Abstract
White shrimp (Litopenaeus setiferus) fisheries-independent and fisheries-dependent landings can be highly variable and may be related to environmental factors that influence growth, mortality, and survival. We used linear regression analysis to look for potential relationships between environmental and white shrimp catch-per-unit-effort (CPUE) data collected from the Ashepoo-Combahee-Edisto (ACE) Basin National Estuarine Research Reserve (NERR) for four critical months in the shrimp life cycle. This analysis used data from white shrimp fisheries-independent CPUE (2002 to 2014) and water quality and meteorological variables for August (juvenile), December (sub-adult), March (adult), and April (spawning adult). The results showed that shrimp CPUE was mainly correlated with water temperature, salinity, and dissolved oxygen concentration collected through the ACE Basin NERR’s System-Wide Monitoring Program (SWMP), but offshore wind, precipitation, and intra-annual CPUEs also partially explained the variability in monthly CPUEs. Black gill prevalence was correlated with water temperature and salinity. Additionally, our analysis found that winter water temperatures of ≤11 °C were correlated with reduced shrimp abundance the following spring. Ultimately, managers would like to successfully predict white shrimp stock abundance throughout fishing seasons based on environmental conditions. This study is a first step in identifying the environmental variables that may be useful in predicting white shrimp CPUE in the South Atlantic Bight. The techniques employed here can serve as a basis for predicting and managing other wild annual fisheries stocks.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Predicting and managing a wild annual fisheries stock can be a challenge for managers, especially when the fishery is large and economically valuable. One such example is the white (Litopenaeus setiferus) and brown (Farfantepenaeus aztecus) shrimp fisheries of the Gulf of Mexico and South Atlantic Bight. Both shrimp species, like other species of penaeid shrimps, have an annual life cycle that is greatly influenced by coastal oceanographic and meteorological processes. Therefore, seasonal and yearly landings can vary widely and are hard to predict; inter- and intra-annual fluctuations in landings are not restricted to only these two species but occur in other penaeid fisheries as well (e.g., Galindo-Bect et al. 2000; Diop et al. 2007; Maynou 2008; Baker et al. 2014).
White shrimp and brown shrimp support the largest and most economically valuable fishery in South Carolina, with a combined average value of over $6.8 million USD annually from 2002 to 2014. However, since 2001, South Carolina landings have been in decline, reaching a record low of 1.7 million lbs. (771,107 kg) and the second lowest value of $5.3 million USD in 2013 (South Carolina Department of Natural Resources, unpublished data). Fisheries-independent sampling by the South Carolina Department of Natural Resources (SCDNR) confirms this trend, showing that one of the lowest catch-per-unit-effort (CPUE) years since 2001 occurred in 2013 (SCDNR, unpublished data).
White shrimp have a life span of ∼9–12 months, and the white shrimp fishery encompasses both a spring “roe” fishery of mature adults as well as a fall and winter fishery of migrating sub-adults. In the late spring and early summer (March–June), adult white shrimp migrate through lower estuaries to nearshore oceanic waters of South Carolina to spawn. Planktonic larvae develop over 10–12 days into postlarvae, which recruit to shallow, muddy, sandy tidal creek habitats with plenty of organic debris between May and July (Bearden 1961; McKenzie 1981; Garcia 1985; Wenner and Beatty 1993). As they mature into juveniles over late summer (July–September), white shrimp occur in a range of estuarine temperatures and salinities (0–45 ppt) but are most abundant in oligohaline and mesohaline habitats (Wenner and Beatty 1993; Longley 1994). As sub-adults, white shrimp migrate into large tidal creeks and rivers and move progressively towards saline waters before migrating offshore to complete development over the winter (December) (Weymouth et al. 1933; Lindner and Anderson 1956). Adults can overwinter in deep, high salinity areas of estuaries and sounds but are restricted by temperature; mortality occurs when water temperatures drop below 8 °C (Joyce 1965; McKenzie 1981).
Variation in white shrimp landings could be due to any number of abiotic or biotic factors, including winter storms, precipitation events, gill fouling ciliate, and wind-driven processes affecting ingress and egress (Farmer et al. 1978; Rothlisberg et al. 1983; Walker and Saila 1986; Wenner et al. 1998, 2005; DeLancey et al. 2003). In addition, these factors can vary on both short- (i.e., days, weeks) and long-term (i.e., months) scales and impact shrimp in a variety of ways. For example, white shrimp landings from North Carolina, South Carolina, Georgia, and Texas have each reported reduced catches associated with cold temperatures (Williams 1969), and Turner (1977) showed that shrimp yield (kg ha−1) was inversely related to degrees of latitude. In both cases, white shrimp are hypothesized to grow and survive better in saltier water and at warmer water temperatures (Williams 1969; Turner 1977; Rozas and Minello 2011). Lower salinities could discourage the presence of some marine fish predators (Salini et al. 1990), while increased rainfall could positively influence nutrient replenishment into coastal ecosystems, which can result in increased productivity (Loneragan 1999).
