Abstract
Marsh pools are present in estuaries throughout the world and provide valuable habitat for fishes and decapod crustaceans (i.e., nekton). The purpose of our study was to examine the species composition and temporal variation of the nekton assemblage within marsh pools of a southeastern US estuary. We conducted weekly sampling of five marsh pools in the North Inlet estuary, SC from May to November 2016. Temporal variation in the nekton assemblage appeared to be related to the life history of individual species, tidal connectivity of pools with adjacent habitats, and environmental conditions within pools. Most transient species, which migrate into the North Inlet estuary as larvae or juveniles, were present primarily in early summer and late fall. Many transient species were absent or occurred in low abundance during July and August when water temperature was highest, salinity most variable, and tidal connectivity with adjacent habitats was lowest. In contrast, most resident species, which can complete their entire life cycle within the North Inlet estuary, were present and relatively abundant throughout the study as juveniles and adults. Based on the limited studies available, species richness and the ratio of transient to resident species in marsh pools at low latitudes (e.g., southeastern US) are higher compared to marsh pools at high latitudes (e.g., east coast of Canada). A more comprehensive understanding of the role of marsh pools in the life history of nekton would be useful for conserving, managing, and restoring salt marshes and the species found in these environments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Marsh pools occur in estuaries throughout the world and are one component within the mosaic of interconnected salt marsh habitats (Minello et al. 2003). Defining characteristics of marsh pools, such as surface area, depth, and connections with adjacent aquatic habitats, can vary greatly. Herein, we focus on high marsh pools that are generally shallow (< 0.5 m), small (surface area = 10s to 100s m2), soft-bottomed depressions embedded in the marsh surface that hold water throughout the tidal cycle and have no permanent connection (i.e., channel) to adjacent water bodies (Harshberger 1916; Nicol 1935; Ingólfsson 1994; Rowe and Dunson 1995; Adamowicz and Roman 2005; MacKenzie and Dionne 2008; Davis et al. 2014a). Pools are only intermittently linked to other aquatic habitats in the salt marsh, such as tidal creeks, primarily during spring high tides and storm events when the marsh surface is flooded. Local weather conditions (e.g., air temperature and precipitation) and the frequency of tidal inundation influences environmental conditions within marsh pools (Nicol 1935; Noël and Chmura 2011). Compared to nearby tidal creeks, environmental conditions within pools can be extreme (Hunter et al. 2007); wide ranges in water temperature (− 1.8 to 41 °C), salinity (0 to 60), and dissolved oxygen (0 to 20 ppm) levels are possible (Rowe and Dunson 1995; Layman et al. 2000; Raposa 2003; Smith and Able 2003).
The fish and decapod crustacean (i.e., nekton) communities of marsh pools have been examined primarily in estuaries along the east coast of North America (see Able et al. 2005 and references therein) and to a lesser extent in Europe (Nicol 1935; Verhoeven and van Vierssen 1978; Frid 1988; Frid and James 1989; Ingólfsson 1994; Hampel et al. 2004), Australia (Davis et al. 2012, 2014a, b), South America (Sampaio and Martinelli-Lemos 2014), and the west coast of North America (Wolf et al. 1983; Barnby et al. 1985). Most of these studies are limited to describing the presence and species richness of nekton within marsh pools; however, detailed studies along the northeastern coast of North America (i.e., north of Cape Hatteras, North Carolina) have found the species richness of nekton to be relatively low compared to adjacent marsh habitats (Rowe and Dunson 1995; Layman et al. 2000; Raposa and Roman 2001; Able et al. 2005) and that pools may function as locations for nekton to overwinter, forage, and reproduce (Chidester 1920; Bleakney and Meyer 1979; Worgan and FitzGerald 1981a, b; Ward and FitzGerald 1983; Talbot and Able 1984; Walsh and FitzGerald 1984; Talbot et al. 1986; Poulin and FitzGerald 1989; Whoriskey and FitzGerald 1989; Smith and Able 1994; Rowe and Dunson 1995; Halpin 2000; Layman et al. 2000; Raposa and Roman 2001; Raposa 2003; Able et al. 2005, 2012; MacKenzie and Dionne 2008; Hunter et al. 2007, 2009; Vincent et al. 2015).
Although marsh pools occur in estuaries along the east coast of North America at lower latitudes (i.e., south of Cape Hatteras, North Carolina), considerably less information is available on nekton use of pools in this region. The presence and species richness of nekton in marsh pools in this region have been examined in few locations (Kilby 1955; Harrington and Harrington 1961; Dahlberg 1972; Subrahmanyam and Coultas 1980), and the function (e.g., feeding, reproduction) of marsh pools has been identified for a few species (Rickards 1966, 1968; Kneib 1978, 1982). From these limited studies, it appears that marsh pools in the southeastern US may provide nursery habitat and offer similar benefits to nekton as has been observed for pools elsewhere. Further, marsh pools in this region may support more species rich nekton assemblages than pools at higher latitudes along the east coast of North America. However, general information on nekton use of marsh pools, as well as information on the biological and physical factors that may cause variation in the presence and composition of nekton assemblages, is lacking for most of this region.
