Abstract
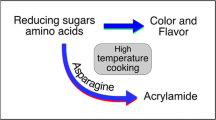
Trace amounts of acrylamide are present in many foods cooked at high temperatures. Acrylamide in processed potato products is formed from reducing sugars and asparagine and is a product of the Maillard reaction; this reaction typically occurs during frying and baking of food products. Processed potato products, including fries and chips, are relatively high in acrylamide compared with other foods and contribute substantially to dietary acrylamide. Acrylamide content in potato products is strongly affected by processing conditions, potato variety, field management, environmental conditions during tuber growth, and tuber storage conditions. Numerous approaches have been described that could potentially reduce the acrylamide content of potato products, but many influence finished product sensory attributes and may be difficult to implement. Health concerns related to acrylamide in food center on its role as a potential carcinogen. Research using feeding studies with rodent models and epidemiological studies with humans are ongoing and are likely to provide future guidance for acceptable amounts of acrylamide in food.
Resumen
Se presentan ligeras cantidades de acrilamida en muchos alimentos cocinados a altas temperaturas. La acrilamida en los productos procesados de papa se forma a partir de los azúcares reductores y asparagina y es un producto de la reacción de Maillard; esta reacción se presenta típicamente durante el freído y cocinado de productos alimenticios. Los productos procesados de la papa, incluyendo hojuelas y a la francesa, son relativamente altos en acrilamida comparados con otros alimentos y contribuyen substancialmente a la acrilamida de la dieta. Su contenido en los productos de papa esta fuertemente afectado por las condiciones del procesamiento, la variedad de papa, el manejo en el campo, condiciones ambientales durante el crecimiento del tubérculo y las condiciones de almacenamiento. Se han descrito numerosas estrategias que podrían potencialmente reducir el contenido de acrilamida de los productos de papa, pero muchas influencian los atributos sensoriales del producto final y pudieran ser difíciles de implementar. Las preocupaciones sobre la salud relacionadas con acrilamida en los alimentos se centran en su papel como un potencial carcinogénico. Esta en proceso la investigación usando estudios de alimentación con modelos de roedores y con estudios epidemiológicos con humanos y es probable que suministren guía a futuro para cantidades aceptables de acrilamida en alimentos.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The potato industry learned in 2002 that french fries, potato chips, hash browns, and other potato products cooked at high temperatures contain trace amounts of acrylamide. Since acrylamide had been identified previously as a neurotoxin and likely carcinogen, this finding raised health concerns that prompted researchers worldwide to investigate the formation, prevalence and safety of acrylamide in food. This review begins with a brief history of acrylamide and its discovery in food. This is followed by a description of the chemical reactions that produce acrylamide during cooking, and how the final amount of acrylamide formed is influenced by the chemical composition of raw potato tubers and by processing methods. Relationships between final acrylamide content and raw product composition or finished product color are discussed. Options for reducing the acrylamide content of processed potato products are discussed next, with an emphasis on the use of potato varieties with low acrylamide-forming potential, careful management of the crop, and appropriate processing and quality control methods. The final section summarizes key findings on dietary exposure to acrylamide and the risk that acrylamide in food poses to human health.
The History of Acrylamide and the Discovery of Acrylamide in Food
Acrylamide (prop-2-enamide) is a small organic compound with high water solubility and moderate reactivity with other organic molecules through binding at either the nitrogen or oxygen atom (Fig. 1). Acrylamide has been used in industry and research since the 1950s, usually in the form of highly hydrated gels produced by linking single acrylamide monomers into linear or branched polymers (Dearfield et al. 1988; IARC 1999). Human health hazards associated with the handling and breathing of acrylamide monomers were first identified in the 1960s (Auld and Bedwell 1967; Garland and Patterson 1967). Chronic exposure to high amounts of acrylamide monomer was shown to result in neurotoxicity that left individuals with muscle weakness, sensory loss and absent reflexes (Garland and Patterson 1967). The possibility that acrylamide might be a carcinogen was raised in the 1970s and 1980s when studies showed that exposure could cause chromosome aberrations in bone marrow and germ line cells of mice (Shiraishi 1978) and tumors in rats exposed to acrylamide through contact or ingestion (Bull et al. 1984b, reviewed in Dearfield et al. 1988, and Rice 2005). Acrylamide was also shown to undergo chemical modification in mammalian tissues to form glycidamide, a mutagen and carcinogen more potent than acrylamide itself (Bergmark et al. 1993; Calleman et al. 1990; Pelucchi et al. 2011; Rice 2005; Tareke et al. 2000).
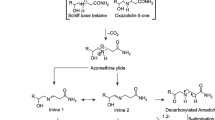
Proposed chemical reactions leading to the formation of acrylamide during cooking. A general series of reactions that begins with reducing sugars and any α-amino acid is found at the top of the figure (a). A reaction sequence beginning with reducing sugars and asparagine as the amino acid is illustrated on the bottom of the figure (b). Figure reproduced with permission from (Parker et al. 2012). American Chemical Society
The possibility that humans might be exposed widely to acrylamide was raised in the 1990s. At that time, N-(2-carbamoylethyl)valine, an adduct of the red blood cell protein hemoglobin and acrylamide, was being use used as a biomarker to monitor occupational exposure to acrylamide (Bergmark et al. 1993). Unexpectedly, the acrylamide-derived adduct was found in individuals with no known exposure to acrylamide in the workplace (Bergmark 1997). This indicated that additional sources of acrylamide existed in the environment. Early suggestions that acrylamide might be found in cooked foods (Tareke et al. 2000) were confirmed in 2002, when it was discovered that many foods, especially those rich in carbohydrates and cooked at high temperatures, had low but detectable amounts of acrylamide (Tareke et al. 2002). Potato products, including french fries, potato chips, and others processed at high temperature were found to contain high concentrations of acrylamide relative to other foods (Stadler and Scholz 2004; Tareke et al. 2002). Other products with relatively high amounts of acrylamide included crackers, cookies, bread and coffee (DiNovi 2006; Stadler and Scholz 2004). Concerns were raised worldwide because of the unknown potential for health problems to develop following long-term dietary exposure to low amounts of acrylamide, and because of the data linking acrylamide and glycidamide to cancer. At the time when it was discovered in food, acrylamide was classified as “probably carcinogenic to humans” by the International Agency for Research on Cancer (IARC 1999; Pelucchi et al. 2011) and a Category 2 carcinogen by the European Union.
Responses to the discovery of acrylamide in food were rapid and occurred on several fronts. Food chemists investigated the mechanism of acrylamide formation during cooking. Food companies and food scientists began identifying changes in processing methods that could reduce acrylamide content in finished products. Regulatory agencies collected data on acrylamide exposure in order to determine if regulations specifically governing acrylamide content in foods were needed. Epidemiologists and laboratory researchers began studies designed to determine if consumption of low amounts of acrylamide, such as those found in food, is a hazard to human health. The activities of these interconnected groups, what they have found, and what remains to be learned are the topic of this review. Additional reviews (Biedermann et al. 2010; Friedman and Levin 2008; Hogervorst et al. 2010; Lineback et al. 2012; Morales et al. 2008; Mucci and Adami 2009; Parzefall 2008; Pelucchi et al. 2011; Seal et al. 2008; Vinci et al. 2011, 2012; Zhang and Zhang 2007) are recommended for those seeking additional detail or alternative perspectives.
Acrylamide Formation in Processed Potato Products
Acrylamide is a Byproduct of the Maillard Reaction
Acrylamide is not present in raw potato tubers and is not present in boiled or steamed potatoes (FDA 2006a; Tareke et al. 2002). Acrylamide is formed at high temperatures such as those used for frying, baking, roasting and in some cases microwave cooking (Michalak et al. 2011). There is general agreement that acrylamide formation begins as a by-product of the Maillard reaction (Mottram et al. 2002; Stadler et al. 2002). The Maillard reaction is better known in the potato industry for the melanoidin pigments it produces that give rise to unacceptably dark-colored chips and fries (Denny and Thornton 1941; Habib and Brown 1956; Kumar et al. 2004).
Acrylamide is Formed from Asparagine and Reducing Sugars
The acrylamide found in potato products is formed almost exclusively from asparagine, glucose and fructose (Knol et al. 2010; Rydberg et al. 2003; Weisshaar 2004; Weisshaar and Gutsche 2002; Zhang and Zhang 2007), although several other compounds have the ability to react with reducing sugars to produce acrylamide (Zhang and Zhang 2007). The chemical steps leading to acrylamide formation are not fully understood, but mechanisms of synthesis have been proposed (Knol et al. 2010; Zhang and Zhang 2007). Parker et al. proposed a framework for acrylamide formation that contains two pathways of acrylamide synthesis (Parker et al. 2012). In the generic amino acid pathway (Fig. 1a), a reducing sugar reacts with any amino acid to generate a Schiff base. This rearranges to form an Amadori compound and these compounds dehydrate and fragment, releasing the amino acid and producing a suite of highly reactive intermediates including deoxyosuloses, dicarbonyls and hydroxycarbonlys. These intermediates react with amino acids to produce the Maillard-reaction compounds that add flavor and color to fried or cooked potato products. However, when the intermediates react with asparagine they initiate a series of reactions that produce acrylamide. An implicit feature of the generic amino acid pathway is that acrylamide formation increases relative to color formation as the molar ratio of asparagine to total amino acids increases (Parker et al. 2012). Alternatively, acrylamide can be formed directly from reducing sugars and asparagine in a reaction sequence that also begins with the formation of a Schiff base (Fig. 1b). Acrylamide is likely to be formed from both pathways in complex systems such as food (Parker et al. 2012).
Asparagine is usually the most abundant free amino acid in potato tubers (Gerendas et al. 2007; Williams 2005), and the high concentration of free asparagine in potatoes relative to other starchy foods (Elmore et al. 2005) is one of the reasons that processed potato products are relatively high in acrylamide (FDA 2006a; Stadler and Scholz 2004). Differences in asparagine content between cultivars have been measured, but for most varieties the range of asparagine contents is fairly narrow. Asparagine content is typically between 4 and 25 mg g−1 dry weight (Amrein et al. 2003; de Meulenaer et al. 2008; Shepherd et al. 2010; Whittaker et al. 2010; Williams 2005; Zhu et al. 2010). In this regard, asparagine is unlike the reducing sugars glucose and fructose that routinely range more widely, from less than 0.04 to 4.8 mg g−1 dry weight in tubers grown and stored commercially for processing (Stark and Love 2003). Tuber contents of asparagine are often far in excess of those for reducing sugars, and when this is the case asparagine is unlikely to be limiting for acrylamide formation (Amrein et al. 2003; de Wilde et al. 2005; Gokmen and Palazoğlu 2008; Knutsen et al. 2009). For example, in a comparison between fry processing varieties and chip processing varieties, a weak correlation was found between tuber asparagine content and acrylamide-forming potential in the fry processing varieties (r = 0.3), but not for the chipping varieties (Halford et al. 2012). In part, this might be due to larger reducing sugar content in the processing potatoes, which averaged 2.4 times that in the chip processing varieties. These data support the idea that asparagine content is a stronger determinant of acrylamide formation as reducing sugar content increases. Lines with low tuber asparagine contents have been created using molecular techniques. These lines produced products with less acrylamide than lines with higher asparagine across a narrow range of reducing sugar concentrations (Rommens et al. 2008).
There is a strong, direct relationship between the reducing sugar content in raw tubers and the amount of acrylamide produced during cooking. The data presented in Fig. 2 for french fries (de Wilde et al. 2005) are representative of several early studies that illustrate this point (Amrein et al. 2003; Chuda et al. 2003; de Wilde et al. 2005; Haase et al. 2003; Ohara-Takada et al. 2005). Potatoes with reducing sugar concentrations ranging from 0 to 2.6 % of dry matter (0 to 26 mg g−1 dry weight) had acrylamide content in finished fries that ranged from 0 to over 2,500 μg kg−1 (Fig. 2). The correlation between acrylamide formed and tuber reducing sugar content was quite high (R2 = 0.84), although tubers used for fry processing rarely have reducing sugar contents above 10 to 15 mg g−1 dry weight. For any given amount of tuber reducing sugar, however, a wide range of acrylamide values was observed (Fig. 2). Thus, reducing sugar content alone gives a very imprecise indication of finished product acrylamide content (de Wilde et al. 2005; Haase et al. 2004). Tuber fructose or glucose, total reducing sugars, and asparagine in the ranges commonly measured in commercial potatoes do not predict acrylamide precisely enough for potential management of processed products (Amrein et al. 2003; Burch et al. 2008; Knutsen et al. 2009; Vinci et al. 2010; Zhu et al. 2010).
