Abstract
The storage of potato tubers at low temperatures leads to the accumulation of sugars in a process called “low-temperature sweetening.” To understand this phenomenon, we measured the sugar contents and the activity of acid invertase over several months in tubers of six Japanese cultivars stored at 4°C or 20°C. At 20°C , few changes in sugar contents took place in any of the tubers. On the other hand, when stored at 4°C, three types of changes were observed among the cultivars: (1) increased levels of reducing sugars during storage; (2) a pattern similar to that of type 1, but with 4- to 6-fold lower levels of reducing sugars throughout storage; and (3) increased sucrose, but not reducing sugars. The activity of vacuolar acid invertase increased in the type-1 cultivars, whereas, in the type-2 and type-3 cultivars, the activities were very low during storage at 4°C. Reverse transcription-polymerase chain reaction analysis of acid invertase showed that the transcripts of the enzyme accumulated in the tubers stored at 4°C in the type-1 cultivars but not in type-3. These results suggest that the activity of vacuolar acid invertase is related to the types of changes that occurred in sugar content during low-temperature storage among the potato cultivars.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In general, potatoes are harvested after they have entered a rest period, and, even after that period ends, their dormancy can be extended forcibly by low-temperature storage. Because of this, potatoes can be supplied for a long period. The low-temperature storage of potato tubers causes an increase in reducing sugars; this phenomenon is known as “low-temperature sweetening” (Isherwood 1973; Burton 1989; Sowokinos 1990). The increase in reducing sugars has negative effects on the quality of fried potato products, because excess sugars react with amino acids during frying, leading to unacceptably dark color and harmful compounds (Burton 1989; Sowokinos 1990; Tareke et al. 2002; Chuda et al. 2003).
Studies of the carbohydrate metabolism in potato tubers have shown that the activities of several enzymes are closely related to the low-temperature sweetening. During low-temperature storage, starch breaks down and sucrose is formed via UDP-glucose pyrophosphorylase and sucrose-phosphate synthase (Pollock and ap Rees 1975; Sowokinos 1994; Hill et al. 1996); the sucrose is subsequently hydrolyzed by soluble acid invertases, yielding the reducing sugars (Pressey 1969a; Richardson et al. 1990; Zrenner et al. 1996). The extent of the sugar accumulation is dependent on the cultivar (Hammond et al. 1990; Richardson et al. 1990; Zrenner et al. 1996). Hammond et al. (1990) reported that inactivation of phosphofructokinase (PFK) at low temperature results in the accumulation of hexose phosphates, leading to increased sucrose synthesis, and that the PFKs from cultivars with different responses in the degree of sugar accumulation show different labilities to low temperature. Richardson et al. (1990) and Zrenner et al. (1996) have reported that the activity of acid invertase increases in tubers stored at low temperatures, and the activity shows different values between cultivars. Zrenner et al. (1996) found that there is no consistent relationship between invertase activity and total sugar accumulation but there is a correlation between this activity and the sucrose/hexose ratio.
We have been attempting to clarify the patterns of changes in sugar contents during storage and the relationship between the changes in the sugar and enzyme activities in each Japanese cultivar. In this study, six cultivars were used: Irish Cobbler, May Queen, and Kita-akari, which are commonly used as table potatoes; Toyoshiro, which is used for potato chips; White Fryer, an advanced breeding cultivar from Toyoshiro; and Inca-no-mezame, which is bred for extensive use and has yellowish orange flesh with a very high content of carotenoids. We measured sugar contents and activity of vacuolar acid invertase (EC 3.2.1.26) in the tubers of these six cultivars during the growing season and storage periods at 4°C and 20°C. We also determined whether the activity of acid invertase was controlled by its gene expression using the reverse transcription-polymerase chain reaction (RT-PCR) method. Here we first report that the patterns of sugar changes in these cultivars are classified into three types, and that these patterns are closely related to the activities of acid invertase.