Disease or parasites may influence shrimp populations in estuaries. One such example is black gill, which was first reported in South Carolina waters in 1999. Black gill, caused by an apostomate ciliate that induces inflammation, melanization, and necrosis of shrimp gill tissue, approaches nearly 100 % prevalence in white shrimp during summer and fall. Recent molecular and morphological analyses to identify the ciliate are contradictory, and its species name remains unknown (M. Fischer, personal communication). How black gill directly impacts shrimp health and survival is poorly understood, but preliminary studies suggest it interferes with oxygen consumption (A. Fowler, unpublished data). This has important ecological implications, as black gill may impair normal physiological functions, which could increase predation rates and vulnerability to changing environmental conditions.
The SCDNR has routinely monitored and regulated the harvest of white shrimp in South Carolina waters since the 1970s, and as part of this effort, the agency conducts fisheries-independent trawl surveys in the Ashepoo-Combahee-Edisto (ACE) Basin National Estuarine Research Reserve (NERR). This survey targets shrimp between 20 and 200 mm total length (TL = tip of the rostrum to tip of the telson). White shrimp collected in the survey are juveniles in August (20–80 mm TL), sub-adults in December (80–120 mm TL), adults in March (121–155 mm TL), and spawning adults (135–200 mm TL) in April (Pérez-Farfante 1969).
Using the data from this established long-term survey, we compared shrimp abundance and prevalence of black gill from 2002 to 2014 with environmental variables (e.g., water temperature, dissolved oxygen, salinity, precipitation, river discharge, wind speed, and direction) collected from the ACE Basin NERR’s System-Wide Monitoring Program (SWMP). We determined which environmental variables and time frames were most influential in explaining the observed trends in white shrimp CPUE and percent prevalence of black gill throughout the ACE Basin NERR. Our study explores the potential effect of environmental variables on a commercial fishery of an annual, migratory species. The insights provided are useful to fishery managers who are our primary intended users but also to commercial shrimpers who are vitally invested in the long-term sustainability of the fishery.
Methods
Biotic Data Collection
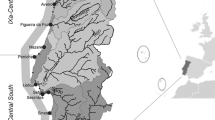
Five stations were sampled within the North and South Edisto River systems and St. Helena Sound of South Carolina (Fig. 1). The SCDNR collected samples at these five sites using the same methods during four times of the year from 2002 to 2014: March, April, August or September (August), and November or December (December). Otter trawls (6.2-m head rope × 2.5-cm stretch mesh) were towed for 15 min, penaeid shrimp were sorted by species and counted, and white shrimp abundance was expressed as CPUE (i.e., the number of individual shrimp collected per 15-min trawl). As growth rates can vary greatly from year to year (Kutkuhn 1962), white shrimp were not assigned to a life stage category based on size. Shrimp, regardless of size, were placed into specific life stage categories based on when they were collected: August = juvenile, December = sub-adult, March = adult, and April = spawning adult. The percent of shrimp exhibiting visible black gill was also noted. Stations were not sampled during December 2006.
Map of the study area showing the Ashepoo-Combahee-Edisto (ACE) Basin National Estuarine Research Reserve outlined in dark gray. Research trawl stations for white shrimp (Litopenaeus setiferus) are denoted by the five stars, the ACE Basin Bennett’s Point meteorological station (ACEBPMET) by a circle, and the ACE Basin Big Bay SWMP water quality station (ACEBBWQ) by a triangle
Abiotic Data Collection
Water quality and environmental data collected through the ACE Basin NERR SWMP between 2001 and 2014 were obtained from the National Oceanic and Atmospheric Administration (NOAA) National Estuarine Research Reserve System (NERRS) Centralized Data Management Office website (NERRS 2015). Water temperature, salinity, dissolved oxygen concentration, and percent oxygen saturation were monitored continuously at the ACE Basin Big Bay station (ACEBBWQ) and reported at 15 to 30 min intervals. Average wind speed, average direction from which the wind originated, and total precipitation were monitored continuously at the ACE Basin Bennett’s Point meteorological station (ACEBPMET) and reported at 15 min intervals. Data with NERRS quality control flags of <−3 > or <1 > (“Data rejected due to QA/QC” and “Suspect data,” respectively) were treated as missing and excluded from analyses.
River discharge and water temperature data were obtained from the United States Geological Survey (USGS) National Water Information System (NWIS) website (USGS 2015). River discharge was monitored continuously at the Givhans, SC Edisto River station (02175000). Continuously monitored water temperature at the Charleston, SC Customs House station (021720710) was used to represent coastal offshore water conditions.