Relationship between tuber reducing sugar content and acrylamide content in french fries. Note that acrylamide content increased linearly with increasing reducing sugar content. Figure reproduced with permission from (de Wilde et al. 2005) American Chemical Society
Antioxidants and Acrylamide Formation
It is likely that tuber constituents other than reducing sugars and asparagine participate in an additive or subtractive fashion to determine final acrylamide content. Antioxidants are one group of compounds that may play a role in modulating the amount of acrylamide produced (Claus et al. 2008; Friedman and Levin 2008; Hedegaard et al. 2008; Morales et al. 2008). A strong correlation was found between total antioxidant capacity and acrylamide content of potato chips (Serpen and Gokmen 2009). In experiments comparing acrylamide formed in heated, freeze-dried potato powder from several varieties of potato tubers (Zhu et al. 2010), high amounts of phenolic compounds (hydroxycinnamoylquinic/hydroxycinnamoyl derivatives) were related to lower levels of acrylamide. Other data indicate that antioxidants may have no effect on acrylamide formation, or increase its production. For example, none of six phenolic antioxidants including ferulic acid, catechin or epicatechin reduced the amount of acrylamide formed in a model system with equal concentrations of glucose and asparagine (Bassama et al. 2010). In a similar model system, proanthocyanadin-enriched fruit extracts inhibited acrylamide formation (Cheng et al. 2010). These conflicting observations may be attributed to differences in the chemical properties of specific antioxidants. Antioxidants in potato tubers fall into several distinct structural categories, including carotenoids, phenolics including chlorogenic acid, flavonoids such as catechin and epicatechin, and anthocyanins (Brown 2005). An important area of research is to characterize specific antioxidants in terms of their potential to inhibit or promote acrylamide formation. Potato cultivars high in phenolics and other antioxidants are being developed by several US potato-breeding programs (Haynes et al. 2010, 2011; Reddivari et al. 2007), and these efforts may have a secondary effect on the acrylamide content of processed products.
Processing Conditions Impact Acrylamide Content of Finished Products
All potato tubers contain reducing sugars and asparagine, but acrylamide formation from these requires specific conditions. Among these are temperatures above ~120 °C and low tissue water content. These conditions are perfectly met during the later stages of chip and fry production.
The influence of temperature on acrylamide formation is illustrated in Fig. 3 (Pedreschi et al. 2004). In this case, fried chips were made from replicate tuber samples from the cultivar Tivoli that were cooked at three oil temperatures. Slices were either fried immediately or blanched in distilled water for 40 or 90 min to reduce the amount of acrylamide precursors prior to frying. In all cases, chips were cooked to final water content of 1.7 %. Final acrylamide content in chips that were not blanched and cooked at 190 °C (375 °F) was almost three times that in chips cooked at 170 °C (338 °C). This finding, that higher temperatures lead to disproportionately higher amounts of acrylamide in finished products, has been demonstrated many times using model systems and fresh potato slices and fries (Amrein et al. 2006; Ehling and Shibamoto 2005; Franke et al. 2009; Granda and Moreira 2005; Granda et al. 2004; Pedreschi et al. 2007b; Rydberg et al. 2003; Williams 2005; Zhang and Zhang 2007).
Acrylamide content of potato chips increased as cooking temperature increased, and decreased with longer times of soaking in water prior to frying. Reprinted with permission from Pedreschi et al. 2004
Water content during cooking has a pronounced influence on acrylamide formation (Amrein et al. 2006; Elmore et al. 2005). As water content drops below ~10 % moisture, the rate of acrylamide production increases and acrylamide accumulation reaches a maximum at <5 % water (Amrein et al. 2006; Knol et al. 2008). The data presented in Fig. 4a illustrate how the final acrylamide content in potato chips varies with cooking time and water content (Knol et al. 2008). In these data, little acrylamide was formed during the first 2.0 min of cooking, when chip temperature increased (Fig. 4b) and water content was still high (Fig. 4a). Rapid acrylamide formation occurred during the next minute. For the data plotted in Fig. 4, two thirds of the acrylamide formed was produced between 2 and 3 min of cooking. Additional cooking at high temperatures leads to a net loss of acrylamide through thermal breakdown (Knol et al. 2009). Over-cooking markedly degrades the taste and texture of the finished product and this makes thermal breakdown an unsuitable method for acrylamide reduction. The degradation of acrylamide that occurs during over-cooking is apparent in Fig. 4a at cooking times greater than 5 min.
Kinetics of acrylamide formation (filled diamonds) and water content (filled circles) in relation to frying time (a) and average temperature changes in the cooking oil and outer cell layer of the chip during the cooking process (b). Figures reproduced from (Knol et al. 2008) with permission
Acrylamide Quantification
Acrylamide Assays are Costly and Technically Demanding
After the discovery of acrylamide in food, many disparate groups concluded that they needed to quantify the amount of acrylamide in potato products. Regulatory agencies began multiyear monitoring efforts to assess consumer exposure to acrylamide and trends in acrylamide content of processed potato products (Becalski et al. 2010; Biedermann et al. 2010; EFSA 2011; FDA 2006a, b; WHO 2011). Processing companies needed to assess acrylamide content as part of emerging quality control strategies. Potato breeding programs needed to assess the acrylamide-forming potential of advance lines, with an eye toward releasing improved germplasm. Unfortunately, accurate quantification of acrylamide in potato products requires technical expertise and sophisticated laboratory equipment. As a result, acrylamide analysis is expensive. The cost per sample can be up to $100 to 150 in a commercial laboratory. Furthermore, the strong influence of processing technique on acrylamide content of finished potato products creates methodological challenges. Variation in starting material, preparation techniques, sample dimensions, cooking temperature and duration, moisture content of finished product, and numerous other steps can contribute to variability in the data and lead to misinterpretation of results. As a consequence, sophisticated, highly controlled approaches for sample preparation must be utilized to generate accurate experimental results. The high cost of analysis and sample preparation means that few organizations can afford to routinely process a large number of samples. This is especially true for potato breeding programs, where direct analysis of acrylamide may be affordable for the most advance clones, but not for early generation material. These limitations have encouraged several researchers to develop models for acrylamide content in finished product based on finished product color or composition of raw product.
Estimating Acrylamide Content Based on Finished Product Color or Raw Product Composition
Acrylamide formation and fried product color both depend primarily on reducing sugar content of raw products. Several groups have investigated the feasibility of using finished product color as an indirect measure of acrylamide content (Burch et al. 2008; Gokmen and Senyuva 2006; Granda and Moreira 2005; Knutsen et al. 2009; Ohara-Takada et al. 2005; Pedreschi et al. 2005, 2006c; Serpen and Gokmen 2009; Silva and Simon 2005; Vinci et al. 2010). These efforts were partially successful. Acrylamide content correlates well with product lightness, measured as the luminosity component of color (L*) across a wide color range (Fig. 5a). The chromatic color component (a*), which is a measure of redness, may correlate better with acrylamide content for both chips (Pedreschi et al. 2005) and fries (Fig. 5b) (Pedreschi et al. 2006c). The variation in L* and a* at acrylamide values of less than 1,000 μg kg−1 may preclude effective use of this approach to predict acrylamide content for most processed potato products. Thus, although there is a general agreement between chip or fry color and acrylamide content, color parameters may not be suitable surrogates for acrylamide measurement because a wide range of acrylamide contents are observed in chips and fries with the same color values. Close relationships between product color and acrylamide content were observed only if samples with the same water content were compared (Amrein et al. 2006). It is important to note that at low water contents, acrylamide-forming reactions are more temperature-sensitive than browning reactions. Hence at higher temperatures there is a disproportionate increase in the amount of acrylamide produced relative to amount of browning that occurs (Amrein et al. 2006). Thus, relationships between color and acrylamide content are likely to be most precise when they are determined for each cooking temperature used.
Acrylamide content in fried potato strips cooked at 3 temperatures was correlated with the color parameters L* (a) and a* (b). Lower values of the lighness parameter L* and higher values of the redness parameter a* correspond to darker colored fried strips. Figure reprinted from (Pedreschi et al. 2006c) with permission
Several acrylamide-prediction models have been developed that focus on the kinetics of acrylamide formation using estimated rates for the underlying chemical reactions (de Vleeschouwer et al. 2008, 2009; Franke et al. 2009; Knutsen et al. 2009; Palazoğlu and Gokmen 2008; Serpen and Gokmen 2007). Models similar to these are useful for testing and optimizing processing operations and understanding the consequences of variation in tuber composition on acrylamide formation, but models must be validated for the range of conditions being examined (Palazoğlu and Gokmen 2008).
Less sophisticated models have been used in attempts to establish correlations between acrylamide in chips or fries and tuber composition, including reducing sugars, asparagine, other sugars and amino acids, dry matter content and protein content (Amrein et al. 2003; de Wilde et al. 2005; Elmore et al. 2005; Granda and Moreira 2005; Knutsen et al. 2009; Whittaker et al. 2010). These models do not capture the chemistry of acrylamide formation, but rather rely on statistical relationships found in the samples used to develop the model. Caution is advised when the tubers used for processing are not representative of those used to develop the model. Variety, growing location, cultural management practices, and storage conditions all influence tuber asparagine and reducing sugar composition and affect acrylamide-forming potential. Because changes in acrylamide content are never explained completely by documented changes in tuber composition, it can be inferred that important yet poorly characterized variation in tuber properties contributes to acrylamide formation. Until these parameters are better defined, models for acrylamide formation need to be validated for individual varieties grown in a particular environment under a limited set of management practices if they are to have high utility.
Analytical Methods of Analysis
The methods used to measure acrylamide have received considerable attention as research groups have sought to develop faster, more accurate, cheaper or simpler approaches (Fernandes and Soares 2007; Gokmen et al. 2005; Rufian-Henares and Morales 2006; Segtnan et al. 2006; Senyuva and Gokmen 2006; Wenzl et al. 2006; Zhou et al. 2007). Acrylamide analysis typically involves aqueous extraction of acrylamide from frozen or fresh samples, concentration or sample cleanup using solid phase extraction, and analysis by gas chromatography and mass spectrophotometry (GC-MS) or high performance liquid chromatography (HPLC). The total time required for analysis is often several days, and this means that product quality assessments based on acrylamide content are not available until days after tubers have been processed into chips, fries or other products. Most improvements to analytical methodologies have been in the ease of sample preparation and the speed of the analysis. None of the methods developed removes the requirements for highly skilled technical staff and sophisticated, expensive equipment. Indeed, in some cases more expensive or complicated equipment has been required. For near-real time sampling, such as might be employed on a process line to make quality control determinations, near infrared spectroscopy (NIR) shows some promise. NIR is used to measure non-destructively the composition of many food products and NIR has been used to measure water content and fat content in potato chips (Shiroma and Rodriguez-Solona 2009). Methods to use NIR to measure acrylamide in potato chips have been investigated (Pedreschi et al. 2010; Segtnan et al. 2006). For chips made from cultivar Saturna, the correlation between acrylamide measured using GC-MS/MS and NIR was high with R2 equal to 0.83 over a wide range of acrylamide contents (Pedreschi et al. 2010). However, a relatively large, average prediction error of 266 μg kg−1 dry weight was observed. An earlier study had a similar level of accuracy (Segtnan et al. 2006), and these data suggest that improvements in methodology are needed before this approach can be used to accurately measure acrylamide in the range of 500 μg kg−1 or less.
Acrylamide Mitigation
Acrylamide Reduction is a System Wide Challenge
Reducing the acrylamide content in processed potato products has become a goal of the potato industry in the US and elsewhere. As noted in a review on this topic, acrylamide reduction requires a comprehensive “farm to fork” approach (Vinci et al. 2012). Consistently, reducing acrylamide in finished products is likely to depend on tailoring variety selection, field and storage management, processing methods and overall quality control to achieve this goal. Changes to any one of these components are not likely to consistently reduce acrylamide content if other components leading to finished product formation are not improved or maintained consistently within narrow limits. The “acrylamide toolbox” (FoodDrinkEurope 2011) and reviews by Seal et al. and Vinci and Mestdagh are recommended for those seeking additional information on this topic (Seal et al. 2008; Vinci et al. 2012).
Potato Varieties Differ in Acrylamide-Forming Potential
As described above, the amount of acrylamide in processed potato products depends on the amounts of tuber fructose, glucose and asparagine, and on the presence of other compounds that accelerate or decelerate the rate or extent of acrylamide-forming reactions. Tuber composition depends on both genotypic and environmental effects, with both contributing substantially to the final acrylamide content of processed potato products.
Potato cultivars have been bred for low reducing sugars for over half a century. Although highly beneficial in terms of acrylamide mitigation, these breeding efforts have been focused primarily on producing light-colored, flavorful processed products from freshly harvested and stored tubers (Douches et al. 1996; Love et al. 1998; Stevenson 1957). Selection for low reducing sugar content has been more successful for chip rather than fry processing potatoes. Chipping potatoes will often have a reducing sugar content (glucose plus fructose) of 0.075 mg g−1 fresh weight or less, whereas fry processing varieties typically have reducing sugar contents of greater than 1 mg g−1 fresh weight, especially for tubers out of storage. Because of this difference, it may be easier to decrease the acrylamide-forming potential of fry processing varieties relative to chip processing varieties through breeding for lower amounts of reducing sugars.
Potato tubers have never been bred for low asparagine, and until recently the range of asparagine in tubers from different cultivars and wild tuber bearing Solanum species was not known. Several recent germplasm surveys have established a substantial range in asparagine content between cultivated potato varieties and wild species relatives (Amrein et al. 2003; Finotti et al. 2006; Matsuura-Endo et al. 2006; McCann et al. 2010; Olsson et al. 2004; Viklund et al. 2008a; Whittaker et al. 2010; Zhu et al. 2010). Tuber asparagine content in most cultivated potato ranged from 4 to 25 mg g−1 dry weight, although data for some varieties are notable for being outside of this range. In one report both Agata and Arinda had very low amounts of tuber asparagine at 156 and 504 mg kg−1 fresh weight (approximately 0.75 and 2.5 mg g−1 dry weight assuming 80 % water content), respectively (Finotti et al. 2006). Conversely, asparagine contents of over 30 mg g−1 dry weight have been reported in potatoes from China, Japan and Italy (Zhu et al. 2010). In S. tuberosum ssp. andigenum, the range of observed tuber asparagine contents was 1.2 to 76.3 mg g−1 dry weight and the range of tuber asparagine content in several wild species relatives of potato was at least that large (McCann et al. 2010). These data suggest that variation in tuber asparagine content is readily available in S. tuberosum and close relatives of cultivated potato, and that individual genotypes with low asparagine contents can be used in breeding efforts aimed at reducing tuber asparagine content. A further indication that breeding for low asparagine content potato tubers is possible comes from an evaluation of clones in a full sib breeding population (Shepherd et al. 2010). Parents had mean tuber asparagine contents of 7.87 and 10.3 mmol kg−1 dry weight. Tuber asparagine content segregated among the progeny with tuber asparagine content in 11 of 43 progeny falling between the two parents, and seven progeny having tuber asparagine less than the lower parent.
