Abstract
Comparisons of natural mortality rates can be used to identify essential habitat and nursery areas for fishery species. We estimated and compared natural mortality rates of juvenile white shrimp Litopenaeus setiferus using length-frequency and mark-recapture data and attempted to identify factors that may affect these mortality rates. Daily instantaneous natural mortality rates (95 % confidence interval (CI)) obtained from length-frequency data by following individual cohorts were 0.043 (0.031–0.054) and 0.014 (0.0–0.039). Combining all length-frequency data, converting to age-frequency data, and using two types of catch-curve analyses yielded estimates of 0.069 (0.042–0.095) and 0.060 (0.046–0.073). Mark-recapture estimates obtained in a separate study from two ponds were 0.129 (0.054–0.203) and 0.014 (−0.048–0.076). These estimates are comparable to previously reported values for this species, but we are the first to report a measure of precision with our estimates. In the mark-recapture study, mortality rates appeared to be related to predator abundance in ponds and flooding patterns of the surrounding marsh. The only mortality rate significantly different from any of the other estimates was the lower of the two length-frequency estimates, but this result should be interpreted with caution because of the uncertainty in that estimate, relative imprecision of our estimates, and confounding factors between the methods we used to estimate mortality. Despite this caveat, the results from our study can be used to improve population models for L. setiferus and our understanding of the role of marsh habitats as nursery areas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Estimates of vital rates such as mortality and growth are useful in identifying essential fish habitat and assessing nursery habitats for marine organisms, such as penaeid shrimps (Beck et al. 2001; NMFS 2010). Coastal wetlands of the northern Gulf of Mexico (nGoM) have been linked to the high productivity of penaeid shrimps in this region (Turner 1977), and tidal marshes in particular are thought to be important nursery habitat for penaeid shrimps and other nekton (Kneib 1997; Zimmerman et al. 2000). These wetlands are disappearing at a relatively rapid rate due to a variety of causes (Turner 1990), and identifying habitat that is valuable for penaeid shrimps and other fishery species (i.e., areas where growth is rapid and mortality is low) should be a priority for both conservation efforts and fisheries management.
In exploited populations, knowledge about mortality is especially important because managing such populations is often based on regulating mortality caused directly by humans. In fisheries applications, mortality is often divided into two main components: (1) fishing mortality, which is due to direct removal of individuals from the population by humans, and (2) natural mortality, which is death due to all other causes (Ricker 1975). Although natural mortality is likely affected by many variables (e.g., life history stage, size, sex, habitat), most fishery population models assume a constant natural mortality rate (Vetter 1988). Results from fishery models can be sensitive to the values used for natural mortality; therefore, the quality of information available to manage exploited populations could be improved by providing more precise, accurate estimates of and identifying causes of variability in natural mortality rates (Vetter 1988).
There is a valuable fishery in the nGoM that targets the white shrimp Litopenaeus setiferus, but few estimates of mortality rates for this species are available in the published literature. Moreover, little is known about variation in natural mortality rates for L. setiferus or the factors that cause this variation. Mortality of L. setiferus during early life history stages has a greater impact on population size than adult mortality or fecundity (Baker et al. 2014), and the abundance of early-stage juveniles is a predictor of late-stage juvenile abundance, which is itself a predictor of adult catch per unit effort (Diop et al. 2007). Estimating the variation in mortality rates of L. setiferus at early life history stages and identifying the factors that cause this variation should be a priority for the management of this species.
Most previous estimates of natural mortality for juvenile penaeid shrimps have been based on length-frequency data (Minello et al. 1989; Haywood and Staples 1993; Wang and Haywood 1999; Pérez-Castañeda and Defeo 2005; Baker and Minello 2010). Generally, two approaches are used with length-frequency data to estimate mortality, but both usually include some type of catch-curve analysis. The first method involves sampling a population of shrimp over time and using length-frequency distributions to identify individual groups (or cohorts) of shrimp. Mortality is estimated as the slope of a linear regression of the natural log of abundance of a cohort against time, or if there are only two sampling times, the formula for instantaneous total mortality in Ricker (1975) is used. Various mechanisms can alter length-frequency distributions and result in very similar looking distributions (e.g., Huston and DeAngelis 1987). This can make it difficult to infer demographic processes from these types of data, especially without any other independent information.
The second approach is to convert the length-frequency data to age-frequency data and then conduct a horizontal catch-curve analysis (Vetter 1988). Using this approach, a sample is taken at a single point or during several points in time, and all the length-frequency data are combined and converted to age-frequency data. Mortality is then calculated as either the slope of a linear regression of the natural log of abundance against age (Ricker 1975) or by using the method described in Chapman and Robson (1960). Mortality rates calculated using this method require even stricter assumptions than the longitudinal catch-curve (Vetter 1988). Additional uncertainty may occur with this method because age is assumed to be known without error, but variation in growth rates can result in relatively large size differences among similarly aged animals.
Mark-recapture studies also have been used, although less frequently, to estimate natural mortality of juvenile penaeid shrimps (Edwards 1977; Gracia and Soto 1986; Knudsen et al. 1989, 1996). Modern mark-recapture techniques may provide higher quality information than traditional catch-effort data for fisheries research because capture probability is explicitly taken into account (Pine et al. 2003). Mark-recapture studies with juvenile shrimps are challenging because return rates (i.e., recapture probability) of marked individuals are generally low (e.g., 1 % by Webb and Kneib 2004) and tag-related mortality may be substantial for small individuals.
The main goal of our study was to estimate variability in natural mortality rates of juvenile L. setiferus and attempt to identify factors that may affect that variability. We also compared natural mortality rates derived from length-frequency and mark-recapture data. The data collected with these two approaches also were used to estimate growth rates of L. setiferus in the study area. Finally, along with our results, we used other studies that estimated natural morality of juvenile L. setiferus to compare mortality estimates derived from mark-recapture and length-frequency data.
Materials and Methods
Study Area
Our study was conducted in the region of coastal Texas and Louisiana known as the Chenier Plain, which stretches 322 km (200 miles) from Vermilion Bay, LA, to East Bay, TX (Gosselink et al. 1979). The Chenier Plain consists mainly of elevated ridges, mudflats, marshes, and inland lakes formed as sediments from the Mississippi River were deposited and reworked by marine processes (Gosselink et al. 1979; Penland and Suter 1989).
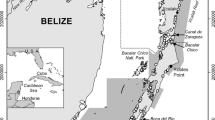
The study area was located within a saline marsh directly west of Sabine Pass in southeast Texas (Fig. 1). The marsh contained numerous shallow ponds (<1-m depth) connected by a subtidal channel (∼1–4-m depth). The mean tidal range at the nearest NOAA tide gauge (Sabine Pass North, station ID 8770570, ∼2.5 km from study area) was 33 cm. Vegetation present at the marsh edge (i.e., marsh shoreline) consisted primarily of the smooth cordgrass Spartina alterniflora, but other species present included Schoenoplectus robustus and Distichlis spicata. No submerged aquatic vegetation was observed in the study area.