Data Analysis
Shrimp CPUE, percent of shrimp with black gill, water quality, river discharge, and environmental data were summarized using the statistical program R (R Core Team 2015) and the R package plyr (Wickham 2011). Each cluster of data from ACE Basin otter trawl samples, usually five samples (one per site, Fig. 1) collected within a 3-day period, was analyzed as a unit; the mean number of white shrimp captured per 15 min trawl (CPUE) and the mean percent of white shrimp with black gill captured were calculated for each trawl cluster. ACE Basin and Edisto River environmental variables were summarized for 7- and 30-day periods preceding the median trawl date for each trawl cluster and for the summer and winter preceding each trawl cluster (Supplementary Table 1). These periods were chosen to explore whether white shrimp CPUE is influenced by ephemeral, localized water quality (e.g., rainfall, tides) changes (7 days) and longer term (30 days, previous summer, previous winter) shifts in environmental variables (e.g., temperature). The potential effect of strong (wind speed >4.47 m s−1) inshore and offshore winds on white shrimp CPUE also was investigated by incorporating these variables in the analyses. Wind metrics were derived from Bennett’s Point. An inshore wind was defined as blowing from 135° to 225°, while an offshore wind was defined as blowing from 315° to 360° or 0° to 45°.
Winter water temperatures are known to impact spring shrimp abundances (Farmer et al. 1978; McKenzie 1981; Daigle 1984; Lam et al. 1989; DeLancey et al. 2008). Therefore, mean coastal offshore water temperature (from the USGS Charleston Customs House), total number of days that mean daily offshore water temperature fell below a threshold temperature, and maximum number of consecutive days that mean daily offshore water temperature remained below a threshold temperature were calculated for the winter preceding each trawl cluster (Supplementary Table 1). Temperature thresholds were set at 7, 8, 9, 10, 11, 12, 13, 14, 15, and 16 °C. The relationship between water temperature and winter survival was explored by graphing the correlations and resulting polynomial best fit line between March and April CPUE and the number of consecutive days and number of total days in which water temperatures were below each temperature threshold. These analyses led to the determination of the temperature at which the strongest correlation occurred.
Statistical Analyses
Because white shrimp is an annual species and its different life cycle stages occur sequentially throughout the year, all analyses were conducted independently for each sampling period: March, April, August, and December. Monthly CPUEs within each shrimp life cycle year were compared linearly to evaluate whether CPUE in one sampling period (e.g., August) correlated with CPUE in a preceding sampling period (e.g., April) throughout the 2002–2014 study period. Any trawl cluster missing 50 % or more of the environmental data was excluded from analyses. If an environmental variable was missing over an entire year, the mean of each month for other years was calculated and used for the missing data; this situation only occurred for total precipitation during 2009. For each sampling month, all pairwise comparisons between biotic and abiotic variables were performed using Spearman rank correlations. In cases of multicollinearity between abiotic variables, only one of the correlated variables was used for the multiple regression analyses. Any relationships between abiotic and biotic variables that had a correlation probability of ≤0.10 were included in the multiple regression analyses. Backward elimination stepwise regressions (i.e., a best fit procedure) were used to determine the relevant independent abiotic variables that contributed most to the individual dependent biotic variables (mean shrimp CPUE and mean percent prevalence of black gill infection) with a correlation probability of ≤0.10 considered statistically significant (SigmaPlot 12.5; Systat Software, Inc., San Jose, CA, USA). This resulted in the most parsimonious, best fit model based on the assumptions and procedures of the backward stepwise regression.
Results
Mean white shrimp CPUE from fisheries-independent sampling at five stations in the ACE Basin NERR varied greatly among months and years (Fig. 2). Juvenile (August) and sub-adult (December) CPUEs were much higher than adults (March and April). The highest mean CPUE of over 4000 white shrimp occurred in December of 2008 (Fig. 2).
Monthly (August, juvenile; December, sub-adult; March, adult; April, spawning adult) mean white shrimp (Litopenaeus setiferus) (CPUE = number of shrimp per 15-min trawl) collected by otter trawl in the ACE Basin between 2002 and 2014. Sampling did not take place December 2006. Mean December 2008 CPUE was 4435 individuals (not shown)
Juveniles
Mean CPUE of juvenile shrimp in August did not correlate significantly with the mean CPUE from any of the other life stages. Juvenile abundance (August CPUE) was negatively correlated with strong offshore wind during the previous 7 days and all 7 days dissolved oxygen variables (Table 1). The backward stepwise multiple regression analysis did not yield a statistically significant model that related mean juvenile shrimp CPUE to the environmental variables considered in our study.
Sub-adults
Mean CPUE of sub-adult shrimp (December) was negatively correlated with 7- and 30-day mean salinity as well as 7-day low salinity (fifth percentile) and positively correlated with the previous 30-day total precipitation (Table 1). The multiple regression analysis did not detect a relationship between mean sub-adult CPUE and environmental variables.