Using Biotechnology to Produce Potato Varieties with Low Acrylamide-Forming Potential
Acrylamide-forming potential, either quantified indirectly by tuber reducing sugar, asparagine and perhaps antioxidant contents or by direct measurement of acrylamide in finished products, is likely to become a standard criterion for evaluating new potato varieties given the importance that the potato industry worldwide has placed on acrylamide mitigation. Selection based on phenotypic expression or molecular markers linked to key traits can be used to develop next-generation genotypes that have lessened acrylamide-forming potential compared with current varieties. Targeted genetic modification is an alternative to conventional potato breeding that also has the potential to reduce the acrylamide content of finished products. A significant advantage of this approach is that it can be used to improve an existing cultivar without altering substantially the agronomic traits of the original variety. Both tuber reducing sugar content and asparagine content have been decreased in popular cultivars using molecular approaches. Asparagine content was reduced in Ranger Russet and in Atlantic using RNA-interference to “turn off”, or silence genes for asparagine synthetase (Chawla et al. 2012; Rommens et al. 2008). These genes encode the proteins that are primarily responsible for asparagine synthesis in potato. Transformed lines varied in the efficiency of gene silencing, but some individual lines had an 80 % reduction in gene expression and a corresponding decrease of up to 95 % in tuber asparagine content. When acrylamide content of fries made from Ranger Russet was quantified, reductions of up to 95 % were observed in transformed lines. For Atlantic, decreasing asparagine content by 80 % resulted in a 90 % reduction in chip acrylamide (Rommens et al. 2008). Double-silencing lines that targeted both asparagine synthetase genes in Ranger Russet had tuber and vine defects under field conditions (Chawla et al. 2012). Silencing only the StAst1 gene, however, was sufficient to substantially lower asparagine in tubers. In this case, tuber yield and quality were comparable to that of non-transformed Ranger Russet (Chawla et al. 2012). These data demonstrate clearly that low tuber asparagine contents can limit acrylamide formation even when the starting material is a processing potato variety with moderately low reducing sugar contents.
Several molecular approaches have been used to decrease tuber reducing sugar contents. In one case, this was achieved by silencing the R1 gene for starch phosphorylase (Rommens et al. 2006). Starch phosphorylase is an important enzyme in the breakdown of stored starch. Reducing expression of R1 resulted in clear reductions in tuber reducing sugar content in storage that corresponded with reduced acrylamide content of fries made from transformed lines of Ranger Russet. An alternative molecular target is vacuolar acid invertase, which functions at the other end of the molecular pathway linking starch breakdown to reducing sugar accumulation. Vacuolar acid invertase hydrolyzes one molecule of sucrose into one molecule of glucose and one of fructose. This reaction takes place in subcellular organelles called vacuoles. The expression of the gene for vacuolar acid invertase has been decreased using RNA interference or antisense expression (Bhaskar et al. 2010; Liu et al. 2011; Wu et al. 2011; Ye et al. 2010). In multiple cultivars with both methods, decreasing the accumulation of the vacuolar acid invertase transcript decreased the accumulation of reducing sugars in cold-stored tubers. In some transformed lines with very low invertase gene expression, chips with acceptable color were produced from tubers that had been stored at 4 °C for up to six months (Bhaskar et al. 2010). At this temperature, which is well below that required for cold-induced sweetening to occur, control tubers accumulated reducing sugars and chips prepared from them were dark brown in color. Compared to untransformed controls, lines with extreme resistance to cold-induced sweetening had reductions of acrylamide in chips of 80 % or more (Bhaskar et al. 2010; Wu et al. 2011; Ye et al. 2010).
Field and Storage Management for Low Asparagine and Low Reducing Sugars
Asparagine and reducing sugars are the two most important substrates for acrylamide formation and hence production practices that consistently minimize the accumulation of asparagine and reducing sugars are effective ways to minimize acrylamide in finished products. Extensive research on the effects of fertilizer type, timing and amount, irrigation timing, year-to-year variation in climate, storage temperature, storage duration and other factors have demonstrated the importance of field and storage management on the accumulation of reducing sugars (Bethke and Busse 2010; Herrman et al. 1995; Kumar et al. 2004; Pisarczyk 1982; Pritchard and Adam 1992; Shock et al. 1993; Sowokinos 1973, 2001; Thompson et al. 2008). These topics have been reviewed extensively elsewhere and are not discussed in depth here. The affect of field management on asparagine accumulation has not been described in comparable detail and much of the data available has been collected since the discovery of acrylamide in food.
The asparagine content of potato tubers at harvest and out of storage depends on varietal differences, production year and location, and field management practices. Storage temperature in the range of 2–10 °C has been shown to have little effect on tuber asparagine content (de Wilde et al. 2005; Matsuura-Endo et al. 2006; Ohara-Takada et al. 2005; Olsson et al. 2004). Tuber asparagine content may change with time in storage, but the magnitude and direction of these changes may be inconsistent from year to year. For example, mean tuber asparagine content for eight varieties stored for 24 weeks did not change with storage duration when asparagine contents for each month were averaged over 3 years (Olsson et al. 2004). During individual years, however, the asparagine content for some varieties increased during storage while for others it decreased. Changes in tuber asparagine content were also observed in a one-year-long study conducted with ten varieties produced by commercial growers in the United Kingdom (Halford et al. 2012). In this case, many of the varieties exhibited a similar pattern in which tuber asparagine content increased from November to December, decreased until February, increased again until March or April, and decreased in May. In other instances, asparagine content varied little during the storage period (Matsuura-Endo et al. 2006). Significant year-to-year variation in tuber asparagine has been observed, with the amount in some years approximately twice as high as in other years (de Meulenaer et al. 2008; Knutsen et al. 2009; Viklund et al. 2008b). Likewise, variation in tuber asparagine occurs when the same variety is grown at different locations during the same year (Knutsen et al. 2009; Whittaker et al. 2010).
Field management practices, especially nitrogen fertilization rates, likely affect free asparagine content in tubers. Several studies have shown that increased application of nitrogen fertilizer increased both the total amount of free amino acids and the amount of free asparagine in potato tubers (Amrein et al. 2003; Gerendas et al. 2007; Hippe 1988; Silva and Simon 2005).
Harvest operations and harvest timing influence tuber composition in several ways that have consequences for acrylamide formation. The most obvious of these is the chemical maturity of the tuber at harvest, which in turn determines the ease with which tubers can be preconditioned and stored with low reducing sugar contents (reviewed in Kumar et al. 2004). Tuber sucrose content is used to quantify chemical maturity, with tubers being chemically mature when sucrose content declines to a minimum value. This state of maturity is typically reached shortly before vine desiccation. Chemical maturity monitoring is an established procedure for scheduling harvest of fry processing and chipping potatoes because of the need to maintain low reducing sugars in tubers, and the success of the approach in meeting this expectation (Sowokinos and Preston 1988). For fry processing varieties such as Russet Burbank that are susceptible to sugar-end defects (Thompson et al. 2008), sucrose and glucose contents at harvest may not adequately indicate potential of the crop for long-term storage with low amounts of reducing sugars (Bethke and Busse 2010).
Harvest timing also influences eventual acrylamide formation in ways that are independent of tuber maturity. Harvests that occur after a period of cold weather are detrimental in that cool soil temperatures can initiate cold-induced sweetening and reducing sugar accumulation. Harvests that impose a temperature or mechanical stress on the tubers are likely to increase tuber sucrose content. Since sucrose production is a common response to many stresses, damage during harvest increases the potential for reducing sugar accumulation.
Acrylamide Mitigation by Changing Processing Method
Identifying superior varieties that have a genetic predisposition for low acrylamide-forming potential will be an important aspect of a complete acrylamide mitigation portfolio. Tailoring processing conditions to minimize acrylamide formation will be another aspect. Numerous technological approaches have been shown to have the potential to reduce acrylamide formation during high temperature processing (Claus et al. 2008; Foot et al. 2007; Friedman and Levin 2008; Morales et al. 2008; Seal et al. 2008; Vinci et al. 2012). Most of these approaches fall in to one of three categories; blanching or presoaking, chemical additions to soaking solutions used prior to cooking, and modifications to cooking conditions.
Presoaking fresh potato slices in solutions with various compositions is one acrylamide mitigation approach that has the goal of reducing the content of acrylamide precursors. Blanching in warm water is the simplest of these approaches that is an extension of the standard fry processing method. Soaking potato products in warm water, typically 50 to 85 °C, decreases the content of reducing sugars and asparagine in the uncooked product (Fig. 3) (Burch et al. 2008; Kita et al. 2004; Pedreschi et al. 2004, 2005, 2007a, b; Viklund et al. 2010; Vinci et al. 2012). Removal of sugars from the cell walls of tuber tissue relies on diffusion alone and for surface layers is likely to be relatively rapid. Removing sugars from the cell cytoplasm and vacuoles is likely to be slower, even when cell membranes have ruptured from increased osmotic pressure. Blanching times of 3 to 10 min at 80 to 85 °C decreased reducing sugar and asparagine in raw product sufficiently that final acrylamide contents decreased by 60 % (Pedreschi et al. 2005; Viklund et al. 2010). This approach is likely to be limited to use in french fries and similar processed products with little application in chips.
Blanching followed by spraying on or soaking in a solution of dextrose (glucose) is used in industry to even out the color of finished fries and provide consistent fried potato flavor in final product. This process was shown to decrease the acrylamide content of finished fries relative to fries prepared without added dextrose when both were cooked to a similar color (Higley et al. 2012). French fries were prepared from tubers with a range of reducing sugar contents to produce products having a range of acrylamide and fried product color. Additional fries were prepared from cut strips that were blanched and then dipped in glucose solutions. Across a wide range of finished product color (approximately 45–80 Agtron) and acrylamide content (approximately 100–1,500 μg kg−1), fries produced by blanching followed by adding back glucose had less acrylamide than fries produced from fresh-cut strips when comparisons were made between products with the same color. It was hypothesized that this could be attributed to a the higher rate of acrylamide formation relative to color development that occurs with fructose relative to glucose (Mestdagh et al. 2007a). Blanching removed both reducing sugars equally well, but only glucose was added back. Additional data support this hypothesis. When blanched fries were dipped for 30 s in solutions with different ratios of glucose to fructose but the same total reducing sugar amount, higher amounts of acrylamide were observed in those cases when the ratio of fructose to glucose was greater than one (Higley et al. 2012). The difference in acrylamide content between fries produced by blanching and dipping in solutions of 100 % glucose or 100 % fructose increased substantially as fry color darkened from 70 to 50 on the Agtron scale (Parker et al. 2012).
Numerous chemicals have been added to presoaking solutions to lower subsequent acrylamide formation. In some cases the goal is to more effectively reduce the amount of acrylamide precursors in the uncooked product. In other cases, the goal is to incorporate compounds into the uncooked products that will interfere with acrylamide-forming reactions. Organic acids such as citric acid, lactic acid and acetic acid have been used in presoaking solutions to decrease the pH of the solution within uncooked products (Mestdagh et al. 2008b, c; Pedreschi et al. 2004, 2006c, 2007a, 2008b). The goal in this case is to increase the protonation of asparagine. This decreases dramatically the reactivity of asparagine in acrylamide-forming reactions and decreases substantially the amount of acrylamide in finished products (de Vleeschouwer et al. 2006).
Soaking or blanching with Na+ or Ca2+ ions has also been shown to reduce acrylamide formation in several instances (Gokmen et al. 2007; Lindsay and Jang 2005; Mestdagh et al. 2008c; Ou et al. 2008; Park et al. 2005; Pedreschi et al. 2007a, b). It has been suggested that they may act by reducing the reactivity of asparagine in acrylamide-forming reactions, and, for Ca2+, a decrease in pH though displacement of protons (Vinci et al. 2012). Soaking or blanching with free amino acids such as glycine, cysteine and lysine also has the potential to reduce acrylamide formation (Brathen et al. 2005; Low et al. 2006; Mestdagh et al. 2008b; Rydberg et al. 2003).
While blanching is an effective way to lower the reducing sugar content in the surface layer of fries, there are difficulties in implementing this approach to achieve large reductions in acrylamide content. The biggest challenge lies in maintaining acceptable sensory characteristics of finished products (Mestdagh et al. 2008c). The adverse effects of blanching, with or without additions, can include loss of starch and consequent increased oil absorption, shrinkage of raw product leading to decreased recovery in finished products and higher input costs, and changes in finished product texture and taste.
