Abstract
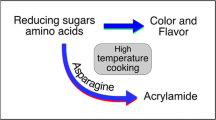
Acrylamide is a probable carcinogen found in processed potato products. The compound is formed at elevated temperatures by the Maillard reaction from two primary precursors - reducing sugars (fructose and glucose) and asparagine. Significant advances have been made in reducing acrylamide formation by selecting varieties with low precursor concentrations through conventional breeding or genetic modification techniques. However, acrylamide in many of the traditional varieties processed for fries or chips is sometimes found at elevated levels. Both agronomic and storage practices can significantly influence glucose, fructose, and asparagine concentrations and therefore the potential to form acrylamide during processing. This summary of a symposium presentation given at the 99th Annual Potato Association of American Meeting is to provide a general overview of previous studies that have examined the effects of agronomic factors such as nutrient and water management and storage factors such as temperature and duration on acrylamide precursors and/or acrylamide in processed potato products. A better understanding of how these factors affect acrylamide precursors is a first step in minimizing acrylamide formation during processing and improving the quality of processed potato products.
Resumen
Acrilamida es un posible carcinogénico encontrado en productos de papa procesada. El compuesto se forma a temperaturas elevadas mediante la reacción de Maillard de dos precursores primarios: azúcares reductores (fructosa y glucosa) y asparagina. Se han hecho avances significativos en reducir la formación de acrilamida con la selección de variedades con bajas concentraciones del precursor a través de mejoramiento convencional o técnicas de modificación genética. No obstante, algunas veces se encuentra la acrilamida a niveles elevados en muchas de las variedades tradicionales procesadas para fritura. Tanto las prácticas agronómicas como las de almacenamiento pueden influenciar significativamente las concentraciones de glucosa, fructosa y asparagina, y en consecuencia el potencial para formar acrilamida durante el procesamiento. Este resumen de la presentación del simposio efectuado durante la 99 Reunión Anual de la asociación Americana de la Papa, es para proporcionar una visión general de estudios previos que han examinado los efectos de los factores agronómicos, tales como el manejo de nutrientes y agua, y factores de almacenamiento como la temperatura y la duración de los precursores de la acrilamida en los productos de papa procesada. Un mejor entendimiento de cómo estos factores afectan a los precursores de la acrilamida, es un primer paso en la minimización de la formación de acrilamida durante el procesamiento y en el mejoramiento de la calidad de los productos de la papa procesada.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acrylamide is a compound formed by the Maillard reaction at high temperatures during potato processing from the precursors asparagine and reducing sugars, glucose and fructose. While acrylamide is found in many processed food products, fries and chips are among the highest contributors of this compound to the diet in the U.S. and Europe (Seal et al. 2008). This is a cause for concern because acrylamide is a neurotoxin and probable carcinogen. In 2005 the World Health Organization stated that “appropriate efforts to reduce acrylamide concentrations in food should continue” and this was reconfirmed in a recent 2015 report by the European Union (WHO 2005; EFSA 2015). Reducing acrylamide concentration in processed potatoes, therefore, has been a priority of the potato industry for many years.
Since its discovery in 2002, numerous reviews have been published on acrylamide formation and mitigation in processed potato as well as other processed food products (Becalski et al. 2003; Silva and Simon 2005; Halford et al. 2007; Morales et al. 2008; Pedreschi 2010; Medeiros Vinci et al. 2012; Halford et al. 2012; Bethke and Bussan 2013; Pal Murugan et al. 2016). These reviews summarized previous studies dealing with how acrylamide is formed, factors affecting its formation, and ways of reducing its occurrence. Factors discussed that affect acrylamide formation include variety selection/genetic modification, production practices and environmental conditions, storage conditions, and processing parameter modifications. While significant improvement can be made with newer varieties/genetic modification as well as processing at lower temperatures, traditional varieties and processing procedures are often preferred by the consumer and the quick serve restaurant industry (Carew et al. 2009). Therefore, production and storage practices that minimize acrylamide-forming potential remain relevant. The overall objective of this article is to augment previous reviews and to focus specifically on agronomic and storage practices that affect acrylamide formation in processed potatoes.
Acrylamide and Acrylamide Precursors
Because acrylamide was only discovered as a compound in processed potato products in 2002, studies prior to that time did not include acrylamide analysis. Additionally, studies since that time are limited to some extent by the high cost of acrylamide analysis using LC/MS or GC/MS techniques. Recently, a lower cost method for acrylamide analysis has been reported and may greatly benefit breeding programs and acrylamide screening for the processing industry (Advant 2016). Despite the limited database on direct acrylamide analysis, numerous studies have been conducted that focus primarily on reducing sugars because of the effect they have on fry color. The obvious question is - can precursors or fry quality be used to predict the potential for acrylamide formation? If they can, then practices that reduce asparagine and reducing sugars or improve fry quality can be used to mitigate acrylamide formation. Studies summarized below indicate that acrylamide precursors and fry quality are for the most part correlated with acrylamide forming potential; however, a direct measurement of acrylamide is preferable whenever possible.
