Abstract
Recruitment is a strong determinant of year class strength and adult population density especially for sessile benthic invertebrates where post-settlement mortality and competition are low or relatively stable over time. A series of surveys were undertaken to characterize recruitment and post-settlement processes for two species of burrowing shrimps, Neotrypaea californiensis and Upogebia pugettensis in order to determine how they influenced broader adult populations in US west coast estuaries. On average, U. pugettensis decapodids settled earlier (April–July), recruited almost exclusively to areas with conspecific adults, and grew more rapidly during their first summer than N. californiensis. Neotrypaea californiensis decapodids settled and recruited over a longer period (June–November) and were distributed across the tidal flat. While initially more abundant in areas with conspecific adults, they also either survived better or redistributed as small juvenile shrimp to areas where adults were absent. Linear relationships were found between abundance of newly recruited (0+ age class) shrimp and that of older 1+ shrimp a year later. Positive slopes were close to one for N. californiensis but less than one for U. pugettensis, suggesting lower survival. Annual recruitment varied dramatically but was more consistent for both species in Yaquina Bay. Patterns in strong recruitment years amongst estuaries, particularly for U. pugettensis, suggest the presence of multi-estuary metapopulations linked via larval dispersal. These results have important implications for shrimp population management including control for shellfish aquaculture, but also conservation of estuarine habitats due to the strong influence of these ecosystem engineers on the benthic community.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Several species of burrowing shrimp inhabit estuaries along the US Pacific coast including two species of ghost shrimp Neotrypaea californiensis and N. gigas (Decapoda: Axiidea: Callianassidae) and the blue mud shrimp Upogebia pugettensis (Decapoda: Gebiidea: Upogebiidae). These shrimp are considered to be ecosystem engineers because they make and maintain extensive galleries in intertidal and subtidal sediments and influence not only sediment biogeochemistry (Webb and Eyre 2004; D’Andrea and DeWitt 2009), but also benthic community composition and the presence of other engineers like seagrass via bioturbation and burrow irrigation (Dumbauld et al. 2001; Dumbauld and Wyllie-Echeverria 2003; Pillay and Branch 2011; Castorani et al. 2014). They play a key role in estuarine food webs not only as consumers of phytoplankton and bacteria (Shimoda et al. 2007; Bosley et al. 2017), but also as prey for other consumers from whales to sturgeon and smaller fish (Feldman et al. 2000; Harada and Tamaki 2004; Dumbauld et al. 2008). From a management perspective, these shrimp have been shown to influence the abundance of important suspension feeding bivalves in estuaries worldwide (Pillay et al. 2007; Takeuchi et al. 2013) and have been actively controlled in US Pacific coast estuaries by shellfish farmers due to the negative effects they have on bivalve, particularly Pacific oyster (Crassostrea gigas) aquaculture (Feldman et al. 2000; Dumbauld et al. 2004; Dumbauld et al. 2006).
Neotrypaea californiensis and U. pugettensis have pelagic larvae that hatch in the estuary, leave as early stage larvae by moving to the surface on ebb tides, and develop in the nearshore coastal ocean (Johnson and Gonor 1982; Breckenridge and Bollens 2010; Shanks et al. 2014). Post-larval or decapodid stages recruit back to estuaries along this coast during two mostly separate periods (April–July for U. pugettensis and August–December for N. californiensis; Dumbauld et al., 1996). Like some of their counterparts elsewhere in the world (Wooldridge and Loubser 1996; Yannicelli et al. 2006b; Pineda et al. 2010; Tamaki et al. 2010; Hernaez et al. 2012; de Oliveira et al. 2016), the pelagic larval durations vary by species (PLD of 3 weeks for U. pugettensis (Hart 1937) and up to 8 weeks for N. californiensis (Bosley and Fritz, unpublished data) and their larvae are generally found within 10 nautical miles of the coastline where they can be one of the most abundant crustaceans in the meroplankton (513 m−3, Fisher et al. 2014; Hameed et al. 2018; T. D’Andrea, unpublished data). Unlike several other US west coast benthic invertebrates with broader distributions and more researched larval recruitment patterns like Dungeness crab and barnacles (Broitman et al. 2008; Shanks et al. 2010; Menge et al. 2011; Woodson et al. 2012) or species with larvae that are mostly retained in estuaries like native oysters (Peteiro and Shanks 2015; Wasson et al. 2016), populations of these shrimp largely depend on larval and post-larval behavior to facilitate their return to small estuaries located at relatively large distances from one another along this open coastline.
Shrimp population density and recruitment to one of these estuaries, Willapa Bay, Washington, have been monitored for several decades and changes in population abundance over this time period previously hypothesized to be a result of fluctuations in larval recruitment (Dumbauld et al. 2004; Dumbauld et al. 2006; Dumbauld et al. 2011). Recruitment to a population is a process that involves decapodid settlement to the benthos, subsequent survival, and potential movement thereafter. Although these shrimp are thought to be sedentary as adults, juveniles of some species have been shown to move and relocate their burrows (Tamaki and Ingole 1993; Feldman 2001). Previous work has shown recruitment to be a strong determinant of adult population density especially for species where both interspecific and intraspecifc competition are low. This has been exhibited in several species of sessile invertebrates that inhabit hard substratum (e.g., barnacles and mussels; Gaines and Roughgarden 1985), but the pattern is less clear for marine soft sediment systems where disturbance and interspecific interactions are important (Woodin 1976; Woodin et al. 1995; Thrush et al. 2012). Evidence from relatively small-scale removal experiments suggested an inverse relationship between recruitment and adult density for N. californiensis (Feldman et al. 2000), yet Tamaki and Ingole (1993) found that a related species, Neotrypaea harmandi, settled broadly and juvenile survival was higher where adults were present.
We examined long-term time series of burrowing shrimp recruitment and adult population density in two estuaries along the US Pacific northwest coast with the goal of determining whether successful recruitment is related to overall population trends in these estuaries. For the purpose of this study, we define recruitment somewhat generally as the abundance of surviving settlers that recruit to the benthos and are quantified during the first year (age 0+). Our primary objectives were to (1) determine if trends in long-term recruitment data at single locations reflected overall population trends within and amongst estuaries and (2) further characterize the recruitment process and broader spatial distribution of burrowing shrimp recruitment within estuaries, taking advantage of 2 years with strong recruitment events. We were also interested in whether recruitment to these two estuaries was temporally synchronous amongst years and therefore might reflect overall trends in a larger coastwide metapopulation. Spatiotemporal trends in recruitment could suggest potential links to oceanographic variables that might eventually be used to evaluate and potentially forecast population size. Our overall goal was not only to inform continued burrowing shrimp population monitoring and management for shellfish aquaculture, but also to inform conservation efforts for these shrimp and other estuarine resources in recognition of the broader role that these deep-dwelling burrowing shrimp play as engineers in these coastal systems.
Methods and Materials
Study Locations
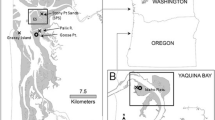
This study was conducted in Yaquina Bay, Oregon (44° N, 124° W), and Willapa Bay, Washington (46° N, 124° W; Fig. 1), drowned river valley estuaries located along the Pacific northwest coast of the continental USA. Forty-eight percent (8.2 km2) of the Yaquina estuary and 68% (227 km2) of Willapa Bay consists of intertidal sand and mudflats that are regularly exposed on semidiurnal low tides. Both U. pugettensis and N. californiensis occur at low density in subtidal areas (< 50 m−2; T.H. DeWitt, unpublished data), but their distribution is thought to be limited mostly to intertidal areas by predation (Posey 1986). Upogebia pugettensis densities are highest in the low to mid intertidal zone and N. californiensis are most abundant in the mid to upper intertidal and densities of both species can exceed 400 shrimp m−2 (Bird 1982; DeWitt et al. 2004; Dumbauld et al. 2011).
Map showing long term monitoring locations for both species of burrowing shrimp in Willapa Bay, Washington and Yaquina Bay, Oregon, USA. Also shown are broad area locations surveyed in 2010–11 (CR Cedar River, ES Ellen Sands, SPS Stony Point Sands, GI Grassy Island, MIS Middle Island Sands, NS N. Stackpole, RB Rhodesia Beach in Willapa Bay, IF Idaho Flats in Yaquina Bay)
Long-Term Population Monitoring
Population structure and local shrimp density were quantified by collecting sediment cores annually within dense shrimp colonies. Neotrypaea californiensis were collected from two locations in Willapa Bay: near the Palix River (1988–2009) and near Stony Point (2009–2016), as well as from one location on Idaho Flats in Yaquina Bay (2005–2016; Fig. 1). These samples were typically taken in September or October. Due to sampling constraints and effort required, cores were initially haphazardly located within a dense shrimp colony at each location. Subsequent sampling efforts from population assessments conducted at a broader spatial scale and utilizing burrow counts have shown that the records obtained from these haphazard sampling protocols adequately track trends in the larger shrimp populations (see the “Discussion” section). Nonetheless, a randomized sample design utilizing a fixed area within the shrimp colony at each location was adopted in 2014 to better reflect the fine-scale variability in shrimp density. Upogebia pugettensis were collected from two locations in Willapa Bay: near the Cedar River (1988 to 2004 and 2007) and near Goose Pt. (2003–2009), as well as from one location on Idaho Flats in Yaquina Bay (2005–2016; Fig. 1). Samples for U. pugettensis were also taken in September and October from 1988 to 2004 and then in June or July from 2005 to present. Ten core samples were taken at each location for both species by inserting a large core (60 cm depth × 40 cm diameter) in the substrate, excavating the contents with a shovel and sieving sediments (3-mm mesh) to collect all shrimps. Species, gender, and size (carapace length, CL in mm) were recorded for all shrimp collected.
