 6 and 7 in this study are likely partially attributable to redistribution of brown shrimp in response to the concurrent drop in salinity (> 30 to < 20). A similar reduction in modal size was observed in tagged brown shrimp in coastal Louisiana impoundments following a rainfall event (Knudsen et al. 1977). Fishery-independent surveys demonstrate the negative correlation between mean size and annual abundance of white shrimp (DeLancey et al. 2008), suggestive of density-dependent growth. However, experimental evidence regarding the degree to which density-dependence impacts growth and mortality rates of juvenile penaeid shrimp within southeastern US estuaries is lacking. Investigation of species-specific susceptibility to density-dependent effects is crucial because even within the penaeid shrimps, environmental preferences can vary (Doerr et al. 2016), which may drive variation in organism density. Related to differences across penaeid shrimp species, white shrimp begin to recruit into the same intertidal creeks used by brown shrimp during early July (Kimball et al. 2023), potentially competing with late-arriving brown shrimp. Resource competition between penaeid shrimp species could serve as an additional source of the reduced growth observed during the latter half of our study period.
Intertidal creeks may be a particularly important nursery ground for brown shrimp because of their relative abundance compared with other suitable habitats within estuaries (e.g., marsh pools and intermittently flooded high marsh). For example, even within the North Inlet estuary, which is dominated by expansive monospecific stands of Spartina alterniflora, tidal creeks and flats make up more than 25% of the available habitat (Dame et al. 1986). In addition to their relative abundance, intertidal creeks are likely key nurseries because juvenile brown shrimp exhibit relatively little movement. Webb and Kneib (2004) found that white shrimp 40–80 mm TL moved an average of 258–373 m and that 93% of tagged animals were recaptured in the same creek. Fry et al. (2003) used a combination of stable isotopes, diet analysis, and quantitative sampling to infer that shrimp exhibited very restricted movement between marsh ponds and marsh channels and that shrimp in optimal areas (shallower, edge habitat) exhibited far less (perhaps even < 10 m) movement than shrimp in suboptimal habitat types. As intertidal creeks are often thought of as corridors for nekton moving between marsh surface and subtidal habitats during periods of tidal inundation (e.g., McIvor and Odum 1988; Rozas et al. 1988), their intermediate position may have led to growth rate estimates only partially overlapping with those observed in other marsh habitats, because shrimp may only use these habitats over short temporal scales. However, juvenile brown shrimp appeared to stay in intertidal creek habitat until reaching near sub-adult sizes (~ 70 mm TL), and our observed growth rates were similar to those from other salt marsh habitats. Thus, juvenile brown shrimp may have an affinity for quality intertidal creek habitat, as has been observed for some juvenile transient fish species that exhibited high site fidelity for particular intertidal creeks during their periods of estuarine residency (Garwood et al. 2019). Considered together, it appears that despite differences in habitat geomorphology, temporal availability, and spatial extent, intertidal creek habitat may function similarly to other habitats in the estuarine seascape for juvenile brown shrimp.
Brown shrimp growth rate estimates from this study are similar to those from other estuaries in the northern Gulf of Mexico, the only other region where published estimates are available, despite substantial differences in estimation method and salt marsh habitat type (Fig. 5). The similarity of estimated rates among regions and habitats suggests that, at least in terms of growth, the relative habitat value of intertidal creeks is comparable to that of salt marsh edge, surface, and pond habitat. The similarity of our rates, derived from sampling free-ranging shrimp at high temporal resolution, also suggests that, at least for the juvenile and sub-adult period, reliable growth rate estimates can be derived for intertidal creek habitats while avoiding some of the limitations of other experimental methods (e.g., caging artifacts; Baker and Minello 2010). Our limited ability to distinguish cohorts, together with a lack of clear increase in total length, during the latter half of the study period, is likely due to the mixing of late-arriving individuals with individuals from the initial cohort, with each group displaying different growth rates (Mace and Rozas 2015). This supports the utility of focusing on the initial cohort of juvenile brown shrimp when using the length-frequency method to estimate growth.