Map of salt marsh study area in southeast Texas where natural mortality rates of juvenile white shrimp Litopenaeus setiferus were estimated. Sample sites where length-frequency data were collected are indicated with solid black symbols representing three different sample dates during 2012 (solid circles = July 18, solid squares = August 1, solid triangles = August 15). The mark-recapture study was carried out in ponds 1 and 2 (within open circles), which were partially blocked. During the mark-recapture study, water quality variables were measured in two adjacent, unaltered ponds (within open triangles) of similar depth and size
Mortality Estimates
Length-Frequency Data
We estimated natural mortality rates by tracking the decline in abundance of individual cohorts of shrimp over time using length-frequency data from samples collected on July 18, August 1, and August 15, 2012. Sample sites were selected before each date from a 1 × 1-km area of saline marsh using random numbers and an aerial photograph of the study area. The area was divided into 16 squares of equal size (0.25 × 0.25-km), and five of these squares were randomly selected for each sampling event. Four samples were collected in each of the five randomly selected squares (20 total samples per sampling event). In each chosen square, we randomly selected sites to collect one sample from each of the following habitat types: marsh ≤1 m from the marsh edge, shallow water ≤1 m from the marsh edge, shallow water 1–5 m from the marsh edge, and shallow water >5 m from the marsh edge. Sample sites for shallow water >5 m were determined by randomly selecting distances between the middle of the water body and >5 m from the nearest shore. These habitat types were chosen to include most locations where shrimp would be present, but also to concentrate sampling effort at sites where shrimp density is high. The density of juvenile L. setiferus varies as a function of distance from the marsh edge; density is highest at the marsh edge and declines sharply with distance on either side of the marsh edge (Minello et al. 2008).
Shrimp were collected using a 1-m2 drop sampler, which is a fiberglass cylinder dropped from a boom mounted on the bow of a boat (Zimmerman et al. 1984). Samples were collected, and water salinity, temperature, depth, dissolved oxygen (DO), and turbidity were measured using the protocol described in Rozas et al. (2012). In the laboratory, all organisms were separated from detritus and plant parts, and juvenile penaeid shrimps were identified to species using the characters from Pérez Farfante (1970), Ditty (2011), and references therein. Carapace length (CL) was measured for all juvenile L. setiferus to the nearest 0.1 mm with an ocular micrometer or calipers. Any L. setiferus too damaged to get a reliable length measurement (n = 50 or 3 % of total) were excluded from further analyses.
Length-frequency distributions were constructed separately for each sampling date and weighted to represent the size distribution of the entire shrimp population within the study area. We first calculated the area of each of the four habitat types within the entire study area. We multiplied this total area of each habitat type by the mean density of each size class of shrimp collected from that habitat type. Length-frequency distributions from all four habitat types were then combined and converted to a relative length-frequency distribution. For the marsh habitat type, we only included marsh within 5 m of the edge because most shrimp occur there (Minello et al. 2008), and we did not collect samples in marsh vegetation >5 m from the edge. Density estimates derived from our marsh sample sites (≤1 m from the edge) were applied to this entire marsh habitat type, although shrimp density may decline from the shoreline to 5 m in the vegetation (Minello et al. 2008). Relative length-frequency distributions for each sampling date were multiplied by the number of shrimp collected on that date to produce weighted length-frequency distributions, which we used in all subsequent analyses.
Individual cohorts were separated from the length-frequency distributions with R (R Core Team 2013) using the mixdist package (MacDonald and Du 2012). The MIX program separates the components (i.e., cohorts) of a finite mixture distribution (i.e., the overall length-frequency distribution), and the components can be modeled using a variety of distributions. We chose to fit normal, lognormal, and gamma distributions to our data based on previous studies (Baxter and Renfro 1967; Williams 1969) and used the Akaike’s information criterion (AIC) value, ΔAIC, and the Akaike weight (w i ) to select the most appropriate model given our data (Online Resource Table 1; Anderson 2008). To assess the fit of a given model to the data, a chi-square goodness-of-fit test (Moore and McCabe 2006) was conducted and hanging root grams were inspected. After selecting a model for each sampling date, we used MIX to estimate the relative proportion of each cohort within the overall length-frequency distribution and the mean ± standard deviation CL of each cohort. The number of individuals in a cohort on a given sample date was then estimated by multiplying the relative proportion of a given cohort by the number of individuals in the overall length-frequency distribution for that sample date.
The difference in abundance of a cohort between two sampling dates was used to calculate the total instantaneous mortality rate (Z) during that time period as:
where N t is the number of shrimp in a cohort at time t and N t + i is the number of shrimp in that cohort at time t + 1 (Ricker 1975). If the relationship between the instantaneous fishing (F) and instantaneous natural (M) mortality rate is additive then Z = F + M. The area we sampled was a small fraction of the total study area and no other fishing mortality was occurring, so we assumed that Z = M. A value for M was calculated over both 2-week sampling periods for any cohorts identified and present on more than one date. Approximate variances for M were estimated using the delta method (Powell 2007) and 95 % confidence intervals (CI) were calculated as: \( M \pm \left(1.96\times \sqrt{\mathrm{var}(M)}\right) \). This method requires the inclusion of the covariance between parameters to get the most accurate approximation of variance. We did not have an estimate of the covariance for the number of shrimp in a cohort on consecutive sampling dates; therefore, this term was dropped from the equation.
As an additional check on the separation of the components of each length-frequency distribution, the mean absolute growth rate (G absolute) of a cohort was calculated as:
where µ t and µ t + 1 are the mean length of a cohort at time t and t + 1, respectively (Isley and Grabowski 2007). Approximate variances and 95 % CI for G absolute were estimated as described above for M. Before calculating growth rates, size in CL was converted to total length (TL = 4.944 × CL; Baker and Minello 2010) to make comparisons with previously published growth rates more convenient. These growth rates were used to determine whether the separation of cohorts by MIX was reasonable because growth rates well outside the range of previous estimates for juvenile L. setiferus would indicate possible problems with the separation of cohorts by MIX. We realize that growth estimates calculated as above could be biased if mean growth rates differ for shrimp that survive and those that do not survive between sampling events.