The enzyme asparaginase, which uses asparagine as a substrate to produce aspartic acid and ammonia, has been used with success to minimize acrylamide formation in products made from dough (Hendriksen et al. 2009). Asparaginase can be incorporated into the dough for cookies, crackers, corn chips, and other products where it enzymatically decreases the asparagine content of the dough in a highly controllable manner. Reducing the acrylamide content of potato products with aspariginase is more difficult because those products are fabricated from fresh vegetable slices instead of dough (Hendriksen et al. 2009). The walls and membranes of the cells within raw chips and fries are barriers to entry for aspariginase that prevent the enzyme from reaching its substrate asparagine, most of which is located in vacuoles and amyloplasts (Farre et al. 2001). Methods that make uncooked or partially cooked tissues more permeable to the enzyme have been slightly more successful (Ciesarova et al. 2006; Hendriksen et al. 2009; Pedreschi et al. 2008c, 2011; Vinci et al. 2011), but create many of the same difficulties in terms of oligoleptic properties as blanching with or without chemical additions.
Lactic acid bacteria, rather than purified enzymes, have also been used to reduce acrylamide production (Baardseth et al. 2006). Raw product is introduced into a soaking solution containing live bacteria prior to processing. The bacteria degrade reducing sugars as part of their metabolism and produce lactic acid in the process. As described above, both of these processes reduce final acrylamide formation. Some lactic acid bacteria also consume asparagine, and this is another potential means for acrylamide reduction.
Whether or not oil composition affects acrylamide formation has been the subject of several studies (Mestdagh et al. 2005, 2007b, 2008a). In general, these studies have not found significant differences in acrylamide formation when the same materials were cooked using a range of oils. Rapeseed, olive, corn, soybean, sunflower, and grapeseed oil, for example, all produced fries with comparable amounts of acrylamide (Mestdagh 2005). This might seem paradoxical in light of the presumed interaction between antioxidants and acrylamide formation. It might be expected that oils differing in antioxidant content would produce different amounts of acrylamide. That differences were not observed suggests that the acrylamide-forming reactions occur in the aqueous milieu of the potato tissue, and have little interaction with the oil/water interface.
Lowering cooking temperature decreases acrylamide content of products in many instances. An extreme case of low-temperature cooking is frying under vacuum (Granda and Moreira 2005; Granda et al. 2004). The theory behind this approach is that water is readily removed from potato chips or fries at low temperatures if the atmospheric pressures is less than that required for water to boil. This method was successful on a small scale (Granda et al. 2004). Chips fried at 118 °C for 4 min at 10 Torr had 84–97 % less acrylamide than those fried under traditional conditions of 165 °C for 3 min.
Quality Control is Essential
Tuber lots used for processing are routinely evaluated at the processing plant for sugar content, specific gravity, fry color and the presence of defects. How acrylamide mitigations efforts affect this process will depend, in part, on the goal of the evaluation process. For example, if there is a requirement that processed products meet specific targets for acrylamide, then loads may be rejected if there is a concern that finished product will exceed those limits. Implementation of quality control in this case would be complicated because near real time measurements of acrylamide are not available. Instead, processors may need to rely on varietal characteristics and fried product color to estimate likely acrylamide content in finished products (FoodDrinkEurope 2011).
Chips, fries and other processed potato products are often not uniform from end to end and side to side. For chips and fries at least, this reflects inherent spatial variation in tuber composition for starch, sucrose, reducing sugars, enzymes and many other compounds (Baijal and Vliet 1966; Cole 1975; Glynne and Jackson 1919; Merlo et al. 1993; Rommens et al. 2010; Sayre et al. 1975). Where differences in reducing sugar content are large, they may result in defects, such as sugar-end defect of fry processing tubers (Thompson et al. 2008) and stem-end chip defect in chip stock (Wang and Bethke 2013; Wang et al. 2012). The importance of defects such as these, as well as those caused by cold-induced sweetening and senescent sweetening, needs to be re-evaluated in the context of acrylamide mitigation. Sugar-related defects are likely to have higher acrylamide contents than defect-free product. For this reason, acrylamide mitigation strategies for potato are likely to contain stringent quality control measures for raw (Vinci et al. 2010) and finished product. These measures may include optical scanners that identify products with defects and remove them from the production line prior to packaging (Pedreschi et al. 2006a, b, 2008a). This technology has already been implemented at many potato processing plants (FoodDrinkEurope 2011).
Acrylamide Mitigation Strategies are Additive
Producing processed potato products with low amounts of acrylamide requires doing many things well. This is especially true in the near term when low asparagine varieties of potato will not be available, and acrylamide content will depend strongly on keeping reducing sugar content to a minimum. Several decades of potato research and processing expertise have made it clear that doing this consistently throughout the year, every year, will be a tremendous challenge for the potato industry. Unfavorable climatic conditions and errors in field management, storage, shipping, and processing have the potential to cause spikes in acrylamide content that will be difficult or expensive to remove later in the supply chain. It remains to be seen how the details of this cooperative process will evolve, but it seems clear that acrylamide will remain a concern for all aspects of the potato industry for many years. What is less clear is how great the risk of acrylamide consumption is to human health. This is an active area of research worldwide, and some of the relevant data are summarized in the following section.
Dietary Exposure to Acrylamide
Most non-smokers in the developed world are exposed to acrylamide primarily through the foods that they eat (Vesper et al. 2010). It has been estimated that more than one-third of the calories consumed each day come from foods with detectable levels of acrylamide (Mucci and Adami 2009). Monitoring programs were initiated in Europe and North America soon after the discovery of acrylamide in food in order to determine which foods contained acrylamide, how much was present at various times throughout the year, and how much acrylamide was being ingested by consumers. Surveys were made over several years to see if the acrylamide content of individual food items decreased, as might be expected if acrylamide mitigation measures had been put into practice. In most cases the foods sampled were processed products purchased from grocery stores and restaurants. Table 1 summarize some of the findings on total acrylamide exposure in several countries and illustrates the range of values found. Also indicated, when available, is the percentage of total exposure that was attributable to potato products. In the US, approximately 38 % of dietary acrylamide comes from processed potato products (DiNovi 2006).
Data for the acrylamide content in processed products including potato chips, french fries, cookies, crackers, and coffee have been collected over several years. The results of the Canadian effort are illustrative of trends and challenges faced in reducing the acrylamide content of potato chips (Becalski et al. 2010) (Fig. 6). The average acrylamide content of potato chips was found to vary within each year and from year to year. Similar data have been reported from Germany (Foot et al. 2007). Chip brand had a significant effect on average acrylamide content. There was a general trend in which some brands were higher in acrylamide content than others (Fig. 6). For french fries baked at home, similar trends in terms of year-to-year variation existed (EFSA 2011). These data are limited to specific locations and a few brands, and it may be that some regions or brands have achieved larger reductions in acrylamide content. However, the data available suggest that it has been difficult to consistently reduce the acrylamide content of processed potato products with existing varieties without incurring unacceptable cost or changes in product quality. That potential acrylamide mitigation measures have not been implemented sucessfully in many potato products (EFSA 2012) has led some to suggest that only measures by the authorities will achieve improvements (Biedermann et al. 2010).
Acrylamide content in three brands of potato chips sampled at multiple times each year from 2002 to 2008. Data extracted from (Becalski et al. 2010). Variation in acrylamide content between brands and sampling dates is apparent
Assessment of Health Risks
Acrylamide as a Potential Carcinogen
Although early health studies related to acrylamide focused almost exclusively on its role as a neurotoxin (LoPachin 2005), the current concern over acrylamide in food has centered on acrylamide as a potential carcinogen (Berger et al. 2011; El-Sayyad et al. 2011a, b; Koyama et al. 2011; Lineback et al. 2012; Tardiff et al. 2010; WHO 2011) or compound that alters pre- or postnatal development (El-Sayyad et al. 2011a, b; Pedersen et al. 2012). This shift in emphasis reflects data from clinical research and epidemiological studies indicating that the margin of safety for neurotoxicity is larger than for carcinogenesis (Hogervorst et al. 2010; Lineback et al. 2012). It also reflects the underlying assumptions about how cancer-causing agents promote disease and how those differ from assumptions about toxicity. The hazards associated with ingestion of toxic substances are typically viewed as increasing in severity as dosage increases. For a toxic compound, very low amounts produce non-detectable effects on health that are routinely ignored, whereas higher amounts lead to acute toxicity symptoms. Safety regulations for such compounds are based on the highest amount of the compound that leads to no-observed effects. Allowable amounts of the compound are less than this amount by some margin of safety (Hogervorst et al. 2010). Carcinogens have traditionally been viewed differently, with the rate of incidence, but not the severity, of cancer increasing with exposure (amount and duration) to the compound. The rational for this difference is that cancer causing agents are stochastic, with each molecule of a carcinogen having a small but equal probability of causing a mutation that leads to cancer. The severity of the resulting cancer depends on the type of cancer and how rapidly it grows. In simplistic terms, carcinogens are managed to limit cumulative exposure whereas toxins are managed to remain below thresholds.
Below we summarize some of the laboratory, clinical and epidemiological data related to acrylamide in food as it relates to cancer or developmental changes. Questions that are invariably raised when evaluating research in this area include the relative sensitivity of rodents and humans to acrylamide-induced cancers, how accurately data derived from experiments that use relatively high doses of acrylamide can be extrapolated to the trace amounts found in food, and how age and other environmental factors influence the susceptibility of humans to acrylamide exposure. A clear answer to these questions is not available, but the numerous research studies on acrylamide and cancer that have been conducted in the past decade shed some light on each of them. The following section reviews some of this data in order to illustrate the current status of this research. The excellent reviews by (Hogervorst et al. 2010; Lineback et al. 2012) are recommended for those who would like additional information in this area.
Acrylamide, Cancer and Developmental Defects in Rodents
Experiments using rat and mouse models have shown clearly that dietary acrylamide can induce benign and cancerous tumors, as well as neurological and developmental defects (Berger et al. 2011; Bull et al. 1984a, b; El-Sayyad et al. 2011a, b; Friedman et al. 1995; Johnson et al. 1986; Koyama et al. 2011; Shiraishi 1978). Doses administered to animals were at least 20 times higher than that found in food (Hogervorst et al. 2010), with one exception, and in some cases several thousand times higher, as is typical of toxicology and carcinogenicity studies that use rodent models. Margins of exposure (MOE) for non-carcinogenic effects, which are a ratio of the maximum level of a compound at which there is no observed adverse effect and actual amount of exposure to that compound, were large enough that risk for neurotoxicity appears to be negligible (Hogervorst et al. 2010). For carcinogenic effects, where accepted margins of safety are larger than they are for non-carcinogenic effects, MOEs were sufficiently small that the animal studies pointed to a risk of cancer due to dietary intake of acrylamide (Hogervorst et al. 2010; Lineback et al. 2012; WHO 2011). In one study that highlights this concern, chronic exposure of mice to acrylamide in drinking water was shown to generate DNA damage in male germ cells (Nixon et al. 2012). Acrylamide was administered at rates that ranged from 0.1 μg kg−1 d−1 to 1,000 μg kg−1 d−1. Dose-dependent increases in DNA damage were observed after 6, 9 and 12 months. At the 12-month sampling period, damage was detected at doses as low as 1 μg kg−1 d−1. Significantly, this dose of acrylamide is comparable to that consumed by humans in food (Table 1).
Epidemiological Studies on Acrylamide and Cancer in Humans
Research conducted with rats and mice has shown clearly that acrylamide administered as a pure compound or as acrylamide-rich food can contribute to the development of benign tumors, cancers and developmental defects. Studies similar to these, where high doses of a potentially toxic or mutagenic compound are fed to subjects and effects on health are monitored for a short period of time, cannot be done with humans. Available options are to extrapolate the findings from short-term, high-dosage rodent experiments to long-term, low-dosage exposures typical for humans, or to conduct epidemiological studies that follow cohorts of subjects over a long period of time and relate health outcomes to specific risk factors. In order to correctly extrapolate data from rodent studies to humans, one needs data on whether or not rodents and human are similar or different with regard to efficiency of acrylamide elimination, amounts of acrylamide converted to glycidamide, modes of action for promoting disease for acrylamide and glycidamide, shape of the dose response curve for each particular disease, susceptibility of different ages and sexes to disease development, and the contribution that other environmental factors make toward promoting or slowing disease progression. These relationships between rodents and humans are not well characterized, in most cases, although progress is being made in some areas (Hogervorst et al. 2010). Epidemiological studies present challenges of their own (Mucci and Adami 2009). For acrylamide research, one of the largest challenges is that exposure rates are based on food frequency questionnaires that are used to calculate daily acrylamide ingestion using average values for acrylamide content of individual food groups. These calculations do not typically take into account variation in acrylamide content that occurs between brands or at different times of year (Fig. 6). Food surveys may also be problematic because they are typically taken during a subset of the years that members of the cohort are being followed, and therefore do not capture changes in diet that might have occurred at earlier or later times.
Early epidemiological studies on occupational (Marsh et al. 1999; Sobel et al. 1986) and dietary exposure to acrylamide (Mucci 2005; Mucci et al. 2003, 2004; Pelucchi et al. 2003, 2004) found no consistent evidence of association with human cancer risk. In order to reduce errors associated with restricted sample sizes and increase the power of the analysis, data from an integrated network of Italian and Swiss hospital-based case-control studies were reanalyzed to assess the relationship between dietary acrylamide intake and cancers of the oral cavity and pharynx, esophagus, large bowel, larynx, breast, ovary and prostate (Pelucchi et al. 2006). In all of the seven studies considered, fried and baked potatoes were the major contributors of acrylamide, accounting for 36–44 % of intake. No association was found between dietary acrylamide intake and risk of any of the cancers investigated. In discussing this finding, Pelucchi et al. (2006) noted that debate continues on the actual risk levels to humans related to acrylamide intake in consideration of discordant findings of animal and epidemiologic studies. They suggest two major lines of reasoning to explain this inconsistency. The first line of reasoning considers the limitations of extrapolating animal data to humans given the uncertain bioavailability of acrylamide after ingestion, as well as differences in the metabolism of acrylamide, and the different level of exposure between laboratory data and human diet. The second line of reasoning criticizes epidemiologic studies for their limited power.