Comparison of published brown shrimp growth rate estimates (mm TL day−1) during April through the beginning of June from studies conducted in northern Gulf of Mexico estuaries with those from this study, both absolute and apparent growth estimates. All growth rates represent weekly estimates except for Leo et al. (2018), which covers the full period (April to June; only start points shown). Published studies used a variety of methods including length-frequency of free-ranging shrimp (St. Amant et al. 1966), caging (Rozas and Minello 2009, 2011, 2015), and mark-recapture (Leo et al. 2018)
Habitat-specific characterization of demographic rates such as growth and mortality, and the relative importance of environmental conditions and density-dependence on those vital rates, will allow for more effective designations of EFH within dynamic estuarine ecosystems (Searcy et al. 2007). Beyond fishery applications, other coastal management decisions could also benefit from improved understanding of fine-scale estimates in nekton growth rates. For example, quantifying nursery value for habitat management (Sheaves et al. 2015), mitigation banking for coastal development (Hough and Robertson 2009), ecosystem restoration projects (Wainger et al. 2017), and environmental impact or injury assessment (e.g., Beyer et al. 2016) could each utilize information on the relative values of specific habitat types. Changes to salt marsh extent, habitat geomorphology, and increased fragmentation due to sea level rise can impact nekton composition, abundance, and species vital rates including growth (James et al. 2021), underscoring the need for detailed information on species- and habitat-specific demographic rates.
References
Allen, D.M., S.S. Haertel-Borer, B.J. Milan, D. Bushek, and R.F. Dame. 2007. Geomorphological determinants of nekton use of intertidal salt marsh creeks. Marine Ecology Progress Series 329: 57–71.
Allen, D.M., V. Ogburn-Matthews, and P.D. Kenny. 2017. Nekton use of flooded salt marsh and an assessment of intertidal creek pools as low-tide refuges. Estuaries and Coasts 40 (5): 1450–1463.
Allen, D.M., W.B. Allen, R.F. Feller, and J.S. Plunket. 2014. Site profile of the North Inlet–Winyah Bay National Estuarine Research Reserve. North Inlet–Winyah Bay National Estuarine Research Reserve. Georgetown, SC. 432 pp.
Baker, R., and T.J. Minello. 2010. Growth and mortality of juvenile white shrimp Litopenaeus setiferus in a marsh pond. Marine Ecology Progress Series 413: 95–104.
Baker, R., B. Fry, L.P. Rozas, and T.J. Minello. 2013. Hydrodynamic regulation of salt marsh contributions to aquatic food webs. Marine Ecology Progress Series 490: 37–52.
Baker, R., M.D. Taylor, K.W. Able, M.W. Beck, J. Cebrian, D.D. Colombano, R.M. Connolly, C. Currin, L.A. Deegan, I.C. Feller, B.L. Gilby, M.E. Kimball, T.J. Minello, L.P. Rozas, C. Simenstad, R.E. Turner, N.J. Waltham, M.P. Weinstein, S.L. Ziegler, P.S.E. zu Ermgassen, C. Alcott, S.B. Alford, M.A. Barbeau, S.C. Crosby, K. Dodds, A. Frank, J. Goeke, L.A.G. Gaines, F.E. Hardcastle, C.J. Henderson, W.R. James, M.D. Kenworthy, J. Lesser, D. Mallick, C.W. Martin, A.E. McDonald, C. McLuckie, B.H. Morrison, J.A. Nelson, GS Norris GS, J. Ollerhead, J.W. Pahl, S. Ramsden, J.S. Rehage, J.F. Reinhardt, R.J. Rezek, L.M. Risse, J.A.M. Smith, E.L. Sparks, and L.W. Staver. 2020. Fisheries rely on threatened salt marshes. Science 370 (6517): 670–671.
Barbier, E.B., S.D. Hacker, C. Kennedy, E.W. Koch, A.C. Stier, and B.R. Silliman. 2011. The value of estuarine and coastal ecosystem services. Ecological Monographs 81 (2): 169–193.