The abundance of small juvenile < 1-year-old shrimp that were presumed to have recently recruited to the sediment was quantified using smaller cores (20 cm depth × 26.5 cm diameter; 1998–2012 and 20 cm depth × 12.3 cm diameter; 2013–2016) at the same locations and in the same months described above (except samples for U. pugettensis recruitment were continued at Goose Pt. in Willapa Bay through 2016). Sediment was excavated from 10 cores (three 12.3-cm diameter cores were combined to represent each of 10 samples from 2013 to 2016) and sieved (1 mm mesh) to retain juvenile shrimp. Small shrimp were placed under a dissecting scope where the size (CL in mm) was measured and recorded for all shrimp collected. These recruit surveys were generally conducted once annually alongside large cores made for each species.
Recruitment Surveys
Several additional surveys were conducted to take advantage of recruitment events that occurred in Yaquina Bay in 2010 and 2011 and in Willapa Bay from 2012 to 2015 to gain further understanding of shrimp recruitment patterns. First, the temporal pattern of shrimp recruitment was assessed by taking 10 samples (20 cm depth × 26.5 cm diameter core, contents sieved on 1 mm mesh) approximately every 2 weeks from June to December 2010 at the established monitoring locations (described above) for N. californiensis and from April to October 2011 for U. pugettensis. In addition, the depth distribution of small shrimp was measured either by excavating samples within 26.5 cm diameter cores to progressive depth intervals (0–5, 5–10, 10–15, and 15–20 cm) or sampling with smaller 12.5 cm diameter cores, extruding contents and then partitioning contents by depth interval (0–20, 20–30, and 30–60 cm).
Second, we characterized the spatial distribution of shrimp recruitment across one large tidal flat known as Idaho Flat in Yaquina Bay (Fig. 1) in October 2010 and July 2011. We used data collected in a broader estuary-wide survey conducted in 2010 to select 50 sampling locations that were classified as being dominated by U. pugettensis (15 locations), N. californiensis (16 locations), or neither species (open habitat, 19 locations). A single 26.5-cm diameter core was excavated to 10 cm depth at each location and contents sieved (1 mm mesh) to retain juvenile shrimp. A follow-up survey was conducted in April 2011 at a subset of locations where N. californiensis recruits had been sampled the previous fall (October 2010, except not at locations where U. pugettensis adults were present) using larger cores (40 cm diameter × 60 cm depth sieved with 1 mm mesh) in order to examine survival rate and quantify the relationship between N. californiensis recruit density and the abundance of older shrimp. The fine-scale distribution of newly recruited shrimp inside and outside of an established N. californiensis colony in Yaquina Bay was also characterized by taking cores (26.5 cm diameter to 20 cm depth) at five locations along four transects located 10 m outside the shrimp colony, along the outside edge of the colony, along the inside edge of the colony, and 10 m within the colony in November 2010.
Finally, to examine recruitment more broadly, we conducted an along estuary survey of N. californiensis recruitment in spring 2012 at 7 locations adjacent to shellfish growing areas in Willapa Bay, but with relatively high adult N. californiensis density (10 samples, each consisting of three 12.3 cm diameter cores to 20 cm depth at each location). Core samples were also taken along a transect across the intertidal gradient at one of these locations in Willapa Bay (Grassy Island,10 samples) in spring 2013 and at three tidal elevations at a location in Yaquina Bay (OSU Dock, 12 samples in spring 2013 and 9 samples in 2014).
Data Analyses
All data from the long-term monitoring locations were entered into an MS Access database and imported into R (R Core Development Team 2015) for the graphical and statistical analyses. The mean density of shrimps per unit area sampled (m2) was calculated for each location and year. Frequency histograms of carapace length were then examined to discern the presence and size of newly recruited (within a year of settlement, 0+) shrimp during years when relatively large recruitment events took place in Yaquina Bay (N. californiensis, 2010 and U. pugettensis, 2011) and the subsequent year to discern 1-year-old (1+) shrimp. We combined shrimp length measurements from small cores with those from large cores and used the “mixdist” package in R (MacDonald 2015) to fit a mixture of lognormal distributions to the resulting combined length frequency distributions and establish breaks between size classes. A bin size of 0.5 mm was used for these analyses because it gave the greatest resolution of modes, and initial starting values were selected based on visual inspection of the modes within the overall length frequency distribution. Visual knife-edge breaks seemed adequate for separating age 0+ shrimp from age 1+ shrimp for both species, so we did not use the mixdist program and/or the resulting distributions to reclassify data from all years but instead used it to establish these visual breaks between presumed age classes. We encountered the most difficulty in establishing the break between 1+ and > 1+ shrimp (adults) but used size class definitions to estimate the density of shrimp collected within three size/age classes (0+, 1+, and > 1+) for all years and estuaries. In several cases, historical collections from Willapa Bay were not made at the same time of year, so size breaks were adjusted slightly for these cases based on ancillary data collected during both periods.
The relationship between the average density of new recruits (0+ age class) and that of older 1+ and > 1+ shrimp for each estuary was quantified with a series of regression models. Initially, simple linear regression was used to relate average 0+ shrimp density lagged 1 year to average 1+ density to examine the relationship of shrimp recruitment in each year to age 1+ shrimp collected the following year. Simple linear models were applied using least squares regression and resulting slopes compared to a 1:1 relationship. Next, we identified relationships between 1+ shrimp abundance estimated from the cohort analysis and older > 1+ shrimp abundance by exploring several lag periods (1–4 years) for these older shrimp to determine whether strong recruitment events resulted in large shrimp populations and over what timescale this increase occurred. Simple linear and quadratic models were applied to each lag period using least squares regression. Best fitting models were selected by visual inspection of model residuals and evaluation of model fit with adjusted r2. Both 0+ and 1+ shrimp numbers were 1 + log-transformed prior to analysis to reduce heteroscedasticity and skewness in the data. All regression analyses were conducted in R (R core development team 2015). Data from 2010 to 2011 recruitment surveys were analyzed using analysis of variance (ANOVA) in a general linear model with fixed factors (e.g., location and habitat; NCSS® statistical software).
Results
Long-Term Population Monitoring
Neotrypaea californiensis density at the Palix River location in Willapa Bay, Washington (Fig. 1) increased between 1988 and 1995 to a high of 472 shrimp m−2 and then declined to 54 shrimp m−2 in 2009 (Fig. 2). Shrimp disappeared from our standard monitoring area and became increasingly patchy at the scale of several hundred meters at this location making coring impractical. In response, we moved our monitoring location to Stony Point Sands (Fig. 1) in 2009, where mean N. californiensis density was comparable, but where the distribution of shrimp was more homogenous and extensive so that cores could still be made. Neotrypaea californiensis density at Stony Point Sands declined from 97 shrimp m−2 in 2009 to 43 shrimp m−2 in 2011 and then increased to 153 shrimp m−2 by 2016. The majority of small (0–3.5 mm CL) newly recruited shrimp likely passed through our 3 mm mesh sieves, but these shrimp were present in separate samples taken with smaller cores and a finer 1-mm mesh sieve (Fig. 3). Strong N. californiensis recruitment events occurred in 1989 and 1993 (88 and 102 shrimp m−2 respectively; no data for 1990–1992) followed by little to no recruitment from 1995 to 2012 and then some moderate events in 2012, 2013, 2015, and 2016 (9–36 shrimp m−2).
Upogebia pugettensis density at the Cedar River location in Willapa Bay exhibited broad fluctuations around an average of 100 shrimp m−2 between 1988 and 2001 but declined to zero in 2003 and remained at zero in 2004 and 2007. In 2003, we moved our sampling location for U. pugettensis to a location near Goose Point where the density was 54 shrimp m−2, but by 2006, this population declined to near zero as well (Fig. 2). Upogebia pugettensis recruitment events also regularly occurred through 2000 (up to 88 shrimp m−2) and then declined to low levels for the remainder of the period monitored with the exception of 2010 (13 shrimp m−2; Fig. 3).