The purpose of our study was to examine the species composition and temporal variation of the nekton assemblage within marsh pools of a southeastern US estuary. We conducted weekly sampling over a 28-week period in five marsh pools within the North Inlet estuary, SC, USA. Our sampling targeted multiple life history stages of nekton to determine if marsh pool use varied among species and life history stage. During this same time period, we measured environmental variables within pools, such as salinity and temperature, and pool connectivity with adjacent habitats to identify abiotic factors that could potentially influence nekton use of marsh pools. We also examined studies from marsh pools in eastern North America and worldwide to identify potential spatial variation in nekton utilization of these habitats in the region and across the globe.
Materials and Methods
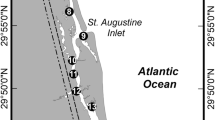
The nekton assemblage in five marsh pools (as defined above) within a 0.1 km2 area in the central portion of the North Inlet estuary was sampled weekly during 6 May to 18 November 2016 (n = 28 weeks). Pools were shallow (< 0.5 m at low tide), relatively small (surface area ranged from 70 to 568 m2; Table 1), and surrounded by either short Spartina alterniflora high marsh or Salicornia–Distichlis marsh described by Teal (1958). All pools were sampled on the same day during low tide when the surrounding marsh was not inundated with water, and pools were not connected to any adjacent aquatic habitats. We chose to sample pool nekton from spring through fall because this is typically the period of highest abundance for fish and decapod crustacean species within the North Inlet estuary (Allen et al. 2014).
Larvae and other small nekton were targeted using a custom Sea Gear rectangular plankton net (20-cm high × 1.4-m wide with 1-mm mesh), which allowed us to sample most of the water column in the shallow pools. The plankton net was pulled once along a fixed transect along the pool’s longest axis for each sampling event. These samples were immediately placed on ice and transported to the laboratory for processing (approximately 2 h after collection). All individuals were identified to the lowest feasible taxonomic level, counted, and up to 20 individuals of each taxon were randomly selected and measured for length (mm): standard length (SL) for fishes, carapace width (CW) for crabs, and total length (TL; tip of rostrum to tip of telson) for shrimps.
After the plankton net sample was collected at each pool, a cast net (1.8-m diameter with 6-mm mesh) was used to target large nekton with three replicate casts thrown from the pool edge. Within the same pool, cast net samples were collected approximately 5 to 10 min after completion of the plankton sample and replicate cast net samples were separated by approximately 2 to 5 min. Cast net samples were sorted in the field where all individuals were identified, counted, and up to 20 individuals of each taxon were randomly selected and measured as described above. All individuals were then released back into the pools except for individuals identified as Megalops atlanticus, which were placed on ice and transported to the laboratory for use in other studies.
Taxon-specific density was calculated for taxa identified from the plankton net samples using the volume of water filtered per sample (height × width of plankton net × length of tow) and standardized to number of individuals per 100 m3. A mean density for each sample date was calculated by averaging densities from all five pools. The number of individuals of a given taxon was determined for each cast net sample (i.e., catch-per-unit-effort; CPUE), then a mean CPUE was calculated for each pool on each sample date. Mean CPUE values for each pool on each sample date were then used to calculate a mean CPUE for each sample date and also a mean CPUE ± standard error over the entire study period.
Environmental conditions within marsh pools were monitored both discretely, during nekton sampling events, and continuously over the entire study period. Prior to collecting samples from each pool on each sample date, water salinity and dissolved oxygen (DO) concentration were measured with a handheld multiparameter meter (YSI, Inc.). Water depth and temperature were monitored continuously at 15-min intervals throughout the study period using a HOBO water level and temperature logger (U20L-01; Onset Computer Corp.) located in the deepest portion of each pool. Water depth measurements taken using a meter stick at the edge of each pool during high tide were used in conjunction with simultaneous data from the HOBO logger to determine a depth reading on the HOBO logger at which the water depth was 5 cm in the surrounding marsh. At this stage of inundation, pools would be connected by a water depth (≥ 5 cm) that we assumed would be sufficient to allow nekton to move between the marsh pool and nearest subtidal creek. Pool connectivity was tracked by creating an index of daily hydrological connectivity, which was calculated for each pool as the percent of daily 15-min intervals (n = 96 intervals per 24 h) when the water depth reading on the HOBO was at a level where water depth on the surrounding marsh was at least 5 cm above the edge of the pool. This resulted in 197 percentage values for each pool (1 per day × 197 days). We then took the mean of these percentage values on each day among all five pools to examine the hydrological connectivity of the pools over the entire study period.