An analysis of epidemiological data published in journal articles prior to June 2009 (Pelucchi et al. 2011) was carried out to determine if associations between acrylamide exposure and cancer could be uncovered when results from multiple studies were combined. Of the papers included in the analysis, 19 publications reported results on dietary intake of acrylamide, two publications reported results on biomarkers of exposure and six publications reported results on occupational exposure. The authors conducted meta-analyses of studies of dietary intake based on random-effects models by calculating pooled relative risks (RR) and the corresponding 95 % confidence intervals (CI). Results of occupational studies were combined according to a fixed-effect model. The authors overall conclusion was that available studies consistently suggest a lack of an increased risk of most types of cancer from exposure to acrylamide. The exception was for kidney cancer, which, the authors suggested, requires further monitoring. Specific attention was given to studies on consumption of potatoes cooked at high temperatures and cancer. These studies looked at potential cancer of the oral cavity and pharynx, esophagus, colorectum, larynx, lung, breast, ovary, prostate, bladder, kidney, and childhood brain. The authors note that significant positive associations have been reported in five case-control studies on cancer risk from intake of acrylamide-rich foods, but that these are vulnerable to selection and report bias. Furthermore, they note that the positive results referred to five different types of cancer and selective reporting of ‘positive’ or ‘significant’ associations can take place in studies assessing large sets of food items.
The association between acrylamide intake and breast cancer risk among 90,628 premenopausal women in the Nurses’ Health Study II (Wilson et al. 2009) was assessed. Acrylamide intake was calculated from 130-item food frequency questionnaires in 1991, 1995, 1999, and 2003. The authors found no associations between intakes of foods high in acrylamide, including french fries, coffee, cereal, potato chips, potatoes, and baked goods, and breast cancer risk. They found no evidence that acrylamide intake within the range of US diets is associated with increased risk of premenopausal breast cancer. Also, there was no association for estrogen receptor (ER)/progesterone receptor (PR) positive or negative cancers or by smoking status.
An analysis of the UK women’s cohort, 33,731 women aged 35–69 who have been followed for a median of 11 years, was conducted to evaluate a possible link between acrylamide consumption in food and breast cancer (Burley et al. 2010). Women in the study completed a 217-item food frequency questionnaire during 1995 to 1998 and this was used to estimate a mean acrylamide intake of 0.23 μg kg−1 day−1, 28 % of which was attributed to french fries and 14 % to potato chips. Overall, there was little evidence of an association between dietary acrylamide intake and breast cancer when premenopausal and postmenopausal cancers were combined. There was some evidence for a positive association with premenopausal breast cancer. Although there was a weak dose–response relationship, the association was particularly apparent in the highest fifth of dietary intake (>23 mg day−1). Given that smoking is not a risk factor for breast cancer and that smokers have substantially higher levels of hemoglobin adducts of acrylamide and glycidamide than non-smokers, and much higher levels than those associated with dietary intake, it was surprising that they found an association between dietary intake and breast cancer. They recommend that it will be important to repeat the analyses amongst a subgroup of subjects who have never smoked.
One of the early prospective cohort studies to address the question of the carcinogenicity of acrylamide in food used data from the Netherlands Cohort Study on diet and food to examine potential associations between endometrial, ovarian and breast cancer in post-menopausal women (Hogervorst et al. 2007). Subjects included in the analysis had average daily exposure to dietary acrylamide of 0.32 ± 0.19 μg kg−1 d−1 with coffee and Dutch spiced cake being the most important contributors to total dietary acrylamide. Increased risks of postmenopausal endometrial and ovarian cancer were observed with increasing dietary acrylamide intake, particularly among never-smokers. Risk of breast cancer was not associated with acrylamide intake. The association with ovarian cancer was not found in a prior case–control study (Pelucchi et al. 2006) and in the Swedish Mammography cohort (Larsson et al. 2009). The range of acrylamide exposure in the Swiss study was narrower than that in the Dutch study, and this could have reduced the possibility of detecting an association if one exists (Larsson et al. 2009).
Biomarkers for Acrylamide Exposure, Disease Incidence and Prenatal Development
Epidemiological studies use food frequency questionnaires to determine acrylamide exposure. This indirect approach may not account for variation in acrylamide content that occurs within and between products of the same type, nor does it account for variation in home cooking methods and personal preferences that may result in higher acrylamide contents in similar items depending on how they are prepared. An alternative method for assessing acrylamide exposure is to use acrylamide and glycidamide adducts of hemoglobin as biomarkers for exposure to acrylamide and it genotoxic metabolite glycidamide. A study of 374 breast cancer cases and 274 controls from a cohort of postmenopausal women found that increased incidence of estrogen receptor positive breast cancer was associated with higher concentrations of acrylamide adducts after adjusting for smoking behavior (Olesen et al. 2008). Women with the highest concentrations of acrylamide adducts of hemoglobin had 2.7 times increased risk of estrogen receptor positive breast cancer when compared to women with the lowest concentrations. The weaker association observed between breast cancer risk and concentrations of gycidamide adducts compared to acrylamide adducts led the authors to suggest that acrylamide might induce cancer by a nongenotoxic mechanism.
Biomarkers for acrylamide ingestion were also used in a pilot study to investigate the possible connection between chronic ingestion of acrylamide-containing potato chips and oxidative stress or inflammation (Naruszewicz et al. 2009). Fourteen healthy volunteers were given 160 g of potato chips containing 157 mg acrylamide daily for 4 week. An increase in acrylamide-hemoglobin adducts in blood was found in all the study subjects. Concurrently, a significant increase in the oxidized LDL, high-sensitivity interleukin-6, high-sensitivity C-reactive protein, and c-glutamyltransferase concentrations was observed in both smokers and nonsmokers. A significant increase in reactive oxygen radical production by monocytes, lymphocytes, and granulocytes and an increase in CD14 expression in macrophages were found after intake of potato chips. Twenty-eight days from the discontinuation of the experiment, the variables under study decreased to some extent. The authors concluded that chronic ingestion of dietary acrylamide might induce oxidative stress in humans through leukocyte activation and increased production of reactive oxygen radicals. They also suggest that long-term ingestion of high-acrylamide doses with food may cause chronic inflammation and contribute to the development of early atherosclerosis as well as increase the risk of coronary artery disease. The need to corroborate this pilot study by others was noted.
The possibility that acrylamide might affect prenatal development of humans was examined in a study that used hemoglobin adducts of acrylamide and glycidamide in umbilical cord blood as biomarkers for acrylamide exposure in utero (Pedersen et al. 2012). The study included 1,101 women from five countries who gave birth from 2006 to 2010. The amounts of acrylamide and glycidamide adducts in cord blood were found to correlate with the amount in maternal blood and with acrylamide intake of mothers based on food frequency questionnaires. Higher levels of acrylamide and glycidamide adducts in cord blood were also associated with a significant decrease in birth weight. The estimated difference in birth weights for infants in the lowest versus highest quartile of acrylamide adducts after adjusting for gestational age and country was 132 g for the study as a whole and 107 g for non-smokers. Adjustments for factors such as infant gender and mother’s pre-pregnancy body mass index, age, previous children, ethnicity, education and dietary pattern did not alter the association between highest acrylamide quartiles and lowest birth weight. These data do not prove that increased acrylamide exposure reduces birth weight, as noted by the authors, since acrylamide adducts may have been acting as proxy markers for another dietary exposure or mix of exposures that were responsible for the associations observed, or for a less healthy diet in general (Pedersen et al. 2012). Despite this caveat, they conclude that, if confirmed, their findings suggest that dietary intake of acrylamide should be reduced among pregnant women.
Summary
In the decade since acrylamide was first discovered in food, considerable progress has been made in our understanding of acrylamide formation chemistry, how potato processing variables influence final product acrylamide content, and how production practices and potato storage conditions influence acrylamide-forming potential of potato tubers. The central importance of raw product characteristics and the need for potato varieties that consistently maintain low tuber reducing sugar and asparagine contents has been demonstrated clearly in numerous research reports. Equally important may be the need to implement potato processing techniques that have the greatest potential to limit acrylamide formation. The consequences of acrylamide ingestion on human health are less well understood despite intensive research efforts in this area. Uncertainty remains with regard to the need for aggressive measures to reduce acrylamide in processed potato products and the potential for regulatory actions to limit acrylamide content in foods. An acrylamide mitigation portfolio will necessarily include deployment of potato varieties that consistently maintain low reducing sugar contents in tubers with few defects in final products. These goals are aligned with long-standing needs of the potato industry; acrylamide mitigation efforts in these areas will provide benefits to potato producers, processors and consumers that do not depend on their ability to produce low acrylamide products.
References
Amrein, T., S. Bachmann, A. Noti, M. Biedermann, M. Barbosa, S. Biedermann-Brem, K. Grob, A. Keiser, P. Realini, F. Escher, and R. Amado. 2003. Potential of acrylamide formation, sugars, and free asparagine in potatoes: a comparison of cultivars and farming systems. Journal of Agriculture and Food Chemistry 51: 5556–5560.
Amrein, T.M., A. Limacher, B. Conde-Petit, R. Amado, and F. Escher. 2006. Influence of thermal processing conditions on acrylamide generation and browning in a potato model system. Journal of Agriculture and Food Chemistry 54: 5910–5916.
Arribas-Lorenzo, G., and F.J. Morales. 2009. Dietary exposure to acrylamide from potato crisps to the Spanish population. Food Additives and Contaminants Part A 26(3): 289–297.
Auld, R.B., and S.F. Bedwell. 1967. Peripheral neuropathy with sympathetic overactivity from industrial contact with acrylamide. Canadian Medical Association Journal 96: 652–654.
Baardseth, P., H. Blom, G. Skrede, L.T. Mydland, A. Skrede, and E. Slinde. 2006. Lactic acid fermentation reduces acrylamide formation and other maillard reactions in french fries. Journal of Food Science 71: C28–C33.
Baijal, B.D., and W.F. Vliet. 1966. The chemical composition in different parts of the potato tuber during storage. European Potato Journal 9: 179–192.
Bassama, J., P. Brat, P. Bohuon, R. Boulanger, and Z. Guenata. 2010. Study of acrylamide mitigation in model system: effect of pure phenolic compounds. Food Chemistry 123: 558–562.
Becalski, A., R. Stadler, S. Hayward, S. Kotello, T. Krakalovich, B.P.Y. Lau, V. Roscoe, S. Schroeder, and R. Trelka. 2010. Antioxidant capacity of potato chips and snapshot trends in acrylamide content in potato chips and cereals on the Canadian market. Food Additives & Contaminants Part A - Chemistry, Analysis, Control, Exposure & Risk Assessment 27: 1193–1198.
Berger, F.I., J. Feld, D. Bertow, G. Eisenbrand, G. Fricker, N. Gerhardt, K.-H. Merz, E. Richling, and M. Baum. 2011. Biological effects of acrylamide after daily ingestion of various foods in comparison to water: a study in rats. Molecular Nutrition & Food Research 55: 387–399.
Bergmark, E. 1997. Hemoglobin adducts of acrylamide and acrylonitrile in laboratory workers, smokers and nonsmokers. Chemical Research in Toxicology 10: 78–84.
Bergmark, E., C.J. Calleman, F. He, and L.G. Costa. 1993. Determination of hemoglobin adducts in humans occupationally exposed to acrylamide. Toxicology and Applied Pharmacology 120: 45–54.
Bethke, P.C., and J.S. Busse. 2010. Vine-kill treatment and harvest date have persistent effects on tuber physiology. American Journal of Potato Research 87: 299–309.
Bhaskar, P.B., L. Wu, J.S. Busse, B.R. Whitty, A.J. Hamernik, S.H. Jansky, C.R. Buell, P.C. Bethke, and J. Jiang. 2010. Suppression of the vacuolar invertase gene prevents cold-induced sweetening in potato. Plant Physiology 154: 939–948.
Biedermann, M., F. Grundbock, K. Fiselier, S. Biedermann, C. Burgi, and K. Grob. 2010. Acrylamide monitoring in Switzerland, 2007–2009: results and conclusions. Food Additives & Contaminants Part A - Chemistry, Analysis, Control, Exposure & Risk Assessment 27: 1352–1362.
Brathen, E., A. Kita, S. Knutsen, and T. Wicklund. 2005. Addition of glycine reduces the content of acrylamide in cereal and potato products. Journal of Agricultural and Food Chemistry 53: 3259–3264.
Brown, C. 2005. Antioxidants in potato. American Journal of Potato Research 82: 163–172.
Bull, R., M. Robinson, and J.A. Stober. 1984a. Carcinogenic activity of acrylamide in the skin and lung of Swiss-ICR mice. Cancer Letters 24: 209–212.
Bull, R.J., M. Robinson, R.D. Laurie, G.D. Stoner, E. Greisiger, J.R. Meier, and J. Stober. 1984b. Carcinogenic effects of acrylamide in Sencar and A/J mice. Cancer Research 44: 107. AACR.
Burch, R.S., A. Trzesicka, M. Clarke, J.S. Elmore, A. Briddon, W. Matthews, and N. Webber. 2008. The effects of low-temperature potato storage and washing and soaking pre-treatments on the acrylamide content of French fries. Journal of the Science of Food and Agriculture 88: 989–995.