Materials and methods
Potatoes (plant material)
Irish Cobbler, May Queen, Kita-akari and Toyoshiro belong to a commonly cultivated tetraploid species, Solanum tuberosum subsp. tuberosum. White Fryer is a progeny of ND 860-2 (North Dakota State University), which is a tetraploid breeding line with S. phureja in its background. Inca-no-mezame is a diploid hybrid derived from a cross between 2 lines: W822229-5 (a cross between haploid S. tuberosum subsp. tuberosum cv. Katahdin and S. phureja) and P10173-5 (a cross between two haploids of S. tuberosum subsp. andigena). These six cultivars were planted in a field of the Department of Upland Agriculture (Memuro, Hokkaido, Japan) in late April, and harvested in early September 1998, 1999, 2000, and 2001. Fertilizer, herbicides, fungicides and all other agronomic measures were used according to the local methods. The weather conditions during the growing seasons were normal, and each cultivar showed the typical growth pattern and yield over the 4-year experiment. After harvesting, tubers were cured for 2 weeks at 15°C in the dark; healthy tubers weighing between 80 g and 100 g were selected from each cultivar and stored at 4°C or 20°C in 90–95% relative humidity in the dark. During the growing season, a total of approximately 300 g fresh weight of immature tubers, of representative size of each stage, was taken from five to ten plants; after harvesting, three selected tubers were used for analyses at each sampling date. The tubers were chopped crosswise into slices (1×1 cm, 1 mm thick) and randomized. For RNA isolation, ca. 1 g of the slices was frozen immediately in liquid nitrogen and stored at −80°C until use. For assay of enzyme activity, ca. 10 g of the slices was used immediately for extracting crude enzyme fractions. The remainder was frozen at −40°C until use for sugar extraction.
Extraction and determination of sugars
Frozen tuber slices (ca. 10 g fresh weight) were homogenized with an Ultra-Turrax disperser (model T25; IKA Works, Selangor, Malaysia) for 5 min in 40 ml 80% (v/v) ethanol, and sugars in the homogenate were extracted at 80°C for one hour. The extract was filtered through four layers of gauze, and the filtrate was centrifuged at 10,000 g at 4°C for 20 min. The resultant supernatant (ethanol extract) was dried under a vacuum, then dissolved in distilled water, and filtered through a 0.2-μm Omnipore membrane filter (Millipore, Tokyo, Japan). The filtrate’s contents of glucose, fructose, and sucrose were determined by high performance liquid chromatography (HPLC) with a TSKgel Amide-80 column (Tosoh, Tokyo, Japan); the mobile phase was 80% (v/v) acetonitrile/water, and the pump was set at a flow rate of 1.0 ml/min. Quantification of sugars in samples was performed by standardization with external glucose, fructose, and sucrose.
Preparation of the crude enzyme fraction
Tuber slices (ca. 10 g fresh weight) were homogenized with an Ultra-Turrax disperser (IKA) for 2 min in a homogenization medium (25 ml) containing 50 mM 4-(2-hydroxyethyl)-1-piperazinepropanesulfonic acid (HEPES)-KOH (pH 7.5), 5 mM MgCl2, 1 mM ethylenediamine-N,N,N′,N′-tetraacetic acid (EDTA), 1 mM ethyleneglycolbis-N,N,N′,N′-tetraacetic acid (EGTA), 1 mM phenylmethylsulphonylfluoride (PMSF), 5 mM dithiothreitol, 0.1% (w/v) Triton X-100, and 10% (w/v) glycerol. The homogenate was filtered through four layers of gauze, and the filtrate was centrifuged at 10,000 g at 4°C for 5 min. The resultant supernatant was desalted using a column of Sephadex G-25 (Amersham Pharmacia, Uppsala, Sweden) equilibrated in the homogenization medium minus PMSF. The eluted samples were stored at −80°C until use.
Enzyme activities
Crude extracts of potato tubers usually contain a small protein that has an inhibitory effect on soluble acid invertase activity (Pressey 1967; Ewing and McAdoo 1971; Bracho and Whitaker 1990). However, Ross and Davies (1992) have pointed out that techniques such as rapid vortexing and foaming of extracts, which were developed to destroy the inhibitor, are severe and that a possible loss of invertase could occur. In this study, the enzyme activity was assayed without destroying the inhibitor effect. The activity of vacuolar acid invertase was assayed by measuring reducing sugar released from sucrose. The reaction mixture (250 μl) contained 100 mM sucrose, 20 mM Na-acetate (pH 4.7), and the desalted sample (ca. 100 μg protein). The mixture was incubated at 30°C for 60 min, and the reaction was terminated by adding 250 μl Somogyi copper solution (Wako, Osaka, Japan) and boiling for 15 min. Then, 250 μl Nelson solution (Wako) and 4 ml distilled water were added to the mixture, and the absorbance of the sample at 660 nm was determined. Controls had the same reaction mixture but were heat-inactivated without incubation. The standard curve was established using fructose.