We also estimated natural morality rates using a horizontal catch-curve analysis, a method commonly used for juvenile penaeid shrimps (Wang and Haywood 1999; Minello et al. 2008; Baker and Minello 2010). We combined L. setiferus data from all the sampling dates and converted the length-frequency data to age-frequency data using a growth rate of 1.0 mm TL day−1 after converting CL to TL as described above. Age was expressed as the estimated number of days since 5 mm TL, which was the smallest shrimp we collected. We estimated Z, or M as explained above, using the Chapman-Robson estimator (Chapman and Robson 1960) with the standard error corrected for overdispersion (Smith et al. 2012). Only ages older than the age group with peak abundance (14 days) were included in our analysis. We also estimated M from the slope of an unweighted ordinary least squares (OLS) linear regression of the natural log of abundance against age (Ricker 1975). With the unweighted OLS regression method, we started the catch curve at the age of peak abundance and used all ages up to but not including the first age with ≤1 individual. Using simulations with a relatively low actual Z (0.2), Smith et al. (2012) found that using the above criteria resulted in no bias for Z estimated with unweighted OLS linear regression. We used this regression approach to directly compare our estimates with previous catch-curve estimates of mortality for juvenile L. setiferus.
Mark-Recapture Data
We also estimated natural mortality of juvenile L. setiferus by conducting a 10-day mark-recapture study from July 19–28, 2012. The mark-recapture study was conducted within the same 1 × 1-km area where we collected samples for length-frequency analyses. We do not believe the mark-recapture study interfered with the collection and interpretation of the length-frequency data because the ponds used for our mark-recapture study were too small to be sampled with a drop sampler. Furthermore, only 1800 shrimp, a small fraction of the entire population, were collected and removed from the study area outside the ponds used for the mark-recapture study.
To estimate natural mortality, we placed marked shrimp (27–50 mm TL) into two ponds (hereafter referred to as pond 1 and pond 2, Fig. 1) that each encompassed approximately 150 m2. Marked shrimp were released into each pond on the first day (300 individuals pond−1) and then every other day (150 individuals pond−1) until the end of the study. This resulted in five separate marking events and a total of 1800 shrimp marked during the study. Shrimp were marked using visible implant elastomer (VIE®), and a different color mark was used for each marking event to indicate when recaptured individuals were released. Shrimp marked in the sixth and third abdominal segments were placed into pond 1 and pond 2, respectively. On the first day of the study, we also marked a subset of shrimp (n = 101) of the same TL to obtain growth rate estimates. These shrimp were marked with the same color as all other shrimp tagged on the first day, but the tag was placed in the first abdominal segment. On each day following marking events, we collected ten samples with a cast net (4.9-m diameter, 4.8-mm mesh) to recapture marked shrimp. Our decision to use ten casts per pond was based on a desire to balance our need to recapture an adequate percentage of marked shrimp each day with a concern that more sampling may have caused too much disturbance in the ponds. All penaeid shrimps captured with the cast net were placed on ice, transferred to 10 % formalin, and taken to the laboratory for processing. Potential predators of L. setiferus collected in these samples were identified, measured, and returned alive to the ponds.
The single intertidal creek leading into each pond was partially blocked to maximize the probability of recovering marked shrimp and to retain a minimum pond depth during low-water events. Untreated plywood was placed across each creek, sunk to the height of the adjacent creek banks, and supported by four posts. We attached two pieces of 6.4-mm wire mesh to the top of the plywood and extended these panels 2 m into the adjacent marsh on each creek bank to impede marked shrimp from leaving the ponds. This partial barrier allowed water to flow into the ponds daily during high tides and to flow out on ebb tides until the water level reached the top of the barrier.
Partially blocking the ponds may have altered the environmental conditions within these ponds. Therefore, we monitored and compared the water quality of these ponds and nearby unaltered ponds. Water level monitors were placed inside both ponds and in two nearby ponds that were similar in size and depth (Fig. 1). Flooding duration (% time water depth ≥5 cm) of the marsh around pond 1 and pond 2 was calculated by measuring water depth at the marsh edge and every meter up to 5 m into the marsh vegetation along six transects around each pond. These water depths were then related to depths recorded hourly over the duration of the study by the water level monitor in each pond. During each day of the study, we also measured water salinity, temperature, DO, and depth twice in all four ponds. In addition to measuring these environmental variables, we caged tagged shrimp (ten shrimp cage−1) within pond 1 and pond 2 to determine whether conditions inside the partially blocked ponds may have caused shrimp mortality. This caging experiment was also used to assess short-term mortality due to capture, handling, and tagging. Shrimp were placed within each cage at the initiation of the study and checked daily until the end of the study. The cages had mesh bottoms, which may have restricted access to benthic infauna (i.e., potential shrimp prey). Therefore, we added food (0.59 ± 0.01 g of Wardley® shrimp pellets) to each cage daily. These pellets contained a minimum of 30 % crude protein, 3 % crude fat, and 10 % crude fiber.
We analyzed the mark-recapture data with the IRATE program (NOAA Fisheries Toolbox 2013), which uses the instantaneous rates version of the Brownie mark-recapture models (Brownie et al. 1985; Hoenig et al. 1998). We compared and selected the best of four possible models (Online Resource Table 2) for each pond using the AIC value, ΔAIC, and the Akaike weight (w i ) as described above. After selecting the best model for pond 1 and pond 2, we evaluated the fit of that model to the data with a chi-square goodness-of-fit test (Moore and McCabe 2006) and by examining the model residuals for any patterns that could indicate violations of the model assumptions (Latour et al. 2001). The mortality rates estimated with these models applied to the period between marking events, which was 2 days, so we converted these to daily rates by dividing the estimates by two. We estimated approximate variances using the delta method and calculated 95 % CI as \( M \pm \left(1.96 \times \sqrt{\mathrm{var}(M)}\right) \).
Comparison of Mortality Estimates
We first compared our estimates calculated from length-frequency data to each of our estimates from mark-recapture data. In these comparisons, the null hypothesis of no difference between the two estimates would be rejected if the associated 95 % CI of the difference did not include zero (the “standard” method in Schenker and Gentleman 2001). We also included our mortality rates in a comparison of published natural mortality rates estimated using length-frequency versus mark-recapture data. In studies that included more than one mortality estimate, we computed a mean value from the multiple estimates derived from length-frequency or mark-recapture data. We then used estimates of natural mortality from different studies as independent replicates to compare mean mortality rates derived from length-frequency and mark-recapture data using an unequal variance t test (Ruxton 2006).
Results
Environmental Variables
During sampling trips to collect length-frequency data, the mean salinity and DO increased from the first to the third trip (Table 1). Mean temperature and turbidity were similar across all three trips, while mean depth was lower on the last two trips than the first trip (Table 1).
