Abstract
The affirmation of acrylamide as a probable carcinogen by the European Food Safety Authority has reinforced the need to lower acrylamide content in fried potato products. Selected for low reducing sugars and acrylamide-forming potential, recently released cultivars ‘Alpine Russet’, ‘Dakota Trailblazer’, and ‘Ivory Crisp’ were evaluated for their processing quality when grown with varying nitrogen (N) fertilizer regimes. The objective of this study was to determine the effects of N fertilizer rate (34, 135, 202, 269 and 336 kg ha−1) on tuber glucose and acrylamide concentration following processing of new cultivars relative to standard cultivars ‘Russet Burbank’ and ‘Snowden’ at harvest, and after 3, 6 and 9 months of storage at 7.2 °C over 2 years. Glucose and acrylamide responses to N rate were similar for chip cultivars, which linearly decreased in 2011, and quadratically increased then decreased in 2012 with increasing N rate. The N rate effect on French fry glucose concentration varied by cultivar and either decreased or did not respond to elevated N rate. Glucose and acrylamide concentrations of chip cultivars generally increased during storage, with a dramatic increase in ‘Snowden’ resulting from senescence sweetening after 9 months of storage. Environmental conditions significantly affected glucose and acrylamide responses of French fry cultivars to storage time. Glucose and acrylamide concentrations of all French fry cultivars generally increased during storage in 2011. In contrast, glucose concentrations of French fry cultivars were stable or increased, while acrylamide concentrations generally decreased during storage in 2012. The relationship between chip color and glucose concentration was significant, but differed by year. Glucose and acrylamide concentrations of French fry and chip cultivars were significantly correlated (R2 = 0.52 and 0.66, in 2011 and 2012, respectively). Generally, acrylamide in fried potato products can be minimized by using low reducing sugar cultivars supplied with a N fertilizer rate that optimizes yield and quality during growing seasons with minimal environmental stress.
Resumen
La afirmación de la acrilamida como probable carcinógeno por la Autoridad Europea de Seguridad Alimentaria ha reforzado la necesidad de bajar el contenido de la acrilamida en los productos de papas fritas. Seleccionadas por sus bajos azucares reductores y el bajo potencial de formación de acrilamida, las variedades recientemente liberadas ‘Alpine Russet’, ‘Dakota Trailblazer’, y ‘Ivory Crisp’, se evaluaron por su cualidad de procesamiento cuando se cultivaron con varios regímenes de fertilizante nitrogenado (N). El objetivo de este estudio fue determinar los efectos del nivel del fertilizante N (34, 135, 202, 269 y 336 kg ha −1) sobre el contenido de glucosa del tubérculo y concentración de acrilamida después del procesamiento de las nuevas variedades en relación con las estándar “Russet Burbank” y “Snowden” a la cosecha, y después de 3, 6, y 9 meses de almacenamiento a 7.2 °C durante dos años. Las respuestas de la glucosa y la acrilamida al nivel de N fueron similares para las variedades de fritura, que disminuyeron linealmente en 2011, y se incrementaron cuadráticamente y después disminuyeron en 2012 con aumentos de nivel de N. El efecto de la dosis nitrogenada en la concentración de glucosa en las papas a la francesa varió por cultivar y disminuyó o no respondió a alto nivel de N. Las concentraciones de glucosa y acrilamida de las variedades de hojuelas generalmente aumentaron durante el almacenamiento, con un aumento dramático en “Snowden” como resultado del endulzamiento por la senectud después de nueve meses de almacenamiento. Las condiciones ambientales afectaron significativamente las respuestas de glucosa y acrilamida de variedades para papas a la francesa respecto al tiempo de almacén. Las concentraciones de glucosa y acrilamida de todas las variedades de papas a la francesa generalmente aumentaron durante el almacenamiento en 2011. En contraste, las concentraciones de glucosa de estas variedades fueron estables o aumentaron, mientras que las concentraciones de acrilamida generalmente disminuyeron durante el almacenamiento en 2012. La relación entre el color de la hojuela y la concentración de glucosa fue significativa, pero difirió por año. Las concentraciones de glucosa y acrilamida de las variedades para francesas y para hojuelas estuvieron correlacionadas significativamente (R2 = 0.52 y 0.66, en 2011 y 2012, respectivamente). Generalmente, la acrilamida en los tubérculos de papa en los productos de papa frita con bajas concentraciones de acrilamida, se pueden minimizar usando variedades de bajos azucares reductores suplementadas con el nivel apropiado de N que optimice la producción de rendimiento y calidad durante los ciclos de crecimiento con mínimo agobio ambiental.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acrylamide can have neurological and reproductive effects on humans and is considered a probable carcinogen by the World Health Organization and the International Agency for Research on Cancer (Friedman 2003). The carcinogenic effect of acrylamide in higher doses has been proven on mice and rats (Johnson et al. 1986), but confirmed association between dietary acrylamide and human cancer occurrence is still lacking (Mucci et al. 2003, 2004, 2006; Mucci and Wilson 2008; Pelucchi et al. 2006; Hogervorst et al. 2007; Weber et al. 2008). Because evidence in human studies is currently limited and inconclusive, the European Food Safety Authority (EFSA) reconfirmed the potential risk of dietary acrylamide consumption for developing cancer in humans of all ages (EFSA 2015). The large consumption of fried potato products contributes a substantial proportion of dietary acrylamide intake in Europe and the United States (EFSA 2011; Elmore et al. 2015). Therefore, reducing acrylamide concentrations in high acrylamide-containing food, such as potato chips and fries (ranging from 170 to 12,000 μg kg−1, Friedman 2003) remains a high priority for the potato industry.
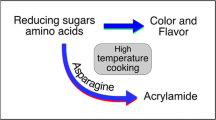
Acrylamide is not present in raw potato tubers, but can be formed from reducing sugars and asparagine in the Maillard reaction during high-temperature processing (Michalak et al. 2011). Reducing sugars are generally considered the limiting factor, because asparagine is often far in excess of reducing sugars in potato tubers, and a significant correlation exists between reducing sugars and acrylamide concentrations (Amrein et al. 2004; Ohara-Takada et al. 2005; Elmore et al. 2015). Raw potato tubers with low concentrations of precursors have been shown effective in reducing acrylamide-forming potential (De Wilde et al. 2005; Lea et al. 2007). For each cultivar, the propensity to contain a high or low concentration of tuber reducing sugars is heritable (Cunningham and Stevenson 1963). Cultivars with low reducing sugar concentrations usually generate less acrylamide in fried potato products, suggesting that cultivar selection would be an effective approach to decrease acrylamide formation (Halford et al. 2012).
Nitrogen (N) management is another practice that can influence sugar content and acrylamide-forming potential in fried potato products. Plants with adequate N supply produce tubers with low reducing sugar content at harvest and through storage (Kumar et al. 2004). Elevated N fertilization has been reported to decrease reducing sugars in tubers and acrylamide concentrations in French fries (De Wilde et al. 2006). Westermann et al. (1994) reported that increasing N application from 0 to 336 kg ha−1 increased reducing sugars in the stem end, but decreased reducing sugars in the bud end for ‘Russet Burbank’. However, Amrein et al. (2003) reported that N fertilization did not significantly influence sugar content and acrylamide formation for 17 potato cultivars in Switzerland. Muttucumaru et al. (2013) tested 3 N treatments (0, 100 and 200 kg ha−1) applied on 13 cultivars (French fry, chip and boiling cultivars), and reported complex effects on glucose and acrylamide concentrations. In that study, the glucose concentrations of each type (French fry, chip and boiling cultivars) were not consistently increased or decreased with increasing N fertilization. The lowest glucose concentrations were reported with 100 kg N ha−1 for French fry and chip cultivars. All but one of the French fry cultivars and the boiling cultivar had acrylamide concentrations that increased with increasing N rate (0 kg ha−1 compared to 200 kg ha−1). In contrast, chip cultivars had acrylamide concentrations that either decreased, slightly increased, or remained unchanged with increasing N rate. Generally, the results of these previous studies suggest that the N fertilization effect on reducing sugars and acrylamide concentrations is not consistent, and is much likely environment, type- (French fry and chips) and cultivar-dependent.