Protein content
Protein content was measured by the method of Lowry et al. (1951) after precipitation with 10% (w/v) trichloroacetic acid.
RNA isolation
Frozen tuber slices were ground into fine powder in liquid nitrogen, and total RNA was isolated from the powder by extraction with phenol/sodium dodecyl sulfate and precipitation with lithium chloride (Shirzadegan et al. 1991).
RT-PCR amplification
Single-stranded cDNAs were synthesized from the total RNA by priming with the adapter-oligonucleic primer, AP (5′-GGC CAC GCG TCG AGT ACT (T)16-3′). One μg total RNA (with added water to a final volume of 5.5 μl) was incubated at 65°C for 10 min and transferred to ice. The following mixture was then added and incubated at 42°C for one hour: 4 μl 5× first-strand buffer (Gibco BRL Life Technologies, UK), 1 μl AP (20 pmol), 8 μl dNTP mixture (1 mM), 0.5 μl RNase inhibitor (20 U; TOYOBO, Osaka, Japan), and 1 μl M-MLV reverse transcriptase (200 units ;Gibco BRL). The reaction was stopped by incubation at 95°C for 5 min. The cDNAs were then used as templates for amplification using a pair of primers, AI-3-M (forward primer, 5′-TGG ATA TAG AAG CCT CAT TT-3′) and AI-3-L (reverse primer, 5′-TAG CCC CTG TGC GAT TGT TG-3′). These primer sequences were designed based on the genomic sequences of the highly conserved regions within the already known vacuolar acid invertase of potatoes (GenBank accession numbers L29099 [Zhou et al. 1994] and X70368 [Zrenner et al. 1996]). PCR was performed with an Expand High Fidelity PCR System (Roche Molecular Biochemicals, Mannheim, Germany) using 5 μl of the synthesized cDNAs and the primer set, AI-3-M and AI-3-L (4 μl, 80 pmol each). After heating at 94°C for 10 min, the PCR reaction proceeded for 25 cycles (94°C, 30 s for denaturation; 55°C, 30 s for annealing; and 72°C, 45 s for extension). Following the last cycle, the reaction mixture was incubated at 72°C for 7 min. The PCR product was analyzed by electrophoresis in 1.5% (w/v) agarose gels. A 433-bp fragment of acid invertase was amplified from the RT-PCR. Sequence analysis using the BLASTN program of the National Center for Biotechnology Information (NCBI) database showed that the 433-bp fragment is 97% identical to the corresponding region of the gene L29099 and also 97% identical to that of the gene X70368 (data not shown).
The data shown in this paper were taken in 1999, as a representative of experiments that were carried out over 4 years. Similar patterns were also observed in the 3 other years.
Results
Changes in the sugar content of potato tubers during growth and storage
Changes in the sugar content of tubers from six cultivars during the growth and the storage are shown in Fig. 1.
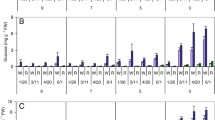
Changes in sugar contents of potato tubers during the growing season and storage period. Seed tubers were planted in late April, and mature tubers were harvested in early September, indicated by arrows. After curing of tubers for 2 weeks, storage was started at the date indicated as “day 0” on the x-axis. During the growing season, approximately 300 g total fresh weight of immature tubers, of representative size for each stage, were taken from five to ten plants; after harvesting, three tubers weighing between 80 g and 100 g were used at each sampling date. Sugars were extracted from tubers in 80% ethanol, and the sugar contents were determined by high performance liquid chromatography (HPLC). Upper panels storage at 20°C; lower panels storage at 4°C. A May Queen. B Irish Cobbler. C Kita-akari. D Toyoshiro. E White Fryer. F Inca-no-mezame. Values are the means of three replicates
Growth period
In young developing tubers (approx. 30 days after emergence, approx. 10 g fresh weight), the content of glucose was high. The content then decreased upon tuber growth and maturation in all cultivars. On the other hand, the fructose content was low throughout the growing period. The ratio of glucose to fructose was high in young tubers, from approximately 30 to 130 among the cultivars examined, and then decreased to the minimum values, which were from 1 to 8 in all cultivars at the harvest date. The sucrose content was high in young tubers; the values were higher than those of glucose, and decreased to a level approximately equivalent to the glucose level up to the harvest date. Burton (1989) reported that the minimum contents of reducing sugars in tubers are reached before the end of the growing season and slight increases occur thereafter. Similar patterns of changes in sugar were obtained in this study.