During the mark-recapture study, salinity and temperature were similar among ponds and varied little over the entire study (Fig. 2). DO varied among ponds (lower in partially blocked than unblocked ponds), within days (lower in morning than later), and over the study. Variation in DO was highest during the first and last few days of the study. Water depth in the four ponds was relatively similar except at low tide, when the plywood barrier retained higher water levels in ponds 1 and 2 (Fig. 2). The marsh edge of both partially blocked ponds was flooded (≥5 cm) continuously, but the mean flooding duration of the marsh ≥2 m from the edge of pond 1 dropped off sharply to <40 % (Fig. 3). In contrast, the mean flooding duration of marsh within 5 m of pond 2 was always >50 %.
Water a salinity, b temperature (°C), c dissolved oxygen (mg/L), and d depth (cm) measured twice daily in mark-recapture study ponds used to estimate natural mortality of juvenile white shrimp Litopenaeus setiferus. Ponds 1 and 2 were partially blocked. Data from two adjacent unaltered ponds of similar size and depth are also shown
Flooding duration of marshes adjacent to ponds 1 and 2 during a mark-recapture study to estimate natural mortality rates of juvenile white shrimp Litopenaeus setiferus. Mean flooding duration was estimated as percentage of time during the study that water depth at a location was ≥5 cm. Each mean (±SE) was calculated from six replicate measurements except for 5 m into the marsh around pond 2, which was based on four replicate measurements
Length-Frequency Data
The number of cohorts and the distributions that fit the data varied among sampling dates (Fig. 4). Three distinct cohorts in the overall length-frequency distribution were identified on the first sampling date (July 18, 2012), all three distributions fit the data, and all three models had approximately the same support (Online Resource Table 1). On the second sampling date (August 1, 2012), two distinct cohorts were identified, and the model with lognormal distributions had the most support followed by the model with gamma distributions (Online Resource Table 1). Two cohorts were identified in the length-frequency distribution from the third sampling date (August 15, 2012), but none of the three distributions fit the data well. More shrimp occurred in the second cohort on this date than on the second sampling date when this cohort was first seen. This was mainly due to many relatively large shrimp collected in two samples from the same 0.25 × 0.25-km square located on the edge of our study area. This apparent increase in cohort size may have represented immigration into the study area and a violation of one of the assumptions, or alternatively, an aggregation of large shrimp emigrating from the area. We could not identify which of these explanations was responsible for the observed data; therefore, we excluded these two samples and repeated the analysis. After excluding the samples, none of the distributions fit the data well, but the model with normal distributions had the most support (Online Resource Table 1). To derive mortality estimates, we used parameters from the model with lognormal distributions for the first two dates and the model with normal distributions for the third date (Table 2, Fig. 4). All results that include parameters from the model on the third sampling date (i.e., growth and mortality rates from the second to third sample dates) should be interpreted cautiously because this model did not fit the data well.
Length-frequency distributions of juvenile white shrimp Litopenaeus setiferus collected on the a first (July 18, 2012), b second (August 1, 2012), and c third (August 15, 2012) sampling dates and used to estimate natural mortality rates. Curves represent separate cohorts of shrimp and were fit to the length-frequency data using the program MIX. Triangles on the x-axes are the mean lengths of each cohort, and n is the total number of shrimp in the length-frequency distribution
Growth rate estimates derived from length-frequency data differed among cohorts. The mean growth rate (95 % CI) calculated from cohort A to the second cohort (A + B) on the second sampling date was 1.58 (1.46–1.69) mm TL day−1 and from cohort B to the second cohort (A + B) was 0.66 (0.53–0.80) mm TL day−1. Both of these mean growth rates are reasonable for juvenile L. setiferus, and we combined cohorts A and B (A + B in Fig. 4) to calculate the mortality rate from the first to second sampling date. The mean growth rate for cohort C between the second and third sampling dates was 1.68 (1.44–1.91) mm TL day−1, which is also reasonable for juvenile L. setiferus.
We used two different approaches to calculate mortality rates from the length-frequency data. In the first approach, we were able to calculate one mortality rate between each of the sampling dates by tracking individual cohorts. The daily instantaneous natural mortality rate (95 % CI) was 0.043 (0.031–0.054) for cohort A + B between the first and second sampling dates and was 0.014 (0.0–0.039) for cohort C between the second and third dates. Using the second approach, we first converted the length-frequency data to age-frequency data and then calculated two mortality rates for both of the catch-curve methods. Using the Chapman-Robson estimator, we obtained a daily instantaneous natural mortality rate (95 % CI) of 0.069 (0.042–0.095) when the two samples from the third sampling date were excluded and 0.062 (0.034–0.090) when they were not excluded. The daily instantaneous natural mortality estimates (95 % CI) we obtained from unweighted OLS regression were 0.060 (0.046–0.073) when the two samples from the third sampling date were excluded and 0.046 (0.032–0.060) when they were not excluded. These results should be considered with caution because the relationship between the natural log of abundance and age does not appear to be linear (Fig. 5).
Natural log of abundance plotted against age for juvenile white shrimp Litopenaeus setiferus collected on three separate dates (July 18, 2012; August 1, 2012; August 15, 2012) and combined to estimate natural mortality using two catch-curve methods. All solid symbols to the right of the square (14 days) represent ages used to estimate mortality with the Chapman-Robson estimator. All solid symbols starting at the square and up to, but excluding, the first triangle were used to estimate mortality with an unweighted ordinary least squares linear regression. Hollow circles represent data points that were excluded when calculating mortality rates with both methods. The age-frequency plot was derived by converting length-frequency data using a growth rate of 1 mm TL day−1. Two samples collected on the last sample date were excluded as explained in the text
Mark-Recapture Data
Survival rates of caged shrimp varied between ponds (Online Resource Fig. 1). We used the proportional mortality of tagged shrimp in the cages over the first 24 h as the value for φ in the mark-recapture models, which was 0.9 for pond 1 and 1.0 for pond 2. Shrimp caged in pond 2 had a relatively high survival rate (90 %) over the entire study, but only 40 % of the shrimp caged in pond 1 were recovered at the end of the study. The 40 % recovery rate of shrimp from the cage in pond 1 was likely due to both mortality of caged shrimp and also the escape of three shrimp. Two large holes through which shrimp could have escaped and a small blue crab (<20 mm CW) not present on July 24 were discovered in this cage on July 25. Moreover, no shrimp body parts, which would indicate mortality due to stress or handling, were present in the cage on July 25. We believe the survival rate for caged shrimp held in pond 1 was actually 70 %, which was the survival rate until July 25 (Online Resource Fig. 1).
We recaptured approximately 14 and 19 % of the marked shrimp released into pond 1 and pond 2, respectively. The mark-recapture model with the most support given our data for both ponds included a constant natural and time-varying fishing mortality rate (Online Resource Table 2). We chose this model to estimate instantaneous natural and fishing mortality rates for tagged shrimp within each pond. The goodness-of-fit tests indicated good agreement between the selected models and the data for pond 1 (χ 2 = 4.06, df = 9, p = 0.91) and pond 2 (χ 2 = 9.36, df = 9, p = 0.40). We found no evidence for non-mixing in either pond, but all negative residuals in rows three and four for the pond 1 data indicate that shrimp tagged and released on July 23 and 25 may have experienced excessive tag-related mortality (Online Resource Table 3; Latour et al. 2001).