Burley, V.J., D.C. Greenwood, S.J. Hepworth, L.K. Fraser, T.M. de Kok, S.G. van Breda, S.A. Kyrtopoulos, M. Botsivali, J. Kleinjans, P.A. McKinney, and J.E. Cade. 2010. Dietary acrylamide intake and risk of breast cancer in the UK women’s cohort. British Journal of Cancer 103: 1749–1754.
Calleman, C.J., E. Bergmark, and L.G. Costa. 1990. Acrylamide is metabolized to glycidamide in the rat: evidence from hemoglobin adduct formation. Chemical Research in Toxicology 3: 406–412.
Chawla, R., R. Shakya, and C.M. Rommens. 2012. Tuber-specific silencing of asparagine synthetase-1 reduces the acrylamide-forming potential of potatoes grown in the field without affecting tuber shape and yield. Plant Biotechnology Journal 10: 913–924.
Cheng, K.-W., J.-J. Shi, S.-Y. Ou, M. Wang, and Y. Jiang. 2010. Effects of fruit extracts on the formation of acrylamide in model reactions and fried potato crisps. Journal of Agricultural and Food Chemistry 58: 309–312.
Chuda, Y., H. Ono, H. Yada, A. Ohara-Takada, C. Matsuura-Endo, and M. Mori. 2003. Effects of physiological changes in potato tubers (Solanum tuberosum L.) after low temperature storage on the level of acrylamide formed in potato chips. Bioscience, Biotechnology, and Biochemistry 67: 1188–1190.
Ciesarova, Z., E. Kiss, and P. Boegl. 2006. Impact of L-asparaginase on acrylamide content in potato products. Journal of Food and Nutrition Research 45: 141–146.
Claus, A., R. Carle, and A. Schieber. 2008. Acrylamide in cereal products: a review. Journal of Cereal Science 47: 118–133.
Cole, C.S. 1975. Variation in dry matter between and within potato tubers. European Potato Journal 18: 28–37.
de Meulenaer, B., T. de Wilde, F. Mestdagh, Y. Govaert, W. Ooghe, S. Fraselle, K. Demeulemeester, C. van Peteghem, A. Calus, J.-M. Degroodt, and R. Verhe. 2008. Comparison of potato varieties between seasons and their potential for acrylamide formation. Journal of the Science of Food and Agriculture 88: 313–318.
de Vleeschouwer, K., I. van der Plancken, A. van Loey, and M.E. Hendrickx. 2006. Impact of pH on the kinetics of acrylamide formation/elimination reactions in model systems. Journal of Agricultural and Food Chemistry 54: 7847–7855. American Chemical Society.
de Vleeschouwer, K., I. van der Plancken, A. van Loey, and M.E. Hendrickx. 2008. Investigation of the influence of different moisture levels on acrylamide formation/elimination reactions using multiresponse analysis. Journal of Agricultural and Food Chemistry 56: 6460–6470.
de Vleeschouwer, K., I. Vanderplancken, A. Vanloey, and M. Hendrickx. 2009. Role of precursors on the kinetics of acrylamide formation and elimination under low moisture conditions using a multiresponse approach—Part II: competitive reactions. Food Chemistry 114: 535–546.
de Wilde, T., B. de Meulenaer, F. Mestdagh, Y. Govaert, S. Vandeburie, W. Ooghe, S. Fraselle, K. Demeulemeester, C. van Peteghem, A. Calus, J. Degroodt, and R. Verhe. 2005. Influence of storage practices on acrylamide formation during potato frying. Journal of Agricultural and Food Chemistry 53: 6550–6557.
Dearfield, K.L., C.O. Abernathy, M.S. Ottley, J.H. Brantner, and P.F. Hayes. 1988. Acrylamide: its metabolism, developmental and reproductive effects, genotoxicity, and carcinogenicity. Mutation Research 195: 45–77.
Denny, F., and N. Thornton. 1941. Potato varieties: sugar-forming characteristics of tubers in cold storage, and suitability for production of potato chips. 12:217–252. Contrib. Boyce Thompson Inst.
DiNovi, M. 2006. Draft action plan: Acrylamide in food. Available online at http://www.fda.gov/food/foodsafety/foodcontaminantsadulteration/chemicalcontaminants/acrylamide/default.htm.
Douches, D., D. Maas, K. Jastrzebski, and R. Chase. 1996. Assessment of potato breeding progress in the USA over the last century. Crop Science 36: 1544–1552.
EFSA. 2011. European Food Safety Authority; results on acrylamide levels in food from monitoring years 2007–2009 and exposure assessment. EFSA Journal 9: 2133–2181. Available online at http://www.efsa.europa.eu/efsajournal.
EFSA. 2012. Update on acrylamide levels in food from monitoring years 2007 to 2010. EFSA Journal 10: 2938. Available online at http://www.efsa.europa.eu/efsajournal.
Ehling, S., and T. Shibamoto. 2005. Correlation of acrylamide generation in thermally processed model systems of asparagine and glucose with color formation, amounts of pyrazines formed, and antioxidative properties of extracts. Journal of Agricultural and Food Chemistry 53: 4813–4819.
Elmore, J., G. Koutsidis, A. Dodson, D. Mottram, and B. Wedzicha. 2005. Measurement of acrylamide and its precursors in potato, wheat, and rye model systems. Journal of Agricultural and Food Chemistry 53: 1286–1293.
El-Sayyad, H.I., H.L. El-Gammal, L.A. Habak, H.M. Abdel-Galil, A. Fernando, R.L. Gaur, and A. Ouhtit. 2011a. Structural and ultrastructural evidence of neurotoxic effects of fried potato chips on rat postnatal development. Nutrition 27: 1066–1075.
El-Sayyad, H.I., M.H. Abou-Egla, F.I. El-Sayyad, H.A. El-Ghawet, R.L. Gaur, A. Fernando, M.H.G. Raj, and A. Ouhtit. 2011b. Effects of fried potato chip supplementation on mouse pregnancy and fetal development. Nutrition 27: 343–350.
Farre, E.M., A. Tiessen, U. Roessner, P. Geigenberger, R.N. Trethewey, and L. Willmitzer. 2001. Analysis of the compartmentation of glycolytic intermediates, nucleotides, sugars, organic acids, amino acids, and sugar alcohols in potato tubers using a nonaqueous fractionation method. Plant Physiology 127: 685–700.
FDA. 2006a. Survey data on acrylamide in food: total diet study results. fda.gov. US Food and Drug Administration. Available online at http://www.fda.gov/Food/FoodSafety/FoodContaminantsAdulteration/ChemicalContaminants/Acrylamide/default.htm.
FDA. 2006b. Survey data on acrylamide in food: individual food products. US Food and Drug Administration. Available online at http://www.fda.gov/Food/FoodSafety/FoodContaminantsAdulteration/ChemicalContaminants/Acrylamide/default.htm.
Fernandes, J.O., and C. Soares. 2007. Application of matrix solid-phase dispersion in the determination of acrylamide in potato chips. Journal of Chromatography. A 1175: 1–6.
Finotti, E., A. Bertone, and V. Vivanti. 2006. Balance between nutrients and anti-nutrients in nine Italian potato cultivars. Food Chemistry 99: 698–701.
FoodDrinkEurope. 2011. Food drink europe acrylamide toolbox 2011. Food Drink Europe. Available online at http://ec.europa.eu/food/food/chemical safety/contaminants/ciaa acrylamide toolbox 09.pdf.
Foot, R.J., N.U. Haase, K. Grob, and P. Gonde. 2007. Acrylamide in fried and roasted potato products: a review on progress in mitigation. Food Additives and Contaminants 24: 37–46.
Franke, K., U. Strijowski, and E.H. Reimerdes. 2009. Kinetics of acrylamide formation in potato powder. Journal of Food Engineering 90: 135–140.
Friedman, M., and C.E. Levin. 2008. Review of methods for the reduction of dietary content and toxicity of acrylamide. Journal of Agricultural and Food Chemistry 56: 6113–6140.
Friedman, M.A., L.H. Dulak, and M.A. Stedham. 1995. A lifetime oncogenicity study in rats with acrylamide. Toxicological Sciences 27: 95–105.
Garland, T.O., and M.W. Patterson. 1967. Six cases of acrylamide poisoning. British Medical Journal 4: 134–138.
Gerendas, J., F. Heuser, and B. Sattelmacher. 2007. Influence of nitrogen and potassium supply on contents of acrylamide precursors in potato tubers and on acrylamide accumulation in french fries. Journal of Plant Nutrition 30: 1499–1516.
Glynne, M.D., and V.G. Jackson. 1919. The distribution of dry matter and nitrogen in the potato tuber. Variety, King Edward. Journal of Agricultural Science 9: 237–258.
Gokmen, V., and T.K. Palazoğlu. 2008. Acrylamide formation in foods during thermal processing with a focus on frying. Food and Bioprocess Technology 1: 35–42. Springer.
Gokmen, V., and H. Senyuva. 2006. Study of colour and acrylamide formation in coffee, wheat flour and potato chips during heating. Food Chemistry 99: 238–243.
Gokmen, V., H. Senyuva, J. Acar, and K. Sarioglu. 2005. Determination of acrylamide in potato chips and crisps by high-performance liquid chromatography. Journal of Chromatography. A 1088: 193–199.
Gokmen, V., B. Akbudak, A. Serpen, J. Acar, Z.M. Turan, and A. Eris. 2007. Effects of controlled atmosphere storage and low-dose irradiation on potato tuber components affecting acrylamide and color formations upon frying. European Food Research and Technology 224: 681–687.
Granda, C., and R. Moreira. 2005. Kinetics of acrylamide formation during traditional and vacuum frying of potato chips. Journal of Food Process Engineering 28: 478–493.
Granda, C., R. Moreira, and S. Tichy. 2004. Reduction of acrylamide formation in potato chips by low-temperature vacuum frying. Journal of Food Science 69: E405–E411.
Haase, N., B. Matthaus, and K. Vosmann. 2003. Acrylamide formation in foodstuffs—Minimising strategies for potato crisps. Deutsche Lebensmittel-Rundschau 99: 87–90.
Haase, N., B. Matthaus, and K. Vosmann. 2004. Aspects of acrylamide formation in potato crisps. Journal of Applied Botany and Food Quality-Angewandte Botanik 78: 144–147.
Habib, A., and H. Brown. 1956. Factors influencing the color of potato chips. Food Technology 10: 322–335. Food Technol.
Halford, N.G., N. Muttucumaru, S.J. Powers, P.N. Gillatt, L. Hartley, J.S. Elmore, and D.S. Mottram. 2012. Concentrations of free amino acids and sugars in nine potato varieties: effects of storage and relationship with acrylamide formation. Journal of Agricultural and Food Chemistry 60: 12044–12055.
Haynes, K.G., B.A. Clevidence, D. Rao, B.T. Vinyard, and J.M. White. 2010. Genotype x environment interactions for potato tuber carotenoid content. Journal of the American Society for Horticultural Science 135: 250–258.
Haynes, K.G., B.A. Clevidence, D. Rao, and B.T. Vinyard. 2011. Inheritance of carotenoid content in tetraploid x diploid potato crosses. Journal of the American Society for Horticultural Science 136: 265–272.
Hedegaard, R.V., K. Granby, H. Frandsen, J. Thygesen, and L.H. Skibsted. 2008. Acrylamide in bread. Effect of prooxidants and antioxidants. European Food Research and Technology 227: 519–525.
Hendriksen, H.V., B.A. Kornbrust, P.R. Østergaard, and M.A. Stringer. 2009. Evaluating the potential for enzymatic acrylamide mitigation in a range of food products using an asparaginase from Aspergillus oryzae. Journal of Agricultural and Food Chemistry 57: 4168–4176.
Herrman, T.J., B. Shafii, S.L. Love, and R.B. Dwelle. 1995. Influence of crop management factors on chipping potato maturity and storage processing performance. Journal of the Science of Food and Agriculture 68: 51–58.
Higley, J., J.-Y. Kim, K.C. Huber, and G. Smith. 2012. Added versus accumulated sugars on color development and acrylamide formation in french-fried potato strips. Journal of Agricultural and Food Chemistry 60: 8763–8771.
Hippe, J. 1988. HPLC-analysis of the concentrations of free asparagine and glutamine in potato tubers grown with varying amounts of nitrogen. Potato Research 31: 535–540.
Hogervorst, J.G., L.J. Schouten, E.J. Konings, R.A. Goldbohm, and P.A. van den Brandt. 2007. A prospective study of dietary acrylamide intake and the risk of endometrial ovarian, and breast cancer. Cancer Epidemiology, Biomarkers & Prevention 16: 2304–2313.
Hogervorst, J.G.F., B.-J. Baars, L.J. Schouten, E.J.M. Konings, R.A. Goldbohm, and P.A. van den Brandt. 2010. The carcinogenicity of dietary acrylamide intake: a comparative discussion of epidemiological and experimental animal research. Critical Reviews in Toxicology 40: 485–512.
IARC. 1999. Acrylamide. IRAC monographs. Vol. 60. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Available online at http://monographs.iarc.fr/ENG/Monographs/vol60/volume60.pdf.
Johnson, K., S. Gorzinski, K. Bodner, R. Campbell, C. Wolf, M. Friedman, and R. Mast. 1986. Chronic toxicity and oncogenicity study on acrylamide incorporated in the drinking-water of Fischer 344 rats. Toxicology and Applied Pharmacology 85: 154–168.