Storage period
At 20°C, there were little changes in the sugar contents during the dormant periods; the contents were below 1 mg/g fresh weight in tubers of all cultivars. This suggests a very low metabolic activity during the dormant period, as reported by Espen et al. (1999). A slight increase in the sucrose content was observed at the time of sprouting in all cultivars, which was in agreement with the results described by Burton (1989).
When stored at 4°C, there were three types of change in the sugar content, as follows: (1) an increased level of reducing sugars during storage; (2) low levels of reducing sugars throughout storage; and (3) increased sucrose but not reducing sugars.
In the type-1 cultivars (May Queen [Fig. 1A], Irish Cobbler [Fig. 1B], Kita-akari [Fig. 1C], and Toyoshiro [Fig. 1D]), firstly, sucrose began to increase rapidly, reaching a peak within 2 weeks (about 4 mg/g fresh weight) before gradually decreasing. Glucose and fructose increased about 1 week later than sucrose, and reached a peak within approximately 60 days; the levels then remained relatively stable at similar levels or increased slightly. The level of reducing sugars was 2- to 4-times higher than the sucrose level after 30 days of storage. After 250 days of storage, the total volume of sugar reached approximately 22 mg/g fresh weight in May Queen (Fig. 1A), 14 mg/g fresh weight in tubers of Irish Cobbler (Fig. 1B) and Kita-akari (Fig. 1C), and 12 mg/g fresh weight in Toyoshiro (Fig. 1D). The pattern of changes shown in the type-1 cultivars was consistent with the results reported by other researchers (Richardson et al. 1990; Hill et al. 1996).
The type-2 cultivar White Fryer (Fig. 1E) showed a pattern that is similar to that of type 1. Sucrose began to increase rapidly and peaked within 2 weeks (about 6 mg/g fresh weight), but then decreased. The reducing sugars also increased about 1 week later than sucrose, and peaked within approximately 60 days, before gradually decreasing. The levels of reducing sugars were 4- to 6-fold lower than those of the type-1 cultivars throughout the storage period. The total sugar content was around 3.6 mg/g fresh weight after 250 days of storage. The White Fryer is the progeny of ND 860-2, which is a so-called, “low sugar-accumulating, cold-tolerant” breeding line. The data obtained in White Fryer were similar to those observed in studies of cold-tolerant lines, ND 860-2 (Blenkinsop et al. 2002), clone 13737 1 and 13676ab 1 (Richardson et al. 1990).
In the type-3 cultivar Inca-no-mezame (Fig. 1F), the sucrose continued to rise after 2 weeks up to around 7.5 mg/g fresh weight within 8 weeks and reached 13 mg/g fresh weight after 250 days of storage. This sucrose content was about 6-fold higher than that of the other two types. The reducing sugars increased gradually; however, they did not exceed approximately 4 mg/g fresh weight. The total sugar content was around 18.5 mg/g fresh weight after 250 days of storage.
Changes in enzyme activity of potato tubers during growth and storage
To examine whether the observed differences in sugar accumulation were caused by differences in the enzyme activities related to sugar metabolism, we next measured the activity of vacuolar acid invertase in tubers of cultivars that represent the three types of sugar accumulation: May Queen (type 1), White Fryer (type 2), and Inca-no-mezame (type 3).
During the growing season, the activity of acid invertase was found to be about 2–5 nmol fructose mg−1 min−1 in the youngest tubers (approx. 10 g fresh weight), then it decreased, maintaining a low level until harvest time in all three cultivars (Fig. 2). In White Fryer the activity increased during the curing period, but during storage, it showed a low level equivalent to that at harvest time (Fig. 2B).