Studies have shown that acrylamide formation increases in processed potato products as concentrations of reducing sugars increase, with correlations (R2) ranging from 0.73 to 0.98 (Amrein et al. 2003; De Wilde et al. 2005). In contrast, the relationship between asparagine and acrylamide formation in most situations is weak because the concentration of asparagine alone in conventionally bred potato tubers is usually much higher than the concentrations of reducing sugars (Lea et al. 2007). Therefore, the Maillard reaction will be controlled more by the concentration of reducing sugars than asparagine. Matsuura-Endo et al. (2006) reported that when the fructose/asparagine ratio was less than 2 (low fructose), the relationship between fructose and acrylamide formation was strong (R2 = 0.81) and the relationship between asparagine and acrylamide was weak (R2 = 0.03). In contrast, when the fructose/asparagine ratio was greater than 2 (high fructose), the relationship between fructose and acrylamide was weak (R2 = 0.12) and the relationship between asparagine and acrylamide was strong (R2 = 0.68). The high fructose in this study was generated by storing the potatoes at cold temperatures, which induced cold sweetening. These results clearly indicate that both precursors can play a role in acrylamide formation, but reducing sugars will control the reaction when potatoes are stored properly for processing requirements.
Another indicator of acrylamide content is fry color (Olsson et al. 2004), which is not too surprising given the strong association between fry color and reducing sugars (Roe et al. 1990). In general, the darker the fry color the higher the acrylamide concentration. The relationship between fry color and acrylamide is highly significant with R2 ranging from 0.79 to 0.95 (Silva and Simon 2005; Pedreschi et al. 2006); however, lower correlations are observed when combined over different varieties (Gokmen et al. 2007). These findings suggest that improvements in fry quality will generally result in lower acrylamide concentrations.
Pre-Harvest Factors Affecting Acrylamide Precursors or Acrylamide
It is well established that the best way of improving fry color is to harvest potatoes when they are chemically mature (Kumar et al. 2004; Sowokinos and Preston 1988). Chemical maturity can be defined as the stage of growth when tuber sucrose and reducing sugars reach a minimum value. This stage occurs during vine senescence and skin set (physical maturity) and when tuber dry matter is at its peak. The best fry quality occurs when sucrose is <1.5 mg/g fw and glucose is less than 0.35 mg/g fw. Even though sucrose is not directly involved in the Maillard reaction, it does serve as source for reducing sugars and some may hydrolyze during frying and therefore lower levels are desirable. Factors affecting chemical maturity include variety, soil moisture, temperature, nutrition, and harvest date. In general, sucrose and reducing sugar concentrations decrease as tubers bulk with higher concentrations of total sugars for frying varieties compared with chipping varieties (Herman et al. 1995; Wohleb et al. 2014).
No studies could be found that directly evaluated the effects of growing season air temperature on acrylamide formation; however numerous studies have evaluated temperature effects on sugars. In general, the optimal temperature for potato growth is between 15 and 20 °C. Below 8 °C, sugars can increase which can be a problem in Northern climates when harvest is delayed to later in the growing season. Likewise, when temperatures increase above 25 to 30 °C, sugars tend to increase. This is particularly a problem for varieties that are susceptible to sugar end defect. Periods of high temperature above 30 °C have been shown to increase sugar ends in varieties like Russet Burbank (Thompson et al. 2008).
In most situations it is difficult to separate the effects of temperature and water stress since these stresses tend to occur simultaneously under field conditions (Shock et al. 1993; Thompson et al. 2008). The timing of water and heat stress affects the amount of sugar accumulation and where the sugars accumulate in the tuber. Stress early in the season causes accumulation of sugars in the stem end and is considered the most sensitive period. Stress late in the season causes sugar accumulation in the bud end (Iritani and Weller 1980; Sowokinos et al. 2000). Short intense stresses over the growing season tend to result in higher sugar concentrations than a continuous stress.
Soil temperature (tuber pulp temperature) can directly affect tuber specific gravity, at-harvest sugar content, and buildup of sugars during storage. The effects of soil temperature depend on stage of tuber development, temperature (Zommick et al. 2014) and duration of heat (Herman et al. 2016b). For example, during the final 53 days of tuber bulking, soil temperatures +7 and + 13 °C above ambient (16 °C) produced tubers with lower gravities and the effect was greatest at the highest soil temperature (29 °C) (Zommick et al. 2014). By contrast, 23 and 29 °C soil temperatures during 29 days of tuber maturation under dead vines at season end had no effect on gravities (Zommick et al. 2014). Following a brief postharvest wound-healing period, sucrose and reducing sugar concentrations were also substantially higher in tubers grown at +7 and + 13 °C soil temperatures during the bulking and maturation stages of growth, respectively. Moreover, high soil (pulp) temperatures during bulking and maturation exacerbated buildup of sucrose and reducing sugars and hastened deterioration of process quality (fry color) during a 24-day period of storage at 4 °C for the cold sweetening-susceptible cultivar Ranger Russet and totally abolished the inherent cold sweetening resistance of Premier Russet. Additional studies demonstrated the importance of duration of high tuber pulp temperatures in these responses and determined that heat stress enhances the cold induction of invertase to invoke sweetening in many cold-sweetening resistant and susceptible varieties (Herman et al. 2016b). In general, cold-sweetening resistant varieties have low acrylamide-forming potential; however, tolerance to heat stress for retention of the low temperature sweetening resistant phenotype is needed in developing varieties with robust low acrylamide forming potential (Herman et al. 2016b).
In a controlled environment study with a chipping potato where a 14-day water stress or temperature stress was imposed, Wang et al. (2012) reported that temperature stress caused glucose accumulation in one out of two years and there was no effect due to water stress. They concluded that moderate stresses did not consistently cause stem end chip defect, a defect similar to sugar end defect in French fry varieties. Similarly, Muttucumaru et al. (2015) reported conflicting results in field and greenhouse water stress studies. In a field study, with no major drought occurring, added irrigation caused an increase in glucose and acrylamide when the tubers were processed. In a greenhouse study, drought caused an increase in acrylamide in one variety and a decrease in another. Surprisingly, glucose and asparagine concentrations increased with drought but acrylamide concentrations in processed tubers were lower. Results from these studies suggest that stress-induced sugar accumulation is strongly influenced by the variety and that more direct measurements of acrylamide following stress is needed to elucidate stress by variety interactions.