Bearden, C.M. 1961. Notes on postlarvae of commercial shrimp (Penaeus) in South Carolina. Contributions of the Bears Bluff Laboratory 33: 1–8.
Beck, M.W., K.L. Heck, K.W. Able, D.L. Childers, D.B. Eggleston, B.M. Gillanders, B. Halpern, C.G. Hays, K. Hoshino, T.J. Minello, R.J. Orth, P.F. Sheridan, and M.P. Weinstein. 2001. The identification, conservation and management of estuarine and marine nurseries for fish and invertebrates. BioScience 51: 633–641.
Beyer, J., H.C. Trannum, T. Bakke, P.V. Hodson, and T.K. Collier. 2016. Environmental effects of the Deepwater Horizon oil spill: A review. Marine Pollution Bulletin 110 (1): 28–51.
Blanco-Martinez, Z., R. Perez-Castaneda, J.G. Sanchez-Martinez, F. Benavides-Gonzalez, J.L. Rabago-Castro, M.L. Vazquez-Sauceda, and L. Garrido-Olvera. 2020. Density-dependent condition of juvenile penaeid shrimps in seagrass-dominated aquatic vegetation beds located at different distance from a tidal inlet. PeerJ 8: e10496.
Colombano, D.D., A.D. Manfree, T.A. O’Rear, J.R. Durand, and P.B. Moyle. 2020. Estuarine-terrestrial habitat gradients enhance nursery function for resident and transient fishes in the San Francisco estuary. Marine Ecology Progress Series 637: 141–157.
Dame, R., M. Alber, D. Allen, M. Mallin, C. Montague, A. Lewitus, A. Chalmers, R. Gardner, C. Gilman, B. Kjerfve, J. Pinckney, and N. Smith. 2000. Estuaries of the south Atlantic coast of North America: Their geographical signatures. Estuaries 23 (6): 793–819.
Dame, R., T. Chrzanowski, K. Bildstein, B. Kjerfve, H. McKellar, D. Nelson, J. Spurrier, S. Stancyk, H. Stevenson, J. Vernberg, and R. Zingmark. 1986. The outwelling hypothesis and North inlet, South Carolina. Marine Ecology Progress Series 33: 217–229.
DeLancey, L.B., J.E. Jenkins, and J.D. Whitaker. 1994. Results of long-term, seasonal sampling for Penaeus postlarvae at Breach Inlet, South Carolina. Fishery Bulletin 92: 633–640.
DeLancey, L.B., E. Wenner, and J.E. Jenkins. 2008. Long-term trawl monitoring of white shrimp, Litopenaeus setiferus (Linnaeus), stocks within the ACE Basin National Estuarine Research Reserve, South Carolina. Journal of Coastal Research S I (55): 193–199.
Ditty, J.G. 2011. Young of Litopenaeus setiferus, Farfantepenaeus aztecus and F. duorarum (Decapoda: Penaeidae): A reassessment of characters for species discrimination and their variability. Journal of Crustacean Biology 31: 458–467.
Doerr, J.C., H. Liu, and T.J. Minello. 2016. Salinity selection by juvenile brown shrimp (Farfantepenaeus aztecus) and white shrimp (Litopenaeus setiferus) in a gradient tank. Estuaries and Coasts 39: 829–838.
Fowler, A.E., J.W. Leffler, S.P. Johnson, L.B. DeLancey, and D.M. Sanger. 2018. Relationships between meteorological and water quality variables and fisheries-independent white shrimp (Litopenaeus setiferus) catch in the ACE Basin NERR, South Carolina. Estuaries and Coasts 41: 79–88.
Fry, B., D.M. Baltz, M.C. Benfield, J.W. Fleeger, A. Gace, H.L. Haas, and Z.J. Quinones-Rivera. 2003. Stable isotope indicators of movement and residency for brown shrimp (Farfantepenaeus aztecus) in coastal Louisiana marshscapes. Estuaries 26 (1): 82–97.
Gardner, L.R., and M. Bohn. 1980. Geomorphic and hydraulic evolution of tidal creeks on a subsiding beach ridge plain, North Inlet, S.C. Marine Geology 34: M91–M97.