Changes in the activity of the acid invertase of potato tubers during the growing season and storage period. The x-axis and tubers used at each sampling date are the same as in Fig. 1. Extracts were prepared from fresh tubers and the activity of acid invertase was measured in desalted extracts. A May Queen. B White Fryer. C Inca-no-mezame. Values are the means of three replicates
During storage at 20°C, the activity was extremely low, slightly under 1 nmol fructose mg−1 min−1 in all three of these cultivars (Fig. 2, open circles). At 4°C, the activity increased rapidly by 2 weeks of storage in May Queen (type-1 cultivar), reached a maximum of 8.3 nmol fructose mg−1 min−1 and then maintained a similar level as storage continued (Fig. 2A, closed circles), reflecting the accumulation of reducing sugars. On the other hand, the activities of White Fryer (type 2) and Inca-no-mezame (type 3) were very low during storage, around 0.3–1.0 nmol fructose mg−1 min−1 (Fig. 2B and C, closed circles). In the case of Inca-no-mezame, the low activity of acid invertase seems to reflect the continuous accumulation of sucrose after 2 weeks of storage. White Fryer showed low activity of the enzyme during storage, although the change in sucrose content had a pattern similar to that observed in the type-1 cultivars; the content increased rapidly and peaked within 2 weeks, before decreasing. At a later stage of storage, the activity of acid invertase increased at the time of sprouting in White Fryer.
Estimation of the relative transcript levels of acid invertase with RT-PCR
To determine specifically the relative transcript levels of the acid invertase gene during the storage, total RNA was isolated from tubers of May Queen, White Fryer, and Inca-no-mezame, and RT-PCR of each cultivar was carried out using gene-specific primers. For May Queen, the transcripts of the acid invertase gene accumulated in the tubers stored at 4°C, while only very weak signals of transcripts could be detected in tubers stored at 20°C (Figs. 3, 4). The results are in agreement with a study reported by Zhou et al. (1994) using northern blot analysis in cv. Russet Burbank, and suggest that the activity of vacuolar acid invertase during storage is regulated at a transcriptional level in the type-1 cultivar May Queen. On the other hand, in tubers of Inca-no-mezame, stored either at 20°C or at 4°C, the transcripts could not be detected clearly (Fig. 4). In this cultivar, these results seem to be convincing since the enzyme activity was very low. In the case of White Fryer, although the enzyme activity was very low during storage, the transcripts accumulated in the tubers stored at 4°C for 1 week in the same way as those of May Queen (Fig. 4).
Expression patterns of the potato acid invertase gene in May Queen during storage. Reverse transcription-polymerase chain reaction (RT-PCR) analysis was performed on RNA isolated from tubers stored at 4°C or 20°C to detect the expression of the acid invertase gene. A 433-bp fragment of the acid invertase gene was amplified from the RT-PCR. The sources of the RNA for each lane are indicated above the picture. Lane 1 day 0 ; lane 2 storage at 4°C for 1 week; lane 3 storage at 20°C for 1 week; lane 4 storage at 4°C for 3 weeks; lane 5 storage at 20°C for 3 weeks; lane 6 storage at 4°C for 5 weeks; lane 7 storage at 20°C for 5 weeks
The differences in expression patterns of the acid invertase gene among cultivars. RT-PCR was performed on RNA isolated from tubers stored for 1 week at 4°C or 20°C to detect the expression of the acid invertase gene. A 433-bp fragment of the acid invertase gene was amplified from the RT-PCR. The sources of the RNA for each lane are indicated above the picture. Lanes 1, 4 and 7 day 0 of May Queen, White Fryer and Inca-no-mezame, respectively; lanes 2, 5 and 8 storage at 4°C for 1 week; lanes 3, 6 and 9 storage at 20°C for 1 week
Discussion
In this study, we monitored the sugar content and enzyme activities of young developing tubers. The contents of sucrose and glucose were high at an early stage, and then decreased with tuber growth in all cultivars. Acid invertase showed considerable activities only in the youngest tubers. Pressey (1969b) and Davies and Viola (1994) reported that in the growing season the activity of sucrose synthase (in the direction of sucrose breakdown) is predominant over that of acid invertase in potato tubers. Furthermore, the high ratio of glucose to fructose in young tubers is due to the activity of fructose-specific hexose kinase, which is higher than that of glucose-specific hexose kinase (Davies and Oparka 1985). As the growing season progresses, there is a decline in the activities of sucrose synthase and fructose kinase, and also glucose-phosphorylating potential approximates that of fructose; these changes might induce the reduction of the ratio of glucose to fructose, from ca. 80:1 to 2:1 (Davies and Viola 1994). The ratio obtained in this study also decreased with tuber maturation, and the values seems to be varied due to the differences of related enzyme activities in the cultivars. We are now determining the activities of the related enzymes in the tubers of these cultivars.