Descriptive statistics, such as mean, maximum, and minimum values, were used to examine nekton and environmental data. Our primary objective was to describe patterns in nekton occurrence and environmental factors that we observed; therefore, we did not test any statistical hypotheses. Nekton taxa were assigned an estuarine status based on whether their entire life cycle (resident) or only a portion (transient) occurred within the estuary. Taxon-specific patterns in marsh pool utilization were examined by plotting mean CPUE and density over time separately for cast net and plankton net sample data, respectively. Before plotting, mean CPUE and density data were scaled by the maximum value observed during the study, so the trend over time can be easily compared among different taxa even if the absolute values vary among taxa. Length-frequency distributions were also examined for nekton taxa for which at least 50 individuals were collected from both sampling gears combined. Larvae collected for a given taxon were examined separately from juveniles. Environmental data were examined by plotting mean, minimum, and maximum values over time for discretely and continuously collected data.
Results
A total of 13,478 individuals distributed among 26 taxa were collected during May through November 2016 (Table 2). Most taxa were fishes (n = 21), but five decapod crustaceans (Acetes americanus, Callinectes sapidus, Farfantepenaeus spp., Litopenaeus setiferus, and Palaemonetes spp.) were also collected. The two most abundant taxa, Palaemonetes spp. and Cyprinodon variegatus, accounted for 75% of all individuals collected. Of the 26 taxa collected, nine were estuarine residents, comprising 88% of all individuals, while 17 were transients and made up 12% of the total catch. Length among all individuals ranged from 4 to 154 mm, but most (99.7%) were < 100 mm. Only two species, M. atlanticus and E. saurus, were collected as larvae.
Temporal patterns in CPUE and density for the most abundant taxa (n = 16; ≥ 50 individuals total collected; 99% of the total catch) were generally related to whether the taxa were an estuarine resident or transient (Fig. 1). Many of the most abundant transient taxa (n = 10) were absent or occurred in relatively low abundance during mid-summer (July and August); however, the most abundant resident taxa (n = 6) were present and relatively abundant throughout the study period, but there were exceptions (Fig. 1). C. sapidus was relatively abundant in summer, and E. saurus (larvae and juveniles) and M. curema were present in low numbers throughout the study period. M. atlanticus (larvae and juveniles) was only present from June through October. In contrast, Palaemonetes spp. was only present during September through November.
Relative CPUE and density for the most abundant nekton taxa (n = 16) collected with cast nets (catch-per-unit-effort, CPUE; individuals/net) and plankton nets (density; individuals/100 m3) from five marsh pools during May to November 2016 in the North Inlet estuary. Mean CPUE and density on a given sample date are relative to the maximum CPUE and density observed during the study period (reported in Table 2). The estuarine status of each taxa is reported in parentheses as either an estuarine resident (R) or transient (T). Taxa with at least 50 individuals collected from both gears combined are presented (totals reported in Table 2)
Length-frequency distribution patterns differed between residents and transients and by gear type (Fig. 2). While the length of resident and transient taxa overlapped; as expected, transient taxa were generally larger than resident taxa. Within taxa, some overlap was observed in the length of individuals between sampling gears, but for transient species in general, individuals collected with the cast net tended to be larger (on average) than individuals collected with the plankton net. For example, the mean length ± standard deviation (SD) of L. setiferus collected with the plankton net was 33 ± 9-mm TL, while the mean length ± SD of L. setiferus collected with the cast net was 57 ± 18-mm TL. In contrast, the size of resident species individuals collected with the two gears was generally similar (Fig. 2). In general, based on the length-frequency distributions, most resident species were represented by both juvenile and adults, while transient species were represented primarily by juveniles (only two transient species were collected as larvae and small juveniles), although some transient individuals may have been or were likely mature individuals, especially the largest C. sapidus.