Adults
Mean CPUE of adult shrimp in March correlated with mean CPUE of sub-adult shrimp in the preceding December (p = 0.047) and with mean CPUE of spawning adult shrimp in the following April (p = 0.021). Winter water temperature was most strongly correlated with mean CPUE in March and April (Table 1); low mean winter water temperature and a large number of days with water temperature below 11 °C (total as well as consecutive) were associated with low mean CPUE (Fig. 3). Adult shrimp (March) mean CPUE was also negatively correlated with dissolved oxygen concentration for the preceding 7 and 30 days, as well as total precipitation for the preceding 7 days. Spawning adult shrimp (April) mean CPUE was negatively associated with total precipitation during the preceding summer (Table 1). Backward stepwise multiple regression indicated that only the total number of days of preceding winter water temperatures below 11 °C was necessary to predict mean adult shrimp CPUE in March (CPUE = 204.391–3.253 (number of winter water temperature days below 11 °C)) and April (CPUE = 86.311–1.221 (number of winter water temperature days below 11 °C)). The model accounted for 52 % of the variability in observed CPUE in March and 36 % in April (Fig. 4).
Strength of the correlations between mean March white shrimp (Litopenaeus setiferus) catch-per-unit-effort (CPUE) from five stations in the ACE Basin between 2002 and 2014 and (a) the number of consecutive winter days below critical temperatures and (b) the total number of winter days when the water was below critical temperatures. In both cases, the strongest correlations occurred at 11 °C
Relationships by month (August, December, March, or April) between observed mean white shrimp catch-per-unit-effort (CPUE) or mean prevalence of black gill and expected CPUE. Only statistically significant (p < 0.1) correlations are shown. Note scale of vertical axis varies among graphs. NS not significant
Black Gill
Black gill prevalence was highest in juvenile shrimp. It was prevalent (i.e., >40 % infection) in seven of the 13 years considered in this analysis. The mean percent prevalence of black gill among juvenile shrimp was positively correlated with high water temperatures (i.e., the 95th percentile over the previous 30 days) and high salinities (i.e., the 95th percentile over the entire summer) (Table 1). Black gill was observed in sub-adult shrimp (December) in only four of the 12 years we sampled and then at levels ranging from only 0.5 to 3.6 %. The mean percent prevalence of black gill in sub-adult shrimp was positively correlated with low, mean, and high water temperatures of the previous 7 days and the mean of the previous 30-day water temperatures (Table 1). Black gill was not observed in adult shrimp. There were no significant correlations between mean shrimp CPUE and the mean prevalence of black gill in shrimp.
Backward stepwise multiple regression indicated a significant potential relationship between total precipitation over 7 days, mean salinity over 30 days, mean percent oxygen saturation over 30 days, and the total number of days of preceding winter water temperatures below 11 °C: y = 494.463–0.455 (7-day total precipitation) + 3.130 (30-day mean salinity) − 7.965 (30-day mean % DO) + 1.022 (number of winter water temperature days below 11 °C). The mean prevalence of black gill in sub-adults was correlated with mean water temperature in the preceding 30 days and mean water temperature during the previous summer: (y = 8.192 + 0.649 (30-day mean water temperature) − 0.640 (previous summer mean water temperature)). These models accounted for 67 % of the variability in observed black gill prevalence in August and 78 % of the variability in December (Fig. 4).
Discussion
Fisheries managers have struggled to predict seasonal white shrimp abundance, which impacts when to open (spring roe season) or close (severe winter cold snaps) the fishery. Our study showed that inter-annual white shrimp abundance was correlated with precipitation, salinity, water temperature, and dissolved oxygen concentration, but offshore wind and intra-annual CPUE also seemed to influence monthly CPUE to a lesser extent. One notable finding was that when the previous winter mean water temperature was below 11 °C, adult and spawning adult white shrimp abundance was significantly reduced. In addition, the appearance of black gill in juvenile white shrimp increased with low dissolved oxygen concentrations and high salinity. Future efforts should expand this analysis to other estuarine areas and work with managers and scientists to examine how white shrimp populations may be affected by environmental variables.
The schedule of the fisheries-independent sampling of white shrimp in the ACE Basin was reflective of the annual life cycle and its migratory nature, both of which are influenced by environmental variables. An examination of relative changes in monthly CPUE found that adult and spawning adult (March and April) mean CPUEs were consistently lower than juveniles (August) and sub-adults (December). This observation is directly related to the white shrimp life cycle. Between April and June, females can produce up to 500,000 eggs (Pérez-Farfante, 1969; Pérez-Velázquez and Gracia, 2000), and the mean August (juvenile) CPUE reflects the large number of juveniles that slowly migrate out of the tidal creeks after post-larval maturation (Weymouth et al. 1933; Lindner and Anderson 1956). By December (sub-adult), the mean CPUE increases as all of the sub-adults have migrated out of the tidal creeks to larger bodies of water, making them more susceptible to capture. At this time, the shrimp are more exposed to predation and, as the winter progresses, colder water temperatures. The population is further reduced between December (sub-adult) and March (adult) due to natural mortality rates, emigration, and harvest, which can lead to fewer adults surviving to become the brood stock the following April (spawning adult). Lam et al. (1989) reported that white shrimp in South Carolina exhibit a spawner-recruit relationship. Therefore, it was hypothesized that the CPUE of the spawning adults in spring (April) could be used as an indicator for the CPUE of juveniles in the fall (August). However, that does not appear to be the case in the ACE Basin, which is in agreement with studies in the southwestern Gulf of Mexico (Gracia 1991) and off the coast of Georgia (Belcher and Jennings 2004).