In our study, three-types of sugar changes among cultivars have been shown during the storage periods at 4°C (Fig. 1). We suggest that the type-1 cultivars belong to “high sugar-accumulating, cold-sensitive cultivars,” which have also been reported by Hammond et al. (1990) and Zrenner et al. (1996), while, the type-2 cultivar White Fryer belongs to “low sugar-accumulating, cold-tolerant cultivars” reported by Blenkinsop et al. (2002) and Richardson et al. (1990). As compared with these two types, the type-3 cultivar Inca-no-mezame showed a new type of sugar change: “increased sucrose, not reducing sugars.” This unique pattern might originate in its genetic background. Inca-no-mezame contains a high carotenoid content (about 530 μg/100 g fresh weight as zeaxanthin) and, therefore, has a yellowish orange flesh (Ishii et al. 1998); these properties seem to come from Solanum tuberosum subsp. andigena. In addition to the six cultivars used in this study, we are currently studying the sugar changes in other cultivars and breeding lines, taking account of their genetic backgrounds. So far, we have investigated some cultivars that can be classified as type 2 (“Hokkaikogane” and “P982”) and some as type 3 (Hokkai No. 88) (data not shown).
It has been well documented that the activity of soluble acid invertase increases in cold-stored tubers (Pressey 1969a; Richardson et al. 1990). Our results showed that in the type-1 cultivar May Queen, the activity of acid invertase was high, while, in the type-3 cultivar Inca-no-mezame, the activity was low in storage at 4°C (Fig. 2). The changes in the transcripts of the enzyme were in agreement with the enzyme activities (Figs. 3, 4). From these results it is suggested that the acid invertase activity reflects the accumulation patterns of sugars in these two types in low-temperature storage. Interestingly, in the type-2 cultivar White Fryer, although the enzyme activity was very low during the storage, the transcripts accumulated in the tubers stored at 4°C (Fig. 4). We are now investigating whether there are factors, such as an enzyme inhibitor, which bring about the low activity of acid invertase during storage in this cultivar, or not. Furthermore, in this cultivar, the sucrose content decreased after a peak at 2 weeks, similarly to that of the type-1 cultivars (Fig. 1E). Our previous study using cultivar “Hokkaikogane” indicated that a high level of sucrose synthase activity, which catalyzes sucrose cleavage, was detected in tubers stored at 4°C for two weeks, compared with tubers stored at 20°C (unpublished data). Detailed analyses of the sucrose synthase in the type-2 cultivars are also needed.
In conclusion, this study revealed that the patterns of sugar changes during low-temperature storage are classified into three types among the cultivars, and those patterns seem to be closely related to the activities of vacuolar acid invertase. The sugar change shown in the type-3 cultivars, increased sucrose but not reducing sugars, is a new pattern. Therefore, the study of sugar changes in these cultivars would provide very useful information for understanding the mechanism(s) of low-temperature sweetening in potato tubers. We are now planning to introduce the sense gene of acid invertase into Inca-no-mezame as well as the anti-sense gene into May Queen in order to investigate the effects of these genetic modifications on sugar changes during low-temperature storage. We hope to utilize the knowledge obtained to design a proper using of cultivars and to develop a control technology for storage quality.