Relative length-frequency distributions of the most abundant nekton taxa (n = 16) collected with a cast net (solid bars) and plankton net (open bars) from five marsh pools during May to November 2016 in the North Inlet estuary. The number of individuals in each length class is relative to the total number of individuals measured (reported in Table 2). Taxa with at least 50 individuals collected from both gears combined are presented (totals reported in Table 2)
Long- and short-term patterns in environmental variables were observed within the pools. Mean water salinity ranged from 14 to 42 and was most variable among sampling dates during July and August (Fig. 3). No clear pattern in discrete DO averages was observed from May through November; however, a daily pattern was evident with values generally lowest at 09:00 (approximately 4 mg/L on average) and highest at 17:00 (approximately 11 mg/L on average). Daily mean water temperature ranged from 13 to 33 °C, and the pattern in water temperature during May to November was typical for this region; highest temperatures occurred during July and August (Fig. 3). Mean daily water depth varied from 0.20 to 0.72 m, but on most (95%) days during May through November, daily mean water depth was between 0.22 and 0.37 m. Marsh pools were hydrologically connected to the nearest subtidal creek (see Table 1) for some amount of time on 167 days during the 197-day study period (85% of the days). Mean percent daily hydrological connectivity was highest during May to June and September to November and lowest during July and August (Fig. 3). During July and August (n = 62 days), the mean percent daily hydrological connectivity was zero (i.e., pools were not connected to nearby subtidal habitats) for 34% of the days. In contrast, during May through June (n = 56 days) and September through November (n = 79 days), the mean percent daily hydrological connectivity was zero for only 5 and 8% of the days, respectively. The peak in water depth and connectivity during October was due to Hurricane Matthew, which made landfall in SC on October 8, 2016 approximately 35 km south of the North Inlet estuary (Fig. 3).
Mean salinity, dissolved oxygen (DO, mg/L), temperature (°C), water depth (m), and percent daily hydrological connectivity observed within five marsh pools during May through November 2016 in the North Inlet estuary. Salinity and DO were measured during weekly sampling with a handheld YSI. Water temperature and depth were recorded every 15 min using a HOBO water level and temperature logger (U20L-01) placed in each pool. Dotted lines represent the minimum and maximum values for each 15-min interval among all five pools. Mean percent daily hydrological connectivity represents the percentage of 15-min intervals within a given day (n = 96 intervals per 24 h) when water depth was at least 5 cm on the marsh surface surrounding each pool, at which point the pool was considered hydrologically connected to the nearest tidal creek
Discussion
A variety of estuarine resident and transient nekton, including larvae, juveniles, and adults, were collected from marsh pools in the North Inlet estuary from May through November. Nekton species richness in these pools (n = 26 taxa) was within the range reported from other marsh pools throughout the southeastern US (Fig. 4, < 35° N latitude) and generally higher than nekton species richness in pools along the northeastern US and Canada Atlantic coasts (Fig. 4, > 35° N latitude). Another difference in nekton species assemblages among marsh pools in eastern North America is a general decrease in the ratio of transient to resident species occurring in marsh pools with increased latitude (Fig. 4). Many of the transient species that occur within marsh pools at lower latitudes include the early life stages of important recreational (e.g., E. saurus, M. atlanticus, Centropomus undecimalis) and commercial fishery (e.g., penaeid shrimps, C. sapidus) species (Kilby 1955; Harrington and Harrington 1961; Rickards 1968; Dahlberg 1972; Subrahmanyam and Coultas 1980). Despite differences in nekton species composition, some species occur in pools along much of the coast, such as F. heteroclitus (Georgia to Nova Scotia; Dahlberg 1972; Bleakney and Meyer 1979) and C. variegatus (Florida to Rhode Island; Harrington and Harrington 1961; Roman et al. 2002). Based on length-frequency distributions, it appears that both juveniles and adults of estuarine residents are present in pools, while it is primarily the juvenile life history stage of estuarine transients that were collected in pools. One notable exception to this was the larval E. saurus and M. atlanticus that appear to recruit directly to these habitats. Larvae of estuarine resident species were likely present in habitats directly adjacent to the marsh pools (e.g., shallow microhabitats on the marsh surface; Kneib 1997), although our sampling effort did not cover these habitats. Along with geographic location, differences in nekton species richness in marsh pools could be due to variation in pool size, depth, distance from the nearest subtidal habitat, and location along the marsh elevation gradient (e.g., low to high marsh); however, where possible, we sought to limit our comparisons to marsh pools as defined in this paper.