The density of N. californiensis on Idaho Flat in Yaquina Bay, Oregon declined from 414 shrimp m−2 in 2004 to 151 shrimp m−2 in 2014 and then increased to 280 shrimp m−2 in 2016 (Fig. 4). Upogebia pugettensis density fluctuated around an average of 200 shrimp m−2 increasing from 157 shrimp m−2 in 2005 to 368 shrimp m−2 in 2007 and declining to 145 shrimp m−2 in 2016. Small peaks in U. pugettensis density observed in 2007 and 2012 and N. californiensis density in 2011 followed similar peaks in the density of small shrimp sampled in separate recruitment samples the previous year (250 and 136 U. pugettensis recruits m−2, and 41 N. californiensis recruits m−2, respectively; Fig. 5).
Recruitment of N. californiensis and U. pugettensis in Yaquina Bay, Oregon. Values represent the mean density of small shrimp taken in 20 cm deep cores (bars represent SE). Note recruitment events for N. californiensis in 2010 and U. pugettensis in 2011, years that we conducted broader surveys across tidal flat
Recruitment Surveys
Significant recruitment events in 2010 and 2011 provided the opportunity to better characterize and understand the recruitment process. Newly recruited N. californiensis (two instars with mean CL of 1.1–1.5 and 2.3–2.7 mm CL, respectively) were noted in Yaquina Bay in late June 2010 and present in samples thereafter through December (Online resource Fig. S1). Though it was difficult to distinguish growth of these small shrimp, two cohorts were found in late September of 2010 and they were primarily found in the top 20 cm of sediment (Fig. 6). The smallest individuals (1–2 mm CL) were found exclusively in the top 10 cm. Newly recruited U. pugettensis (instar with mean CL of 1.9 mm; Online resource Fig. S2) were first present in Yaquina Bay in mid-May 2011. These small recruits were also primarily found in the top 5 cm, and all were found in the top 20 cm in June 2011. While a second cohort recruited in July, this first cohort grew to a mean size of 6.9 mm CL and remained near the surface (top 20 cm) through September of that year (Fig. 7). A broad area survey conducted across Idaho Flat in Yaquina Bay during October 2010 revealed a significantly higher density of N. californiensis recruits in habitat where adult N. californiensis were present than in open habitat where no shrimp were present, and no N. californiensis recruits were found in areas where U. pugettensis dominated (ANOVA, habitat, F = 6.23, df = 2, p = 0.004; Fig. 8a). Upogebia pugettensis recruits were much larger (\( \overline{x} \) = 8.2 mm CL, SE = 0.46) and only found in habitat where adult U. pugettensis were present. Results of a follow-up survey conducted in April 2011 for N. californiensis using a larger core (but sieving to 1 mm) showed no difference in the abundance of surviving recruits (\( \overline{x} \) = 4.19 ± 0.1 mm CL) in open habitat and areas where older N. californiensis were present (Fig. 8b). Neotrypaea californiensis also recruited to Willapa Bay in 2010, but they were not found at our long-term monitoring location at Stony Point Sands (Fig. 3). After a report from another researcher confirmed that recruits were present in the estuary (Jacob Moore, pers. comm.), we re-surveyed our long-term monitoring location and several other locations in April 2011. While recruits were still not found at our Stony Point Sands monitoring location or a nearby location, we documented low but variable abundance at several other locations along an estuarine gradient with statistically higher abundance at a location near Grassy Island (\( \overline{x} \) = 14.5 shrimp m−2, ANOVA on log transformed density, F = 2.51, df = 6, p = 0.033) and similar density at our Yaquina Bay location (7.25 shrimp m−2; Fig. 8c).
Average density of a small recruits (both species) from open habitat (no adult shrimp) and that with each species of adults present in a wide area tidal flat survey in Yaquina Bay during fall 2010, b older 1+ shrimp from same locations in Spring 2011 (only in open habitat and that dominated by N. californiensis adults, c N. californiensis recruits from a broader area survey in Willapa Bay during spring 2011 (CR Cedar River, ES Ellen Sands, SPS Stony Point Sands, GI Grassy island, MIS Middle island Sands, NS N Stackpole, RB Rhodesia Beach; see Fig. 1, also shown is Idaho flats from Yaquina Bay = IF), and d shrimp density from Yaquina Bay tidal flat surveys in summer 2011, small recruits (both species) from open habitat (no adult shrimp) and that with each species of adults present in broad tidal flat survey
In a second survey conducted in Yaquina Bay in July 2011, small N. californiensis recruits (CL < 3.5 mm) were found in all three habitats including the area with adult U. pugettensis (no significant difference in abundance, ANOVA, habitat, F = 0.33, df = 2, p = 0.72; Fig. 8d). Upogebia pugettensis recruits (\( \overline{x} \) = 4.0 ± 0.08 mm CL) were again most abundant where adults were present, though a few were also found in open habitat (ANOVA, habitat, F = 10.70, df = 2, p < 0.001; Fig. 8d). We found significantly more N. californiensis recruits along the edge in both open habitat and within a dense shrimp colony in Yaquina Bay, but no significant difference between habitats themselves in November 2011 (ANOVA, habitat, F = 0.20, df = 1, p = 0.023, Edge/Interior F = 6.35, df = 1, p = 0.662, Online resource Fig. S3A). This difference was not significant when only small < 2 mm CL shrimp were selected, but the power to detect a difference was low. Density of N. californiensis recruits and the subsequent year class of 1 year old shrimp varied with tidal elevation or at least distance to channel but was highest near the middle of the tidal flat at locations where this gradient was easily measured in Yaquina Bay (ANOVA, location, F = 5.88, df = 2, p = 0.012 for 0+ shrimp and location, F = 15.19, df = 2, p < 0.001 for 1+ shrimp; Online resource Fig. S3B) and a similar trend was observed in Willapa Bay (Online resource Fig. S3C).
Size Cohort Analyses 2010–2012
A closer examination of size cohorts of shrimp sampled in Yaquina Bay during significant recruitment events and subsequent years (2010–2012) suggests that there are often at least two cohorts of new 0+ shrimp recruits (both species) present in a given year. The cohort analysis also illustrates the difficulty in discerning a size range for older 1+ animals, particularly the break between these and potentially older animals (Table 1; Fig. 9). Juvenile U. pugettensis recruits grew much faster than N. californiensis with juvenile shrimp reaching a mean size of 9.6 to 12.1 mm CL 1 year post-settlement, whereas 1-year-old N. californiensis were smaller at 4.6 to 6.6 mm CL (note that the 1+ age class of shrimp in the recruitment event year (2010) were mostly absent and thus their estimated mean size (8.4 mm CL) is misleading). These discrete recruitment events, however, enabled us to use these size breakdowns to establish visual size breaks between 0+, 1+, and > 1+ shrimp in order to estimate densities and examine recruitment relationships for the long-term database (Table 2).
Results of cohort analyses on combined data for recruits from small cores and older shrimp from large cores using the “mixdist” package in R to fit a mixture of log normal distributions to combined length frequency distributions. Dashed lines represent cutoffs for 0+ and 1+ shrimp (see also Table 1). Data are shown for years when significant recruitment occurred and the subsequent year (2010, 2011 for N. californiensis, left and 2011, 2012 for U. pugettenis, right)
Correlation of Recruitment with Juvenile and Adult Density
Linear relationships between newly recruited (0+ age class) shrimp lagged 1 year with that of older 1+ shrimp were significant with positive slopes and best fit using a multiplicative model (Fig. 10; Table 3). Slopes of these relationships for N. californiensis were very close to 1 (Willapa Bay, 0.92 ± 0.22; Yaquina Bay, 0.83 ± 0.28), while those for U. pugettensis were less (Willapa Bay, 0.76 ± 0.39; Yaquina Bay, 0.63 ± 0.09), suggesting lower first-year survival for U. pugettensis in both Willapa Bay and Yaquina Bay. There were also obvious outliers in these relationships where recruitment was either not observed at all or was relatively low, yet the older 1+ age class of shrimp was found at higher density the following year. This was particularly the case for N. californiensis in Willapa Bay, suggesting either sampling error or post-recruitment movement/immigration of these small shrimp. Explorations of relationships between the 1+ year class and older > 1+ shrimp (grouped together as one “adult” age group) revealed significant relationships for both species in Willapa Bay with the best relationship achieved using a 2-year lag for U. pugettensis and longer 4-year lag for N. californiensis (Fig. 11; Table 4; see also Online resource Figs. S4 and S6). Linear relationships were not significant for either species of shrimp in Yaquina Bay, although a quadratic relationship with a 2-year lag of adults fit the N. californiensis numbers reasonably well (r2 = 0.57, p = 0.017) and there was a slight positive linear trend with a 2-year lag for U. pugettensis. (Fig. 11; Table 4; see also Online resource Figs. S5 and S6).
Relationships between 1+ shrimp density and older age classes (> 1+) for both species and estuaries. Labels represent the recruitment year. Coefficients and model statistics are presented in Table 4
Discussion
Our results confirm that recruitment of two species of burrowing shrimps, N. californiensis and U. pugettensis, is highly variable from year to year but is directly related to subsequent abundance of larger shrimp and therefore directly influences population size of these important ecosystem engineers in US west coast estuaries. We found interspecific differences in seasonal timing of the return of decapodids from the ocean to these coastal estuaries, spatial patterns of recruitment to the benthos within the estuary, and growth of these small recruits and therefore the depth of their burrows in the sediment. These results have significant management implications, particularly for shellfish aquaculture operations where they are considered to be pests, but also for broader ecosystem management goals and conservation efforts in these estuaries.