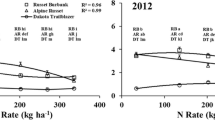
The effects of nitrogen (N) fertility on acrylamide precursors have been extensively studied (Roe et al. 1990; Herman et al. 1995). Nitrogen fertilization can affect reducing sugars and asparagine in opposite ways. For example, adequate N fertilization tends to decrease tuber sugar concentrations at harvest, although there are some studies showing no effect or sometimes increasing reducing sugar concentrations depending on the part of the tuber (Westermann et al. 1994; Brandt et al. 2016). In contrast, asparagine in tubers almost always increases with increasing N fertilization (Lea et al. 2007) as shown by the average response of six late season frozen processing cultivars grown in the Columbia Basin of Washington (Fig. 1). One explanation for lower reducing sugars with increasing N fertilizer is that the biosynthesis of asparagine competes for available carbon (Morales et al. 2008). It appears however, this effect depends on variety and harvest management. In a three-year study, Knowles et al. (2015a, b) reported that increasing N rate delayed physiological maturity by approximately 8 days in Sage Russet and Alpine Russet. Physiological maturity in these studies was defined as the average days after planting to reach maximum yield and specific gravity, and minimum sucrose and reducing sugar concentrations in tubers (Knowles et al. 2010; Wohleb et al. 2014). Delaying harvest after tubers achieved physiological maturity increased reducing sugars with this effect being more pronounced with low N (Fig. 2), which in turn can affect process quality at harvest (e.g. sugar ends). Further studies demonstrated that reducing sugars in over mature tubers often continue to increase postharvest, particularly in the stem ends of tubers, resulting in earlier deterioration of process quality in storage (Knowles et al. 2015b; Wohleb et al. 2014). These results therefore suggest that if N fertility is limiting, an earlier harvest date is warranted to limit over maturation of tubers under dead vines, which advances tuber age and leads to reducing sugar accumulation and accelerated loss of process quality during storage in some varieties.
Changes in tuber asparagine and total nitrogen concentrations with increasing N fertility. Data are averages of six cultivars (Russet Burbank, Ranger Russet, Umatilla Russet, Classic Russet, Owyhee Russet and Teton Russet) grown under late season management at Othello, WA in 2011 and 2012 (Knowles, N.R., unpublished). N rates include residual soil N, pre-plant incorporated N and that added in-season through fertigation
Changes in reducing sugars (glucose + fructose), specific gravity and days after planting (DAP) to physiological maturity (PM) of Alpine Russet tubers grown with relatively low (213 kg ha−1) and high (409 kg ha−1) rates of N (averaged over 3 seasons, 2011–13) at Othello, WA (Knowles et al. 2015b). The recommended N rate for production of Alpine Russet in this region is 409 kg ha−1. Physiological maturity was determined as the average days after planting to reach maximum yield, maximum specific gravity and minimum sucrose and reducing sugar concentrations in tubers (Wohleb et al. 2014)
While numerous studies have reported on the effects of N fertility on acrylamide precursors, there are fewer studies that have directly measured acrylamide. In studies that have measured acrylamide directly, the results are variable. Muttucumaru et al. (2013) reported that N fertilization tended to increase acrylamide although the effect was cultivar dependent. If N management results in an immature crop at harvest reducing sugars are likely to be elevated. Other studies have reported that N fertilization decreased acrylamide formation (De Wilde et al. 2006). In general, N fertility appears to influence acrylamide formation by affecting reducing sugars and asparagine; however, the specific effects depend on variety, growing conditions, harvest date, and storage conditions (Amrein et al. 2003; Muttucumaru et al. 2017; Sun et al. 2018). Because so many variables are involved, general recommendations regarding N fertility and potential acrylamide formation need to be variety and growing region specific.
No studies could be found that evaluated phosphorus (P) fertility directly on acrylamide formation. However, a few studies have reported P effects on reducing sugars. These studies have shown that low P fertility increases tuber reducing sugars and increases susceptibility to sugar ends (Kolbe et al. 1995), although a couple of studies have shown no effect of P fertility on reducing sugars (Herlihy and Carroll 1969; Whittaker et al. 2010). It is likely that effects of P fertility on reducing sugars will depend on the soil type, organic matter content and initial soil P status. Soils initially testing medium to high in P will have a lower probability of response to increasing levels of P fertility than those testing low in P.
Potassium (K) fertility has been shown to have significant effects on reducing sugars and fry quality. In general, most studies indicate that K application results in lower reducing sugars and lighter chip color (Murphy and Goven 1966; Herlihy and Carroll 1969; Stanley and Jewell 1989) although, a couple of studies have reported no effect on reducing sugars (Kumar et al. 2007). Gerendás et al. (2007) reported that high N and low K fertility resulted in higher acrylamide than high N and high K fertility. Gause (2014), however, found no effect of increasing K application from 0 to 280 kg K ha−1 on acrylamide or fry color. As with P, if soil test K is in the medium to high range, the likelihood of affecting reducing sugars and acrylamide formation with added K may be low. In general, the potential for higher acrylamide formation in processed potatoes exists when potatoes are grown under low K conditions.