Species richness and the ratio of transient to resident species reported from published studies on marsh pool nekton assemblages in eastern North America. Numbers represent an individual study and are presented in order of increasing latitude (south to north): 1. Harrington and Harrington (1961), 2a-b. Kilby (1955), 3. Subrahmanyam and Coultas (1980), 4. Dahlberg (1972), 5. Rickards (1968), 6. present study, 7. Rowe and Dunson (1995), 8. Able et al. (2005), 9. Raposa and Roman (2001), 10. Bleakney and Meyer (1979), and 11. Worgan and FitzGerald (1981b). We treated the two locations from Kilby (1955), Bayport (2a) and Cedar Key (2b), as separate data points because they are separated by approximately 1° of latitude, represented different environmental conditions, and had different nekton assemblages
Tidal flooding of the marsh surface ultimately controls the connectivity of pools with nearby subtidal creeks; therefore, spatial and temporal variation in tidal regime governs nekton access to marsh pools (Rozas 1995). All of the marsh pools included in our study were relatively similar in their elevation along the intertidal gradient, but we observed differences in the patterns of nekton CPUE or density over time that appeared to be related to tidal connectivity. During July and August, when pools and nearby creeks were infrequently hydrologically connected, the relative abundance (density or CPUE) of many estuarine transient taxa was low or zero. These transient taxa were primarily larvae and juveniles of marine species that migrated into the North Inlet estuary. In contrast, most resident species were present throughout the summer period. Tidal connectivity is important for transient species because a hydrological connection to adjacent habitats allow these species to emigrate from pools to complete their life cycle and avoid potentially lethal conditions, such as low temperatures during winter (Mace et al. 2017). The effect of tidal connectivity on individual organisms over short time scales (e.g., consecutive tidal cycles) has rarely been examined, and therefore, residence times for individuals of resident or transient species using marsh pools are not well understood (but see Hunter et al. 2009; Able et al. 2012 for Fundulus heteroclitus). Future studies using mark-recapture techniques would be useful in determining residence time and site fidelity for taxa in marsh pools and how these metrics vary among estuarine resident and transient species.
Environmental conditions, such as water temperature, salinity, and DO concentration, may influence marsh pool nekton by affecting the growth and survival of individuals present in marsh pools. We observed a large range for all environmental variables and a relatively large degree of variation in environmental variables over time. For example, DO varied from 1.04 to 13.45 mg/L and tended to be lowest in the morning and highest in the afternoon, which is similar to diel patterns observed in other locations (Nicol 1935; Rowe and Dunson 1995; Smith and Able 2003). Salinity also varied greatly (14 to 42) within marsh pools over time, as reported by others (Nicol 1935; Smith and Able 2003; Noël and Chmura 2011). Environmental variables, such as temperature and salinity, may also interact with hydrological connectivity (Nicol 1935). In our study, variation in salinity was highest during July and August when hydrological connectivity was lowest, which was likely due to combinations of an increase in evaporation, variation in precipitation, and infrequent tidal flooding during this period. Relatively extreme environmental conditions within pools likely limit the number and relative abundance of nekton species present in marsh pools. Species capable of tolerating a wide range of environmental conditions, such as resident cyprinodontoid fishes (Nordlie 2006), and species that have adapted to deal with potentially lethal conditions (e.g., low DO) via specialized behaviors (e.g., M. atlanticus breathing air at the water’s surface) are better able to withstand large fluctuations in environmental conditions that are likely to occur in pool habitats.
Factors other than those we directly examined in our study could also affect the presence and abundance of nekton in marsh pools. Competition for food resources among and within species could affect nekton presence, abundance, and growth in marsh pools (Layman et al. 2000); especially during periods when connectivity is low and individuals are not able to migrate from pools. Predation may also affect nekton within marsh pools (Kneib 1982). For example, juvenile M. atlanticus are known to feed on estuarine resident fishes and crustaceans (Rickards 1966), and C. variegatus has been observed in the stomach contents of M. atlanticus collected from North Inlet estuary pools (M. Mace, unpublished data). During September, when M. atlanticus abundance was highest, C. variegatus abundance was lowest, possibly reflecting the impact of predation by M. atlanticus. Future studies involving manipulative experiments and examination of stomach contents could help to elucidate the effect of biological interactions among nekton on observed nekton assemblage patterns.
Our examination of other studies on marsh pool use by nekton globally revealed several interesting patterns. Nekton species richness is low in marsh pools at high latitudes. This pattern was consistent for pools within estuaries of Europe (Nicol 1935; Ingólfsson 1994) and the west coast of North America (Wolf et al. 1983), which is similar to the US and Canada Atlantic coasts (Fig. 4). Pools in estuaries at low latitudes, such as those in North America (Rickards 1968; Subrahmanyam and Coultas 1980) and Australia (Davis et al. 2012), appear to support more speciose and diverse nekton communities, however, relatively few studies have been conducted in this zone. Similar families of nekton were also found utilizing marsh pools from different areas around the world. Fishes from the family Gasterosteidae (sticklebacks) reside in marsh pools along the east and west coasts of North America and in Europe (Verhoeven and van Vierssen 1978; Worgan and FitzGerald 1981a, b; Wolf et al. 1983; Barnby et al. 1985; Ingólfsson 1994), and shrimp from the family Palaemonidae are present in marsh pools of Europe and the east coast of North America (present study; Rickards 1968; Verhoeven and van Vierssen 1978; Frid 1988; Hampel et al. 2004). In addition, larvae and juveniles of economically important fishes (present study, Rickards 1968; Wolf et al. 1983; Frid 1988; Davis et al. 2012), shrimps (present study; Rickards 1968; Frid 1988; Hampel et al. 2004; Sampaio and Martinelli-Lemos 2014), and crabs (present study; Rickards 1968; Subrahmanyam and Coultas 1980) occur in marsh pools in many regions around the globe; this finding suggests that marsh pools may provide valuable nursery habitat for estuarine transient species worldwide.