While our data incorporated both short- (7 days) and long- (30 days, previous summer and winter) term environmental variables, it is important to note that the CPUE data may reflect natural shrimp movement into and out of the sampling areas rather than actual stock size at any given point in time. However, the correlations of white shrimp CPUE and various environmental variables found in our study are in agreement with other studies relating white shrimp CPUE to precipitation, salinity and river discharge (Walker and Saila 1986; Lam et al. 1989; Garcia 1991; Belcher and Jennings 2004), offshore winds (Walker and Saila 1986), water temperature (Williams 1969; Farmer et al. 1978; McKenzie 1981; Daigle 1984; Lam et al. 1989; Belcher and Jennings 2004; DeLancey et al. 2005, 2008), and dissolved oxygen (DeLancey et al. 2008). In particular, an analysis of white shrimp CPUE between 1989 and 2005 in the ACE Basin NERR showed that the previous winter water temperatures and dissolved oxygen best explained white shrimp abundance, as low winter water temperatures caused shrimp mortality and shrimp avoided areas with low dissolved oxygen (DeLancey et al. 2008).
In our study, mean sub-adult, adult, and spawning adult white shrimp CPUE (December, March, and April) was correlated with precipitation, as well as salinity in December. High precipitation and low salinity, possibly also associated with high river discharge, may force shrimp out from marshes into deeper water, which increases their catchability. While white shrimp can tolerate salinities as low as 0.8 ppt for short periods of time (Gunter and Hildebrand 1954; McFarland and Lee 1963), it is possible that excessive rainfall and river discharge could consistently dilute estuarine and nearshore waters to below tolerance levels for an extended period of time (Barrett and Gillespie 1973, 1975; Barrett and Ralph 1976, 1977). River discharge on the South Edisto River was not an important factor in the ACE Basin; however, river discharge may be an important factor in explaining shrimp abundances in other estuarine systems. Indeed, in an earlier white shrimp study on the South Carolina coast, Lam et al. (1989) found white shrimp landings to be inversely related to salinity such that increased rainfall and river discharge reduced estuarine waters to an optimal salinity for nursery habitat. Similar correlations have been shown between brown shrimp landings in Louisiana and rainfall and discharge of the Mississippi River (Barrett and Gillespie 1973, 1975; Barrett and Ralph 1976, 1977).
The previous winter mean water temperature and days with water temperature below 11 °C were both significantly correlated with adult and spawning adult white shrimp abundance (i.e., cold temperatures over long periods of time are associated with low mean CPUE). Previous studies from North Carolina, South Carolina, Georgia, and Texas show that severe winters can cause high mortality rates in overwintering shrimp and significantly alter the spawning shrimp landings the following spring (Williams 1969; Farmer et al. 1978; McKenzie 1981; Daigle 1984; Lam et al. 1989; DeLancey et al. 2005, 2008). White shrimp mortality in South Carolina increases in years with >10 consecutive days below 8.5 °C (Farmer et al. 1978). Currently, if water temperatures dip below 9 °C for seven consecutive days, the South Carolina fishery may be closed in the exclusive economic zone to protect shrimp brood stock (SAFMC 2013). Therefore, the SCDNR currently monitors coastal offshore water temperature in December, January, and February at the USGS Charleston Customs House to determine whether and when to close the white shrimp fishery. The South Carolina exclusive economic zone was closed in 2001, 2011, and 2014 due to cold winter water temperatures, which occurred during January in all 3 years. Our analysis suggests that the critical mean winter temperature for white shrimp in the ACE Basin is not 9 °C but rather 11 °C, which has the strongest correlations with adult and spawning adult (March and April) CPUE.
Adequate levels of dissolved oxygen (DO) are critical to the survival and growth of estuarine invertebrates and fish. Low DO levels (<4 mg L−1) can negatively impact respiratory efficiency and energy available for white shrimp growth (Rosas et al. 1998). White shrimp are mobile and have the ability to detect and avoid low DO areas (Stickle et al. 1989; Burnett and Stickle 2001). This behavior, also observed during high precipitation and low salinity events as noted above, may force shrimp out from tidal creeks into deeper water, increasing their catchability. This may explain why dissolved oxygen concentration and saturation measurements for the previous week in our study were negatively correlated with mean juvenile and adult CPUEs. The aggregation of white shrimp in oxygenated water, during localized low DO events, has been observed in populations of white shrimp in the Gulf of Mexico (Renaud 1986).
Wind patterns were an important factor for juvenile white shrimp. In our analysis, strong offshore (northwest) winds over the previous 7 days were associated with low juvenile shrimp (August) CPUE. Previous models to relate Gulf of Mexico shrimp commercial landings to environmental factors have shown positive correlations with summer river discharge and negative correlations with strong northwest winds during spring and summer, which transport larvae away from estuaries (Walker and Saila 1986). The combination of diel vertical migration and tidal wind-forced currents is also thought to influence the spatial dynamics and movement patterns of sub-adult shrimp in Australian (Penaeus latisulcatus) and South Carolinian (L. setiferus) estuaries (Rothlisberg et al. 1983; Wenner et al. 1998).