Garwood, J.A., D.M. Allen, M.E. Kimball, and K.M. Boswell. 2019. Site fidelity and habitat use by young-of-the-year transient fishes in salt marsh intertidal creeks. Estuaries and Coasts 42(5): 1387–1396.
Haas, H.L., E.C. Lamon III., K.A. Rose, and R.F. Shaw. 2001. Environmental and biological factors associated with stage specific abundance of brown shrimp (Penaeus aztecus) in Louisiana: Applying a new combination of statistical techniques to long-term monitoring data. Canadian Journal of Fisheries and Aquatic Sciences 58: 2258–2270.
Hackney, C.T., and W.D. Burbanck. 1976. Some observations on the movement and location of juvenile shrimp in coastal waters of Georgia. Bulletin of the Georgia Academy of Science 34: 129–136.
Hough, P., and M. Robertson. 2009. Mitigation under Section 404 of the Clean Water Act: Where it comes from, what it means. Wetlands Ecology and Management 17: 15–33
Hunter, J., and R.J. Feller. 1987. Immunological dietary analysis of two penaeid shrimp species from a South Carolina tidal creek. Journal of Experimental Marine Biology and Ecology 107: 61–70.
James, W.R., Z.M. Topor, and R.O. Santos. 2021. Seascape configuration influences the community structure of marsh nekton. Estuaries and Coasts 44: 1521–1533.
Kimball, M.E., B.W. Pfirrmann, D.M. Allen, V. Ogburn-Matthews, and P.D. Kenny. 2023. Intertidal creek pool nekton assemblages: Longterm patterns in diversity and abundance in a warm-temperate estuary. Estuaries and Coasts 46(3): 860–877.
Knudsen, E.E., W.H. Herke, and J.M. Mackler. 1977. The growth rate of marked juvenile brown shrimp, Penaeus aztecus, in a semi-impounded Louisiana coastal marsh. Proceedings of the Gulf and Caribbean Fisheries Institute 29: 144–159.
Knudsen P.A., W. H. Herke, and E.E. Knudsen. 1985. Emigration of brown shrimp from a low-salinity shallow-water marsh. Proceedings of the Louisiana Academy of Sciences 48: 30–40.
Leo, J.P., T.J. Minello, and W.E. Grant. 2018. Assessing variability in juvenile brown shrimp growth rates in small marsh ponds: An exercise in model evaluation and improvement. Marine and Coastal Fisheries 10 (3): 347–356.
Levin, P.S., and G.W. Stunz. 2005. Habitat triage for exploited fishes: Can we identify essential “Essential Fish Habitat?” Estuarine, Coastal and Shelf Science 64: 70–78.
Mace, M.M., III., and L.P. Rozas. 2015. Estimating natural mortality rates of juvenile white shrimp Litopenaeus setiferus. Estuaries and Coasts 38: 1580–1592.
Mace, M.M., III., and L.P. Rozas. 2017. Population dynamics and secondary production of juvenile white shrimp (Litopenaeus setiferus) along an estuarine salinity gradient. Fishery Bulletin 115: 74–88.
Mace, M.M., III., M.E. Kimball, and E.R. Haffey. 2019. The nekton assemblage of salt marsh pools in a southeastern United States estuary. Estuaries and Coasts 42 (1): 264–273.
McIvor, C.C., and W.E. Odum. 1988. Food, predation risk, and microhabitat selection in a marsh fish assemblage. Ecology 69 (5): 1341–1351.
Miller, J.R., and L.R. Gardner. 1981. Sheet flow in a salt-marsh basin, North Inlet, South Carolina. Estuaries 4 (3): 234–237.
Minello, T.J. 1999. Nekton densities in shallow estuarine habitats of Texas and Louisiana and the identification of essential fish habitat. American Fisheries Society Symposium 22: 43–75.
Minello, T.J. 2017. Fishery habitat in estuaries of the Gulf of Mexico: reflections on geographical variability in salt marsh value and function. Gulf and Caribbean Research 28(1): ii-xi.