The extent of marsh pools in southeastern US estuaries and their function in the life history of nekton is not well known. Therefore, it is difficult to assess the overall value of habitat provided by high marsh pools on nekton populations in this region. In contrast, their extent has been well documented in some regions (e.g., New England; Adamowicz and Roman 2005), where these pools are a common feature of the marsh landscape and are important habitats for critical life history functions of fishes (e.g., Able et al. 2005) and also serve as foraging habitats for numerous waterbirds (Erwin et al. 2006). Although our results are based on one location in one year, when compared with observations from earlier studies focused on southeastern US estuaries our results support the conclusion that marsh pools appear to serve as valuable habitat for common resident and transient salt marsh nekton species in this region. For example, E. saurus, M. atlanticus, and C. sapidus use marsh pools extensively during their larval and juvenile stages as do juvenile and adult stages of resident nekton. It is unclear, however, whether or how individuals of these transient species contribute to adult populations. A more comprehensive understanding of the function of marsh pools for nekton, their function relative to other habitats in the estuarine ecosystem, and how these functions vary geographically would be useful for supporting efforts to conserve, manage, and restore salt marshes and the species that depend on these systems.
References
Able, K.W., K.J. Smith, and S.M. Hagan. 2005. Fish composition and abundance in New Jersey salt marsh pools: sampling technique effects. Northeastern Naturalist 12 (4): 485–502.
Able, K.W., D.N. Vivian, G. Petruzzelli, and S.M. Hagan. 2012. Connectivity among salt marsh subhabitats: residency and movements of the mummichog (Fundulus heteroclitus). Estuaries and Coasts 35 (3): 743–753.
Adamowicz, S.C., and C.T. Roman. 2005. New England salt marsh pools: a quantitative analysis of geomorphic and geographic features. Wetlands 25 (2): 279–288.
Allen, D.M., W.B. Allen, R.F. Feller, and J.S. Plunkett, eds. 2014. Site profile of the North Inlet-Winyah Bay National Estuarine Research Reserve, 432 pp. Georgetown, SC: North Inlet-Winyah Bay National Estuarine Research Reserve.
Barnby, M.A., J.N. Collins, and V.H. Resh. 1985. Aquatic macroinvertebrate communities of natural and ditched potholes in a San Francisco Bay salt marsh. Estuarine, Coastal and Shelf Science 20 (3): 331–347.
Bleakney, J.S., and K.B. Meyer. 1979. Observations on saltmarsh pools, Minas Basin, Nova Scotia 1965–1977. Proceedings of the Nova Scotian Institute of Science 29: 353–371.
Chidester, F.E. 1920. The behavior of Fundulus heteroclitus on the salt marshes of New Jersey. The American Naturalist 54 (635): 551–557.
Dahlberg, M.D. 1972. An ecological study of Georgia coastal fishes. Fishery Bulletin 70: 323–353.
Davis, B., R. Johnston, R. Baker, and M. Sheaves. 2012. Fish utilisation of wetland nurseries with complex hydrological connectivity. PLoS One 7 (11): e49107.
Davis, B., C. Mattone, and M. Sheaves. 2014a. Bottom-up control regulates patterns of fish connectivity and assemblage structure in coastal wetlands. Marine Ecology Progress Series 500: 175–186.
Davis, B., R. Baker, and M. Sheaves. 2014b. Seascape and metacommunity processes regulate fish assemblage structure in coastal wetlands. Marine Ecology Progress Series 500: 187–202.
Erwin, R.M., D.R. Cahoon, D.J. Prosser, G.M. Sanders, and P. Hensel. 2006. Surface elevation dynamics in vegetated Spartina marshes versus unvegetated tidal ponds along the Mid-Atlantic coast, USA, with implications to waterbirds. Estuaries and Coasts 29 (1): 96–106.
Frid, C.L.J. 1988. The marine fauna of the north Norfolk salt marshes, and their ecology. Transactions of the Norfolk and Norwich Naturalists’ Society 28: 46–50.
Frid, C.L.J., and R. James. 1989. The marine invertebrate fauna of a British coastal salt marsh. Holarctic Ecology 12: 9–15.
Halpin, P.M. 2000. Habitat use by an intertidal salt-marsh fish: trade-offs between predation and growth. Marine Ecology Progress Series 198: 203–214.