Although our analysis detected no statistically significant difference in daily instantaneous natural mortality rates between ponds, the estimate (M, 95 % CI) for pond 1 (0.129, 0.054–0.203) was higher than pond 2 (0.014, −0.048–0.076). We also captured more potential predators in pond 1 than pond 2 (Online Resource Table 4).
We estimated growth rates from the subset of recaptured shrimp marked for growth estimates. Thirteen of these shrimp were recaptured during the study, but four of these individuals were excluded from the analysis because they were recaptured the day after being released. The nine shrimp included in the growth analysis were recaptured 6–10 days after being released. Their mean (95 % CI) growth rate was 0.60 (0.45–0.74) mm TL day−1, and individual growth rates ranged from 0.20 to 0.86 mm TL day−1.
Comparison of Mortality Estimates
Both catch-curve estimates ± standard error (SE) (0.060 ± 0.007 and 0.069 ± 0.013) and one of the mark-recapture estimates ± SE (0.129 ± 0.038) were significantly higher than the lower of the two length-frequency estimates ± SE (0.014 ± 0.013). Our analysis detected only three significant differences among the mortality estimates, but these results should be interpreted with caution. The lower of the two length-frequency estimates was calculated using parameters from the normal distribution model for the third sampling date, which did not fit the data well. There may be more differences among our mortality rates because the confidence intervals around all estimates were relatively wide, and the statistical power to detect a significant difference between estimates was low. Furthermore, if these differences among estimates do exist, they could be biologically significant.
The daily instantaneous natural mortality rates we estimated for juvenile L. setiferus are within the range of previous estimates (Table 3). Daily instantaneous natural mortality estimates for juvenile L. setiferus from the literature are comparable and mostly range from 0.01 to 0.09 despite differences in locations, methods, and shrimp size (Table 3). Furthermore, these estimates do not appear to differ between the two types of data (mark-recapture, length-frequency) commonly used to estimate these values. No significant difference could be detected (unequal variance t test, t = −0.4246, df = 6, p = 0.6859) between the means (M ± SE) calculated from mark-recapture data (0.05 ± 0.01, n = 3) and length-frequency data (0.06 ± 0.02, n = 5).
Discussion
The natural mortality rates we estimated for juvenile L. setiferus derived from two independent types of data were similar and comparable to previous published estimates, and we are the first to report a measure of precision with our estimates. Although we did not find any strong evidence for differences in mortality rates derived from the two types of data, given the uncertainty in our estimates and the limited number of previous studies, we cannot conclusively state that the two methods are equally efficacious. In the mark-recapture study, mortality rates appeared to be related to predator abundance in ponds and flooding patterns of the surrounding marsh, which supports previous evidence recognizing predation as an important cause of mortality for juvenile penaeid shrimps (Minello et al. 1989; Dall et al. 1990; Salini et al. 1990) and identifying the vegetated marsh surface as a predator refuge (Minello and Zimmerman 1983; Minello et al. 1989; Minello 1993).
Mortality rates for fishery species are difficult to estimate, and where estimates are available, they may not be very precise or accurate. Accuracy can be assessed by comparing estimates of mortality derived from different methods or by determining whether estimates vary with environmental variables known to influence mortality (Miranda and Bettoli 2007). Few studies have directly compared total mortality rates (Z) derived from length-frequency, mark-recapture, or catch-curve analyses (e.g., Fabrizio et al. 1997), while even fewer have focused only on natural mortality (M), and only one study has focused specifically on natural mortality of juvenile penaeid shrimps (Gracia and Soto 1986). Gracia and Soto (1986) estimated a slightly lower natural mortality rate for large (75–120 mm TL) juvenile L. setiferus when using length-frequency data (0.029) versus mark-recapture data (0.031). All methods used to estimate mortality have potential limitations and biases (Miranda and Bettoli 2007), but the methods we used to calculate mortality rates for juvenile L. setiferus seem to provide similar and reasonable estimates.
The mortality estimates from our mark-recapture study were also consistent with our current understanding of how access to marsh vegetation and predator abundance influence mortality rates (Minello and Zimmerman 1983; Minello et al. 1989; Minello 1993). The most likely explanation for the higher mortality rate for shrimp in our pond 1 was that predator density was higher and the area of flooded marsh was less in pond 1 than pond 2. Differences in water quality between ponds and excessive tag-related mortality in pond 1 are also possible explanations, but these seem less likely. Access to the marsh surface has long been hypothesized to influence production of juvenile penaeid shrimps in the nGoM by providing protection from predators and abundant prey resources (Minello et al. 1989; Zimmerman et al. 2000). Marsh flooding has been correlated with the importance of trophic support from marsh production for juvenile penaeid shrimps across the nGoM (Baker et al. 2013) and with inshore commercial landings of penaeid shrimps in Louisiana (Childers et al. 1990). Moreover, individual-based models that simulate juvenile brown shrimp Farfantepenaeus aztecus production within estuarine nursery areas incorporate the assumption that shrimp are less vulnerable to predation when residing in marsh vegetation than in open water (Haas et al. 2004; Roth et al. 2008). Identifying variables that affect the production of juvenile shrimp populations (i.e., rates of mortality, growth, and migration) is important, because this information would inform estimates of the adult population available in the coastal fishery (Haas et al. 2001; Diop et al. 2007).
Uncertainty exists in all parameters (e.g., number of shrimp in a cohort, mean size of shrimp in a cohort) that are estimated when fitting distributions to size-frequency data. This uncertainty should be presented and, where feasible, incorporated into demographic rates derived from these parameters (Grant et al. 1987); however, this has not been done in previous studies on growth and mortality of juvenile penaeid shrimps (Minello et al. 1989; Haywood and Staples 1993; O’Brien 1994; Pérez-Castañeda and Defeo 2003, 2005). We used the delta method (Powell 2007) to estimate approximate variances for our mortality rates, and these variance estimates were used to calculate confidence intervals. Even so, the accuracy of our confidence intervals could have been improved had we been able to include a measure of the covariance between the numbers of shrimp in a cohort on consecutive sampling dates. We agree with Grant et al. (1987) that studies using length-frequency data to estimate demographic parameters should report not only point estimates but also the precision associated with those parameters. Presenting only a point estimate of mortality with no measure of precision is misleading and suggests confidence in the mortality estimate, which is likely not supported by the data.