The number of burrowing shrimp recruiting to the benthos at our long-term monitoring locations in two estuaries was highly variable from year to year and annual patterns clearly differed between the two shrimp species. Variable recruitment and lack of a significant adult spawner–juvenile recruitment relationship displayed by marine invertebrates and fish have intrigued ecologists and fishery managers for over a century (Houde 2008) but are hallmark features of these organisms with pelagic planktotrophic larvae that are subject to broad dispersal by currents, variable feeding conditions, and high predation in the ocean. While there has been extensive research on larval development and behaviors of individual invertebrate species including seminal original work by Thorson (1950, 1966), relatively few long-term recruitment records exist. This is particularly true for the US west coast where most of these records are for commercially fished or important managed species (Hannah 2011; Shanks 2013; Wasson et al. 2016), for those that inhabit hard substrates along the open coast (Broitman et al. 2008; Menge et al. 2011), or in another case, the introduced European green crab, Carcinus maenas (Yamada and Kosro 2010).
Recruitment limitation has been the established paradigm, especially for benthic invertebrates in regions like the US west coast, where strong seasonal upwelling dominates nearshore oceanography resulting in Eckman transport of surface waters equatorward and away from the shore (Connolly and Roughgarden 1998; Leslie et al. 2005; Shanks and Shearman 2009; Pineda et al. 2010). Until recently, it was expected that episodic recruitment was the result of storms and/or relaxation events where these currents reverse (Dudas et al. 2009; Garcia-Reyes and Largier 2012). Many estuarine invertebrate larvae exhibit behavior which allows them to be retained in estuaries (Strathmann 1982; Sulkin and Van Heukelem 1982; Ogburn et al. 2009; Kunze et al. 2013; Peteiro and Shanks 2015); however, recent evidence suggests that larvae of some marine invertebrates and fish also have behavioral adaptations that allow them to remain in the nearshore zone along the coast and recruit to the shore despite the influence of these predominant currents (Morgan et al. 2009; Morgan et al. 2012; Shanks et al. 2014). The estuarine populations of both burrowing shrimp species we studied have early-stage larvae that are exported from the estuary and then return to the relatively small entrances to these estuaries after development in coastal ocean waters. Research examining larvae from other species of burrowing shrimp along the upwelling coasts of South Africa and South America (Wooldridge and Loubser 1996; Yannicelli et al. 2006a; Teske et al. 2008), as well as N. californiensis (Morgan and Fisher 2010; Morgan et al. 2014; Hameed et al. 2018), suggests that shrimp larvae are either always present in deeper water or have diel vertical migration patterns which keep them both close to shore and in deeper water where they avoid dominant surface transport patterns. Though not in regular upwelling systems, similar behavior has been noted for late-stage larvae and decapodids of Lepidophthalmus siriboia and Upogebia vasquezi in South America and Nihonotrypaea harmandi in Japan where they take advantage of rapid flood tide transport to return to shore (Tamaki et al. 2010; de Oliveira et al. 2012; Tamaki et al. 2013). We have yet to directly relate our annual recruitment data to long-term records for these physical transport mechanisms in the coastal ocean, but marked interannual differences in shrimp recruitment were observed in both estuaries clearly suggesting that recruitment limitation occurs in some locations and years. Recruitment of both species was more consistent to Yaquina Bay, and there were a few coherent patterns amongst these two estuaries which are 250 km apart (Fig. 12). These patterns may indicate links between these populations and to nearshore coastal oceanography. For example, most of the highest N. californiensis recruitment occurred after 2010 in both estuaries and the only strong recruitment years for U. pugettensis in Willapa Bay, where an adult spawning population is virtually absent, ocurred in 2006, 2010, and 2015 which were also strong recruitment years in Yaquina Bay. While not conclusive, this suggests that shrimp from individual estuaries might contribute larvae and subsequent recruits to a larger multi-estuary metapopulation (Camus and Lima 2002; Kritzer and Sale 2004; Lipcius et al. 2008; Watson et al. 2012), but the number of larvae returning to individual estuaries is still subject to variable ocean conditions along this coast.
Annual recruitment estimates for N. californiensis (top) and U. pugettensis (bottom) ranked from highest to lowest within each estuary shaded with colors representing four groups (0 = light blue, low (1–4) = light green, moderate (5–8) = medium green, high (> 8) = dark green (nd= no data). Both species displayed more consistent recruitment to Yaquina Bay and strong recruitment years differed by species. Circled are years with highest recruitment and some coherence between estuaries (color figure online)
Despite variable recruitment from year to year, we show that the number of 0+ recruits of both shrimp species was directly related to the density of 1+ and older shrimp present at the same locations in subsequent years. This is not surprising because recruitment rate is a necessary parameter that appears in all population dynamics models and the expectation for most benthic marine invertebrates is that mortality decreases exponentially with age (i.e., Type III survivorship). Unfortunately, it is also difficult to determine age for these shrimp especially using size alone, though efforts have been made to independently assess this using the pigment lipofuscin (Bosley and Dumbauld 2011; Bosley 2016). Simple linear models suggest that mortality is higher for U. pugettensis than for N. californiensis during this first year. We speculate that this is, in part, a result of seasonal timing as U. pugettensis recruit to intertidal locations during the summer months when known predators such as Pacific staghorn sculpin (Leptocottus armatus) and juvenile Dungeness crab (Carcinus magister; Armstrong et al. 1995; Feldman et al. 1994; Feldman 2001) are most abundant and active.
We also found significant differences between these two shrimp species with respect to seasonal timing and patterns of settlement and post-settlement mortality that corroborate their previously documented life histories (Bird 1982; Dumbauld et al. 1996; Feldman et al. 1997; Feldman 2001), but this work further clarifies observed recruitment patterns across broader estuarine intertidal areas. Neotrypaea californiensis have been documented to reproduce and extrude eggs in the spring and summer (March–July) which results in larvae developing in the nearshore coastal ocean during summer and decapodids returning during late summer and fall (Dumbauld et al. 1996). Conversely, U. pugettensis extrude eggs in the fall (Oct–Dec), larvae develop in the ocean earlier in the spring, and decapodids return to estuaries in late spring and early summer (April–July). We demonstrated that two cohorts of each species recruited to Yaquina Bay in 2010 and 2011, but numbers differed markedly by species and year. These patterns were preceded by similar peaks in abundance of decapodids sampled in plankton tows from a dock near the mouth of the estuary (J. Chapman et al., unpublished data). Upogebia pugettensis recruits grew rapidly over the summer reaching a mean size of 9.6 to 12.1 mm CL in 1 year compared to N. californiensis which recruited later and grew slower reaching only 4.6 to 6.6 mm CL by the following summer. While mud shrimp grew faster, both species built relatively shallow burrows at first and were still present primarily in the top 20 cm by September. Faster growth also resulted in these two separate U. pugettensis cohorts remaining distinguishable the following year. It was more difficult, however, to track N. californiensis cohorts and distinguish this 0+ age class from larger/older shrimp due to their slower and highly variable growth and our inability to use other aging techniques on these small shrimp (Bosley 2016; Bosley et al., submitted).
While settlement and early post-settlement mortality or movement are separate processes and often decoupled (Olafsson et al. 1994; Etherington and Eggleston 2000; Pineda et al. 2010), we could not distinguish these processes for shrimp decapodids in our field collections (but see Tamaki et al. 2013 for methods to make such distinctions). Our broader sampling efforts across the tidal flat in Yaquina Bay revealed that N. californiensis decapodids and small juveniles were present across habitats, but the numbers were higher where N. californiensis adults were present in 2011 (a strong recruitment year). We could not detect a difference, however, in abundance of older 1+ juveniles across habitats the following spring. Neotrypaea californiensis recruits were again found in both habitats including locations where adult U. pugettensis were present later in 2012 (a moderate recruitment year). In contrast, U. pugettensis either appeared to be more selective and did not settle to or perhaps failed to survive in open areas where adult shrimp were absent or in areas where adult N. californiensis were present. Results of relatively small-scale field and laboratory experiments conducted by Feldman et al. (1997) demonstrated that N. californiensis preferentially settled in mud/sand substrate versus habitat with mollusk shells present and that post-settlement mortality was higher in shell substrates where they were exposed to predation by juvenile Dungeness crabs, C. magister. Posey (1986) also documented recruitment of N. californiensis outside established adult colonies and subsequent mortality, especially at seaward ends of the adult beds due to predation by both crab and sculpins which resulted in higher juvenile abundance in established colonies. Similarly, Tamaki and Ingole (1993) found that decapodids of a sister species, Nihonotrypaea harmandi, settled broadly but appeared to survive better in areas where adult shrimp were present. Though Tamaki et al. (1992) documented the use of adult burrows by juveniles of N. harmandi, no settlement cues aside from the presence of suitable sand substrate have been identified for these axiid shrimp. Whether or not this result is due to facilitation by adult shrimp is not clear because competition for food resources may also occur. We found a trend of higher abundance of N. californiensis recruits at the edge of the established colony in Yaquina Bay suggesting that at least some initial settlement cue may be present. Feldman (2001) also conducted settlement experiments with U. pugettensis, which revealed active settlement choice of habitats with epibenthic shell present versus open mud in the field, but a laboratory experiment suggested that they did not appear to actively cue in on larger conspecifics. In contrast with her results for N. californiensis, she found that juvenile Dungeness crabs were not a significant source of post-settlement mortality for this species. Thus, it seems that distribution and recruitment of both species are ultimately linked to post-settlement processes that not only include mortality due to predation but may also involve post-settlement dispersal of juvenile shrimp after metamorphosis, a process often overlooked for many benthic invertebrates (Pilditch et al. 2015). Feldman (2001) observed small-scale movement of both 0+ and even older 1+ U. pugettensis into experimental settlement trays and larger shell plots but did not directly observe this movement for N. californiensis. The patterns we observed in Willapa Bay where the 1+ age class of N. californiensis appeared at our long-term monitoring location without documented settlement the previous year suggest that post-settlement movement might occur for this species as well and there is evidence that it occurs for other congeners (Tamaki and Ingole 1993; de Oliveira et al. 2012; Tamaki et al. 2013). Variable but consistently higher numbers of N. californiensis recruits at locations close to the estuary mouth and at middle tidal elevations in both estuaries agree with results from previous studies and other recent surveys (Bird 1982; Patten and Norelius 2016).