Most of the acrylamide research related to sulfur (S) nutrition has been conducted in the United Kingdom (Elmore et al. 2007, 2010; Muttucumaru et al. 2013). Sulfur effects on acrylamide formation are contradictory and depended on how the study was conducted. In a pot study, yield was severely limited in plants not receiving S. In low S plants, asparagine increased in one variety and decreased in two other varieties. In all varieties, acrylamide was lower in processed tubers from S-deprived plants and the correlation between acrylamide and precursors was poor; which supports direct acrylamide measurement instead of relying solely on precursor measurements to determine acrylamide forming potential. In a N by S factorial field experiment with 13 varieties, the effects of N on acrylamide formation were variety dependent. Acrylamide formation increased with increasing N in 11 varieties and decreased in 2 varieties. Yield increased with increasing N for all varieties. The effects of S on acrylamide depended on N. Sulfur reduced the effect of high N on acrylamide formation similar to the response with N and K (Muttucumaru et al. 2013). Yields were not affected by S application indicating that adequate levels of S were present in the soil. These results suggest that under conditions when S is not limiting, added S may be beneficial in reducing acrylamide formation. Reasons for this benefit are not entirely clear but may be due to changes in the amino acid profile with S addition.
In a broad survey of potato nutrient composition, reducing sugars, and acrylamide formation in Italy, tuber copper and zinc (Zn) were positively correlated with reducing sugars (Whittaker et al. 2010) when tubers were analyzed within 29 days after harvest and not exposed to temperatures below 8 °C. Zinc was singled out as having a significant effect on increasing acrylamide in three potato varieties. Further investigation as to how Zn affects carbohydrate metabolism is warranted.
As indicated above, potatoes should ideally be harvested when tubers are physiologically mature, which occurs during vine senescence and coincides with maximum dry matter (specific gravity) and minimum sucrose and reducing sugar concentrations. However, a number of situations may occur to cause elevated sugars, two of which can be somewhat reversed (Sowokinos and Preston 1988). For example, elevated sugars can occur if tubers are harvested when they are immature due to a shortened growing season. Another example is when harvest occurs during a cold period, which induces cold sweetening. In both of these situations preconditioning is recommended before processing or storage (Pritchard and Adam 1992). Preconditioning involves maintaining tubers post-harvest at temperature between 13 °C and 16 °C to promote wound healing and lower reducing sugars. At least 14 days of preconditioning is recommended if potatoes are harvested in temperatures less than about 12.8 °C. Extended preconditioning may be required for immature potatoes (Pritchard and Adam 1992). Herman et al. (1995) reported that harvesting tubers when they are slightly immature can favor processing performance during long-term storage. However, early harvest without vine killing can result in elevated glucose even with preconditioning (Bethke and Busse 2010), but this may be variety dependent (Woodell et al. 2004). Mechanical stresses/physical damage can increase sucrose concentrations at harvest and levels may continue to increase in storage. These increased sugar levels may be related to increased respiration rate due to damage (Kumar et al. 2004).
Storage Practices Affecting Acrylamide Precursors or Acrylamide
Storage practices can significantly influence processing quality and acrylamide formation by directly affecting the level of reducing sugars (Chuda et al. 2003; Matsuura-Endo et al. 2006). For most varieties grown for processing, storage temperature exerts significant control over sugar accumulation and thus acrylamide forming potential through a process called cold-induced sweetening. Resistance to cold sweetening is therefore a major goal in developing new varieties for processing. In contrast to reducing sugars, storage temperature has minimal effect on tuber asparagine concentration as depicted in Fig. 3 for eighteen clones/varieties possessing varying degrees of resistance to cold sweetening. In-season stress and poor storage conditions can intensify cold sweetening in susceptible varieties and diminish the inherent resistance to cold sweetening in resistant varieties, resulting in high sugar accumulation during storage even if sugar levels are acceptable at harvest (Zommick et al. 2014; Herman 2016b).
Effects of storage temperature on tuber reducing sugar (glucose plus fructose) and asparagine concentrations of clones and cultivars from the 2015 Washington Late Regional Russet Trial of the Northwest Variety Development Program. Tubers were grown for 153 days (planting to vine kill), wound healed for 7 days (9 °C, 95% RH) and then stored for an additional 60 days at the indicated temperatures prior to analysis (Pavek and Knowles 2016)
De Wilde et al. (2005) clearly showed that when three varieties Bintje, Ramos, and Saturna were stored at 4 °C, glucose concentrations rose from less than 0.2% to over 6% on a dry weight basis over a 24-week storage period. In contrast, when stored for the same time frame at 8 °C, levels remained relatively constant at less than 0.2%. Following processing, acrylamide concentrations mirrored glucose concentrations with elevated concentration above 1500 μg kg−1 after 24 weeks of storage at 4 °C in all three varieties and concentrations less than 500 μg kg−1 when stored at 8 °C. Similar results were reported by Brandt and Olsen (2013). When storage temperatures increased from 5.6 °C to 8.9 °C, acrylamide concentrations decreased. In that study, significant positive correlations between glucose and acrylamide and fry color and acrylamide were found in 5 of 6 storage times. A positive relationship between asparagine and acrylamide was found in only one of six storage times.