After harvest, potato tubers from some cultivars can be stored for up to 1 year to assure a year-round supply for fresh market and the processing industry. Low temperature (4 °C) is ideal to prevent tubers from sprouting and weight loss during storage. However, it can greatly increase the activity of invertase that could hydrolyze sucrose to glucose and fructose, and cause reducing sugar accumulation (Sowokinos 1990; Matsuura-Endo et al. 2006). Consequently, a storage temperature of 8 to 9 °C is typically recommended to ensure desirable sugar levels for French fry processing (Knowles et al. 2009), and 10–13 °C for chip processing (Rowe and Curwen 1993) in the potato industry. Storage time has been reported to influence reducing sugar levels, but this effect usually depends on storage temperature and cultivar (Matsuura-Endo et al. 2004). Knowles et al. (2009) reported that reducing sugars in ‘Ranger Russet’ increased during 230 days of storage, while reducing sugars in ‘Umatilla Russet’ and ‘Russet Burbank’ initially leveled off and then decreased following 111 days storage at 4.5 °C. When storage temperature was raised to 9 °C, reducing sugars levels of all three cultivars stopped increasing at 48 days in storage, and then started decreasing. Elmore et al. (2015) stored 20 potato cultivars at 8 °C for 6 months, with reducing sugars and acrylamide concentrations determined after 2 and 6 months of storage. Five cultivars, ‘Lady Rosetta’, ‘Daisy’, ‘Innovator’, ‘Markies’ and ‘Ramos’, showed a significant increase in reducing sugars, while reducing sugars of other cultivars did not change with storage. Acrylamide formation in response to storage time was cultivar dependent as well, with either an increase (‘Innovator’), a decrease (‘Saturna’), or no significant change (all other cultivars). Therefore, the post-harvest storage condition is a major management factor that can affect sugar content and acrylamide-forming potential, but also appears to be cultivar dependent.
Recently released French fry cultivars ‘Alpine Russet’, ‘Dakota Trailblazer’, and a chip cultivar ‘Ivory Crisp’, have a common characteristic of low tuber reducing sugars and low acrylamide-forming potential. The agronomic performance (tuber yield and quality) of these cultivars in response to N rate has been previously reported (Sun et al. 2017). In the present study, the effects of N fertilization on tuber reducing sugars and acrylamide concentrations of French fries and chips were investigated. The specific objectives of this study were to (1) characterize the interactive effects of cultivar, N fertilization and storage on tuber glucose, acrylamide formation and fry color; and (2) determine the relationships between fry color ratings, glucose concentrations and acrylamide concentrations in the standard and new cultivars.
Materials and Methods
This study was conducted at the Sand Plain Research Farm in Becker, Minnesota, on a Hubbard loamy sand (sandy, mixed, frigid Entic Hapludolls) soil in 2011 and 2012. A randomized complete block design was adopted with four replicates using a factorial treatment arrangement of N rate and cultivar. Three French fry cultivars ‘Russet Burbank’, ‘Alpine Russet’, and ‘Dakota Trailblazer’, and two chip cultivars ‘Snowden’ and ‘Ivory Crisp’ were subjected to 5 N fertilizer treatments, 34, 135, 202, 269 and 336 kg ha−1. The recommended N rate for full season potato production in the region is generally between 202 and 269 kg ha−1 N. All plots received 31 kg N ha−1 as monoammonium phosphate and 3 kg N ha−1 as ammonium sulfate applied in a band 8 cm to the side and 5 cm below the seed tuber at planting. The rest of the N fertilizer (0, 101, 168, 235 and 302 kg ha−1) was side-dressed as Environmentally Smart Nitrogen (Agrium Inc., 44–0-0), a controlled release polymer-coated urea fertilizer, and hilled in at emergence. At equivalent N rates, ESN has been shown to perform as well or better than conventional fertigation treatments (Wilson et al. 2009). Soil properties and further cultural practices used in this study can be found in Sun et al. (2017).
Sample Collection and Analysis
Harvest date was scheduled according to weather conditions, and differed each year. Vines were killed using a mechanical vine chopper on 15 September 2011 and 10 September 2012 for chip cultivars, 23 September 2011 and 21 September 2012 for French fry cultivars. All plots were planted on 3 May 2011, and 17 April 2012, and machine harvested on 29 September 2011, and 2 October 2012. After harvest, approximately 23 kg of tubers weighing between 170 and 283 g from each plot were shipped to the USDA-ARS Potato Research Worksite in East Grand Forks, Minnesota, preconditioned at 10 °C for 2 weeks, and stored at 7.2 °C for 9 months. From the 23 kg subsample, five tubers from each plot were randomly sampled for glucose determination at harvest, and after 3, 6 and 9 months of storage. With a 2 cm cork borer, the center core from the longitudinal axis or proceeding from stem to bud end from each tuber was sampled and the peel removed. After the peel was removed, the core was cut lengthwise into strips. To ensure a representative sample, approximately 40 g of core strips were collected from each of five tubers and transferred to a 600 mL beaker with a final weight of 200 g tuber tissue per sample. Samples were juiced using an ACME™ Supreme JUICERator in 50 mM sodium phosphate buffer with a pH of 7.2. Sucrose and glucose concentrations were determined using an YSI 2700 Biochemistry Analyzer (YSI Life Sciences, Yellow Springs, OH).
To process the frying cultivars into French fries, tubers were steam-pealed for 30 s, washed at high pressure, cut on an Urschel cutter (9.5 mm by 9.5 mm), and blanched at 77 °C for 7 to 10 min. After drying at 60 °C until 9 to 11% of their weight was lost, they were par-fried at 185 °C for 90 s, then at 191 °C for 35 to 50 s, and flash-frozen at −26 °C. After 1 week, the frozen fries were fried at 177 °C for 165 to 180 s. Fry color of the finished fried samples was estimated by visual comparison with the Munsell USDA Frozen French Fry Standard (Munsell Color) Color Chart, with a higher number indicating darker color.
For chip cultivars, tubers were steam-pealed for 30 s, followed by pressure washing and slicing (chip thickness 1.5 mm). These slices were rinsed in cold water, and fried at 185 °C for 90 s. Finished chipswere then de-oiled, crushed, and scanned with an Agtron (AGT) Analyzer (Agtron Inc., Reno, NV) to quantify chip color; a reading with a higher number indicating a lighter color.
Acrylamide was extracted using the following procedure. For each plot, three fries or 1.0 to 2.0 g of chips were ground for 30 s in a coffee grinder, and 0.8 to 1.0 g (for fries) or 0.20 to 0.25 g (for chips) of ground sample were placed in 15 ml Falcon ™ Conical Centrifuge tubes with 10 parts distilled and deionized water for fries and 40 parts distilled and deionized water for chips (8 to 10 mL), then vortexed for 30 s. The resulting suspension was placed on the table for an hour and then centrifuged. Three ml of the aqueous fraction was then pipetted away from the fatty and solid fractions into two 1.5 ml Eppendorf ™ Snap-Cap Microcentrifuge Safe-Lock ™ tubes. The centrifugation-isolation step was repeated, after which 0.5 ml of purified aqueous solution from each of two tubes of the same sample was then pipetted into a 1.5 ml Eppendorf ™ Snap-Cap Microcentrifuge Safe-Lock ™ tube, with an addition of 100 pg of heavy acrylamide (Cambridge Isotope Laboratories, INC Andover MA; Acrylamide, 2, 3, 3-D3, 98%). Samples were then subjected to solid phase extraction with a Phenomenex Strata ™-X-C 33 μm Polymeric Strong Cation column.