Minello, T.J., K.W. Able, M.P. Weinstein, and C.G. Hays. 2003. Salt marshes as nurseries for nekton: Testing hypotheses on density, growth and survival through meta-analysis. Marine Ecology Progress Series 246: 39–59.
Minello, T.J., L.R. Rozas, and R. Baker. 2012. Geographic variability in salt marsh flooding patterns may affect nursery value for fishery species. Estuaries and Coasts 35: 501–514.
Parrack, M.L. 1979. Aspects of brown shrimp, Penaeus aztecus, growth in the northern Gulf of Mexico. Fishery Bulletin 76 (4): 827–833.
Perez-Castaneda, R., J.G. Sánchez-Martínez, and G. Aguirre-Guzmán. 2012. Growth and survival of brown shrimp (Farfantepenaeus aztecus) in a closed recirculation seawater system at different salinities. Thai Journal of Veterinary Medicine 42 (1): 95–99.
Perez-Castaneda, R., and O. Defeo. 2005. Growth and mortality of transient shrimp populations (Farfantepenaeus spp.) in a coastal lagoon of Mexico: role of the environment and density-dependence. ICES Journal of Marine Science 62 (1): 14–24.
R Core Team 2021. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
Rosenberg, A., T.E. Bigford, S. Leathery, R.L. Hill, and K. Bickers. 2000. Ecosystem approaches to fishery management through essential fish habitat. Bulletin of Marine Science 66 (3): 535–542.
Rozas, L.P., and T.J. Minello. 2009. Using nekton growth as a metric for assessing habitat restoration by marsh terracing. Marine Ecology Progress Series 394: 179–193.
Rozas, L.P., and T.J. Minello. 2011. Penaeid shrimp growth rates vary along an estuarine salinity gradient: Implications for managing river diversions. Journal of Experimental Marine Biology and Ecology 397: 196–207.
Rozas, L.P., and T.J. Minello. 2015. Small-scale nekton density and growth patterns across a saltmarsh landscape in Barataria Bay, Louisiana. Estuaries and Coasts 38: 2000–2018.
Rozas, L.P., C.C. McIvor, and W.E. Odum. 1988. Intertidal rivulets and creekbanks: Corridors between tidal creeks and marshes. Marine Ecology Progress Series 47: 303–307.
RStudio Team 2021. RStudio: integrated development for R. RStudio, PBC, Boston, MA. http://www.rstudio.com/.
SAFMC 1998. Comprehensive amendment addressing essential fish habitat in fishery management plans of the South Atlantic Region. South Atlantic Fishery Management Council, Charleston, South Carolina.
Saoud, I.P., and D.A. Davis. 2003. Salinity tolerance of brown shrimp Farfantepenaeus aztecus as it relates to postlarval and juvenile survival, distribution, and growth in estuaries. Estuaries 26 (4A): 970–974.
Searcy, S.P., D.B. Eggleston, and J.A. Hare. 2007. Is growth a reliable indicator of habitat quality and essential fish habitat for a juvenile estuarine fish? Canadian Journal of Fisheries and Aquatic Sciences 64 (4): 681–691.
Sheaves, M., R. Baker, I. Nagelkerken, and R.M. Connolly. 2015. True value of estuarine and coastal nurseries for fish: Incorporating complexity and dynamics. Estuaries and Coasts 38 (2): 401–414.
Shervette, V.R., and F. Gelwick. 2008. Relative nursery function of oyster, vegetated marsh edge, and nonvegetated bottom habitats for juvenile white shrimp Litopenaeus setiferus. Wetlands Ecology and Management 16: 405–419.
Shoup, D.E., and P.H. Michaletz. 2017. Growth estimation: Summarization. In Age and Growth of Fishes: Principles and Techniques, ed. M.C. Quist and D.A. Isermann, 233–264. Bethesda, MD: American Fisheries Society.
Simpson, G. 2021. gratia: Graceful 'ggplot'-Based Graphics and Other Functions for GAMs Fitted Using 'mgcv'. R package version 0.6.0.