Kita, A., E. Bråthen, S.H. Knutsen, and T. Wicklund. 2004. Effective ways of decreasing acrylamide content in potato crisps during processing. Journal of Agriculture and Food Chemistry 52: 7011–7016.
Knol, J.J., G.A.I. Viklund, J.P.H. Linssen, I.M. Sjoholm, K.I. Skog, and M.A.J.S. van Boekel. 2008. A study on the use of empirical models to predict the formation of acrylamide in potato crisps. Molecular Nutrition & Food Research 52: 313–321.
Knol, J.J., G.A.I. Viklund, J.P.H. Linssen, I.M. Sjoholm, K.I. Skog, and M.A.J.S. van Boekel. 2009. Kinetic modelling: a tool to predict the formation of acrylamide in potato crisps. Food Chemistry 113: 103–109.
Knol, J.J., J.P.H. Linssen, and M.A.J.S. van Boekel. 2010. Unravelling the kinetics of the formation of acrylamide in the Maillard reaction of fructose and asparagine by multiresponse modelling. Food Chemistry 120: 1047–1057.
Knutsen, S.H., S. Dimitrijevic, E.L. Molteberg, V.H. Segtnan, L. Kaaber, and T. Wicklund. 2009. The influence of variety, agronomical factors and storage on the potential for acrylamide formation in potatoes grown in Norway. Lwt-Food Science and Technology 42: 550–556.
Koyama, N., M. Yasui, A. Kimura, S. Takami, T. Suzuki, K. Masumura, T. Nohmi, S. Masuda, N. Kinae, T. Matsuda, T. Imai, and M. Honma. 2011. Acrylamide genotoxicity in young versus adult gpt delta male rats. Mutagenesis 26: 545–549.
Kumar, D., B. Singh, and P. Kumar. 2004. An overview of the factors affecting sugar content of potatoes. Annals of Applied Biology 145: 247–256.
Larsson, S.C., A. Akesson, and A. Wolk. 2009. Long-term dietary acrylamide intake and risk of epithelial ovarian cancer in a prospective cohort of Swedish women. Cancer Epidemiology, Biomarkers & Prevention 18: 994–997.
Lindsay, R., and S. Jang. 2005. Chemical intervention strategies for substantial suppression of acrylamide formation in fried potato products. Chemistry and Safety of Acrylamide in Food 561: 393–404.
Lineback, D.R., J.R. Coughlin, and R.H. Stadler. 2012. Acrylamide in foods: a review of the science and future considerations. Annual Review of Food Science and Technology 3: 15–35.
Liu, X., C. Zhang, Y. Ou, Y. Lin, B. Song, C. Xie, J. Liu, and X.-Q. Li. 2011. Systematic analysis of potato acid invertase genes reveals that a cold-responsive member, StvacINV1, regulates cold-induced sweetening of tubers. Molecular Genetics and Genomics 286: 109–118.
LoPachin, R. 2005. Acrylamide Neurotoxicity: neurological, morhological and molecular endpoints in animal models. In Chemistry and safety of acrylamide in food. Vol. 561, Advances in experimental medicine and biology. Chemistry and safety of acrylamide in food, eds. M.A. Friedman and D.S. Mottram, 21–37.
Love, S.L., J. Pavek, A. Thompson-Johns, and W. Bohl. 1998. Breeding progress for potato chip quality in North American cultivars. American Journal of Potato Research 75: 27–36.
Low, M.Y., G. Koutsidis, J.K. Parker, J.S. Elmore, A.T. Dodson, and D.S. Mottram. 2006. Effect of citric acid and glycine addition on acrylamide and flavor in a potato model system. Journal of Agricultural and Food Chemistry 54: 5976–5983.
Marsh, G.M., L.J. Lucas, A.O. Youk, and L.C. Schall. 1999. Mortality patterns among workers exposed to acrylamide: 1994 follow up. Occupational and Environmental Medicine 56: 181–190.
Matsuura-Endo, C., A. Ohara-Takada, Y. Chuda, H. Ono, H. Yada, M. Yoshida, A. Kobayashi, S. Tsuda, S. Takigawa, T. Noda, H. Yamauchi, and M. Mori. 2006. Effects of storage temperature on the contents of sugars and free amino acids in tubers from different potato cultivars and acrylamide in chips. Bioscience, Biotechnology, and Biochemistry 70: 1173–1180.
McCann, L.C., P.C. Bethke, and P.W. Simon. 2010. Extensive variation in fried chip color and tuber composition in cold-stored tubers of wild potato (solanum) germplasm. Journal of Agricultural and Food Chemistry 58: 2368–2376.
Merlo, L., P. Geigenberger, M.-R. Hajirezaei, and M. Stitt. 1993. Changes of carbohydrates, metabolites and enzyme activities in potato tubers during development, and within a single tuber along a stolon-apex gradient. Journal of Plant Physiology 142: 392–402.
Mestdagh, F., B. de Meulenaer, C. van Poucke, C. Cromphout, and C. van Peteghem. 2005. Influence of oil type on the amounts of acrylamide generated in a model system and in french fries. Journal of Agricultural and Food Chemistry 53(15): 6170–6174.
Mestdagh, F., T. Wilde, P. Castelein, O. Nemeth, C. Peteghem, and B. Meulenaer. 2007a. Impact of the reducing sugars on the relationship between acrylamide and Maillard browning in French fries. European Food Research and Technology 227: 69–76.
Mestdagh, F., B. de Meulenaer, and C. van Peteghem. 2007b. Influence of oil degradation on the amounts of acrylamide generated in a model system and in French fries. Food Chemistry 100(3): 1153–1159.
Mestdagh, F., P. Castelein, C. van Peteghem, and B. de Meulenaer. 2008a. Importance of oil degradation components in the formation of acrylamide in fried foodstuffs. Journal of Agricultural and Food Chemistry 56(15): 6141–6144.
Mestdagh, F., J. Maertens, T. Cucu, K. Delporte, C. van Peteghem, and B. de Meulenaer. 2008b. Impact of additives to lower the formation of acrylamide in a potato model system through pH reduction and other mechanisms. Food Chemistry 107: 26–31.
Mestdagh, F., T. de Wilde, K. Delporte, C. van Peteghem, and B. de Meulenaer. 2008c. Impact of chemical pre-treatments on the acrylamide formation and sensorial quality of potato crisps. Food Chemistry 106: 914–922.
Michalak, J., E. Gujska, and J. Klepacka. 2011. The effect of domestic preparation of some potato products on acrylamide content. Plant Foods for Human Nutrition 66: 307–312.
Mills, C., C. Tlustos, R. Evans, and W. Matthews. 2008. Dietary acrylamide exposure estimates for the United Kingdom and Ireland: comparison between semiprobabilistic and probabilistic exposure models. Journal of Agricultural and Food Chemistry 56(15): 6039–6045.
Mojska, H., I. Gielecińska, L. Szponar, and M. Ołtarzewski. 2010. Estimation of the dietary acrylamide exposure of the Polish population. Food and Chemical Toxicology 48(8–9): 2090–2096.
Morales, F., E. Capuano, and V. Fogliano. 2008. Mitigation strategies to reduce acrylamide formation in fried potato products. Maillard Reaction: Recent Advances in Food and Biomedical Sciences 1126: 89–100.
Mottram, D.S., B. Wedzicha, and A. Dodson. 2002. Acrylamide is formed in the Maillard reaction. Nature 419: 448–449.
Mucci, L.A. 2005. Acrylamide intake and breast cancer risk in Swedish women. JAMA : The Journal of the American Medical Association 293: 1326–1327.
Mucci, L.A., and H.-O. Adami. 2009. The plight of the potato: is dietary acrylamide a risk factor for human cancer? Journal of the National Cancer Institute 101: 618–621.
Mucci, L.A., P.W. Dickman, G. Steineck, H.O. Adami, and K. Augustsson. 2003. Dietary acrylamide and cancer of the large bowel, kidney, and bladder: absence of an association in a population-based study in Sweden. British Journal of Cancer 88: 84–89. Nature Publishing Group.
Mucci, L.A., P. Lindblad, G. Steineck, and H.-O. Adami. 2004. Dietary acrylamide and risk of renal cell cancer. International Journal of Cancer. Journal International du Cancer 109: 774–776.
Naruszewicz, M., D. Zapolska-Downar, A. Kosmider, G. Nowicka, M. Kozłowska-Wojciechowska, A.S. Vikstrom, and M. Tornqvist. 2009. Chronic intake of potato chips in humans increases the production of reactive oxygen radicals by leukocytes and increases plasma C-reactive protein: a pilot study. American Journal of Clinical Nutrition 89: 773–777.
Nixon, B., S. Stanger, B. Nixon, and S. Roman. 2012. Chronic exposure to acrylamide induces DNA damage in male germ cells of mice. Toxicological Sciences In press.
Ohara-Takada, A., C. Matsuura-Endo, Y. Chuda, H. Ono, H. Yada, M. Yoshida, A. Kobayashi, S. Tsuda, S. Takigawa, T. Noda, H. Yamauchi, and M. Mori. 2005. Change in content of sugars and free amino acids in potato tubers under short-term storage at low temperature and the effect on acrylamide level after frying. Bioscience, Biotechnology, and Biochemistry 69: 1232–1238.
Olesen, P.T., A. Olsen, H. Frandsen, K. Frederiksen, K. Overvad, and A. Tjonneland. 2008. Acrylamide exposure and incidence of breast cancer among postmenopausal women in the Danish Diet, Cancer and Health study. International Journal of Cancer. Journal International du Cancer 122: 2094–2100.
Olsson, K., R. Svensson, and C. Roslund. 2004. Tuber components affecting acrylamide formation and colour in fried potato: variation by variety, year, storage temperature and storage time. Journal of the Science of Food and Agriculture 84: 447–458.
Ou, S., Q. Lin, Y. Zhang, C. Huang, X. Sun, and L. Fu. 2008. Reduction of acrylamide formation by selected agents in fried potato crisps on industrial scale. Innovative Food Science & Emerging Technologies 9: 116–121.
Palazoğlu, T.K., and V. Gokmen. 2008. Development and experimental validation of a frying model to estimate acrylamide levels in French fries. Journal of Food Science 73: E109–E114.
Park, Y., H. Yang, J. Storkson, K. Albright, W. Liu, R. Lindsay, and M. Pariza. 2005. Controlling acrylamide in french fry and potato chip models and a mathematical model of acrylamide formation - Acrylamide: acidulants, phytate and calcium. In Vol. Advances in experimental medicine and biology 561. Chemistry and safety of acrylamide in food, eds. M. Friedman and D.S. Mottram, 343–356.
Parker, J.K., D.P. Balagiannis, J. Higley, G. Smith, B.L. Wedzicha, and D.S. Mottram. 2012. Kinetic model for the formation of acrylamide during the finish-frying of commercial french fries. Journal of Agricultural and Food Chemistry 60: 9321–9331.
Parzefall, W. 2008. Minireview on the toxicity of dietary acrylamide. Food and Chemical Toxicology 46: 1360–1364.
Pedersen, M., H. von Stedingk, M. Botsivali, S. Agramunt, J. Alexander, G. Brunborg, L. Chatzi, S. Fleming, E. Fthenou, B. Granum, K.B. Gutzkow, L.J. Hardie, L.E. Knudsen, S.A. Kyrtopoulos, M.A. Mendez, D.F. Merlo, J.K. Nielsen, P. Rydberg, D. Segerbäck, J. Sunyer, J. Wright, M. Tornqvist, J.C. Kleinjans, M. Kogevinas, and NewGeneris Consortium. 2012. Birth weight, head circumference, and prenatal exposure to acrylamide from maternal diet: the European prospective mother-child study (NewGeneris). Environmental Health Perspectives 120: 1739–1745.
Pedreschi, F., K. Kaack, and K. Granby. 2004. Reduction of acrylamide formation in potato slices during frying. Lebensmittel-Wissenschaft Und-Technologie-Food Science and Technology 37: 679–685.
Pedreschi, F., P. Moyano, K. Kaack, and K. Granby. 2005. Color changes and acrylamide formation in fried potato slices. Food Research International 38: 1–9.
Pedreschi, F., D. Mery, F. Mendoza, and J.M. Aguilera. 2006a. Classification of potato chips using pattern recognition. Journal of Food Science 69: E264–E270.
Pedreschi, F., J. Leon, D. Mery, and P. Moyano. 2006b. Development of a computer vision system to measure the color of potato chips. Food Research International 39: 1092–1098.
Pedreschi, F., K. Kaack, and K. Granby. 2006c. Acrylamide content and color development in fried potato strips. Food Research International 39: 40–46.
Pedreschi, F., K. Kaack, K. Granby, and E. Troncoso. 2007a. Acrylamide reduction under different pre-treatments in French fries. Journal of Food Engineering 79: 1287–1294.
Pedreschi, F., O. Bustos, D. Mery, P. Moyano, K. Kaack, and K. Granby. 2007b. Color kinetics and acrylamide formation in NaCl soaked potato chips. Journal of Food Engineering 79: 989–997.
Pedreschi, F., D. Mery, and T. Marique. 2008. Quality evaluation and control of potato chips and french fries. In Computer vision technology for food quality evaluation, ed. D.-W. Sun, 545–566. Elsevier Inc.
Pedreschi, F., K. Kaack, and K. Granby. 2008b. Acrylamide mitigation procedures in fried potatoes. Agro Food Industry Hi-Tech 19: 32–36.
Pedreschi, F., K. Kaack, and K. Granby. 2008c. The effect of asparaginase on acrylamide formation in French fries. Food Chemistry 109: 386–392.