To determine acrylamide concentrations: 20 μl of extracted samples were subjected to LC-MS/MS (SCIEX) using an Agilent autosampler with an analytical Thermo Hypercarb (100 L × 1.0 mm I.D. Columns, 5 μm particle size) column connected to the Applied Biosystem 4000 ion trap fitted with a turbo V electrospray source. The samples were subjected to a linear gradient of 0 to 100% acetonitrile for 15 min at a column flow rate of 150 μl minute−1. Transitions monitored were m/z 72 greater than m/z 44 and m/z 72 greater than 55 for the light acrylamide, and m/z 75 greater than 44 m/z and m/z 75 greater than m/z 58 for the heavy acrylamide. The data were analyzed using MultiQuant (ABI) providing the peak area ratio for the m/z 58// m/z 55 transition. Standard curves for quantification were constructed using 100 pg heavy acrylamide/ml with light acrylamide ranging from 5 pg–1500 pg ml−1. The amount of acrylamide was determined and expressed as ng acrylamide g−1 (ppb) on a fresh weight basis. All analysis was conducted at the Center for Mass Spectrometry and Proteomics at the University of Minnesota. Because of different frying methods, acrylamide concentrations were generally much higher for tubers prepared as chips, compared to French fries.
Statistical Analysis
Analysis of variance (ANOVA) of fry color, sugars and acrylamide concentrations as functions of N rate, cultivar, storage time, and year was conducted using PROC ANOVA in SAS 9.4 statistical software package (SAS Institute Inc., Cary NC. USA). Repeated measures were used for storage time. A square root transformation was used when necessary to account for the heterogeneity of variance. All data were presented separately for French fry and chip cultivars, due to the high acrylamide concentrations in chip cultivars resulting from different frying methods. Means of fry color, glucose, and acrylamide concentrations in 2011 and 2012 were compared among French fry and chip cultivars using PROC GLM with the LSMEANS statement, or at each N rate using PROC GLM, MEANS statement, and the least significant difference (LSD) at 5% probability level in SAS. Linear or quadratic responses of glucose, acrylamide and fry color to N rate and storage time within each year and/or cultivar were determined using PROC GLM and the CONTRAST statement in SAS. A probability of 0.05 was considered significant and the quadratic function prior to the linear function. AGT scores with glucose or acrylamide concentrations and within each treatment were plotted for ‘Ivory Crisp’ and ‘Snowden’ in 2011 and 2012, respectively, with linear regressions determined for each cultivar and each year in Excel (Microsoft). The regressions between fry color and glucose or acrylamide concentrations within each treatment, cultivar and year were determined using PROC REG, and then plotted in Excel for French fry cultivars. Acrylamide and glucose concentrations within each treatment were plotted for French fry and chip cultivars, respectively, with linear regressions determined for each market type.
Results and Discussion
Interactive Effects on Glucose Concentrations
Tuber glucose concentrations were significantly affected by the interaction of N rate by cultivar by year for French fry cultivars (Table 1, Fig. 1). The effect of N rate was not influenced by storage time. ‘Russet Burbank’ glucose concentrations decreased quadratically with increasing N rate in 2011 and 2012. For ‘Alpine Russet’, glucose concentrations decreased quadratically in 2011, but linearly in 2012, with increasing N rate. The glucose concentrations of ‘Dakota Trailblazer’ were not affected by N supply in 2011, but minimally responded quadratically with increasing N rate in 2012. Compared to the standard cultivar ‘Russet Burbank’, ‘Dakota Trailblazer’ had significantly lower glucose concentrations at all N rates both years. As for ‘Alpine Russet’, glucose concentrations were in the same range as ‘Russet Burbank’ at the low (34 kg ha−1) and high N (≥ 269 kg ha−1) rates in both years. At medium N rates, ‘Alpine Russet’ had glucose concentrations higher than Russet Burbank in 2011, but lower than ‘Russet Burbank’ in 2012, suggesting a significant effect of environmental conditions during the growing season on glucose concentrations for some cultivars.
Glucose concentrations as affected by the three-way interaction of cultivar, N rate and year for ‘Russet Burbank’ (RB), ‘Alpine Russet’ (AR) and ‘Dakota Trailblazer’ (DT). Each point represents the mean of four replications averaged over storage time. Mean values followed by the same letter are not significantly different α = 0.05. Equations: ‘Russet Burbank’, 2011: y = 2.66 × 10−5 × 2−1.34 × 10−2 x + 3.27; 2012: y = −2.58 × 10−5 × 2 + 5.95 × 10−3x + 3.37. ‘Alpine Russet’, 2011: y = −1.82 × 10−5 × 2 + 1.52 × 10−3 x + 2.91; 2012: y = −4.38 × 10−3 x + 3.72. ‘Dakota Trailblazer’, 2012: y = −1.15 × 10−5 × 2 + 5.18 × 10−3 x + 0.4
The N rate by year and N rate by cultivar interactions were significant for glucose concentrations in chip cultivars (Table 1, Figs. 2 and 3). Similar to French fry cultivars, the N rate effect on glucose concentration was not affected by storage time for chip cultivars. Glucose concentrations in ‘Snowden’ were not affected by N supply. In contrast, glucose concentrations in ‘Ivory Crisp’ linearly decreased with increasing N rate (Fig. 2). When averaged over cultivar, glucose concentrations linearly decreased in 2011, but responded quadratically to N rate in 2012, with an increase at low N rates from 34 to 162 kg ha−1, followed by a decrease at rates higher than 162 kg ha−1 (Fig. 3).
Glucose concentrations in 2011 and 2012 as affected by the nitrogen rate by year interaction. Each point represents the mean of four replications averaged over the chip cultivars (‘Ivory Crisp’ and ‘Snowden’) and storage time. Equation: 2011: y = −1.35 × 10−5 × 2 + 4.21 × 10−3 x + 0.65; 2012: y = −1.80 × 10−3 x + 1.08
Glucose response to N rate was cultivar dependent in this study. Decreases in glucose concentrations with increasing N rate were observed for ‘Russet Burbank’, ‘Alpine Russet’ and ‘Ivory Crisp’, but minimally affected by N rate for ‘Snowden’ and ‘Dakota Trailblazer’. In addition, the N rate effect on glucose concentration was not affected by storage time for any of the cultivars (Table 1). Long et al. (2004) reported that increasing N fertilizer from 200 to 400 kg ha−1 had no effect on glucose concentrations for ‘Snowden’ on an irrigated soil in central Michigan, which is consistent with the results in this study. Knowles et al. (2015) reported decreased reducing sugar concentrations in the stem and bud end for ‘Alpine Russet’, with increasing N rate with a delayed harvest at Othello, WA. Increasing N application from 0 to 336 kg ha−1 caused an increase or a decrease in reducing sugars in the bud and stem end for ‘Russet Burbank’, respectively, in Utah (Westermann et al. 1994). However, Zebarth et al. (2004) reported that increased N fertilizer from 0 to 160 kg ha−1 had little effect on reducing sugars concentration for ‘Russet Burbank’ in New Brunswick, Canada. The contrasting results suggest that growing conditions (environmental conditions and growing locations) also play a critical role in glucose concentrations of ‘Russet Burbank’. The effect of environmental conditions was confirmed in this study as well, such as the elevated glucose concentrations for French fry cultivars in 2012, and different glucose response to N rate for ‘Alpine Russet’ in 2011 and 2012. The effect of N rate on glucose concentration has not been previously reported for ‘Dakota Trailblazer’ and ‘Ivory Crisp’.