While higher temperature reduces cold sweetening after harvest, eventually tubers in storage will undergo senescent sweetening, which is characterized by an accumulation of sugars when dormancy is broken (Sowokinos and Preston 1988). The timing of senescent sweetening is cultivar dependent and is related to dormancy requirements and sprout growth. Once senescent sweetening occurs, it is not reversible, which eliminates reconditioning as a management option for improving process quality and reducing acrylamide formation. Reconditioning involves raising the storage temperature to facilitate a drop in reducing sugar levels through stimulating respiration and starch synthesis. An example of cultivar differences in senescent sweetening is clearly shown in a comparison of Snowden and Ivory Crisp when stored at 8 °C over nine months (Fig. 4; Sun et al. 2018). Glucose increased dramatically for Snowden after six months of storage but not for Ivory Crisp. The increase in tuber glucose in Snowden was associated with an increase in acrylamide concentrations in the chips. Acrylamide in Ivory Crisp chips remained relatively constant throughout nine months of storage, indicating that substantial reductions in acrylamide can be attained through variety selection.
a Senescent sweetening in Snowden and Ivory Crisp tubers as a function of storage time at 8.3 °C (b) Acrylamide concentrations in chips processed from Snowden and Ivory Crisp tubers as a function of storage time. Each data point is the mean of four replications averaged over five N rates ± one standard deviation. (Sun et al. 2018)
Gaseous composition of the storage atmosphere can also affect tuber sugar content although the effect is somewhat temperature and cultivar dependent. Workman and Twomey (1970) reported that low oxygen can suppress sugar accumulation at 0 °C and 5 °C. Low O2 stimulated buildup in sucrose and diminished the cold-induced accumulation of reducing sugars and associated deterioration of process quality in Russet Burbank and Innovator tubers during storage at 4 °C, but little if any effect was observed at 8 °C where sweetening was minimal through 7 months of storage (Herman et al. 2016a). Moreover, the effect of hypoxia (2.5 kPa O2) on limiting cold sweetening and loss of process quality was greater for Innovator than Russet Burbank tubers during the first 3 months of storage only. Cold sweetening then resumed in Innovator tubers with reducing sugar concentration increasing to equal that of Russet Burbank tubers stored at 4 °C and 21 kPa O2. Low O2 inhibited the cold induction of invertase to limit the extent of sweetening in these two cultivars. Increased carbon dioxide was found to increase tuber sugars at 5 °C but not at 3.3 °C (Daniels-Lake and Prange 2009). At 8 °C, Gokmen et al. (2007) reported that increasing CO2 and decreasing O2 prolonged potato storage life, but increased reducing sugars and acrylamide when processed. High ethylene and CO2 have been shown to increase sugars (Daniels-Lake 2013), but in most storage situations ethylene does not reach high enough levels to cause problems (Bethke 2014).
Conclusions
Agronomic factors that affect sugars and asparagine will affect the potential for acrylamide formation in processed potatoes. However, the interactive effects of variety, nutrition, and environmental variables often make it difficult to draw specific conclusions. In most conventionally bred potato cultivars, the concentration of reducing sugars appears to be the controlling factor. Temperature and water stress outside the optimal ranges for potato production will increase sugars and therefore acrylamide forming potential. The effects of N fertility are complex because chemical/physiological maturity, sugars and asparagine are altered but often in opposite directions and the effects are often cultivar dependent. Nitrogen can affect the potential for acrylamide formation directly through influencing amino acid concentrations and indirectly through affecting tuber maturity and thus reducing sugar levels at harvest and their subsequent buildup in storage. Other nutrients can also be involved, but under field conditions, they are not involved to the same extent as N. In general, any nutrient stress will increase sugars, but conflicting results are often reported. Of the micronutrients, the effect of Zn on acrylamide formation requires further study.
Storage conditions strongly affect reducing sugar concentrations. Temperatures greater than 8 °C will reduce cold-induced sweetening during storage. However, higher temperatures promote aging and may induce senescent sweetening. Lowering oxygen concentration during storage will reduce sugars at low temperatures, but the effect may only be temporary for some cultivars and low O2 storage is not an economical practice. Increasing CO2 in storage may increase reducing sugars in storage as temperatures increase.
One of the best acrylamide mitigation strategies is to choose a cold-sweetening resistant cultivar with inherently low levels of asparagine. Additionally, implementation of agronomic management and storage recommendations to reduce acrylamide include the following: manage irrigation to minimize water and heat stress during the growing season; adjust N rates and other nutrients to optimize yield, avoiding excessive N application rates; harvest as close to physiological/chemical maturity as possible. If tubers are immature or have been exposed to cold temperatures prior to harvest, precondition tubers at higher temperatures initially during storage; and handle tubers appropriately to minimize mechanical damage. Finally, store tubers at temperatures that do not cause cold-induced sweetening in the chosen cultivar.
References
Advant, S. 2016. A quicker way to detect acrylamide in French fries. USDA Ag Research Magazine. https://agresearchmag.ars.usda.gov/2016/nov/frenchfries/#printdiv. Accessed 17 December 2016.
Amrein, T.M., S. Bachmann, A. Noti, M. Biedermann, M.F. Barbosa, S. Biedermann-Brem, K. Grob, A. Keiser, P. Realini, F. Escher, and R. Amado. 2003. Potential of acrylamide formation, sugars, and free asparagine in potatoes: A comparison of cultivars and farming systems. Journal of Agricultural and Food Chemistry 51:5556-5560.
Becalski, A., B.P. Lau, D. Lewis, and S.W. Seaman. 2003. Acrylamide in foods: Occurrence, sources, and modeling. Journal of Agricultural and Food Chemistry 51: 802–808. https://doi.org/10.1021/jf020889y.
Bethke, P.C. 2014. Ethylene in the atmosphere of commercial potato (Solanum tuberosum) storage bins and potential effects on tuber respiration rate and fried chip color. American Journal of Potato Research 91: 688–695. https://doi.org/10.1007/s12230-014-9400-1.
Bethke, P.C., and A.J. Bussan. 2013. Acrylamide in processed potato products. American Journal of Potato Research 90: 403–424. https://doi.org/10.1007/s12230-013-9321-4.
Bethke, P.C., and J.S. Busse. 2010. Vine-kill treatment and harvest date have persistent effects on tuber physiology. American Journal of Potato Research 87: 299–309. https://doi.org/10.1007/s12230-010-9137-4.