Hampel, H., A. Cattrijsse, and J. Mees. 2004. Changes in marsh nekton communities along the salinity gradient of the Schelde river, Belgium and The Netherlands. Hydrobiologia 515 (1-3): 137–146.
Harrington, R.W., Jr., and E.S. Harrington. 1961. Food selection among fishes invading a high subtropical salt marsh: from onset of flooding through the progress of a mosquito brood. Ecology 42 (4): 646–666.
Harshberger, J.W. 1916. The origin and vegetation of salt marsh pools. Proceedings of the American Philosophical Society 55: 481–484.
Hunter, K.L., M.G. Fox, and K.W. Able. 2007. Habitat influences on reproductive allocation and growth of the mummichog (Fundulus heteroclitus) in a coastal salt marsh. Marine Biology 151 (2): 617–627.
Hunter, K.L., M.G. Fox, and K.W. Able. 2009. Influence of flood frequency, temperature and population density on migration of Fundulus heteroclitus in semi-isolated marsh pond habitats. Marine Ecology Progress Series 391: 85–96.
Ingólfsson, A. 1994. Species assemblages in saltmarsh ponds in western Iceland in relation to environmental variables. Estuarine, Coastal and Shelf Science 38 (3): 235–248.
Kilby, J.D. 1955. The fishes of two Gulf coastal marsh areas of Florida. Tulane Studies in Zoology 2: 175–247.
Kneib, R.T. 1978. Habitat, diet, reproduction and growth of the spotfin killifish, Fundulus luciae, from a North Carolina salt marsh. Copeia 1: 164–168.
Kneib, R.T. 1982. The effects of predation by wading birds (Ardeidae) and blue crabs (Callinectes sapidus) on the population size structure of the common mummichog, Fundulus heteroclitus. Estuarine, Coastal and Shelf Science 14 (2): 159–165.
Kneib, R.T. 1997. Early life stages of resident nekton in intertidal marshes. Estuaries 20 (1): 214–230.
Layman, C.A., D.E. Smith, and J.D. Herod. 2000. Seasonally varying importance of abiotic and biotic factors in marsh-pond fish communities. Marine Ecology Progress Series 207: 155–169.
Mace, M.M., III, E.R. Haffey, and M.E. Kimball. 2017. Low-temperature tolerance of juvenile tarpon Megalops atlanticus. Environmental Biology of Fishes 100 (8): 913–922.
MacKenzie, R.A., and M. Dionne. 2008. Habitat heterogeneity: importance of salt marsh pools and high marsh surfaces to fish production in two Gulf of Maine salt marshes. Marine Ecology Progress Series 368: 217–230.
Minello, T.J., K.W. Able, M.P. Weinstein, and C.G. Hays. 2003. Salt marshes as nurseries for nekton: testing hypotheses on density, growth and survival through meta-analysis. Marine Ecology Progress Series 246: 39–59.
Nicol, E.A.T. 1935. The ecology of a salt-marsh. Journal of the Marine Biological Association of the United Kingdom 20 (2): 203–261.
Noël, P.E., and G.L. Chmura. 2011. Spatial and environmental variability of pools on a natural and a recovering salt marsh in the Bay of Fundy. Journal of Coastal Research 27: 847–856.
Nordlie, F.G. 2006. Physicochemical environments and tolerances of cyprinodontoid fishes found in estuaries and salt marshes of eastern North America. Reviews in Fish Biology and Fisheries 16 (1): 51–106.
Poulin, R., and G.J. FitzGerald. 1989. Early life histories of three sympatric sticklebacks in a salt-marsh. Journal of Fish Biology 34 (2): 207–221.
Raposa, K.B. 2003. Overwintering habitat selection by the mummichog, Fundulus heteroclitus, in a Cape Cod (USA) salt marsh. Wetlands Ecology and Management 11 (3): 175–182.
Raposa, K.B., and C.T. Roman. 2001. Seasonal habitat-use patterns of nekton in a tide-restricted and unrestricted New England salt marsh. Wetlands 21 (4): 451–461.
Rickards, W.L. 1966. A study of the ecology of first-year tarpon, Megalops atlantica Valenciennes, in a Georgia salt-marsh, with laboratory studies of growth rates and ecological growth efficiencies, 67 pp. Athens, GA: M.S. Thesis. University of Georgia.
Rickards, W.L. 1968. Ecology and growth of juvenile tarpon, Megalops atlanticus, in a Georgia salt marsh. Bulletin of Marine Science 18: 220–239.
Roman, C.T., K.B. Raposa, S.C. Adamowicz, M.-J. James-Pirri, and J.G. Catena. 2002. Quantifying vegetation and nekton response to tidal restoration of a New England salt marsh. Restoration Ecology 10 (3): 450–460.