The three-way interaction of cultivar by storage time by year was significant for glucose concentration of both French fry and chip cultivars (Table 1). In 2011, glucose concentrations in tubers of French fry cultivars tended to increase over the 9-month storage period, linearly for ‘Dakota Trailblazer’ and ‘Alpine Russet’, but quadratically for ‘Russet Burbank’ (Fig. 4). Glucose concentrations increased linearlyfor ‘Ivory Crisp’ during storage (Fig. 5), while glucose in ‘Snowden’ increased quadratically, with dramatically higher concentrations after 9 months of storage (3.67 fold higher than the previous test after 6 months of storage). This increase in tuber glucose concentrations for ‘Snowden’ is due to senescent sweetening, which is irreversible, cultivar dependent, and occurs after an extended period of storage (Wiltshire and Cobb 1996).
Glucose concentrations as affected by the three-way interaction of cultivar, storage time and year for the fry cultivars ‘Russet Burbank’ (RB), ‘Alpine Russet’ (AR) and ‘Dakota Trailblazer’ (DT). Each point represents the mean of four replications averaged over nitrogen rate. Mean values followed by the same letter are not significantly different α = 0.05. Equations: 2011: RB y = −0.015 × 2 + 0.175 x + 1.523, AR y = 0.146 x + 1.685, DT y = 0.056 x + 0.402. 2012: RB y = 0.040 × 2–0.296 x + 3.299, DT y = 0.025 × 2–0.217 x + 1.116
Glucose concentrations as affected by the three-way interaction of cultivar, storage time and year for the chip cultivars ‘Ivory Crisp’ (IC) and ‘Snowden’ (S). Each point represents the mean of four replications averaged over nitrogen rate. Mean values followed by the same letter are not significantly different α = 0.05. Equations: 2011: S y = 0.060 × 2–0.269 x + 0.299, IC y = 0.066 x + 0.187. 2012: S y = 0.049 × 2–0.256 x + 0.577, IC y = 0.023 × 2–0.168 x + 0.638
In 2012, all French fry and chip cultivars had a higher level of glucose at harvest than in 2011 (Figs. 4 and 5). The storage time effect on glucose concentrations differed by cultivar during 2012. Glucose concentrations in ‘Alpine Russet’ tubers were not affected by storage time, while for ‘Russet Burbank’, they were not affected during the first 6 months of storage, but then significantly increased (Fig. 4). At 3 months of storage, glucose concentrations decreased in ‘Dakota Trailblazer’ (Fig. 4), ‘Snowden’ and ‘Ivory Crisp’ (Fig. 5), compared to glucose concentrations at harvest. After 3 months, glucose concentrations of ‘Dakota Trailblazer’, ‘Snowden’ and ‘Ivory Crisp’ increased quadratically during storage, with senescent sweetening observed for ‘Snowden’ at 9 months similar to 2011 for this cultivar.
The higher glucose concentrations at harvest in all cultivars in 2012 compared with 2011 may have been induced by weather conditions during the growing season, mechanical stress at harvest, or chemical maturity (Kumar et al. 2004; Knowles et al. 2015). Because harvesting method, post-harvest handling and shipping were the same in 2011 and 2012, mechanical stress was not likely the reason. Chemical maturity is the state when tubers have the highest dry matter content and lowest sugar concentrations, and usually occurs prior to vine desiccation (Sowokinos and Preston 1988). Tubers harvested at chemical maturity often have lower reducing sugar concentrations during storage (Kumar et al. 2004). When harvested before or post chemical maturity, tubers often accumulate high reducing sugar concentrations (Sowokinos 1971; Knowles et al. 2009; Knowles et al. 2015). Knowles et al. (2009) reported a growing season length of 146 days and 154 days for ‘Alpine Russet’ to reach chemical maturity under low N and high N supply, respectively, in a three-year study. Reducing sugar concentrations continued to increase in over-mature tubers, with higher concentrations at the stem end and in the low N treatment. In this study, tubers were harvested 149 and 168 days after planting in 2011 and 2012, respectively. Because a period of cold weather did not occur in either year, cold-sweetening was likely not the reason for the higher glucose concentrations at harvest in 2012. Over-maturity due to a longer growing season in 2012 was the likely reason for the high sugar concentrations that year. In-season temperature conditions might be another possible cause. Thompson et al. (2008) reported that sugars tend to increase when air temperatures increase above 25 to 30 °C during the growing season. In places like Minnesota, July and August are the times for tuber bulking and dry matter accumulation. The average maximum air temperature and numbers of days with maximum air temperature > 30 °C in July 2011 and 2012, was generally the same (data not shown). However, in August, there were 14 days of maximum air temperature > 30 °C in 2012, and 5 days of maximum air temperature > 30 °C in 2011 (with a monthly average maximum air temperature 27.4 °C in 2011 and 28.7 °C in 2012). The high temperature in August might be another reason for the high sugar levels in 2012. Reconditioning is a phenomenon known in the potato industry whereby starch is resynthesized from free sugars in potato tubers when stored under proper temperature (Elbashir and Saeed 2014). Among the five cultivars, the elevated glucose concentrations were reconditioned during storage to a lower level for ‘Dakota Trailblazer’, ‘Snowden’ and ‘Ivory Crisp’, but not ‘Russet Burbank’ and ‘Alpine Russet’ (Figs. 4 and 5). The results above suggest that growing season should be limited to a length when tubers are neither immature nor over mature to prevent the accumulation of reducing sugars. In addition, proper use of irrigation should be considered to lower soil temperature during the maximum air temperature > 30 °C days and reduce the incidence of dark-end French fries (Kincaid et al. 1993).
Glucose concentration was significant increased in the nine-month storage under the conditions in this study (7.2 °C) for the tested cultivars (Figs. 4 and 5), with exceptions of ‘Alpine Russet’ in 2012, and the first 3 months of storage in 2012 for ‘Dakota Trailblazer’, ‘Snowden’ and ‘Ivory Crisp’. Variable results due to storage temperature and cultivar have also been reported in previous studies (Matsuura-Endo et al. 2006; Knowles et al. 2009; Muttucumaru et al. 2013). Generally, a storage temperature of 8 °C or above is recommended to avoid a significant increase of reducing sugars (Blenkinsop et al. 2002; De Wilde et al. 2005; Vinci et al. 2012). However, this is not always the case. Elmore et al. (2015) stored 20 potato cultivars at 8 °C for 6 months, with reducing sugars and acrylamide concentrations determined after 2 and 6 months of storage. Five cultivars ‘Lady Rosetta’, ‘Daisy’, ‘Innovator’, ‘Markies’ and ‘Ramos’ showed a significant increase in reducing sugars, while reducing sugars of the other cultivars did not change with storage time. Muttucumaru et al. (2014) determined glucose in five chip cultivars and seven French fry cultivars at harvest and after storage of 5–6 months at 9 °C. They found that glucose concentration in ‘Lady Claire’ tubers decreased, while glucose concentrations increased in tubers of all other cultivars.