Brandt, T., and N. Olsen. 2013. Acrylamide concentrations in six potato cultivars at harvest and in storage. American Journal of Potato Research 90: 127.
Brandt, T., N. Olsen, and M. Thornton. 2016. Effects of nitrogen fertilizer management on postharvest performance of Russet Burbank and three low acrylamide potato clones. American Journal of Potato Research 93: 123–124.
Carew, R., M. Khakbazan, and R. Mohr. 2009. Cultivar developments, fertilizer inputs, environmental conditions, and yield determination for potatoes in Manitoba. American Journal of Potato Research 86: 442–455. https://doi.org/10.1007/s12230-009-9099-6.
Chuda, Y., H. Ono, H. Yada, A. Ohara-Takada, C. Matsuura-Endo, and M. Mori. 2003. Effects of physiological changes in potato tubers (Solanum tuberosum L.) after low temperature storage on the level of acrylamide formed in potato chips. Bioscience, Biotechnology, and Biochemistry 67: 1188–1190. https://doi.org/10.1271/bbb.67.1188.
Daniels-Lake, B.J. 2013. The combined effect of CO2 and ethylene sprout inhibitor on the fry colour of stored potatoes (Solanum tuberosum L.). Potato Research 56: 115–126. https://doi.org/10.1007/s11540-013-9234-0.
Daniels-Lake, B.J., and R.K. Prange. 2009. The interaction effect of carbon dioxide and ethylene in the storage atmosphere on potato fry color is dose-related. Hortscience 44: 1641–1644.
De Wilde, T., B. De Meulenaer, F. Mestdagh, Y. Govaert, S. Vandeburie, W. Ooghe, S. Fraselle, K. Demeulemeester, C. Van Peteghem, A. Calus, J.M. Degroodt, and R. Verhe. 2005. Influence of storage practices on acrylamide formation during potato frying. Journal of Agricultural and Food Chemistry 53: 6550–6557. https://doi.org/10.1021/jf050650s.
De Wilde, T., B. De Meulenaer, F. Mestdagh, Y. Govaert, S. Vandeburie, W. Ooghe, S. Fraselle, K. Demeulemeester, C. Van Peteghem, A. Calus, J.M. Degroodt, and R. Verhe. 2006. Influence of fertilization on acrylamide formation during frying of potatoes harvested in 2003. Journal of Agricultural and Food Chemistry 54: 404–408. https://doi.org/10.1021/jf0521810.
Elmore, J.S., D.S. Mottram, N. Muttucumaru, A.T. Dodson, M.A. Parry, and N.G. Halford. 2007. Changes in free amino acids and sugars in potatoes due to sulfate fertilization and the effect on acrylamide formation. Journal of Agricultural and Food Chemistry 55: 5363–5366. https://doi.org/10.1021/jf070447s.
Elmore, J.S., A.T. Dodson, N. Muttucumaru, N.G. Halford, M.A. Parry, and D.S. Mottram. 2010. Effects of sulphur nutrition during potato cultivation on the formation of acrylamide and aroma compounds during cooking. Food Chemistry 122: 753–760. https://doi.org/10.1016/j.foodchem.2010.03.049.
European Food Safety Authority. 2015. Acrylamide. https://www.efsa.europa.eu/en/topics/topic/acrylamide. Accessed 12 December 2016.
Gause, K. 2014. Effect of nitrogen and potassium on potato yield, quality and acrylamide forming potential. Electronic Theses and Dissertations. Paper 2170. http://digitalcommons.library.umaine.edu/etd/2170.
Gerendás, J., F. Heuser, and B. Sattelmacher, B. 2007. Influence of nitrogen and potassium supply on contents of acrylamide precursors in potato tubers and on acrylamide accumulation in French fries. Journal of Plant Nutrition 30:1499–1516. doi: https://doi.org/10.1080/01904160701555846.
Gokmen, V., B. Akbudak, A. Serpen, J. Acar, Z.M. Turan, and A. Eris. 2007. Effects of controlled atmosphere storage and low-dose irradiation on potato tuber components affecting acrylamide and color formations upon frying. European Food Research and Technology 224: 681–687. https://doi.org/10.1007/s00217-006-0357-2.
Halford, N.G., N. Muttucumaru, T.Y. Curtis, and M.A. Parry. 2007. Genetic and agronomic approaches to decreasing acrylamide precursors in crop plants. Food Additives and Contaminants 24 (Suppl. 1): 26–36. https://doi.org/10.1080/02652030701403093.
Halford, N.G., T.Y. Curtis, N. Muttucumaru, J. Postles, J.S. Elmore, and D.S. Mottram. 2012. The acrylamide problem: A plant and agronomic science issue. Journal of Experimental Botany 63: 2841–2851. https://doi.org/10.1093/jxb/ers011.
Herlihy, M., and P.J. Carroll. 1969. Effects of N, P, and K and their interactions on yield, tuber blight and quality of potatoes. Journal of the Science of Food and Agriculture 20: 513–517. https://doi.org/10.2134/agronj1977.00021962006900030023x.
Herman, T.J., B. Shafii, S.L. Love, and R.B. Dwelle. 1995. Influence of crop management factors on chipping potato maturity and storage processing performance. Journal of the Science of Food and Agriculture 68: 51–58.
Herman, D.J., L.O. Knowles, and N.R. Knowles. 2016a. Low oxygen storage modulates invertase activity to attenuate cold-induced sweetening and loss of process quality in potato (Solanum tuberosum L.). Postharvest Biology and Technology 121: 106–117.