St. Amant, L.S., J.G. Broom, and T.B. Ford. 1966. Studies of the brown shrimp, Penaeus aztecus, in Barataria Bay, Louisiana, 1962–1965. Proceedings of the Gulf and Caribbean Fisheries Institute 18: 1–17.
Tuckey, T.D., J.L. Swinford, M.C. Fabrizio, H.J. Small, and J.D. Shields. 2021. Penaeid shrimp in Chesapeake Bay: Population growth and black gill disease syndrome. Marine and Coastal Fisheries 13: 159–173.
Wainger, L.A., D.H. Secor, C. Gurbisz, W.M. Kemp, P.M. Glibert, E.D. Houde, J. Richkus, and M.C. Barber. 2017. Resilience indicators support valuation of estuarine ecosystem restoration under climate change. Ecosystem Health and Sustainability 3 (4): e01268.
Webb, S., and R.T. Kneib. 2004. Individual growth rates and movement of juvenile white shrimp (Litopenaeus setiferus) in a tidal marsh nursery. Fishery Bulletin 102: 376–388.
Webb, S.R., and R.T. Kneib. 2002. Abundance and distribution of juvenile white shrimp Litopenaeus setiferus within a tidal marsh landscape. Marine Ecology Progress Series 232: 213–223.
Wenner, E.L., and H.R. Beatty. 1993. Utilization of shallow estuarine habitats in South Carolina, USA, by postlarval and juvenile stages of Penaeus spp. (Decapoda: Penaeidae). Journal of Crustacean Biology 13 (2): 280–295.
Wicker, A.M., B.M. Currin, and J.M. Miller. 1988. Migration of brown shrimp in nursery areas of Pamlico Sound, North Carolina, as indicated by changes in length-frequency distributions. Transactions of the American Fisheries Society 117 (1): 92–94.
Williams, A.B. 1955. A contribution to the life histories of commercial shrimps (Penaeidae) in North Carolina. Bulletin of Marine Science 5 (2): 116–146.
Williams, A.B. 1969. A ten-year study of meroplankton in North Carolina estuaries: Cycles of occurrence among penaeidean shrimps. Chesapeake Science 10 (1): 36–47.
Wood, S.N. 2017. Generalized additive models: an introduction with R (2nd edition). Chapman and Hall/CRC.
Yu, Y. 2022. mixR: An R package for finite mixture modeling for both raw and binned data. Journal of Open Source Software 7 (69): 4031.
Zein-Eldin, Z.P., and M.L. Renaud. 1986. Inshore environmental effects on brown shrimp, Penaeus aztecus, and white shrimp, P. setiferus, populations in coastal waters, particularly of Texas. Marine Fisheries Review 48 (3): 9–19.
Zink, I.C., J.A. Browder, D. Lirman, and J.E. Serafy. 2017. Review of salinity effects on abundance, growth, and survival of nearshore life stages of pink shrimp (Farfantepenaeus duorarum). Ecological Indicators 81: 1–17.
Acknowledgements
We would like to thank faculty, staff, and students from the USC Baruch Marine Field Laboratory for their help with this research (Nate Adams, Dennis Allen, Tyler Gobert, Nadya Gutierrez, Andrea Landaverde, Cate O’Brien).
Funding
Support for KMG was provided by the University of South Carolina Magellan program. Funding for MMP and RPD was provided by the Office for Coastal Management within the National Oceanic and Atmospheric Administration via the operations award to the North Inlet-Winyah Bay National Estuarine Research Reserve (Grants NA20NOS4200030 and NA21NOS420039).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
This research was conducted in accordance with the guidelines set forth in University of South Carolina IACUC Animal Care and Use Protocol #2484-101488-030420.
Additional information
Communicated by Ronald Baker
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Glover, K.M., Kimball, M.E., Pfirrmann, B.W. et al. Juvenile Brown Shrimp (Farfantepenaeus aztecus) Use of Salt Marsh Intertidal Creeks as Nursery Habitat. Estuaries and Coasts 46, 1895–1906 (2023). https://doi.org/10.1007/s12237-023-01251-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-023-01251-5