Conservation and Management Implications
Upogebia pugettensis populations have recently declined dramatically to very low levels in many US west coast estuaries where they were once abundant raising conservation concerns for the shrimp themselves (Chapman et al. 2012). This decline has been linked to very high prevalence of an introduced bopyrid isopod, Orthione griffenis, that directly affects recruitment by rendering adult female shrimp incapable of producing eggs (Dumbauld et al. 2011; Repetto and Griffen 2012; Asson et al. 2017). As a result, this species is no longer ecologically important in Washington state coastal estuaries including Willapa Bay where the population at our Goose Pt. monitoring location was reduced to a level after 2010 where we could no longer use cores to quantitatively sample adults. Upogebia pugettensis are still present at relatively high density in Yaquina Bay, but this decline has also occurred in Oregon estuaries and the spatial extent of their populations has decreased in Yaquina Bay (Bosley 2016; Dumbauld et al. unpublished manuscript). The data we present here suggest that recruitment to Willapa Bay has been less since the mid-1990s and that while there is a positive relationship between the number of 0+ shrimp and older 1+ shrimp, the slope of this relationship is less than one and these new recruits may experience relatively high mortality. Furthermore, they appear to only settle and recruit to areas where conspecific adults are present which would enhance declines and restrict population expansions. It is not yet clear why U. pugettensis populations near the center of their coastwide distribution in Oregon estuaries have persisted, while those further north and south near the ends of that distribution have declined even though O. griffenis continues to be recorded at high prevalence in all extant populations (Chapman et al. 2012; Chapman and Carter 2014, B. Dumbauld unpublished data). The data we collected here show that higher recruitment years for U. pugettensis in Willapa Bay were also strong recruitment years in Yaquina Bay (though not necessarily vice versa). This suggests that these populations are part of a larger metapopulation, so the proximity of nearby estuaries and potential for larval return could be one factor sustaining them.
Population density of N. californiensis also declined dramatically in both of the estuaries we studied beginning in the mid-1990s in Willapa Bay and from the outset of our monitoring program in Yaquina Bay in 2004, and these trends are reflected in the spatial extent of these populations as well (Dumbauld et al. unpublished manuscript). Neotrypaea californiensis population trajectories reversed from 2012 to 2014 in both estuaries, and we show that this is directly correlated to strong recruitment events beginning in 2010/2011. The slope of the relationship between 0+ shrimp density and that of older age classes was close to one suggesting that either mortality of these new recruits is low or that it is more difficult to track them as they have a broader recruitment window and post-settlement movement of juveniles occurs. It is not known whether there are other diseases or parasites that affect N. californiensis, but their population dynamics are not likely to be influenced by their native bopyrid isopod parasite Ione cornuta, which has always been observed at low prevalence (< 10%). Neotrypaea californiensis are longer lived than U. pugettensis (Dumbauld et al. 2011; Bosley 2016) which is likely linked to the longer, 4-year lag period and the lack of a linear relationship between 1+ and older shrimp in Yaquina Bay. Neotrypaea californiensis decapodids may also be initially attracted to settle where adults are present, but they appear to ultimately recruit more broadly across the estuarine landscape and the period over which this occurs is temporally variable.
As estuarine ecosystem engineers, these burrowing shrimp have clearly been shown to influence sediment biogeochemistry, benthic community composition, the presence of other suspension feeders, and even other engineers like seagrass via their bioturbation and burrow irrigation (Feldman et al. 2000; Pillay and Branch 2011; Takeuchi et al. 2013). They are also recognized for their role in estuarine food webs as both consumers and as prey. While some of these consumers with threatened populations like green sturgeon have raised recent concern (Dumbauld et al. 2008; Borin et al. 2017), only the shrimp’s effect on shellfish aquaculture operations has received direct management attention in the US west coast estuaries we studied.
Both species of shrimp have caused a significant problem for oyster culture operations that occur in these estuaries because oysters are often seeded directly on the sediment surface. As sessile organisms, they succumb readily to bioturbation and sediment turnover caused by the shrimp (Dumbauld et al. 2004; Dumbauld et al. 2006). Oyster growers in Washington State applied a pesticide (carbaryl, n-methyl carbamate) at low tide to treat shellfish beds in coastal estuaries and remove these shrimp beginning in the early 1960s (Feldman et al. 2000). The growers signed an agreement to pursue integrated pest management (IPM) in 2000 and participated in an out-of-court settlement in 2002 where they agreed to stop using this pesticide by 2013. Research on alternative treatments resulted in continued studies and a recent evaluation of a less toxic pesticide, Imidacloprid (Washington State Dept. Ecology 2015, 2018). The tenets of IPM include knowledge of the pest’s life history in order to target treatment at the most opportune point in time for control as well as knowledge of pest population dynamics in order to treat and control them when the level of pest abundance reaches a threshold (Kogan 1998; Lefebvre et al. 2015). These concepts have been little applied for aquaculture pests (see Rae 2002), and the threshold model has also proven difficult to apply for shellfish aquaculture in the case of these shrimp (Dumbauld et al. 2006). This is because the shrimp have a complex life cycle, and shellfish are also a perennial crop, grown in a spatially and temporally variable estuarine environment, and subject to economic market volatility.
Monitoring pest abundance is an essential component of IPM and the ability to predict shrimp abundance, even 1 year or one season before treatment would be valuable, especially for grow-out or fattening beds where shellfish are only present for a short period of time (Dumbauld et al. 2006). Here we document annual monitoring that has occurred in Willapa Bay for over two decades and show that annual recruitment patterns clearly influence resulting shrimp populations in areas with dense shrimp adjacent to shellfish beds. While shrimp density is lower on most culture beds, it is clearly useful to maintain such a monitoring program and understand shrimp recruitment ecology, regardless of the ultimate fate and nature of a control program. Several results from our research are insightful in this regard:
-
1)
The only practical way to assess the abundance of larger shrimp across culture beds is via counts of burrow openings on the surface. Due to their small size, burrow openings of new recruits are only recognizable at a minimum of 1 year of age and it is likely that only 1+ U. pugettensis are counted in most surveys. For N. californiensis, burrow openings are smaller and more easily disturbed so this species may be 2 years old before they are counted in population assessments. Continuing to monitor the abundance of 0+ shrimp (and perhaps 1+ N. californiensis because small 0+ shrimp settle over a longer period and move to new areas after settlement) at long-term monitoring locations outside the culture beds will provide a window of opportunity for growers to anticipate and seek control.
-
2)
Differences in recruitment behavior amongst species suggest that N. californiensis decapodids and small juveniles are more likely to recruit to shellfish beds even where previous treatment has recently removed adults. They distribute broadly and do not appear to be as selective in their behavior as U. pugettensis (but see Feldman 2001 who found that U. pugettensis also select areas where shell is present).
-
3)
Due to their greatly reduced populations, U. pugettensis are currently less of an issue for shellfish growers, but they also appear to experience higher natural mortality during their first year of benthic life than N. californiensis. Juvenile N. californiensis grow more slowly and thus remain in relatively shallow burrows over the summer and fall (July–November) when they too are potentially more susceptible to control methods which could include enhanced biocontrol via predation and alternative physical control measures.
Our results thus provide important insight for managers that seek not only to maintain shrimp populations as native bioengineers that shape the ecology of the predominantly soft sediment ecosystems in these US Pacific coast estuaries, but also to control them and grow shellfish which play an equally important role in that ecology (D'Andrea and DeWitt 2009; Dumbauld et al. 2009; Ferraro and Cole 2010; Ferraro and Cole 2011; Volkenborn et al. 2012; Dumbauld and McCoy 2015).