Herman, D.J., L.O. Knowles, and N.R. Knowles. 2016b. Heat stress affects carbohydrate metabolism during cold-induced sweetening of potato (Solanum tuberosum L.). Planta 245: 1–20. https://doi.org/10.1007/s00425-016-2626-z.
Iritani, W.M. and L.D. Weller. 1980. Sugar development in potatoes. Washington State University Cooperative Extension Bulletin no. 0717, 16 pp.
Knowles, N.R., M.J. Pavek, C. Hiles, L.O. Knowles and Z. Holden. 2010. Nitrogen management affects tuber physiological maturity and retention of processing quality. Proceedings of the 94th Annual Meeting of the Potato Association of America. Am. J. Pot. Res. 88:49.
Knowles, N.R., M.J. Pavek, and L.O. Knowles. 2015a. Nitrogen modulates physiological maturity and tuber N content to affect postharvest processing and nutritional qualities. Proceedings of the 98th Annual Meeting of the Potato Association of America. Am. J. Pot. Res 92:196.
Knowles, N.R. Pavek, M.J. and Knowles, L.O. 2015b. Developmental profiles, nitrogen use and postharvest quality of alpine and sage russet potatoes in the Columbia Basin. Annual Washington and Oregon potato conference, Jan. 27–30, Kennwick, WA. pp. 37–50. http://www.nwpotatoresearch.com/IPMStuff/PDFs/Proceedings2015.pdf. Accessed 12 December 2016.
Kolbe, H., K. Muller, G. Olteanu, and T. Gorea. 1995. Effects of nitrogen, phosphorus and potassium fertilizer treatments on weight loss and changes in chemical composition of potato tubers stored at 4°C. Potato Research 38: 97–107. https://doi.org/10.1007/BF02358074.
Kumar, D., B.P. Singh, and P. Kumar. 2004. An overview of the factors affecting sugar content of potatoes. Annals of Applied Biology 145: 247–256. https://doi.org/10.1111/j.1744-7348.2004.tb00380.x.
Kumar, P., S.K. Pandey, B.P. Singh, S.V. Singh, S. V, and D. Kumar. 2007. Influence of source and time of potassium application on potato growth, yield, economics and crisp quality. Potato Research 50: 1–13. https://doi.org/10.1007/s11540-007-9023-8.
Lea, P.J., L. Sodek, M.A. Parry, P.R. Shewry, and N.G. Halford. 2007. Asparagine in plants. Annals of Applied Biology 150: 1–26. https://doi.org/10.1111/j.1744-7348.2006.00104.x.
Matsuura-Endo, C., A. Ohara-Takada, Y. Chuda, H. Ono, H. Yada, M. Yoshida, A. Kobayashi, S. Tsuda, S. Takigawa, T. Noda, H. Yamauchi, and M. Mori. 2006. Effects of storage temperature on the contents of sugars and free amino acids in tubers from different potato cultivars and acrylamide in chips. Bioscience, Biotechnology, and Biochemistry 70: 1173–1180. https://doi.org/10.1271/bbb.70.1173.
Medeiros Vinci, R., F. Mestdagh, and B. De Meulenaer. 2012. Acrylamide formation in fried potato products - present and future, a critical review on mitigation strategies. Food Chemistry 133: 1138–1154. https://doi.org/10.1016/j.foodchem.2011.08.001.
Morales, F., E. Capuano, and V. Fogliano. 2008. Mitigation strategies to reduce acrylamide formation in fried potato products. Annals of the New York Academy of Sciences 1126: 89–100. https://doi.org/10.1196/annals.1433.051.
Murphy, H.J., and M.J. Goven. 1966. The last decade in 38 years of potash studies for potato fertilizersin Maine. American Potato Journal 43: 122–127.
Muttucumaru, N., S.J. Powers, J.S. Elmore, D.S. Mottram, and N.G. Halford. 2013. Effects of nitrogen and sulfur fertilization on free amino acids, sugars, and acrylamide-forming potential in potato. Journal of Agricultural and Food Chemistry 61: 6734–6742. https://doi.org/10.1021/jf401570x.
Muttucumaru, N., S.J. Powers, J.S. Elmore, D.S. Mottram, and N.G. Halford. 2015. Effects of water availability on free amino acids, sugars, and acrylamide-forming potential in potato. Journal of Agricultural and Food Chemistry 63: 2566–2575. https://doi.org/10.1021/jf506031w.
Muttucumaru, N., S.J. Powers, J.S. Elmore, A. Dodson, A. Briddon, D.S. Mottram, and N.G. Halford. 2017. Aryclamide-forming potential of potatoes grwn at different locaitons, and thenratio of free asparagine to reducing sugars at which free asparagine becomes a limiting facotr for acrylamide formation. Food Chemistry 220: 76–86.
Olsson, K., R. Svensson, and C.A. Roslund. 2004. Tuber components affecting acrylamide formation and colour in fried potato: variation by variety, year, storage temperature and storage time. Journal of the Science of Food and Agriculture 84: 447–458. https://doi.org/10.1002/jsfa.1681.
Pal Murugan, M., G. Agathian, A.D. Semwal, and G.K. Sharma. 2016. A review on acrylamide mitigation strategies in various processed foods. International Journal of Advanced Research 4: 1025–1040.
Pavek, M.J. and N.R. Knowles. 2016. WSU potato cultivar yield and postharvest quality evaluations for 2015. Washington State University Special Report, 114 pp. (http://potatoes.wsu.edu/wp-content/uploads/2016/01/Potato-Cultivar-Yield-and-Postharvest-Quality-Evaluations-Research-Edition-2015.pdf) accessed 13 December 2016.