A few studies have reported on storage effects using the same cultivars as this research. Knowles et al. (2009) reported that reducing sugars in ‘Russet Burbank’ tubers stored at 6.7 °C and 9 °C significantly increased during 17–48 days of storage, and then decreased for the next 182 days, but they never reached the initial glucose concentrations. ‘Russet Burbank’ tubers stored at 9 °C had lower reducing sugars than those stored at 6.7 °C after 111 days of storage. Whitworth et al. (2011) reported that glucose concentrations of ‘Alpine Russet’ generally increased and then leveled off during storage at 7.2 °C. During the 250 days of storage, ‘Alpine Russet’ glucose concentrations remained near or below 0.05% on a fresh weight basis, which was significantly lower than ‘Russet Burbank’. Glucose concentrations of ‘Snowden’ tubers did not change significantly when stored at 6 and 8 °C for 18 weeks, but the glucose concentration was higher when tubers were stored at 6 °C versus 8 °C (Matsuura-Endo et al. 2006). The glucose concentrations of ‘Alpine Russet’ in this study were higher than those concentrations reported by Whitworth et al. (2011). Although the glucose concentrations were higher, the effects of storage on glucose concentrations were similar (Fig. 4). In contrast, storage effects on glucose concentrations of ‘Russet Burbank’ and ‘Snowden’ in our study (Figs. 4 and 5) differed from those reported by Knowles et al. (2009) and Matsuura-Endo et al. (2006). These results indicate that the growing environment has a significant influence on tuber reducing sugar concentrations and changes in concentration that occur during storage.
Interactive Effects on Acrylamide Concentrations
The effect of N rate on acrylamide concentrations was influenced by year for both processing types (Table 1). Acrylamide concentrations for French fry cultivars responded quadratically to N rate (increased up to 212 kg N ha−1 and then decreased) in 2011, but did not respond to N rate in 2012 (Fig. 6). However, for chip cultivars, acrylamide concentrations linearly decreased with increasing N rate in 2011, but responded quadratically to increasing N rate in 2012 (increased up to 212 kg N ha−1 and then decreased). Acrylamide concentrations in 2012 were higher than in 2011 for French fry cultivars at all N rates, and for chip cultivars at N rates greater than 66.4 kg ha−1.
Acrylamide concentrations in 2011 and 2012 as affected by the nitrogen rate by year interaction for French fry and chip cultivars. Each point represents the mean of four replications averaged over storage time and the chip cultivars (‘Ivory Crisp’ and ‘Snowden’) or storage time and the French fry cultivars (‘Russet Burbank’, ‘Alpine Russet’ and ‘Dakota Trailblazer’. Equations: French fry cultivars 2011: y = −5.11 × 10−3 × 2 + 2.16 x + 408.00. Chip cultivars 2011: y = −5.74 x + 4462.80; 2012: y = −4.23 × 10−2 × 2 + 17.79 x + 3087.12
Variable results (increase, decrease or quadratic responses) for N rate effects on acrylamide concentrations were observed for the processing types evaluated in this study. Similar to glucose concentrations, the N rate effect on acrylamide was not affected by storage time (Table 1). For chip cultivars, glucose and acrylamide concentrations responded similarly to increasing N rate both years (Fig. 3 and Fig. 6). However, glucose concentrations in French fry cultivars generally decreased in both years (Fig. 1), while acrylamide concentrations increased and then decreased in 2011, but were not affected by N rate in 2012 (Fig. 6). This inconsistent response has been previously reported by Muttucumaru et al. (2013). They reported increased acrylamide concentrations in six French fry cultivars (including ‘Russet Burbank’), and decreased acrylamide concentrations in cultivar Pentland Dell with increasing N supply from 0 to 200 kg ha−1, while glucose concentrations changed minimally. Amrein et al. (2003) reported that N fertilization did not significantly influence acrylamide formation in 17 potato cultivars. In contrast, De Wilde et al. (2006) reported an increase of acrylamide-forming potential with increased N application in French fries produced from ‘Bintje’, ‘Ramos’ and ‘Saturna’. Gerendás et al. (2007) reported an increase in acrylamide concentrations for the French fry cultivar Agria when grown with adequate N and inadequate K supply. In other studies, acrylamide concentrations either decreased, slightly increased, or remained unchanged with increasing N rate for chip cultivars ‘Hermes’, ‘Lady Clarie’, ‘Lady Rosetta’, ‘Saturna’, and ‘Verdi’ (Muttucumaru et al. 2013). No studies have reported the N rate effect on acrylamide concentrations of the rest of test cultivars used in this study. Based on our results and those in the literature, glucose and acrylamide concentrations are not consistently affected by N supply, but are affected by cultivar regardless of N supply as discussed below. Therefore, to reduce the acrylamide-forming potential, low reducing sugar cultivars should be selected and N should be managed to optimize yield and quality for each cultivar.
Changes in acrylamide concentrations during storage depended on year for all cultivars (Figs. 7 and 8). For French fry cultivars, acrylamide concentrations increased linearly during storage in 2011, but decreased during storage in a linear or quadratic trend in 2012 (Fig. 7). ‘Dakota Trailblazer’ had consistently lower acrylamide concentrations than ‘Russet Burbank’ and ‘Alpine Russet’. The changes in acrylamide concentrations in chip cultivars during storage were different from those for French fry cultivars (Fig. 8). Acrylamide concentrations in chip cultivars at harvest were generally higher than after 3 months of storage both years. For ‘Ivory Crisp’ in 2011, acrylamide concentrations changed quadratically; decreased until 6 months of storage, and then slightly increased after 9 months of storage. However, for ‘Snowden’ in 2011 and 2012, and ‘Ivory Crisp’ in 2012, acrylamide concentrations increased quadratically during the 3 to 9 months of storage period. The large increase in acrylamide for ‘Snowden’ after 9 months of storage corresponds with senescent sweetening (Fig. 5). In general, the changes in acrylamide concentrations for all cultivars tended to be similar to changes in glucose concentrations, except for French fry cultivars in 2012 (Figs. 4 and 5).
Acrylamide concentrations as affected by the three-way interaction of cultivar, storage time and year for the fry cultivars ‘Russet Burbank’ (RB), ‘Alpine Russet’ (AR) and ‘Dakota Trailblazer’ (DT). Each point represents the mean of four replications averaged over nitrogen rate. Mean values followed by the same letter are not significantly different α = 0.05. Equations: 2011: RB y = 35.40 x + 591.44, AR y = 56.83 x + 432.19, DT y = 19.85 x + 137.60. 2012: RB y = −60.39 x + 1308.93, AR y = −44.70 x + 1191.54, DT y = 6.67 × 2–88.58 x + 503.89
Acrylamide concentrations as affected by the three-way interaction of cultivar, storage time and year for the chip cultivars ‘Ivory Crisp’ (IC) and ‘Snowden’ (S). Each point represents the mean of four replications averaged over nitrogen rate. Mean values followed by the same letter are not significantly different α = 0.05. Equations: 2011: S y = 225.60 × 2–1341.60 x + 3082.86, IC y = 33.80 × 2–42.80 x + 3323.72. 2012: S y = 266.00 × 2–1709.77 x + 5403.60, IC y = 120.21 × 2–1251.96 x + 5309.30
The effect of cultivar on glucose and acrylamide concentrations was consistent throughout this study. For example, ‘Dakota Trailblazer’ had lower glucose and acrylamide concentrations than ‘Russet Burbank’ and ‘Alpine Russet’. A similar trend was observed for ‘Ivory Crisp’ and ‘Snowden’. In contrast, the effect of storage time on glucose and acrylamide concentrations depended on both year and cultivar, making it difficult to generalize about storage effects. Comparable results were observed in previous studies as well (Matsuura-Endo et al. 2006; Elmore et al. 2015). Matsuura-Endo et al. (2006) stored five cultivars at 6 °C for 18 weeks, and reported that acrylamide concentrations decreased for ‘Snowden’ and ‘Hokkai’, but increased for ‘Toyoshiro’, ‘Inca-no-mezame’, and ‘Irish Cobbler’. ‘Snowden’ was stored for 9 months in this study, and acrylamide decreased after the first 12 weeks of storage, which is similar to the results reported in the Matsuura-Endo et al. (2006) study. Powers et al. (2013) determined acrylamide concentrations in over 40,000 fresh sliced potato chips from 2002 to 2011, and reported that the lowest acrylamide concentration occurred in July, which corresponds to when new season potatoes were used. Acrylamide concentrations increased from October to January (about 3 to 6 months after harvest), and remained high until the following July. Similar results have also been observed in Japan between 2006 and 2010 (Tsukakoshi et al. 2012). The effect of storage time on acrylamide concentration in the Powers et al. (2013) and Tsukakoshi et al. (2012) studies is similar to our results with ‘Ivory Crisp’ in 2012, and ‘Snowden’ prior to senescence sweetening occurring in both years of this study. Acrylamide concentrations in ‘Russet Burbank’ were not significantly affected during storage at 8 °C in the Elmore et al. (2015) study, while in our study, acrylamide increased in 2011, and decreased in 2012, during storage at 7.2 °C. Differences between the two studies may have been due to differences in storage temperature or growing conditions. Selecting cultivars such as ‘Dakota Trailblazer’ or ‘Ivory Crisp’ that maintain low reducing sugars over long periods of storage is an effective approach to keeping acrylamide concentrations low.