References
Armstrong, J.L., D.A. Armstrong, and S.B. Mathews. 1995. Food habits of estuarine staghorn sculpin, Leptocottus armatus, with focus on consumption of juvenile Dungeness crab, Cancer magister. Fishery Bulletin 93: 456–470.
Asson, D., J.W. Chapman, and B.R. Dumbauld. 2017. No evidence that the introduced parasite Orthione griffenis Markham, 2004 causes sex change or differential mortality in the native mud shrimp, Upogebia pugettensis (Dana, 1852). Aquatic Invasions 12 (2): 213–224.
Bird, E.M. 1982. Population dynamics of thalassinidean shrimps and community effects through sediment modification. Ph. D. dissertation, University of Maryland, College Park, Maryland.
Borin, J., M.L. Moser, A. Hansen, D.A. Beauchamp, S. Corbett, B.R. Dumbauld, C. Pruitt, J. Ruesink, and C. Donohue. 2017. Energetic requirements of green sturgeon (Acipensier medirostris) feeding on burrowing shrimp (Neotrypaea californiensis) in estuaries: importance of temperature, reproductive investment, and residence time. Environmental Biology of Fishes 100 (12): 1561–1573.
Bosley, K. 2016. An integrated approach to the investigation of age, growth and population dynamics of burrowing thalassinidean shrimps in a US West Coast estuary, Ph. D. dissertation, Oregon State University, Corvallis, Oregon.
Bosley, K.M., L.A. Copeman, B.R. Dumbauld, and K.L. Bosley. 2017. Identification of burrowing shrimp food sources along an estuarine gradient using fatty acid analysis and stable isotope ratios. Estuaries and Coasts 40 (4): 1113–1130.
Bosley, K.M., and B.R. Dumbauld. 2011. Use of extractable lipofuscin to estimate age structure of ghost shrimp populations in west coast estuaries of the USA. Marine Ecology-Progress Series 428: 161–176.
Breckenridge, J.K., and S.M. Bollens. 2010. Biological thin layer formation: interactions between the larval decapod, Neotrypaea californiensis, haloclines and light. Journal of Plankton Research 32 (7): 1097–1102.
Broitman, B.R., C.A. Blanchette, B.A. Menge, J. Lubchenco, C. Krenz, M. Foley, P.T. Raimondi, D. Lohse, and S.D. Gaines. 2008. Spatial and temporal patterns of invertebrate recruitment along the West Coast of the United States. Ecological Monographs 78 (3): 403–421.
Camus, P.A., and M. Lima. 2002. Populations, metapopulations, and the open-closed dilemma: the conflict between operational and natural population concepts. Oikos 97 (3): 433–437.
Castorani, M.C.N., K.A. Hovel, S.L. Williams, and M.L. Baskett. 2014. Disturbance facilitates the coexistence of antagonistic ecosystem engineers in California estuaries. Ecology 95 (8): 2277–2288.
Chapman, J.W., and C.S. Carter. 2014. A rapid intertidal megafauna survey method applied to Upogebia pugettensis, and its introduced parasite, Orthione griffensis. Journal of Crustacean Biology 34 (3): 349–356.
Chapman, J.W., B.R. Dumbauld, G. Itani, and J.C. Markham. 2012. An introduced Asian parasite threatens northeastern Pacific estuarine ecosystems. Biological Invasions 14 (6): 1221–1236.
Connolly, S.R., and J. Roughgarden. 1998. A latitudinal gradient in northeast Pacific intertidal community structure: evidence for an oceanographically based synthesis of marine community theory. American Naturalist 151 (4): 311–326.
D'Andrea, A.F., and T.H. DeWitt. 2009. Geochemical ecosystem engineering by the mud shrimp Upogebia pugettensis (Crustacea: Thalassinidae) in Yaquina Bay, Oregon: density-dependent effects on organic matter remineralization and nutrient cycling. Limnology and Oceanography 54 (6): 1911–1932.
de Oliveira, D.B., J.M. Martinelli-Lemos, A.S. de Souza, J.R. da Costa, and F.A. Abrunhosa. 2016. Does retention or exportation occur in the larvae of the mud shrimp Upogebia vasquezi (Decapoda, Gebiidea)? Implications for the reproductive strategy of the species on the Amazon coast. Hydrobiologia 773 (1): 241–252.
de Oliveira, D.B., D.C. Silva, and J.M. Martinelli. 2012. Density of larval and adult forms of the burrowing crustaceans Lepidophthalmus siriboia (Callianassidae) and Upogebia vasquezi (Upogebiidae) in an Amazon estuary, northern Brazil. Journal of the Marine Biological Association of the United Kingdom 92 (02): 295–303.
DeWitt, T.H., A.F. D'Andrea, C.A. Brown, B.D. Griffen, and P.M. Eldridge. 2004. Impact of burrowing shrimp populations on nitrogen cycling and water quality in western north American temperate estuaries. In Symposium on “Ecology of large bioturbators in tidal flats and shallow sublittoral sediments-from individual behavior to their role as ecosystem engineers”, ed. A. Tamaki, 107–118. Nagasaki: Nagasaki University.
Dudas, S.E., B.A. Grantham, A.R. Kirincich, B.A. Menge, J. Lubchenco, and J.A. Barth. 2009. Current reversals as determinants of intertidal recruitment on the central Oregon coast. ICES Journal of Marine Science 66: 396–407.
Dumbauld, B.R., D.A. Armstrong, and K.L. Feldman. 1996. Life-history characteristics of two sympatric thalassinidean shrimps, Neotrypaea californiensis and Upogebia pugettensis, with implications for oyster culture. Journal of Crustacean Biology 16 (4): 689–708.
Dumbauld, B.R., S. Booth, D. Cheney, A. Suhrbier, and H. Beltran. 2006. An integrated pest management program for burrowing shrimp control in oyster aquaculture. Aquaculture 261 (3): 976–992.
Dumbauld, B.R., K.M. Brooks, and M.H. Posey. 2001. Response of an estuarine benthic community to application of the pesticide carbaryl and cultivation of Pacific oysters (Crassostrea gigas) in Willapa Bay, Washington. Marine Pollution Bulletin 42 (10): 826–844.
Dumbauld, B.R., J.W. Chapman, A.M. Kuris, and M.E. Torchin. 2011. Is the collapse of mud shrimp (Upogebia pugettensis) populations along the Pacific coast of North America caused by outbreaks of a previously unknown bopyrid isopod parasite (Orthione griffenis)? Estuaries and Coasts 34 (2): 336–350.
Dumbauld, B.R., K. Feldman, and D. Armstrong. 2004. A comparison of the ecology and effects of two species of thalassinidean shrimps on oyster aquaculture operations in the eastern North Pacific. In Symposium on “Ecology of large bioturbators in tidal flats and shallow sublittoral sediments-from individual behavior to their role as ecosystem engineers”, ed. A. Tamaki, 53–61. Nagasaki: Nagasaki University.
Dumbauld, B.R., D.L. Holden, and O.P. Langness. 2008. Do sturgeon limit burrowing shrimp populations in Pacific Northwest estuaries. Environmental Biology of Fishes 83 (3): 283–296.
Dumbauld, B.R., and L.M. McCoy. 2015. The effect of oyster aquaculture on seagrass (Zostera marina) at the estuarine landscape scale in Willapa Bay, Washington (USA). Aquaculture Environment Interactions 7 (1): 29–47.
Dumbauld, B.R., J.L. Ruesink, and S.S. Rumrill. 2009. The ecological role of bivalve shellfish aquaculture in the estuarine environment: a review with application to oyster and clam culture in West Coast (USA) estuaries. Aquaculture 290 (3-4): 196–223.
Dumbauld, B.R., and S. Wyllie-Echeverria. 2003. The influence of burrowing thalassinid shrimps on the distribution of intertidal seagrasses in Willapa Bay, Washington, USA. Aquatic Botany 77 (1): 27–42.
Etherington, L., and D. Eggleston. 2000. Large-scale blue crab recruitment: linking postlarval transport, post-settlement planktonic dispersal, and multiple nursery habitats. Marine Ecology Progress Series 204: 179–198.
Feldman, K. 2001. Contrasting patterns of habitat-specific recruitment success in sympatric species of thalassinidean shrimp: effects of epibenthic bivalve shell with implications for population control in areas with commercial oyster aquaculture. Ph. D. Thesis, University of Washington Seattle, Washington.
Feldman, K.L., D.A. Armstrong, B.R. Dumbauld, T.H. DeWitt, and D.C. Doty. 2000. Oysters, crabs, and burrowing shrimp: review of an environmental conflict over aquatic resources and pesticide use in Washington State's (USA) coastal estuaries. Estuaries 23 (2): 141–176.
Feldman, K.L., D.A. Armstrong, D.B. Eggleston, and B.R. Dumbauld. 1994. Ghost shrimp recruitment to intertidal shell and mud habitats: effects of substrate selection and post settlement survival on distribution of young-of-the-year5699.