The appearance of “black gill” in white shrimp arises between July and August and lasts until November or December. The appearance of black gill in the fall sub-adult fishery has been postulated to be related to stress from high summer temperatures and excessive rainfall (DeLancey et al. 2003; Geer 2003). In the ACE Basin, the prevalence of black gill in juvenile shrimp collected in August increased with low dissolved oxygen concentrations and high salinity over the previous month. In contrast, heavy precipitation in the preceding week and month decreased black gill prevalence. Therefore, it is possible that high salinities and low dissolved oxygen concentrations over a prolonged period of time (at least a month) are synergistic stressors that lower the resistance of shrimp to black gill. However, precipitation may in fact offset both low dissolved oxygen concentration and high salinity, thus reducing the stressors and allowing the shrimp to combat black gill. A small proportion of sub-adult white shrimp (December) also exhibited black gill, and prevalence increased with warm water temperature and low dissolved oxygen concentrations during the preceding week and month. This is not surprising as black gill prevalence is thought to be positively related to water temperatures, and the ciliate that causes black gill may survive longer and remain infective during periods of warm water temperature (DeLancey et al. 2003; Geer 2003). In addition, periods of low dissolved oxygen levels may make the shrimp more susceptible to infection (L. Burnett, personal communication).
Managers would like to consistently predict stock abundance throughout the fishing seasons based on environmental conditions. As high variability in landings is a hallmark of penaeid shrimp fisheries globally, it is a challenge for managers to create predictive models of future stock landings (Diop et al. 2007). Our study is a first step in elucidating which environmental variables may be useful in predicting white shrimp seasonal abundances in the ACE Basin NERR. No single abiotic measure can explain CPUE across all sampling periods. Rather a combination of various environmental factors contributes to the growth and survival of white shrimp throughout the year. Our study suggests that overwintering temperature is an important variable defining the spring CPUE of adult and spawning adult shrimp. Therefore, we suggest revisiting the critical overwintering water temperature currently used for white shrimp management to protect the remaining brood stock, as it may need to be adjusted from 9 to 11 °C. Further investigation using laboratory experiments to elucidate cold tolerance of white shrimp is needed to confirm our findings. We were unable to construct a predictive model for juvenile and sub-adult CPUE using abiotic variables. This may be due, in part, to the relatively recent appearance of black gill and lack of information about its possible effects on individual growth and survival rates. Therefore, additional physiological and biochemical analyses exploring how black gill affects white shrimp are needed. Due to the complex abiotic dynamics of estuarine and oceanic systems, influenced by a changing climate, the scale at which these environmental variables act is also important to consider. Future efforts should expand these analyses to other estuarine areas and work with managers and scientists to examine how white shrimp populations may be affected by a changing climate.
References
Baker, R., M. Fujiwara, and T.J. Minello. 2014. Juvenile growth and mortality effects on white shrimp Litopenaeus setiferus population dynamics in the northern Gulf of Mexico. Fisheries Research 155: 74–82.
Barrett, B. B. and M. C. Gillespie. 1973. Primary factors which influence commercial shrimp production in coastal Louisiana. Louisiana Department of Wildlife and Fisheries, Technical Bulletin 9, New Orleans.
Barrett, B. B. and M. C. Gillespie. 1975. 1975 environmental conditions relative to shrimp production in coastal Louisiana. Louisiana Department of Wildlife and Fisheries, Technical Bulletin 15, New Orleans.
Barrett, B. B. and E. J. Ralph. 1976. 1976 environmental conditions relative to shrimp production in coastal Louisiana. Louisiana Department of Wildlife and Fisheries, Technical Bulletin 21, New Orleans.
Barrett, B. B. and E. J. Ralph. 1977. 1977 environmental conditions relative to shrimp production in coastal Louisiana along with white shrimp catch data for the Gulf of Mexico. Louisiana Department of Wildlife and Fisheries, Technical Bulletin 26, New Orleans.
Bearden, C.M. 1961. Notes on postlarvae of commercial shrimp (Penaeus) in South Carolina. Contributions from Bears Bluff Laboratories 33: 1–8.
Belcher, C.N., and C.A. Jennings. 2004. Evaluation of stock-recruitment curves for white shrimp in Georgia. North American Journal of Fisheries Management 24: 654–661.
Burnett, L.E., and W.B. Stickle. 2001. Physiological responses to hypoxia. In In: Coastal hypoxia: consequences for living resources and ecosystems, ed. N.N. Rabalais, and R.E. Turner, 101–114. Washington, D. C: American Geophysical Union.
Daigle, R. 1984. 1984 commercial shrimping season may rank as worst in recent times. Coastlines Georgia 7(3): 3–4.