Interactive Effects on Fry Color
The Maillard reaction not only produces acrylamide, but also generates melanoidin pigments that give a darker color to fried potato products (Bethke and Bussan 2013). The N fertilization effect was significant for color of French fries and chips. Lighter color of French fries and chips was observed with increasing N supply. While the effect was minimal (Table 1, Supplementary Figs. 1 and 2), fry color is an important quality parameter for the potato processing industry and therefore these results suggest that an adequate N supply is needed to ensure high quality processed potatoes. Knowles et al. (2015) also reported lighter fry color from tubers grown under high N fertilizer application.
French fry and chip color was significantly affected by the interaction of storage time by cultivar by year (Table 1, supplementary Figs. 3 and 4). Fry color of French fry cultivars generally increased during storage in 2011 and decreased in 2012, which was consistent with acrylamide response to storage time. Darker color chips during storage were observed for ‘Ivory Crisp’ in 2012, and ‘Snowden’ in 2011 and 2012, which generally corresponded with glucose and acrylamide responses to storage time.
Relationships between Glucose, Acrylamide and Fry Color
The relationship between fry color and glucose concentration was not significant individually for French fry cultivars within each year, except for ‘Alpine Russet’ (R2 = 0.44) and ‘Dakota Trailblazer’ (R2 = 0.29) in 2011. However, combined over all three French fry cultivars and both years, an increase in fry color was observed with increasing glucose concentration (R2 = 0.40, Fig. 9).
The relationship between glucose and AGT score was significant for chip cultivars (R2 = 0.89 and 0.81 in 2011 and 2012, respectively, Fig. 10). Chips with AGT color readings >45 are considered acceptable (Santerre et al. 1986). ‘Ivory Crisp’ was bred as a low reducing sugars cultivar and demonstrated lighter color (50 to 60 in 2011, and 43 to 60 in 2012) compared to ‘Snowden’ during the 9-month storage. Produced from tubers with the same amount of glucose, chip color was darker in 2012, than in 2011. The results indicate that while glucose and chip color are correlated, the correlation can differ with growing season, suggesting that glucose is not the only component determining chip color.
The correlations between acrylamide and glucose concentrations for chip and French fry cultivars had R2 values of 0.66 and 0.52, respectively (Fig. 11). This suggests that even though the relationships were significant, there was still variability in acrylamide concentrations for the same glucose concentration. A similar result has been previously reported by Muttucumaru et al. (2014). They measured glucose concentrations in 5 chip cultivars and 7 French fry cultivars at harvest and after 5–6 months of storage at 9 °C, and found ‘Lady Claire’ had a low glucose concentration with a high acrylamide concentration. In contrast, cultivars ‘Hermes’, ‘Verdi’ and ‘Markies’ had a high glucose concentration, but low acrylamide concentration. Similar results were also reported by Elmore et al. (2015).
Relationships between acrylamide and glucose concentrations over cultivars and 2 years (The left Y axis is for chip acrylamide reading, and the right Y axis is for French fry acrylamide reading.). Each point represents the value from an individual plot. Equations: French fry cultivars: y = 251.73 x + 191.15; Chip cultivars: y = 3045.49 x + 1694.20
Significant relationships between acrylamide and reducing sugars, glucose or fructose have been reported before (Amrein et al. 2003; Chuda et al. 2003; De Wilde et al. 2005; Zhu et al. 2010; Parker et al. 2012; Muttucumaru et al. 2014). Tuber asparagine and fructose were not determined in this study. In this study, we assumed that fructose concentration would be equal to glucose concentration since sucrose breaks down to equal molar concentrations of fructose and glucose. Based on results reported by Amrein et al. (2003) and Matsuura-Endo et al. (2006), an improved correlation between acrylamide and glucose might be obtained when asparagine and possibly fructose are considered. For example, a strong correlation of acrylamide-forming potential as a function of (0.5 * glucose + fructose) * asparagine with R2 of 0.91 was reported for 17 cultivars, including both French fry and chip cultivars (Amrein et al. 2003). Matsuura-Endo et al. (2006) reported that R2 value for acrylamide and fructose correlation could be improved to 0.81 when only plotted for samples with high asparagine concentrations (fructose/asparagine molar ratios <2). For those samples with high sugar concentrations, acrylamide was correlated to asparagine rather than fructose, with R2 = 0.68.
The relationship between fry color and acrylamide was significant for all French fry cultivars over the 2 years, except for ‘Russet Burbank’ in 2011 with R2 = 0.01 (supplementary Fig. 5). Darker French fries generally contained higher amounts of acrylamide. The AGT score was highly correlated with acrylamide concentration for ‘Snowden’ and ‘Ivory Crisp’ in 2011, with R2 = 0.88 (supplementary Fig. 6). However, the relationship between AGT score and acrylamide differed by cultivar and sampling time. In 2012, for the same chip color, ‘Snowden’ and ‘Ivory Crisp’ contained higher acrylamide concentrations at harvest than during storage. If harvest data is excluded, AGT score was negatively correlated with acrylamide concentrations for ‘Snowden’ and ‘Ivory Crisp’, with R2 = 0.96 and 0.81, respectively. Therefore, optimizing conditions to improve fry color will also result in lower acrylamide concentrations.
Conclusions
Darker French fries and chips were often produced from tubers containing higher glucose concentrations. Fry color was highly correlated to glucose concentration for chip cultivars and was moderately correlated for French fry cultivars, but the equation used for 1 year could not be used to predict the fry color for another year. The positive relationship between glucose and acrylamide was significant, but with great variability. Including asparagine and possibly fructose data might be helpful for a better prediction of acrylamide formation.
The most consistent finding for glucose and acrylamide formation in this study was the effect of cultivar. Regardless of N rate or storage time, ‘Dakota Trailblazer’ had lower glucose and acrylamide concentrations than ‘Russet Burbank’ and ‘Alpine Russet’. For the chipping cultivars, storage time played an important role. ‘Ivory Crisp’ and ‘Snowden’ have similar glucose and acrylamide concentrations early in storage, but ‘Snowden’ was more susceptible to senescent sweetening resulting in a spike in glucose and acrylamide formation at 9 months of storage.