Pedreschi, F., V.H. Segtnan, and S.H. Knutsen. 2010. On-line monitoring of fat, dry matter and acrylamide contents in potato chips using near infrared interactance and visual reflectance imaging. Food Chemistry 121: 616–620.
Pedreschi, F., S. Mariotti, K. Granby, and J. Risum. 2011. Acrylamide reduction in potato chips by using commercial asparaginase in combination with conventional blanching. Lwt-Food Science and Technology 44: 1473–1476.
Pelucchi, C., S. Franceschi, F. Levi, D. Trichopoulos, C. Bosetti, E. Negri, and C. La Vecchia. 2003. Fried potatoes and human cancer. International Journal of Cancer. Journal International du Cancer 105: 558–560.
Pelucchi, C., C. La Vecchia, S. Franceschi, and F. Levi. 2004. Fried potatoes and human cancer. International Journal of Cancer. Journal International du Cancer 108: 636–637.
Pelucchi, C., C. Galeone, F. Levi, E. Negri, S. Franceschi, R. Talamini, C. Bosetti, A. Giacosa, and C. La Vecchia. 2006. Dietary acrylamide and human cancer. International Journal of Cancer. Journal International du Cancer 118: 467–471.
Pelucchi, C., C. La Vecchia, C. Bosetti, P. Boyle, and P. Boffetta. 2011. Exposure to acrylamide and human cancer-a review and meta-analysis of epidemiologic studies. Annals of Oncology 22: 1487–1499.
Pisarczyk, J. 1982. Field harvest damage affects potato tuber respiration and sugar content. American Potato Journal 59: 205–211.
Pritchard, M., and L. Adam. 1992. Preconditioning and storage of chemically immature Russet Burbank and Shepody potatoes. American Potato Journal 69: 805–815.
Reddivari, L., A.L. Hale, and J.C.J. Miller. 2007. Determination of phenolic content, composition and their contribution to antioxidant activity in specialty potato selections. American Journal of Potato Research 84: 275–282.
Rice, J.M. 2005. The carcinogenicity of acrylamide. Mutation Research 580: 3–20.
Rommens, C.M., J. Ye, C. Richael, and K. Swords. 2006. Improving potato storage and processing characteristics through all-native DNA transformation. Journal of Agriculture and Food Chemistry 54: 9882–9887.
Rommens, C.M., H. Yan, K. Swords, C. Richael, and J. Ye. 2008. Low-acrylamide French fries and potato chips. Plant Biotechnology Journal 6: 843–853.
Rommens, C.M., R. Shakya, M. Heap, and K. Fessenden. 2010. Tastier and healthier alternatives to French fries. Journal of Food Science 75: H109–H115.
Rufian-Henares, J., and F. Morales. 2006. Determination of acrylamide in potato chips by a reversed-phase LC-MS method based on a stable isotope dilution assay. Food Chemistry 97: 555–562.
Rydberg, P., S. Eriksson, E. Tareke, P. Karlsson, L. Ehrenberg, and M. Tornqvist. 2003. Investigations of factors that influence the acrylamide content of heated foodstuffs. Journal of Agricultural and Food Chemistry 51: 7012–7018.
Sayre, R.N., M. Nonaka, and M.L. Weaver. 1975. French fry quality related to specific gravity and solids content variation among potato strips within the same tuber. American Potato Journal 52: 73–82. Springer-Verlag.
Seal, C.J., A. de Mul, G. Eisenbrand, A.J. Haverkort, K. Franke, S.P.D. Lalljie, H. Mykkänen, E. Reimerdes, G. Scholz, V. Somoza, S. Tuijtelaars, M. van Boekel, J. van Klaveren, S.J. Wilcockson, and L. Wilms. 2008. Risk-benefit considerations of mitigation measures on acrylamide content of foods–a case study on potatoes, cereals and coffee. The British Journal of Nutrition 99(Suppl 2): S1–S46.
Segtnan, V.H., A. Kita, M. Mielnik, K. Jorgensen, and S.H. Knutsen. 2006. Screening of acrylamide contents in potato crisps using process variable settings and near-infrared spectroscopy. Molecular Nutrition & Food Research 50: 811–817.
Senyuva, H., and V. Gokmen. 2006. Interference-free determination of acrylamide in potato and cereal-based foods by a laboratory validated liquid chromatography-mass spectrometry method. Food Chemistry 97: 539–545.
Serpen, A., and V. Gokmen. 2007. Modeling of acrylamide formation and browning ratio in potato chips by artificial neural network. Molecular Nutrition & Food Research 51: 383–389.
Serpen, A., and V. Gokmen. 2009. Evaluation of the Maillard reaction in potato crisps by acrylamide, antioxidant capacity and color. Journal of Food Composition and Analysis 22: 589–595.
Shepherd, L.V.T., J.E. Bradshaw, M.F.B. Dale, J.W. McNicol, S.D.A. Pont, D.S. Mottram, and H.V. Davies. 2010. Variation in acrylamide producing potential in potato: Segregation of the trait in a breeding population. Food Chemistry 123: 568–573.
Shiraishi, Y. 1978. Chromosome aberrations induced by monomeric acrylamide in bone marrow and germ cells of mice. Mutation Research 57: 313–324.
Shiroma, C., and L. Rodriguez-Solona. 2009. Application of NIR and MIR spectroscopy in quality control of potato chips. Journal of Food Composition and Analysis 22: 596–605.
Shock, C., Z. Holmes, T. Stieber, E. Eldredge, and P. Zhang. 1993. The effect of timed water stress on quality, total solids and reducing sugar content of potatoes. American Potato Journal 70: 227–241.
Silva, E., and P. Simon. 2005. Genetic, physiological, and environmental factors affecting acrylamide concentration in fried potato products. Chemistry and Safety of Acrylamide in Food 561: 371–386.
Sobel, W., G. Bond, T.W. Parsons, and F. Brenner. 1986. Acrylamide cohort mortality study. British Journal of Industrial Medicine 43: 785–788.
Sowokinos, J.R. 1973. Maturation of Solanum tuberosum.1. comparative sucrose and sucrose synthetase levels between several good and poor processing varieties. American Potato Journal 50: 234–247.
Sowokinos, J.R. 2001. Biochemical and molecular control of cold-induced sweetening in potatoes. American Journal of Potato Research 78: 221–236.
Sowokinos, J.R., and D.A. Preston. 1988. Maintenance of potato processing quality by chemical maturity monitoring (CMM). Minnesota Agricultural Experiment Station Bulletin AD-SB-3411, pp. 1–11.
Stadler, R., and G. Scholz. 2004. Acrylamide: An update on current knowledge in analysis, levels in food, mechanisms of formation, and potential strategies of control. Nutrition Reviews 62: 449–467.
Stadler, R., I. Blank, N. Varga, F. Robert, J. Hau, P. Guy, M. Robert, and S. Riediker. 2002. Acrylamide from Maillard reaction products. Nature 419: 449–450.
Stark, J., and S. Love. 2003. Potato production systems. University of Idaho. Center for Potato Research and Education, University of Idaho. Extension.
Stevenson, F.J. 1957. Red Dot Foods, Inc. and its potato research program. American Potato Journal 34: 136–141.
Tardiff, R.G., M.L. Gargas, C.R. Kirman, M.L. Carson, and L.M. Sweeney. 2010. Estimation of safe dietary intake levels of acrylamide for humans. Food and Chemical Toxicology 48: 658–667. Elsevier Ltd.
Tareke, E., P. Rydberg, P. Karlsson, S. Eriksson, and M. Tornqvist. 2000. Acrylamide: a cooking carcinogen? Chemical Research in Toxicology 13: 517–522. ACS Publications.
Tareke, E., P. Rydberg, P. Karlsson, S. Eriksson, and M. Tornqvist. 2002. Analysis of acrylamide, a carcinogen formed in heated foodstuffs. Journal of Agricultural and Food Chemistry 50: 4998–5006.
Thompson, A.L., S.L. Love, J.R. Sowokinos, M.K. Thornton, and C.C. Shock. 2008. Review of the sugar end disorder in potato (Solanum tuberosum, L.). American Journal of Potato Research 85: 375–386.
Vesper, H.W., S.P. Caudill, J.D. Osterloh, T. Meyers, D. Scott, and G.L. Myers. 2010. Exposure of the US population to acrylamide in the National Health and Nutrition Examination Survey 2003–2004. Environmental Health Perspectives 118: 278–283.
Viklund, G.A.I., K.M. Olsson, I.M. Sjoholm, and K.I. Skog. 2008a. Variety and storage conditions affect the precursor content and amount of acrylamide in potato crisps. Journal of the Science of Food and Agriculture 88: 305–312.
Viklund, G.A.I., K.M. Olsson, I.M. Sjoholm, and K.I. Skog. 2008b. Impact of harvest year on amino acids and sugars in potatoes and effect on acrylamide formation during frying. Journal of Agricultural and Food Chemistry 56: 6180–6184.
Viklund, G.A.I., K.M. Olsson, I.M. Sjoholm, and K.I. Skog. 2010. Acrylamide in crisps: effect of blanching studied on long-term stored potato clones. Journal of Food Composition and Analysis 23: 194–198.
Vinci, R.M., F. Mestdagh, N. de Muer, C. van Peteghem, and B. de Meulenaer. 2010. Effective quality control of incoming potatoes as an acrylamide mitigation strategy for the French fries industry. Food Additives & Contaminants Part A - Chemistry, Analysis, Control, Exposure & Risk Assessment 27: 417–425.
Vinci, R.M., F. Mestdagh, C. Van Poucke, B. Kerkaert, N. de Muer, Q. Denon, C. van Peteghem, and B. de Meulenaer. 2011. Implementation of acrylamide mitigation strategies on industrial production of French fries: challenges and pitfalls. Journal of Agricultural and Food Chemistry 59: 898–906.
Vinci, R.M., F. Mestdagh, and B. de Meulenaer. 2012. Acrylamide formation in fried potato products—present and future, a critical review on mitigation strategies. Food Chemistry 133: 1138–1154. Elsevier Ltd.
Wang, Y., and P.C. Bethke. 2013. Effects of infection on stem-end chip defect development in potatoes. Crop Science.
Wang, Y., A.J. Bussan, and P.C. Bethke. 2012. Stem-end defect in chipping potatoes (Solanum tuberosum L.) as influenced by mild environmental stresses. American Potato Journal 89: 392–399.
Weisshaar, R. 2004. Acrylamide in heated potato products—analytics and formation routes. European Journal of Lipid Science and Technology 106: 786–792.
Weisshaar, R., and B. Gutsche. 2002. Formation of acrylamide in heated potato products—model experiments pointing to asparagine as precursor. Deutsche Lebensmittel-Rundschau 98: 397–400.
Wenzl, T., L. Karasek, J. Rosen, K.-E. Hellenaes, C. Crews, L. Castle, and E. Anklam. 2006. Collaborative trial validation study of two methods, one based on high performance liquid chromatography-tandem mass spectrometry and on gas chromatography–mass spectrometry for the determination of acrylamide in bakery and potato products. Journal of Chromatography. A 1132: 211–218.
Whittaker, A., I. Marotti, G. Dinelli, L. Calamai, S. Romagnoli, M. Manzelli, E. Palchetti, V. Vecchio, and S. Benedettelli. 2010. The influence of tuber mineral element composition as a function of geographical location on acrylamide formation in different Italian potato genotypes. Journal of the Science of Food and Agriculture 90: 1968–1976.
WHO. 2011. Safety evaluation of certain contaminants in food. FAO JECFA Monographs 8. 7
Williams, J. 2005. Influence of variety and processing conditions on acrylamide levels in fried potato crisps. Food Chemistry 90: 875–881.
Wilson, K.M., L.A. Mucci, E. Cho, D.J. Hunter, W.Y. Chen, and W.C. Willett. 2009. Dietary acrylamide intake and risk of premenopausal breast cancer. American Journal of Epidemiology 169: 954–961.
Wu, L., P.B. Bhaskar, J.S. Busse, R. Zhang, P.C. Bethke, and J. Jiang. 2011. Developing cold-chipping potato varieties by silencing the vacuolar invertase gene. Crop Science 51: 981–990.
Ye, J., R. Shakya, P. Shrestha, and C.M. Rommens. 2010. Tuber-specific silencing of the acid invertase gene substantially lowers the acrylamide-forming potential of potato. Journal of Agricultural and Food Chemistry 58: 12162–12167.
Zhang, Y., and Y. Zhang. 2007. Formation and reduction of acrylamide in Maillard reaction: a review based on the current state of knowledge. Critical Reviews in Food Science and Nutrition 47: 521–542.
Zhou, X., L.-Y. Fan, W. Zhang, and C.-X. Cao. 2007. Separation and determination of acrylamide in potato chips by micellar electrokinetic capillary chromatography. Talanta 71: 1541–1545.
Zhu, F., Y.-Z. Cai, J. Ke, and H. Corke. 2010. Compositions of phenolic compounds, amino acids and reducing sugars in commercial potato varieties and their effects on acrylamide formation. Journal of the Science of Food and Agriculture 90: 2254–2262.
Acknowledgments
This review is part of NIFA SCRI research project 2011-51181-30829 “Improved breeding and variety evaluation methods to reduce acrylamide content and increase quality in processed potato products”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bethke, P.C., Bussan, A.J. Acrylamide in Processed Potato Products. Am. J. Potato Res. 90, 403–424 (2013). https://doi.org/10.1007/s12230-013-9321-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12230-013-9321-4