DeLancey, L. B., J. E. Jenkins, M. B. Maddox, E. L. Wenner, R. P. Webster, and A. L. Segars. 2003. “Black gill” infection in penaeid shrimp in South Carolina. Abstract. The Crustacean Society summer meeting program. Gloucester Point, Virginia. Virginia Institute of Marine Science.
DeLancey, L.B., J.E. Jenkins, M.B. Maddox, J.D. Whitaker, and E.L. Wenner. 2005. Field observations on white shrimp, Litopenaeus setiferus, during spring spawning season in South Carolina, U.S.A., 1980-2003. Journal of Crustacean Biology 25: 212–218.
DeLancey, L.B., E.L. Wenner, and J.E. Jenkins. 2008. Long-term trawl monitoring of white shrimp, Litopenaeus setiferus (Linnaeus), stocks within the ACE Basin National Estuarine Research Reserve, South Carolina. Journal of Coastal Research 55: 193–199.
Diop, H., W.R. Keithly, R.F. Kazmierczak, and R.F. Shaw. 2007. Predicting the abundance of white shrimp (Litopenaeus setiferus) from environmental parameters and previous life stages. Fisheries Research 86(1): 31–41.
Farmer, C. H., J. D. Whitaker, and N. L. Chipley. 1978. Pilot study to determine the overwintering patterns of white shrimp. Final report for National Marine Fisheries Service Contract 03-6-042-35105, South Carolina Wildlife and Marine Resources Department, Charleston, South Carolina.
Galindo-Bect, M.S., E.P. Glenn, H.M. Page, K. Fitzsimmons, L.A. Galindo-Bect, J.M. Hernandez-Ayon, R.L. Petty, J. Garcia-Hernandez, and D. Moore. 2000. Penaeid shrimp landings in the upper Gulf of California in relation to Colorado River freshwater discharge. Fishery Bulletin 98: 222–225.
Garcia, S. 1985. Reproduction, stock assessment models and population parameters in exploited penaeid shrimp populations. In Second Australian National Prawn Seminar, ed. P. C. Rothlisberg, B. J. Hill, and D. J. Staples, 139–158. Cleveland, Queensland Australia.
Geer, P. 2003. Black gill disease in the Georgia shrimp fishery. Abstract. The Crustacean Society summer meeting program. Gloucester Point, Virginia. Virginia Institute of Marine Science.
Gracia, A. 1991. Spawning stock-recruitment relationships of white shrimp in the Southwestern Gulf of Mexico. Transactions of the American Fisheries Society 120: 519–527.
Gunter, G., and H.H. Hildebrand. 1954. The relation of total rainfall of the state and catch of the marine shrimp (Penaeus setiferus) in Texas waters. Bulletin of Marine Science of the Gulf and Caribbean 4: 95–103.
Joyce, E. A. Jr 1965. The commercial shrimps of the northeast coast of Florida. Florida Board of Conservation Marine Laboratory, St. Petersburg, Florida, Professional Papers Series No. 6, 224p.
Kutkuhn, J.H. 1962. Gulf of Mexico commercial shrimp populations—trends and characteristics, 1956-59. United States Fish and Wildlife Service Fisheries Bulletin 62: 343–402.
Lam, C., J.D. Whitaker, and F. Lee. 1989. Model for white shrimp landings for the central coast of South Carolina. North American Journal of Fisheries Management 9: 12–22.
Lindner, M.J., and W.W. Anderson. 1956. Growth, migrations, spawning and size distribution of shrimp Penaeus setiferus. Fishery Bulletin (United States) 56: 555–645.
Loneragan, N.R. 1999. River flows and estuarine ecosystems: implications for coastal fisheries from a review and a case study of the Logan River, southeast Queensland. Australian Journal of Ecology 24: 431–440.
Longley, W.L. 1994. Freshwater inflows to Texas bays and estuaries: ecological relationships and methods for determination of needs. Texas Water Development Board and Texas Parks and Wildlife Department. Austin, Texas. 386 p.
Maynou, F. 2008. Environmental causes of the fluctuations of red shrimp (Aristeus antennatus) landings in the Catalan Sea. Journal of Marine Systems 71: 294–302.
McFarland, W.N., and B.D. Lee. 1963. Osmotic and ionic concentrations of penaeidean shrimp of the Texas coast. Bulletin of Marine Science of the Gulf and Caribbean 13: 391–417.
McKenzie, M. D. 1981. Profile of the penaeid shrimp fishery in the South Atlantic. South Atlantic Fishery Management Council, Charleston, South Carolina.
National Estuarine Research Reserve System (NERRS). 2015. System-wide monitoring program. Data accessed from the NOAA NERRS Centralized Data Management Office website: http://www.nerrsdata.org/. Accessed 7 Apr 2015.
Pérez-Farfante, I. 1969. Western Atlantic shrimps of the genus Penaeus. Fishery Bulletin 67: 461–591.
Pérez-Velázquez, M., and A. Gracia. 2000. Fecundity of Litopenaeus setiferus, Farfantepenaeus aztecus and F. duorarum, in the southwestern Gulf of Mexico. Gulf and Caribbean. Research 12: 1–9.