Nitrogen rate and storage time significantly affected glucose and acrylamide concentrations in the cultivars tested in this study. However, the effects were often influenced by cultivar and growing conditions. For example, increased N supply resulted in a decrease in glucose concentration in ‘Russet Burbank’, ‘Alpine Russet’ and ‘Ivory Crisp’, but did not affect glucose concentrations in ‘Dakota Trailblazer’ and ‘Snowden’. Glucose and acrylamide concentrations were not consistently affected by N supply and were cultivar dependent. Acrylamide responses to N supply generally corresponded with responses of glucose to N rate for chip cultivars (lower acrylamide as N rate increased), but not for French fry cultivars.
The effect of time in storage on glucose and acrylamide concentrations appeared to be influenced by growing conditions. In a year with less environmental stress (2011), both glucose and acrylamide concentrations increased with storage period for all cultivars tested at 7.2 °C. However, when heat stress occurred more frequently during the bulking and maturation period (2012), tubers contained higher glucose at harvest and had higher acrylamide concentrations compared with those grown in a season with minimal heat stress. In a year with greater frequency of heat stress, glucose response to storage time differed by cultivar. The difference between years may have also been due to differences in physiological maturity associated with a later harvest date and longer growing season in 2012. Storage time effects on acrylamide and glucose concentrations were similar for chip cultivars, but not for French fry cultivars.
Because of complex interactions, we conclude that while N supply and storage time can affect acrylamide concentration in fried potato products, the direction of response will depend upon cultivar and growing conditions, often precluding the ability to generally predict their effects on acrylamide formation. To minimize acrylamide in fried potato products, cultivars should be selected with low reducing sugars and resistance to cold sweetening.
References
Amrein, T.M., S. Bachmann, A. Noti, M. Biedermann, M.F. Barbosa, S. Biedermann-Brem, K. Grob, A. Keiser, P. Realini, F. Escher, and R. Amadó. 2003. Potential of acrylamide formation, sugars, and free asparagine in potatoes: A comparison of cultivars and farming systems. Journal of Agriculture and Food Chemistry 51: 5556–5560.
Amrein, T.M., B. Schönbächler, F. Rohner, H. Lukac, H. Schneider, A. Keiser, F. Escher, and R. Amadò. 2004. Potential for acrylamide formation in potatoes: Data from the 2003 harvest. European Food Research and Technology 219: 572–578.
Bethke, P.C., and A.J. Bussan. 2013. Acrylamide in processed potato products. American Journal of Potato Research 90: 403–424.
Blenkinsop, R.W., L.J. Copp, R.Y. Yada, and A.G. Marangoni. 2002. Changes in compositional parameters of tubers of potato (Solanum tuberosum) during low-temperature storage and their relationship to chip processing quality. Journal of Agricultural and Food Chemistry 50: 4545–4553.
Chuda, Y.H., H. Yada Ono, A. Ohara-Takada, C. Matsuura-Endo, and M. Mori. 2003. Effects of physiological changes in potato tubers (Solanum tuberosum L.) after low temperature storage on the level of acrylamide formed in potato chips. Bioscience, Biotechnology and Biochemistry 67: 1188–1190.
Cunningham, C.E., and F.J. Stevenson. 1963. Inheritance of factors affecting potato chip color and their association with specific gravity. American Potato Journal 40: 253–265.
De Wilde, T.B., Meulenaer De, F. Mestdagh, Y. Govaert, S. Vandeburie, W. Ooghe, S. Fraselle, K. Demeulemeester, C. Van Peteghem, A. Calus, and J.M. Degroodt. 2005. Influence of storage practices on acrylamide formation during potato frying. Journal of Agricultural and Food Chemistry 53: 6550–6557.
De Wilde, T.B., Meulenaer De, F. Mestdagh, Y. Govaert, S. Vandeburie, W. Ooghe, S. Fraselle, K. Demeulemeester, C. Van Peteghem, A. Calus, and J.M. Degroodt. 2006. Influence of fertilization on acrylamide formation during frying of potatoes harvested in 2003. Journal of Agricultural and Food Chemistry 54: 404–408.
Elbashir, H.A. and I. K. Saeed. 2014. Reconditioning of cold stored potato varieties (Solanum tuberosum L.) Kondor and Markies. Journal of Agri-Food and Applied Sciences 2: 230.
Elmore, J.S., A. Briddon, A.T. Dodson, N. Muttucumaru, N.G. Halford, and D.S. Mottram. 2015. Acrylamide in potato crisps prepared from 20 UK-grown varieties: Effects of variety and tuber storage time. Food Chemistry 182: 1–8.
European Food Safety Authority. 2011. EFSA publishes report on monitoring and exposure assessment of acrylamide. http://www.efsa.europa.eu/en/press/news/datex110420. Accessed on 20 December 2016.
European Food Safety Authority. 2015. Acrylamide in food is a public health concern. http://www.efsa.europa.eu/en/press/news/150604. Accessed on 20 December 2016.
Friedman, M. 2003. Chemistry, biochemistry and safety of acrylamide. Journal of Agricultural and Food Chemistry 51: 4504–4526.
Gerendás, J., F. Heuser, and B. Sattelmacher. 2007. Influence of nitrogen and potassium supply on contents of acrylamide precursors in potato tubers and on acrylamide accumulation in French fries. Journal of Plant Nutrition 30: 1499–1516.
Halford, N.G., T.Y. Curtis, N. Muttucumaru, J. Postles, J.S. Elmore, and D.S. Mottrom. 2012. The acrylamide problem: A plant and agronomic science issue. Journal of Experimental Botany 63: 2841–2851.
Hogervorst, J.G., L.J. Schouten, E.J. Konings, R.A. Goldbohm, and P.A. van den Brandt. 2007. A prospective study of dietary acrylamide intake and the risk of endometrial, ovarian, and breast cancer. Cancer Epidemiology Biomarkers & Prevention 16: 2304–2313.
Johnson, K.A., S.J. Gorzinski, K.M. Bodner, R.A. Campbell, C.H. Wolf, M.A. Friedman, and R.W. Mast. 1986. Chronic toxicity and oncogenicity study on acrylamide incorporated in the drinking water of Fischer 344 rats. Toxicology and Applied Pharmacology 85: 154–168.
Kincaid, D.C., D.T. Westermann, and T.J. Trout. 1993. Irrigation and soil temperature effects on russet Burbank quality. American Potato Journal 70: 711–723.
Knowles, N.R., E.P. Driskill, and L.O. Knowles. 2009. Sweetening responses of potato tubers of different maturity to conventional and non-conventional storage temperature regimes. Postharvest Biology and Technology 52: 49–61.
Knowles, N.R., M.J. Pavek and L.O. Knowles. 2015. Developmental profiles, nitrogen use and postharvest quality of Alpine and Sage Russet potatoes in the Columbia Basin. Annual Washington and Oregon Potato Conference, Jan. 27–30, Kennwick, WA. 37–50. http://www.nwpotatoresearch.com/IPMStuff/PDFs/Proceedings2015.pdf. Accessed 12 December 2016.
Kumar, D., B.P. Singh, and P. Kumar. 2004. An overview of the factors affecting sugar content of potatoes. Annals of Applied Biology 145: 247–256.
Lea, P.J., L. Sodek, M.A. Parry, P.R. Shewry, and N.G. Halford. 2007. Asparagine in plants. Annals of Applied Biology 150: 1–26.
Long, C.M., S.S. Snapp, D.S. Douches, and R.W. Chase. 2004. Tuber yield, storability, and quality of Michigan cultivars in response to nitrogen management and seed piece spacing. American Journal of Potato Research 81: 347–357.