As with all statistical analyses, the validity of the results we obtained depends on meeting the underlying assumptions of our methods. The length-frequency method assumes no net migration into or out of the study area once a cohort of shrimp arrives or at least migration is random with respect to size (Online Resource Table 5). We excluded two samples collected the same day on the border of our study area because they contained an unusually high number of large shrimp that may have recently immigrated to, or were about to migrate from, the study area. This method also assumes that growth rates among shrimp that differ in size are the same or do not vary substantially. If growth rates vary substantially within cohorts of shrimp, then fast-growing shrimp from a newly arrived cohort may quickly reach a size similar to that of slow-growing shrimp from a previous cohort. Also, if mean growth rates differ among cohorts, then faster growing cohorts may merge with slower growing cohorts. Finally, separating components of an overall length-frequency distribution may be problematic with sample sizes typically used in marine ecology studies (Grant et al. 1987). We could not assess the accuracy of our length-frequency distributions by comparing them to the true, but unknown length-frequency distributions of all shrimp in our study area, although 20 drop samples appeared to be enough to get an adequate estimate of mean length on each sample date (Online Resource Fig. 2). We determined this by randomly selecting 20 drop samples with replacement, calculating a cumulative mean size, and repeating this ten times. Cumulative mean size reached an asymptote well before a sample size of 20 on each sampling date (Online Resource Fig. 2).
The mark-recapture method assumes that the marked population is representative of the unmarked population (Online Resource Table 5). The mortality rates we estimated for marked shrimp can be extrapolated to the unmarked population only if the survival of marked shrimp was not affected by tagging. It seems unlikely that shrimp tagged with small VIE® marks in our study were more vulnerable to predators than unmarked shrimp, and laboratory studies show no effects of non-predation mortality or altered behavior from tagging (Kneib and Huggler 2001; Baker and Minello 2010). Therefore, we are confident our mortality estimates can be applied to untagged shrimp. Our analysis indicated some evidence for excessive tag-induced mortality (Online Resource Table 3, Latour et al. 2001); however, plausible reasons for this cannot be easily identified. All personnel that tagged shrimp in our study had previous experience using VIE® tags on L. setiferus, and no tagged shrimp that appeared distressed or dead were released into the ponds. Unfavorable environmental conditions could also be an explanation, but conditions in pond 2 were similar to pond 1 throughout the study, and we saw no evidence for excessive tag-induced mortality for shrimp in pond 2. Even assuming a 20 % higher mortality for shrimp in pond 1 than pond 2 (based on our caging results) due to unmeasured environmental conditions, the mortality rate for shrimp would still be higher in pond 1 (0.10) than in pond 2 (0.01). As with previous studies using mark-recapture techniques to estimate juvenile shrimp mortality, our estimates from ponds 1 and 2 may not be applicable to the entire population of shrimp within the larger study area. The entire shrimp population may not have been exposed to the same conditions affecting shrimp mortality within our partially blocked ponds (e.g., predator abundance, marsh flooding patterns).
The catch-curve method is likely the most problematic in terms of meeting underlying assumptions for calculating mortality rates. First, the method assumes that historical mortality has been the same for all shrimp used in the analysis (Online Resource Table 5). Determining whether this assumption is met would be difficult, but it seems unlikely in our study. Second, this method assumes that recruitment over time is relatively constant. Although Baker and Minello (2010) did not find evidence for pulsed recruitment by L. setiferus, data from Baxter and Renfro (1967) indicate this species does recruit in pulses. Third, this method assumes no error in aging individuals, but we know that growth rates vary among individual shrimp, and this variation in growth introduces error into the aging of individuals. Based on simple numerical models, however, Ricker (1975) concluded that errors in aging would not bias mortality estimates derived from catch curves if the true mortality rate is constant among ages and either (1) the proportion of positive and negative errors in aging are equal in both directions at all ages or (2) positive and negative errors are the same at a given age but increase or decrease with age. We have no reason to believe that positive and negative errors in aging L. setiferus were not equal at a given age; therefore, this source of error may not be biasing our mortality estimates. Finally, the catch-curve method also assumes that mortality for different aged individuals is relatively constant over time. Baker and Minello (2010) reported higher mortality rates for small (<28 mm TL) versus large (28–70 mm TL) juvenile L. setiferus. The convex relationship between the natural log of abundance and age we observed for juvenile L. setiferus may indicate an increase in natural mortality with age (Ricker 1975) but more likely represents the migration of older, larger individuals from our study area. We may have violated some of the assumptions for estimating mortality using the catch-curve method, but our estimates from this method were comparable to mortality rates we derived from the same length-frequency data using a different procedure and separately from our mark-recapture data.
Mortality is one of the key vital rates that regulate populations (Rockwood 2006). Although notoriously difficult to estimate, the natural mortality rate is an important parameter in models of exploited animal populations (Vetter 1988). We directly calculated natural mortality rates of juvenile white shrimp L. setiferus using two different types of data that provided comparable estimates, and our estimates are similar to those from previous studies of juvenile L. setiferus. Mortality rates in the mark-recapture study appeared to be influenced by shrimp access to emergent vegetation and predator abundance in ponds. Estimating variability in mortality and identifying factors that affect this variability are crucial to understanding population dynamics. Population models for L. setiferus and other marine organisms may be improved by incorporating this variability to account for the uncertainty in estimates of natural mortality.
References
Anderson, D.R. 2008. Model based inference in the life sciences: A primer on evidence. New York, NY: Springer.
Baker, R., and T.J. Minello. 2010. Growth and mortality of juvenile white shrimp Litopenaeus setiferus in a marsh pond. Marine Ecology Progress Series 413: 95–104.
Baker, R., B. Fry, L.P. Rozas, and T.J. Minello. 2013. Hydrodynamic regulation of salt marsh contributions to aquatic food webs. Marine Ecology Progress Series 490: 37–52.
Baker, R., M. Fujiwara, and T.J. Minello. 2014. Juvenile growth and mortality effects on white shrimp Litopenaeus setiferus population dynamics in the northern Gulf of Mexico. Fisheries Research 155: 74–82.
Baxter, K.N., and W.C. Renfro. 1967. Seasonal occurrence and size distribution of postlarval brown and white shrimp near Galveston, Texas, with notes on species identification. Fishery Bulletin 66: 149–158.
Beck, M.W., K.L. Heck Jr., K.W. Able, D.L. Childers, D.B. Eggleston, B.M. Gillanders, B. Halpern, C.G. Hays, K. Hoshino, T.J. Minello, R.J. Orth, P.F. Sheridan, and M.P. Weinstein. 2001. The identification, conservation, and management of estuarine and marine nurseries for fish and invertebrates. BioScience 51: 633–641.
Brownie, C., D. R. Anderson, K. P. Burnham, and D. S. Robson. 1985. Statistical inference from band recovery data—a handbook. 2nd ed. U.S. Fish and Wildlife Service Resource Publication No. 156, 305 pp.