R Core Team. 2015. R: a language and environment for statistical computing. R Foundation for Statistical Computer, Vienna, Austria. URL http://www.R-project.org.
Renaud, M.L. 1986. Detecting and avoiding oxygen deficient sea water by brown shrimp, Penaeus aztecus (lves), and white shrimp, Penaeus setiferus (Linnaeus. Journal of Experimental Marine Biology and Ecology 98: 283–292.
Rothlisberg, P.C., J.A. Church, and A.M.G. Forbes. 1983. Modeling the advection of vertically migrating shrimp larvae. Journal of Marine Research 41: 511–554.
Rosas, C., E. Martinez, G. Gaxiola, R. Brito, E. Diaz-Inglesia, and L.A. Soto. 1998. Effect of dissolved oxygen on the energy balance and survival of Penaeus setiferus juveniles. Marine Ecology Progress Series 174: 67–75.
Rozas, L.P., and T.J. Minello. 2011. Variation in penaeid shrimp growth rates along an estuarine salinity gradient: implications for managing river diversions. Journal of Experimental Marine Biology and Ecology 397: 196–207.
Salini, J.P., S.J.M. Blaber, and D.T. Brewer. 1990. Diets of piscivorous fishes in a tropical Australian estuary, with special reference to predation on penaeid prawns. Marine Biology 105(3): 363–374.
South Atlantic Fishery Management Council (SAMFC). 2013. Fishery management plan for the shrimp fishery of the South Atlantic region. Amendment 9. South Atlantic Fishery Management Council. Charleston, S.C.
Stickle, W.B., M.A. Kapper, L.L. Liu, E. Gnaiger, and S.Y. Wang. 1989. Metabolic adaptations of several species of crustaceans and molluscs to hypoxia: tolerance and microcalorimetric studies. Biological Bulletin 177: 303–312.
Turner, R.E. 1977. Intertidal vegetation and commercial yields of penaeid shrimp. Transactions of the American Fisheries Society 106: 411–416.
U.S. Geological Survey (USGS). 2015. National Water Information System (NWIS Web): U.S. Geological Survey database. http://waterdata.usgs.gov/nwis. Accessed 7 Apr 2015.
Walker, H.A., and S.B. Saila. 1986. Incorporating climatic and hydrographic information into shrimp yield forecasts using seasonal climatic component models. In In: Proceedings of the shrimp yield prediction workshop, ed. Andre M. Landry, and Edward F. Klima, 56–99. Galveston: Texas A&M University at Galveston.
Wenner, E.L., and H.R. Beatty. 1993. Utilization of shallow estuarine habitats in South Carolina, U.S.A., by postlarval and juvenile stages of Penaeus spp. (Decapoda: Penaeidae. Journal of Crustacean Biology 13: 280–295.
Wenner, E., D. Knott, J. Blanton, C. Barans, and J. Amft. 1998. Roles of tidal and wind-generated currents in transporting white shrimp (Penaeus setiferus) postlarvae through a South Carolina (USA) inlet. Journal of Plankton Research 20: 2333–2356.
Wenner, E., D. Knott, C. Barans, S. Wilde, J. Blanton, and J. Amft. 2005. Key factors influencing transport of white shrimp (Litopenaeus setiferus) post-larvae into the Ossabaw Sound system, Georgia, USA. Fisheries Oceanography 14(3): 175–194.
Weymouth, F.W., M. Lindner, and W.W. Anderson. 1933. Preliminary report on the life history of the common shrimp Penaeus setiferus (Linn.). Fishery Bulletin (U.S.) 48: 1–26.
Wickham, H. 2011. The split-apply-combine strategy for data analysis. Journal of Statistical Software 40(1): 1–29.
Williams, A.B. 1969. Penaeid shrimp catch and heat summation, an apparent relationship. FAO (Food and Agriculture Organization of the United Nations) Fisheries Reports 57(3): 643–656.
Acknowledgments
We thank the staff at the SCDNR who maintain the ACE Basin NERR meteorological and water quality sampling locations. We also thank C. Farmer, C. Bearden, and J.D. Whitaker for instituting the SCDNR crustacean trawling monitoring program and the various crustacean program staff members and vessel captains who have contributed to the acquisition of the data. We also thank two anonymous reviewers and the associate editor (Lawrence P. Rozas) for comments that significantly improved the manuscript. This is contribution No. 754 from the South Carolina Department of Natural Resources Marine Resources Research Institute. Funding was provided, in part, by South Carolina Saltwater Recreational Fishing License Funds and NOAA’s Office of Coastal Management.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Lawrence P. Rozas
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 34.9 kb)
Rights and permissions
About this article
Cite this article
Fowler, A.E., Leffler, J.W., Johnson, S.P. et al. Relationships Between Meteorological and Water Quality Variables and Fisheries-Independent White Shrimp (Litopenaeus setiferus) Catch in the ACE Basin NERR, South Carolina. Estuaries and Coasts 41, 79–88 (2018). https://doi.org/10.1007/s12237-016-0171-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-016-0171-4