Feldman, K.L., D.A. Armstrong, D.B. Eggleston, and B.R. Dumbauld. 1997. Effects of substrate selection and post-settlement survival on recruitment success of the thalassinidean shrimp Neotrypaea californiensis to intertidal shell and mud habitats. Marine Ecology Progress Series 150: 121–136.
Ferraro, S.P., and F.A. Cole. 2010. Ecological periodic tables for nekton usage of four US Pacific Northwest estuarine habitats. Canadian Journal of Fisheries and Aquatic Sciences 67 (12): 1957–1967.
Ferraro, S.P., and F.A. Cole. 2011. Ecological periodic tables for benthic macrofaunal usage of estuarine habitats in the US Pacific Northwest. Estuarine Coastal and Shelf Science 94 (1): 36–47.
Fisher, J.L., W.T. Peterson, and S.G. Morgan. 2014. Does larval advection explain latitudinal differences in recruitment across upwelling regimes? Marine Ecology Progress Series 503: 123–137.
Gaines, S., and J. Roughgarden. 1985. Larval settlement rate—a leading determinant of structure in an ecological community of the marine intertidal zone. Proceedings of the National Academy of Sciences of the United States of America 82 (11): 3707–3711.
Garcia-Reyes, M., and J.L. Largier. 2012. Seasonality of coastal upwelling off central and northern California: new insights, including temporal and spatial variability. Journal of Geophysical Research-Oceans 117 (C3).
Hameed, S.O., M.L. Elliott, S.G. Morgan, and J. Jahnke. 2018. Interannual variation and spatial distribution of decapod larvae in a region of strong upwelling. Marine Ecology Progress Series 587: 55–71.
Hannah, R.W. 2011. Variation in the distribution of ocean shrimp (Pandalus jordani) recruits: links with coastal upwelling and climate change. Fisheries Oceanography 20 (4): 305–313.
Harada, K., and A. Tamaki. 2004. Assessment of the predation impact of the stingray Dasytis akajei (Muller and Henle, 1841) on the population of the ghost shrimp Nihonotrypaea harmandi (Bouvier, 1901) on and intertidal sandflat (preliminary report). In Symposium on “Ecology of large bioturbators in tidal flats and shallow sublittoral sediments-from individual behavior to their role as ecosystem engineers”, ed. A. Tamaki, 81–85. Nagasaki: Nagasaki University.
Hart, J.F.L. 1937. Larval and adult stages of British Columbia Anomura. Canadian Journal of Research 15: 179–219.
Hernaez, P., E. Villegas-Jimenez, F. Villalobos-Rojas, and I.S. Wehrtmann. 2012. Reproductive biology of the ghost shrimp Lepidophthalmus bocourti (A. Milne-Edwards, 1870) (Decapoda: Axiidea: Callianassidae): a tropical species with a seasonal reproduction. Marine Biology Research 8 (7): 635–643.
Houde. 2008. Emerging from Hjort’s shadow. Journal of Northwest Atlantic Fisheries Science 41: 53–70.
Johnson, G.E., and J.J. Gonor. 1982. The tidal exchange of Callianassa californiensis (Crustacea, Decapoda) larvae between the ocean and Salmon River estuary, Oregon. Estuarine, Coastal and Shelf Science 14 (5): 501–516.
Kogan, M. 1998. Integrated pest management: historical perspectives and contemporary developments. Annual Review of Entomology 43 (1): 243–270.
Kritzer, J.P., and P.F. Sale. 2004. Metapopulation ecology in the sea: from Levins’ model to marine ecology and fisheries science. Fish and Fisheries 5 (2): 131–140.
Kunze, H.B., S.G. Morgan, and K.M. Lwiza. 2013. Field test of the behavioral regulation of larval transport. Marine Ecology Progress Series 487: 71–87.
Lefebvre, M., S.R.H. Langrell, and S. Gomez-Y-Paloma. 2015. Incentives and policies for integrated pest management in Europe: a review. Agronomy for Sustainable Development 35 (1): 27–45.
Leslie, H.M., E.N. Breck, F. Chan, J. Lubchenco, and B.A. Menge. 2005. Barnacle reproductive hotspots linked to nearshore ocean conditions. Proceedings of the National Academy of Sciences of the United States of America 102 (30): 10534–10539.
Lipcius, R.N., D.B. Eggleston, S.J. Schreiber, R.D. Seitz, J. Shen, M. Sisson, W.T. Stockhausen, and H.V. Wang. 2008. Importance of metapopulation connectivity to restocking and restoration of marine species. Reviews in Fisheries Science 16 (1-3): 101–110.
MacDonald, P. 2015. Mixdist: finite mixture distribution models. R package version 0.5–4 https://cran.r-project.org/web/packages/mixdist/mixdist.pdf.
Menge, B.A., T.C. Gouhier, T. Freidenburg, and J. Lubchenco. 2011. Linking long-term, large-scale climatic and environmental variability to patterns of marine invertebrate recruitment: toward explaining “unexplained” variation. Journal of Experimental Marine Biology and Ecology 400 (1-2): 236–249.
Morgan, S.G., and J.L. Fisher. 2010. Larval behavior regulates nearshore retention and offshore migration in an upwelling shadow and along the open coast. Marine Ecology-Progress Series 404: 109–126.
Morgan, S.G., J.L. Fisher, S.T. McAfee, J.L. Largier, and C.M. Halle. 2012. Limited recruitment during relaxation events: larval advection and behavior in an upwelling system. Limnology and Oceanography 57 (2): 457–470.
Morgan, S.G., J.L. Fisher, S.T. McAfee, J.L. Largier, S.H. Miller, M.M. Sheridan, and J.E. Neigel. 2014. Transport of crustacean larvae between a low-inflow estuary and coastal waters. Estuaries and Coasts 37 (5): 1269–1283.
Morgan, S.G., J.L. Fisher, S.H. Miller, S.T. McAfee, and J.L. Largier. 2009. Nearshore larval retention in a region of strong upwelling and recruitment limitation. Ecology 90 (12): 3489–3502.
Ogburn, M.B., H. Diaz, and R.B. Forward. 2009. Mechanisms regulating estuarine ingress of blue crab Callinectes sapidus megalopae. Marine Ecology-Progress Series 389: 181–192.
Olafsson, E.B., C.H. Peterson, and W.G.J. Ambrose. 1994. Does recruitment limitation structure populations and communities of macro-invertebrates in marine soft sediments: the relative significance of pre- and post-settlement processes. Oceanography and Marine Biology: An Annual Review 32: 65–109.
Patten, K., and S. Norelius. 2016. Burrowing shrimp recruitment survey for Willapa Bay late summer 2016. Progress report to the Washington Department of Fish and Wildlife, from Washington State University, Long Beach Research and Extension Unit, 6p.
Peteiro, L.G., and A.L. Shanks. 2015. Up and down or how to stay in the bay: retentive strategies of Olympia oyster larvae in a shallow estuary. Marine Ecology Progress Series 530: 103–117.
Pilditch, C.A., S. Valanko, J. Norkko, and A. Norkko. 2015. Post-settlement dispersal: the neglected link in maintenance of soft-sediment biodiversity. Biology Letters 11 (2): 20140795.
Pillay, D., and G.M. Branch. 2011. Bioengineering effects of burrowing thalassinidean shrimps on marine soft-bottom ecosystems. Oceanography and Marine Biology: An Annual Review 49: 137–191.
Pillay, D., G.M. Branch, and A.T. Forbes. 2007. The influence of bioturbation by the sandprawn Callianassa kraussi on feeding and survival of the bivalve Eumarcia paupercula and the gastropod Nassarius kraussianus. Journal of Experimental Marine Biology and Ecology 344 (1): 1–9.
Pineda, J., F. Porri, V. Starczak, and J. Blythe. 2010. Causes of decoupling between larval supply and settlement and consequences for understanding recruitment and population connectivity. Journal of Experimental Marine Biology and Ecology 392 (1-2): 9–21.
Posey, M.H. 1986. Predation on burrowing shrimp: distribution and community consequences. Journal of Experimental Marine Biology and Ecology 103 (1-3): 143–161.
Develoment Core Team, R. 2015. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
Rae, G.H. 2002. Sea louse control in Scotland, past and present. Pest Management Science 58 (6): 515–520.
Repetto, M., and B.D. Griffen. 2012. Physiological consequences of parasite infection in the burrowing mud shrimp, Upogebia pugettensis, a widespread ecosystem engineer. Marine and Freshwater Research 63 (1): 60–67.
Shanks, A., G.C. Roegner, and J. Miller. 2010. Using megalopae abundance to predict future commercial catches of Dungeness crabs (Cancer magister) in Oregon. California Cooperative Oceanic Fisheries Investigations Reports 51: 106–118.
Shanks, A.L. 2013. Atmospheric forcing drives recruitment variation in the Dungeness crab (Cancer magister), revisited. Fisheries Oceanography 22 (4): 263–272.