Chapman, D.G., and D.S. Robson. 1960. The analysis of a catch curve. Biometrics 16: 354–368.
Childers, D.L., J.W. Day Jr., and R.A. Muller. 1990. Relating climatological forcing to coastal water levels in Louisiana estuaries and the potential importance of El Niño-Southern Oscillation events. Climate Research 1: 31–42.
Dall, W., B.J. Hill, P.C. Rothlisberg, and D.J. Sharples. 1990. The biology of the Penaeidae. Advances in Marine Biology 27: 1–484.
Diop, H., W.R. Keithly Jr., R.F. Kazmierczak Jr., and R.F. Shaw. 2007. Predicting the abundance of white shrimp (Litopenaeus setiferus) from environmental parameters and previous life stages. Fisheries Research 86: 31–41.
Ditty, J.G. 2011. Young of Litopenaeus setiferus, Farfantepenaeus aztecus and F. duorarum (Decapoda: Penaeidae): A re-assessment of characters for species discrimination and their variability. Journal of Crustacean Biology 31: 458–467.
Edwards, R.R.C. 1977. Field experiments on growth and mortality of Penaeus vannamei in a Mexican coastal lagoon complex. Estuarine and Coastal Marine Science 5: 107–121.
Fabrizio, M.C., M.E. Holey, P.C. McKee, and M.L. Toneys. 1997. Survival rates of adult lake trout in northwestern Lake Michigan, 1983–1993. North American Journal of Fisheries Management 17: 413–428.
Gosselink, J. G., C. L. Cordes, and J. W. Parsons. 1979. An ecological characterization study of the Chenier Plain coastal ecosystem of Louisiana and Texas. 3 vols. U.S. Fish and Wildlife Service, Office of Biological Services. FWS/OBS-78/9 through 78/11.
Gracia, A., and L.A. Soto. 1986. Estimación del tamaño de la población, crecimiento y mortalidad de los juveniles de Penaeus setiferus (Linnaeaus, 1767) mediante marcado recaptura en la Laguna Chacahito, Campeche, México. Anales del Centro de Ciencias del Mar y Limnología 13: 217–230.
Grant, A., P.J. Morgan, and P.J.W. Olive. 1987. Use made in marine ecology of methods for estimating demographic parameters from size/frequency data. Marine Biology 95: 201–208.
Haas, H.L., E.C. Lamon III, K.A. Rose, and R.F. Shaw. 2001. Environmental and biological factors associated with the stage-specific abundance of brown shrimp (Penaeus aztecus) in Louisiana: Applying a new combination of statistical techniques to long-term monitoring data. Canadian Journal of Fisheries and Aquatic Sciences 58: 2258–2270.
Haas, H.L., K.A. Rose, B. Fry, T.J. Minello, and L.P. Rozas. 2004. Brown shrimp on the edge: Linking habitat to survival using an individual-based simulation model. Ecological Applications 14: 1232–1247.
Haywood, M.D.E., and D.J. Staples. 1993. Field estimates of growth and mortality of juvenile banana prawns (Penaeus merguiensis). Marine Biology 116: 407–416.
Hoenig, J.M., N.J. Barrowman, W.S. Hearn, and K.H. Pollock. 1998. Multiyear tagging studies incorporating fishing effort data. Canadian Journal of Fisheries and Aquatic Sciences 55: 1466–1476.
Huston, M.A., and D.L. DeAngelis. 1987. Size bimodality in monospecific populations: A critical review of potential mechanisms. American Naturalist 129: 678–707.
Isley, J.J., and T.B. Grabowski. 2007. Age and growth. In Analysis and interpretation of freshwater fisheries data, ed. C.S. Guy and M.L. Brown, 187–228. Bethesda, Maryland: American Fisheries Society.
Kneib, R.T. 1997. The role of tidal marshes in the ecology of estuarine nekton. Oceanography and Marine Biology: An Annual Review 35: 163–220.
Kneib, R.T., and M.C. Huggler. 2001. Tag placement, mark retention, survival and growth of juvenile white shrimp (Litopenaeus setiferus Pérez Farfante, 1969) injected with coded wire tags. Journal of Experimental Marine Biology and Ecology 266: 109–120.
Knudsen, E.E., R.F. Paille, B.D. Rogers, and W.H. Herke. 1989. Effects of a fixed-crest weir on brown shrimp Penaeus aztecus growth, mortality, and emigration in a Louisiana coastal marsh. North American Journal of Fisheries Management 9: 411–419.
Knudsen, E.E., B.D. Rogers, R.F. Paille, and W.H. Herke. 1996. Juvenile white shrimp growth, mortality, and emigration in weired and unweired Louisiana marsh ponds. North American Journal of Fisheries Management 16: 640–652.
Laney, R.W. and B.J. Copeland. 1981. Population dynamics of penaeid shrimp in two North Carolina tidal creeks. Raleigh, NC: Report 81-1 Carolina Power & Light Co.
Latour, R.J., J.M. Hoenig, J.E. Olney, and K.H. Pollock. 2001. Diagnostics for multiyear tagging models with application to Atlantic striped bass (Morone saxatilis). Canadian Journal of Fisheries and Aquatic Sciences 58: 1716–1726.
MacDonald, P., and J. Du. 2012. Mixdist: Finite mixture distribution models. R package version 0.5-4. http://CRAN.R-project.org/package=mixdist.
Minello, T.J. 1993. Chronographic tethering: A technique for measuring prey survival time and testing predation pressure in aquatic habitats. Marine Ecology Progress Series 101: 99–104.
Minello, T.J., and R.J. Zimmerman. 1983. Fish predation on juvenile brown shrimp, Penaeus aztecus Ives: The effect of simulated Spartina structure on predation rates. Journal of Experimental Marine Biology and Ecology 72: 211–231.
Minello, T.J., R.J. Zimmerman, and E.X. Martinez. 1989. Mortality of young brown shrimp Penaeus aztecus in estuarine nurseries. Transactions of the American Fisheries Society 118: 693–708.
Minello, T.J., G.A. Matthews, P.A. Caldwell, and L.P. Rozas. 2008. Population and production estimates for decapod crustaceans in wetlands of Galveston Bay, Texas. Transactions of the American Fisheries Society 137: 129–146.
Miranda, L.E., and P.W. Bettoli. 2007. Mortality. In Analysis and interpretation of freshwater fisheries data, ed. C.S. Guy and M.L. Brown, 229–277. Bethesda, Maryland: American Fisheries Society.
Moore, D.S., and G.P. McCabe. 2006. Introduction to the practice of statistics, 5th ed. New York: W. H. Freeman and Company.