References
Blenkinsop RW, Copp LJ, Yada RY, Marangoni AG (2002) Changes in compositional parameters of tubers of potato (Solanum tuberosum) during low-temperature storage and their relationship to chip processing quality. J Agric Food Chem 50:4545–4553
Bracho GE, Whitaker JR (1990) Characteristics of the inhibition of potato (Solanum tuberosum) invertase by an endogenous proteinaceous inhibitor in potatoes. Plant Physiol 92:381–385
Burton WG (1989) The potato, 3rd edn. Longman Scientific and Technical, New York
Chuda Y, Ono H, Yada H, Ohara-Takada A, Matsuura-Endo C, Mori M (2003) Effect of physiological change in potato tuber (Solanum tuberosum L.) after low temperature storage on the level of acrylamide formed in potato chips. Biosci Biotechnol Biochem 67:1188–1190
Davies HV, Oparka KJ (1985) Hexose metabolism in developing tubers of potato (Solanum tuberosum L.) cv Maris Piper. J Plant Physiol 119:311–316
Davies HV, Viola R (1994) Control of sugar balance in potato tubers. In: Belknap WR, Vayda ME, Park WD (eds) The molecular and cellular biology of the potato, 2nd edn. CAB International, Wallingford, UK, pp 67–80
Espen L, Morgutti S, Abruzzese A, Negrini N, Rivetta A, Quattrini MM, Cocucci M, Cocucci SM (1999) Changes in the potato (Solanum tuberosum L.) tuber at the onset of dormancy and during storage at 23°C and 3°C. I. Biochemical and physiological parameters. Potato Res 42:189–201
Ewing EE, McAdoo MH (1971) An examination of methods used to assay potato tuber invertase and its naturally occurring inhibitor. Plant Physiol 48:366–370
Hammond JBW, Burrell MM, Kruger NJ (1990) Effect of low temperature on the activity of phosphofructokinase from potato tubers. Planta 180:613–616
Hill LM, Reimholz R, Schröder R, Nielsen TH, Stitt M (1996) The onset of sucrose accumulation in cold-stored potato tubers is caused by an increased rate of sucrose synthesis and coincides with low levels of hexose-phosphates, an activation of sucrose phosphate synthase and the appearance of a new form of amylase. Plant Cell Environ 19:1223–1237
Isherwood FA (1973) Starch-sugar interconversion in Solanum tuberosum. Phytochemistry 12:2579–2591
Ishii G, Mori M, Ohara A, Umemura Y (1999) Food chemical properties of a new potato with orange flesh. In: Hagg M, Ahvenainen R, Evers AM, Tiilikkala K (eds) Agri-food quality. II. Quality management of fruits and vegetables. RSC, Wallingford, UK, pp 357–359
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Pollock C, ap Rees T (1975) Activities of enzymes of sugar metabolism in cold-stored tubers of Solanum tuberosum. Phytochemistry 14:613–617
Pressey R (1967) Invertase inhibitor from potatoes: purification, characterization, and reactivity with plant invertases. Plant Physiol 42:1780–1786
Pressey R (1969a) Role of invertase in the accumulation of sugars in cold-stored potatoes. Am Potato J 46:291–297
Pressey R (1969b) Potato sucrose synthetase: purification, properties, and changes in activity associated with maturation. Plant Physiol 44:759–764
Richardson DL, Davies HV, Ross HA, Mackay GR (1990) Invertase activity and its relation to hexose accumulation in potato tubers. J Exp Bot 41:95–99
Ross HA, Davies HV (1992) Sucrose metabolism in tubers of potato (Solanum tuberosum L.): effect of sink removal and sucrose flux on sucrose-degrading enzymes. Plant Physiol 98:287–293
Shirzadegan M, Christie P, Seemann JR (1991) An efficient method for isolation of RNA from tissue cultured plant cells. Nucleic Acids Res 19:6055
Sowokinos JS (1990) Stress-induced alterations in carbohydrate metabolism. In: Vayda ME, Park WD (eds) The molecular and cellular biology of the potato. CAB International, Wallingford, UK, pp 137–158
Sowokinos J (1994) Post-harvest regulation of sucrose accumulation in transgenic potatoes: role and properties of potato tuber UDP-glucose pyrophosphorylase. In: Belknap WR, Vayda ME, Park WD (eds) The molecular and cellular biology of the potato, 2nd edn. CAB International, Wallingford, UK, pp 81–106
Tareke E, Rydberg P, Karlsson P, Eriksson S, Törnqvist M (2002) Analysis of acrylamide, a carcinogen formed in heated foodstuffs. J Agric Food Chem 50:4998–5006
Zhou D, Mattoo A, Li N, Imaseki H, Solomos T (1994) Complete nucleotide sequence of potato tuber acid invertase cDNA. Plant Physiol 106:397–398
Zrenner R, Schuler K, Sonnewald U (1996) Soluble acid invertase determines the hexose-to-sucrose ratio in cold-stored potato tubers. Planta 198:246–252
Acknowledgement
This work was partially supported by a Grant-in-Aid for the Research and Development Program for New Bio-industry Initiatives from the Bio-oriented Technology Research Advancement Institution (BRAIN), Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matsuura-Endo, C., Kobayashi, A., Noda, T. et al. Changes in sugar content and activity of vacuolar acid invertase during low-temperature storage of potato tubers from six Japanese cultivars. J Plant Res 117, 131–137 (2004). https://doi.org/10.1007/s10265-003-0137-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-003-0137-z