Shanks, A.L., S.G. Morgan, J. MacMahan, A.J.H.M. Reniers, M. Jarvis, J. Brown, A. Fujimura, and C. Griesemer. 2014. Onshore transport of plankton by internal tides and upwelling-relaxation events. Marine Ecology Progress Series 502: 39–51.
Shanks, A.L., and R.K. Shearman. 2009. Paradigm lost? Cross-shelf distributions of intertidal invertebrate larvae are unaffected by upwelling or downwelling. Marine Ecology-Progress Series 385: 189–204.
Shimoda, K., Y. Aramaki, J. Nasuda, H. Yokoyama, Y. Ishihi, and A. Tamaki. 2007. Food sources for three species of Nihonotrypaea (Decapoda: Thalassinidea: Callianassidae) from western Kyushu, Japan, as determined by carbon and nitrogen stable isotope analysis. Journal of Experimental Marine Biology and Ecology 342 (2): 292–312.
Strathmann, R.R. 1982. Selection for retention or export of larvae in estuaries. In Estuarine Comparisons, ed. V.S. Kennedy, 521–536. New York: Academic Press.
Sulkin, S.D., and W.V. Van Heukelem. 1982. Larval recruitment in the crab Callinectes sapidus Rathbun: amendment to the concept of larval retention in estuaries. In Estuarine comparisons, ed. V.S. Kennedy, 459–475. New York: Academic Press.
Takeuchi, S., Y. Takahara, Y. Agata, J. Nasuda, F. Yamada, and A. Tamaki. 2013. Response of suspension-feeding clams to natural removal of bioturbating shrimp on a large estuarine intertidal sandflat in western Kyushu, Japan. Journal of Experimental Marine Biology and Ecology 448: 308–320.
Tamaki, A., K. Ikebe, K. Muramatsu, and B. Ingole. 1992. Utilization of adult burrows by juveniles of the ghost shrimp, Callianassa japonica Ortmann: evidence from resin casts of burrows. Researches on Crustacea 217: 113–120.
Tamaki, A., and B. Ingole. 1993. Distribution of juvenile and adult ghost shrimps, Callianassa japonica Ortmann (Thalassinidea), on an intertidal sand flat: intraspecific facilitation as a possible pattern-generating factor. Journal of Crustacean Biology 13 (1): 175–183.
Tamaki, A., S. Mandal, Y. Agata, I. Aoki, T. Suzuki, H. Kanehara, T. Aoshima, Y. Fukuda, H. Tsukamoto, and T. Yanagi. 2010. Complex vertical migration of larvae of the ghost shrimp, Nihonotrypaea harmandi, in inner shelf waters of western Kyushu, Japan. Estuarine Coastal and Shelf Science 86 (1): 125–136.
Tamaki, A., Y. Saitoh, J. Itoh, Y. Hongo, S. Sen-Ju, S. Takeuchi, and S. Ohashi. 2013. Morphological character changes through decapodid-stage larva and juveniles in the ghost shrimp Nihonotrypaea harmandi from western Kyushu, Japan: clues for inferring pre- and post-settlement states and processes. Journal of Experimental Marine Biology and Ecology 443: 90–113.
Teske, P.R., I. Papadopoulos, B.K. Newman, P.C. Dworschak, C.D. McQuaid, and N.P. Barker. 2008. Oceanic dispersal barriers, adaptation and larval retention: an interdisciplinary assessment of potential factors maintaining a phylogeographic break between sister lineages of an African prawn. BMC Evolutionary Biology 8: 1–14.
Thorson, G. 1950. Reproductive and larval ecology of marine bottom invertebrates. Biological Reviews 25 (1): 1–45.
Thorson, G. 1966. Some factors influencing recruitment and establishment of marine benthic communities. Netherlands Journal of Sea Research 33: 267–293.
Thrush, S.F., J.E. Hewitt, and A.M. Lohrer. 2012. Interaction networks in coastal soft-sediments highlight the potential for change in ecological resilience. Ecological Applications 22 (4): 1213–1223.
Volkenborn, N., L. Polerecky, D.S. Wethey, T.H. DeWitt, and S.A. Woodin. 2012. Hydraulic activities by ghost shrimp Neotrypaea californiensis induce oxic-anoxic oscillations in sediments. Marine Ecology-Progress Series 455: 141–156.
Washington State Dept. of Ecology. 2015. Final environmental impact statement control of burrowing shrimp using imidacloprid on commercial oyster and clam beds in Willapa Bay and Grays Harbor, Washington, 389 p. https://www.ecology.wa.gov/burrowingshrimp
Washington State Dept. of Ecology. 2018. Final supplemental environmental impact statement control of burrowing shrimp using imidacloprid on commercial oyster and clam beds in Willapa Bay and Grays Harbor, Washington, 885 p. https://www.ecology.wa.gov/burrowingshrimp
Wasson, K., B.B. Hughes, J.S. Berriman, A.L. Chang, A.K. Deck, P.A. Dinnel, C. Endris, M. Espinoza, S. Dudas, M.C. Ferner, E.D. Grosholz, D. Kimbro, J.L. Ruesink, A.C. Trimble, D.V. Schaaf, C.J. Zabin, and D.C. Zacherl. 2016. Coast-wide recruitment dynamics of Olympia oysters reveal limited synchrony and multiple predictors of failure. Ecology 97 (12): 3503–3516.
Watson, J.R., B.E. Kendall, D.A. Siegel, and S. Mitarai. 2012. Changing seascapes, stochastic connectivity, and marine metapopulation dynamics. American Naturalist 180 (1): 99–112.
Webb, A.P., and B.D. Eyre. 2004. Effect of natural populations of burrowing thalassinidean shrimp on sediment irrigation, benthic metabolism, nutrient fluxes and denitrification. Marine Ecology-Progress Series 268: 205–220.
Woodin, S.A. 1976. Adult-larval interactions in dense infaunal assemblages: patterns of abundance. Journal of Marine Research 34: 25–41.
Woodin, S.A., S.M. Lindsay, and D.S. Wethey. 1995. Process-specific recruitment cues in marine sedimentary systems. Biological Bulletin 189 (1): 49–58.
Woodson, C.B., M.A. McManus, J.A. Tyburczy, J.A. Barth, L. Washburn, J.E. Caselle, M.H. Carr, D.P. Malone, P.T. Raimondi, B.A. Menge, and S.R. Palumbi. 2012. Coastal fronts set recruitment and connectivity patterns across multiple taxa. Limnology and Oceanography 57 (2): 582–596.
Wooldridge, T.H., and H. Loubser. 1996. Larval release rhythms and tidal exchange in the estuarine mudprawn, Upogebia africana. Hydrobiologia 337 (1-3): 113–121.
Yamada, S.B., and P.M. Kosro. 2010. Linking ocean conditions to year class strength of the invasive European green crab, Carcinus maenas. Biological Invasions 12 (6): 1791–1804.
Yannicelli, B., L.R. Castro, W. Schneider, and M. Sobarzo. 2006a. Crustacean larvae distribution in the coastal upwelling zone off Central Chile. Marine Ecology-Progress Series 319: 175–189.
Yannicelli, B., L.R. Castro, A. Valle-Levinson, L. Atkinson, and D. Figueroa. 2006b. Vertical distribution of decapod larvae in the entrance of an equatorward facing bay of central Chile: implications for transport. Journal of Plankton Research 28 (1): 19–37.
Acknowledgements
The authors especially thank Lee McCoy and John Chapman for their dedicated assistance and help with field work, data analysis, and interpretation particularly during the 2010–2012 surveys in Yaquina Bay. We are grateful to a host of field assistants that are too numerous to mention that assisted with the long-term monitoring program but include most significantly Kristine Feldman who served over most of the program’s life and in more recent years efforts by Daniel Sund, Dacey Mercer, Jonathan Minch, Samantha Bund, Cara Fritz, Roy Hildenbrand, and Roxanna Hintzman. The Willapa Bay/Grays Harbor Shellfish growers provided significant in-kind assistance including use of their beds and consultation including assistance from integrated pest management coordinators Steve Booth, Jacob Moore, and David Beugli. We also frequently collaborated with and acknowledge similar Willapa Bay surveys and support from Kim Patten at the Washington State University extension station in Long Beach. Previous versions of the manuscript were greatly improved by comments from several reviewers including Dany de Oliveira, Dacey Mercer, and one anonymous reviewer. Mention of trade names or commercial products does not constitute endorsement or recommendation for use.
Funding
This research was funded by the U.S. Department of Agriculture, Agricultural Research Service (CRIS Project 2072-63000-004-00D) and several other institutions and granting agencies over the life of the long-term monitoring program including the Washington State Department of Fisheries, the Western Regional Aquaculture Center and Washington Sea Grant.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Marianne Holmer
Rights and permissions
About this article
Cite this article
Dumbauld, B.R., Bosley, K.M. Recruitment Ecology of Burrowing Shrimps in US Pacific Coast Estuaries. Estuaries and Coasts 41, 1848–1867 (2018). https://doi.org/10.1007/s12237-018-0397-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-018-0397-4