Pedreschi, F. 2010. Acrylamide formation and reduction in fried potatoes. In Processing effects on safety and quality of foods chapter 9, ed. E. Ortegas-Rivas, 231–252. Boca Raton FL: CRC Press.
Pedreschi, F., K. Kaack, and K. Granby. 2006. Acrylamide content and color development in fried potato strips. Food Research International 39: 40–46. https://doi.org/10.1016/j.foodres.2005.06.001.
Pritchard, M.K., and L.R. Adam. 1992. Preconditioning and storage of chemically immature russet Burbank and Shepody potatoes. American Potato Journal 69: 805–815. https://doi.org/10.1007/BF02854188.
Roe, M.A., R.M. Faulks, and J.L. Belsten. 1990. Role of reducing sugars and amino acids in fry colour of chips from potatoes grown under different nitrogen regimes. Journal of the Science of Food and Agriculture 52: 207–214. https://doi.org/10.1002/jsfa.2740520207.
Seal, C.J., A. de Mul, G. Eisenbrand, A.J. Haverkort, K. Franke, S.P. Lalljie, H. Mykkänen, E. Reimerdes, G. Scholz, V. Somoza, S. Tuijtelaars, M. van Boekel, J. van Klaveren, S.J. Wilcockson, and L. Wilms. 2008. Risk-benefit considerations of mitigation measures on acrylamide content of foods--a case study on potatoes, cereals and coffee. The British Journal of Nutrition 99 (2): S1–S46. https://doi.org/10.1017/S0007114508965314.
Shock, C.C., Z.A. Holmes, T.D. Stieber, E.P. Eldredge, and P. Zhang. 1993. The effect of timed water stress on quality, total solids, and reducing sugar content of potatoes. American Potato Journal 70: 227–241.
Silva, E. M. and P. W. Simon. 2005. Genetic, physiological, and environmental factors affecting acrylamide concentration in fried potato products. in M. Friedman and D. Moltram eds. Chemistry and Safety of Acrylamide in Food. Advances in Experimental Medicine and Biology. 561:371–386. Springer US, New York.
Sowokinos, J.R. and D.A. Preston. 1988. Maintenance of potato processing quality by chemical maturity monitoring (CMM). Station Bulletin 586–1988 (Item No. AD-SB-3441) Minn. Agric. Expt. University of Minnesota.
Sowokinos, J.R., C.C. Shock, T.D. Stieber, and E.P. Eldredge. 2000. Compositional and enzymatic changes associated with the sugar-end defect in Russet Burbank potatoes. American Journal of Potato Research 77: 47–56. https://doi.org/10.1007/BF02853661.
Stanley, R., and S. Jewell. 1989. The influence of source and rate of potassium fertilizer on the quality of potatoes for french fry production. Potato Research 32: 439–446.
Sun, N., C.J. Rosen, and A.L. Thompson. 2018. Acrylamide formation in processed potates as affected by cultivar, nitroegn fertilization and storage time. Amercian Journal of Potato Research. https://doi.org/10.1007/s12230-018-9647-z.
Thompson, A.L., S.L. Love, J.R. Sowokinos, M.K. Thornton, and C.C. Shock. 2008. Review of the sugar end disorder in potato (Solanum tuberosum L.). American Journal of Potato Research 85: 375–386. https://doi.org/10.1007/s12230-008-9034-2.
Wang, Y., A.J. Bussan, and P.C. Bethke. 2012. Stem-end defect in chipping potatoes (Solanum tuberosum L.) as influenced by mild environmental stresses. American Journal of Potato Research 89: 392–399. https://doi.org/10.1007/s12230-012-9259-y.
Westermann, D.T., D.W. James, T.A. Tindall, and R.L. Hurst. 1994. Nitrogen and potassium fertilization of potatoes: Sugars and starch. American Potato Journal 71: 433–453. https://doi.org/10.1007/BF02849098.
Whittaker, A., I. Marotti, G. Dinelli, L. Calamai, S. Romagnoli, M. Manzelli, E. Palchetti, V. Vecchio, and S. Benedettelli. 2010. The influence of tuber mineral element composition as a function of geographical location on acrylamide formation in different Italian potato genotypes. Journal of the Science of Food and Agriculture 90: 1968–1976. https://doi.org/10.1002/jsfa.4026.
Woodell L., N. Olsen, T.L. Brandt and G.E. Kleinkopf. 2004. Vine-kill and long-term storage of Ranger Russet potatoes. CIS 1119. University of Idaho Extension, Moscow, ID.
Wohleb, C.H., N.R. Knowles, and M.J. Pavek. 2014. Plant growth and development. In D.A. Navarre and M.J. Pavek eds., The Potato: Botany, Production and Uses, 1st edition, Chapter 5 pp. 64–82. CABI Press.
Workman, M., and J. Twomey. 1970. The influence of storage on the physiology and productivity of Kennebec seed potatoes. American Potato Journal 47: 372–378.
World Health Organization. 2005. Acrylamide levels in food should be reduced because of public health concern says UN expert. http://www.who.int/mediacentre/news/notes/2005/np06/en/ accessed 12 December 2016.
Zommick, D.H., L.O. Knowles, M.J. Pavek, and N.R. Knowles. 2014. In-season heat stress compromises postharvest quality and low-temperature sweetening resistance in potato (Solanum tuberosum L.). Planta 239: 1243–1263. https://doi.org/10.1007/s00425-014-2048-8.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rosen, C., Sun, N., Olsen, N. et al. Impact of Agronomic and Storage Practices on Acrylamide in Processed Potatoes. Am. J. Potato Res. 95, 319–327 (2018). https://doi.org/10.1007/s12230-018-9659-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12230-018-9659-8