NMFS. 2010. Marine fisheries habitat assessment improvement plan. Report of the National Marine Fisheries Service Habitat Assessment Improvement Plan Team. U.S. Dep. Commer., NOAA Tech. Memo. NMFS-F/SPO-108, 115 pp.
NOAA Fisheries Toolbox. 2013. Instantaneous Rates (IRATE) Program, Version 2.0. http://nft.nefsc.noaa.gov.
O’Brien, C.J. 1994. Population dynamics of juvenile tiger prawns Penaeus esculentus in south Queensland, Australia. Marine Ecology Progress Series 104: 247–256.
Penland, S., and J.R. Suter. 1989. The geomorphology of the Mississippi River Chenier Plain. Marine Geology 90: 231–258.
Pérez Farfante, I. 1970. Diagnostic characters of juveniles of the shrimps Penaeus aztecus aztecus, P. duorarum duorarum, and P. brasiliensis (Crustacea, Decapoda, Penaeidae). U.S. Fish and Wildlife Service Special Scientific Report, Fisheries No. 599. 26 pp.
Pérez-Castañeda, R., and O. Defeo. 2003. A reciprocal model for mortality at length in juvenile pink shrimps (Farfantepenaeus duorarum) in a coastal lagoon of Mexico. Fisheries Research 63: 283–287.
Pérez-Castañeda, R., and O. Defeo. 2005. Growth and mortality of transient shrimp populations (Farfantepenaeus spp.) in a coastal lagoon of Mexico: role of the environment and density-dependence. ICES Journal of Marine Science 62: 14–24.
Pine, W.E., K.H. Pollock, J.E. Hightower, T.J. Kwak, and J.A. Rice. 2003. A review of tagging methods for estimating fish population size and components of mortality. Fisheries 28: 10–23.
Powell, L.A. 2007. Approximating variance of demographic parameters using the delta method: A reference for avian biologists. The Condor 109: 949–954.
R Core Team. 2013. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/.
Ricker, W.E. 1975. Computation and interpretation of biological statistics of fish populations. Bulletin of the Fisheries Research Board of Canada 191: 1–382.
Rockwood, L.L. 2006. Introduction to population ecology. Malden, MA: Blackwell Publishing.
Roth, B.M., K.A. Rose, L.P. Rozas, and T.J. Minello. 2008. Relative influence of habitat fragmentation and inundation on brown shrimp Farfantepenaeus aztecus production in northern Gulf of Mexico salt marshes. Marine Ecology Progress Series 359: 185–202.
Rozas, L.P., T.J. Minello, and D.D. Dantin. 2012. Use of shallow lagoon habitats by nekton of the northeastern Gulf of Mexico. Estuaries and Coasts 35: 572–586.
Ruxton, G.D. 2006. The unequal variance t-test is an underused alternative to Student’s t-test and the Mann-Whitney U test. Behavioral Ecology 17: 688–690.
Salini, J.P., S.J.M. Blaber, and D.T. Brewer. 1990. Diets of piscivorous fishes in a tropical Australian estuary, with special reference to predation on penaeid prawns. Marine Biology 105: 363–374.
Schenker, N., and J.F. Gentleman. 2001. On judging the significance of differences by examining the overlap between confidence intervals. The American Statistician 55: 182–186.
Smith, M.W., A.Y. Then, C. Wor, G. Ralph, K.H. Pollock, and J.M. Hoenig. 2012. Recommendations for catch-curve analysis. North American Journal of Fisheries Management 32: 956–967.
Turner, R.E. 1977. Intertidal vegetation and commercial yields of penaeid shrimp. Transactions of the American Fisheries Society 106: 411–416.
Turner, R.E. 1990. Landscape development and coastal wetland losses in the northern Gulf of Mexico. American Zoologist 30: 89–105.
Vetter, E.F. 1988. Estimation of natural mortality in fish stocks: A review. Fishery Bulletin 86: 25–43.
Wang, Y.-G., and M.D.E. Haywood. 1999. Size-dependent natural mortality of juvenile banana prawns Penaeus merguiensis in the Gulf of Carpentaria, Australia. Marine and Freshwater Research 50: 313–317.
Webb, S., and R.T. Kneib. 2004. Individual growth rates and movement of juvenile white shrimp (Litopenaeus setiferus) in a tidal marsh nursery. Fishery Bulletin 102: 376–388.
Williams, A.B. 1969. A ten-year study of meroplankton in North Carolina estuaries: Cycles of occurrence among Penaeidean shrimps. Chesapeake Science 10: 36–47.
Zimmerman, R.J., T.J. Minello, and G. Zamora Jr. 1984. Selection of vegetated habitat by brown shrimp, Penaeus aztecus, in a Galveston Bay salt marsh. Fishery Bulletin 82: 325–336.
Zimmerman, R.J., T.J. Minello, and L.P. Rozas. 2000. Salt marsh linkages to productivity of penaeid shrimps and blue crabs in the Northern Gulf of Mexico. In Concepts and controversies in tidal marsh ecology, ed. M.P. Weinstein and D.A. Kreeger, 293–314. Dordrecht, The Netherlands: Kluwer Academic Publishers.
Acknowledgments
This research was conducted through the NOAA National Marine Fisheries Service (NMFS) Southeast Fisheries Science Center (SEFSC) by personnel from the Galveston Laboratory and the Estuarine Habitats and Coastal Fisheries Center in Lafayette, Louisiana. In particular, we thank Shawn Hillen, Juan Salas, Jennifer Doerr, Rick Hart, and Lainey Broussard for help in the field and Alex Cummings for help processing samples in the laboratory. We would also like to thank David Richard at Stream Wetland Services and the staff at Murphree Wildlife Management Area for providing lodging during the study and the staff of the Texas Point National Wildlife Refuge for access to the field site. We thank Rick Hart for information on white shrimp stock assessments and Phil Caldwell for conducting the GIS analysis needed to weight the length-frequency distributions and for creating Fig. 1. Tom Minello, Ronnie Baker, and two anonymous reviewers provided helpful suggestions that improved the original manuscript. A NOAA Habitat Assessment Improvement Program grant, a Teaching Assistantship from the University of Louisiana at Lafayette, a Rockefeller Wildlife Scholarship, a Coastal Conservation Association/Ted Beaulieu Sr. Scholarship, a research grant from the Wetland Foundation, and the NMFS SEFSC provided funding to support this project. The findings and conclusions in this paper are those of the authors and do not necessarily represent the views of the NOAA NMFS.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Wim J. Kimmerer
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 89 kb)
Rights and permissions
About this article
Cite this article
Mace, M.M., Rozas, L.P. Estimating Natural Mortality Rates of Juvenile White Shrimp Litopenaeus setiferus . Estuaries and Coasts 38, 1580–1592 (2015). https://doi.org/10.1007/s12237-014-9901-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-014-9901-7