Matsuura-Endo, C., A. Kobayashi, T. Noda, S. Takigawa, H. Yamauchi, and M. Mori. 2004. Changes in sugar content and activity of vacuolar acid invertase during low-temperature storage of potato tubers from six Japanese cultivars. Journal of Plant Research 117: 131–137.
Matsuura-Endo, C., A. Ohara-Takada, Y. Chuda, H. Ono, H. Yada, M. Yoshida, A. Kobayashi, S. Tsuda, S. Takigawa, T. Noda, and H. Yamauchi. 2006. Effects of storage temperature on the contents of sugars and free amino acids in tubers from different potato cultivars and acrylamide in chips. Bioscience, Biotechnology and Biochemistry 70: 1173–1180.
Michalak, J., E. Gujska, and J. Klepacka. 2011. The effect of domestic preparation of some potato products on acrylamide content. Plant Foods for Human Nutrition 66: 307–312.
Mucci, L.A., and K.M. Wilson. 2008. Acrylamide intake through diet and human cancer risk. Journal of Agricultural and Food Chemistry 56: 6013–6019.
Mucci, L.A., P.W. Dickman, G. Steineck, H.O. Adami, and K. Augustsson. 2003. Dietary acrylamide and cancer of the large bowel, kidney, and bladder: Absence of an association in a population-based study in Sweden. British Journal of Cancer 88: 84–89.
Mucci, L.A., P. Lindblad, G. Steineck, and H.O. Adami. 2004. Dietary acrylamide and risk of renal cell cancer. International Journal of Cancer 109: 774–776.
Mucci, L.A., H.O. Adami, and A. Wolk. 2006. Prospective study of dietary acrylamide and risk of colorectal cancer among women. International Journal of Cancer 118: 169–173.
Muttucumaru, N., S.J. Powers, J.S. Elmore, D.S. Mottram, and N.G. Halford. 2013. Effects of nitrogen and sulfur fertilization on free amino acids, sugars, and acrylamide-forming potential in potato. Journal of Agricultural and Food Chemistry 61: 6734–6742.
Muttucumaru, N., S.J. Powers, J.S. Elmore, A. Briddon, D.S. Mottram, and N.G. Halford. 2014. Evidence for the complex relationship between free amino acid and sugar concentrations and acrylamide-forming potential in potato. Annals of Applied Biology 164: 286–300.
Ohara-Takada, A., C. Matsuura-Endo, Y. Chuda, H. Ono, H. Yada, M. Yoshida, A. Kobayashi, S. Tsuda, S. Takigawa, T. Noda, and H. Yamauchi. 2005. Change in content of sugars and free amino acids in potato tubers under short-term storage at low temperature and the effect on acrylamide level after frying. Bioscience, Biotechnology, and Biochemistry 69: 1232–1238.
Parker, J.K., D.P. Balagiannis, J. Higley, G. Smith, B.L. Wedzicha, and D.S. Mottram. 2012. Kinetic model for the formation of acrylamide during the finish-frying of commercial French fries. Journal of Agricultural and Food Chemistry 60: 9321–9331.
Pelucchi, C., C. Galeone, F. Levi, E. Negri, S. Franceschi, R. Talamini, C. Bosetti, A. Giacosa, and C. La Vecchia. 2006. Dietary acrylamide and human cancer. International Journal of Cancer 118: 467–471.
Powers, S.J., D.S. Mottram, A. Curtis, and N.G. Halford. 2013. Acrylamide concentrations in potato crisps in Europe from 2002 to 2011. Food Additives & Contaminants: Part A 30: 1493–1500.
Rowe, R.C. and D. Curwen. 1993. Potato health management. APS Press.
Santerre, C.R., J.N. Cash, and R.W. Chase. 1986. Influence of cultivar, harvest-date and soil nitrogen on sucrose, specific gravity and storage stability of potatoes grown in Michigan. American Potato Journal 63: 99–110.
Sowokinos, J.R. 1971. Relationship of sucrose synthetase cleavage activity to the chemical and physical maturity of Norchip and Kennebec potatoes. American Potato Journal 48: 37–46.
Sowokinos, J. 1990. Effect of stress and senescence on carbon partitioning in stored potatoes. American Journal of Potato Research 67: 849–857.
Sowokinos, J.R. and D.A. Preston. 1988. Maintenance of potato processing quality by chemical maturity monitoring (CMM). Minnesota Agricultural Experiment Station. Retrieved from the University of Minnesota Digital Conservancy. http://hdl.handle.net/11299/123027. Accessed on 21 December 2016.
Sun, N., C.J. Rosen, and A.L. Thompson. 2017. Nitrogen response of French fry and chip cultivars selected for low reducing sugars. American Journal of Potato Research 94: 606–616. https://doi.org/10.1007/s12230-017-9599-8.
Thompson, A.L., S.L. Love, J.R. Sowokinos, M.K. Thornton, and C.C. Shock. 2008. Review of the sugar end disorder in potato (Solanum tuberosum, L.). American Journal of Potato Research 85: 375–386.
Tsukakoshi, Y., H. Ono, N. Kibune, S. Isagawa, K. Yamazaki, M. Watai and M., Yoshida. 2012. Monitoring of acrylamide concentrations in potato chips in Japan between 2006 and 2010. Food Additives & Contaminants: Part A, 29: 1212–1218.
Vinci, R.M., F. Mestdagh, and B. De Meulenaer. 2012. Acrylamide formation in fried potato products–present and future, a critical review on mitigation strategies. Food Chemistry 133: 1138–1154.
Weber, E.A., S. Graeff, W.D. Koller, W. Hermann, N. Merkt, and W. Claupein. 2008. Impact of nitrogen amount and timing on the potential of acrylamide formation in winter wheat (Triticum aestivum L.). Field Crops Research 106: 44–52.
Westermann, D.T., D.W. James, T.A. Tindall, and R.L. Hurst. 1994. Nitrogen and potassium fertilization of potatoes: Sugars and starch. American Potato Journal 71: 433–453.
Whitworth, J.L., R.G. Novy, J.C. Stark, J.J. Pavek, D.L. Corsini, S.L. Love, N. Olsen, S.K. Gupta, T. Brandt, M.I. Vales, and A.R. Mosley. 2011. Alpine russet: A potato cultivar having long tuber dormancy making it suitable for processing from long-term storage. American Journal of Potato Research 88: 256–268.
Wilson, M.L., C.J. Rosen, and J.F. Moncrief. 2009. Potato response to a polymer-coated urea on an irrigated, coarse-textured soil. Agronomy Journal 101: 897–905.
Wiltshire, J.J.J., and A.H. Cobb. 1996. A review of the physiology of potato tuber dormancy. Annals of Applied Biology 129: 553–569.
Zebarth, B.J., Y. Leclerc, G. Moreau, and E. Botha. 2004. Rate and timing of nitrogen fertilization of russet Burbank potato: Yield and processing quality. Canadian Journal of Plant Science 84: 855–863.
Zhu, F., Y.Z. Cai, J. Ke, and H. Corke. 2010. Compositions of phenolic compounds, amino acids and reducing sugars in commercial potato varieties and their effects on acrylamide formation. Journal of the Science of Food and Agriculture 90: 2254–2262.
Acknowledgements
We thank the Minnesota Department of Agriculture and the North Dakota Department of Agriculture for supporting this research through grants from the USDA/NIFA Specialty Crops Block program, Martin Glynn for assistance with sugar analysis, and Bruce Witthuhn for assistance with acrylamide analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, N., Rosen, C.J. & Thompson, A.L. Acrylamide Formation in Processed Potatoes as Affected by Cultivar, Nitrogen Fertilization and Storage Time. Am. J. Potato Res. 95, 473–486 (2018). https://doi.org/10.1007/s12230-018-9647-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12230-018-9647-z