Rowe, C.L., and W.A. Dunson. 1995. Individual and interactive effects of salinity and initial fish density on a salt marsh assemblage. Marine Ecology Progress Series 128: 271–278.
Rozas, L.P. 1995. Hydroperiod and its influence on nekton use of the salt marsh: a pulsing ecosystem. Estuaries 18 (4): 579–590.
Sampaio, H.A., and J.M. Martinelli-Lemos. 2014. Use of intertidal areas by shrimps (Decapoda) in a Brazilian Amazon estuary. Annals of the Brazilian Academy of Sciences 86 (1): 333–345.
Smith, K.J., and K.W. Able. 1994. Salt-marsh tide pools as winter refuges for the mummichog, Fundulus heteroclitus, in New Jersey. Estuaries 17 (1): 226–234.
Smith, K.J., and K.W. Able. 2003. Dissolved oxygen dynamics in salt marsh pools and its potential impacts on fish assemblages. Marine Ecology Progress Series 258: 223–232.
Subrahmanyam, C.B., and C.L. Coultas. 1980. Studies on the animal communities in two North Florida salt marshes. Part III. Seasonal fluctuations of fish and macroinvertebrates. Bulletin of Marine Science 30: 790–818.
Talbot, C.W., and K.W. Able. 1984. Composition and distribution of larval fishes in New Jersey high marshes. Estuaries 7 (4): 434–443.
Talbot, C.W., K.W. Able, and J.K. Shisler. 1986. Fish species composition in New Jersey salt marshes: effects of marsh alterations for mosquito control. Transactions of the American Fisheries Society 115 (2): 269–278.
Teal, J.M. 1958. Distribution of fiddler crabs in Georgia salt marshes. Ecology 39 (2): 185–193.
Verhoeven, J.T.A., and W. van Vierssen. 1978. Distribution and structure of communities dominated by Ruppia, Zostera and Potamogeton species in the inland waters of ‘De Bol’, Texel, The Netherlands. Estuarine and Coastal Marine Science 6 (4): 417–428.
Vincent, R.E., M. Dionne, D.M. Burdick, and E.A. Hobbie. 2015. Fish productivity and trophic transfer in created and naturally occurring salt marsh habitat. Estuaries and Coasts 38 (4): 1233–1250.
Walsh, G., and G.J. FitzGerald. 1984. Resource utilization and coexistence of three species of sticklebacks (Gasterosteidae) in tidal salt-marsh pools. Journal of Fish Biology 25 (4): 405–420.
Ward, G., and G.J. FitzGerald. 1983. Fish predation on the macrobenthos of tidal salt marsh pools. Canadian Journal of Zoology 61 (6): 1358–1361.
Whoriskey, F.G., and G.J. FitzGerald. 1989. Breeding-season habitat use by sticklebacks (Pisces: Gasterosteidae) at Isle Verte, Quebec. Canadian Journal of Zoology 67 (9): 2126–2130.
Wolf, E.G., B. Morson, and K.W. Fucik. 1983. Preliminary studies of food habits of juvenile fish, China Poot Marsh and Potter Marsh, Alaska, 1978. Estuaries 6 (2): 102–114.
Worgan, J.P., and G.J. FitzGerald. 1981a. Diel activity and diet of three sympatric sticklebacks in tidal salt marsh pools. Canadian Journal of Zoology 59 (12): 2375–2379.
Worgan, J.P., and G.J. FitzGerald. 1981b. Habitat segregation in a salt marsh among adult sticklebacks (Gasterosteidae). Environmental Biology of Fishes 6 (1): 105–109.
Acknowledgments
We thank A. Adams and J. Wilson from the Bonefish & Tarpon Trust for their suggestions and guidance with the development and execution of this project. We would also like to thank faculty, staff, and students from the USC Baruch Marine Field Laboratory (D. Allen, S. Forehand, M. Kennedy, P. Kenny, T. Thomas), Cornell College Rogers Fellowship in Environmental Studies program (R. Bulger, J. Dean, J. Tesensky), and Wofford College (K. Dickson, D. Kusher, K. Moorhouse) for their assistance with this study. The suggestions of L. Rozas, the associate editor, and two anonymous reviewers improved the manuscript. This research was conducted in accordance with the guidelines set forth in University of South Carolina IACUC Animal Care and Use Protocols #2154-100810-040814, #2264-101032-080315, and #2273-101047-093015.
Funding
Funding for this research was provided by the Bonefish & Tarpon Trust.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Charles T. Roman
Rights and permissions
About this article
Cite this article
Mace, M.M., Kimball, M.E. & Haffey, E.R. The Nekton Assemblage of Salt Marsh Pools in a Southeastern United States Estuary. Estuaries and Coasts 42, 264–273 (2019). https://doi.org/10.1007/s12237-018-0450-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-018-0450-3