Abstract
Silica particles were modified with 3-methacryloxypropyl trimethoxysilane (MPS) as a carrier material. The tetracycline (TC) molecular imprinting polymer based on the surface of modified silica (MPS/SiO2) was prepared by adopting the novel surface molecular imprinting technique with tetracycline as template molecule and ethylene glycol diglycidyl ether (EGDE) as crosslinker. The rebinding capacity of molecularly imprinted polymer (MIP) was evaluated according to the adsorption kinetics and the adsorption isotherm. The two distinct linear portions in Scatchard analysis indicate that two classes of binding sites existed in the imprinted polymer. The MIP obtained was used as a selective sorbent for molecularly imprinted solid-phase extraction (MISPE) prior to HPLC analysis. The results showed that good linearity was observed in the range of 150∼950 μg/L for TC, with a correlation coefficient of 0.9972. Adding level of 300∼700 μg/L, the average of spiked recoveries of TC is about 81.1 %, with a relative standard deviation (RSD) range from 3.0 to 4.4 %, and the limit of detection (LOD) reached 25 μg/L. The proposed method is easy to operate, reliable, and applicable to analyze the TC residues in milk samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tetracycline (TC) antibiotics are commonly used as antibiotics both for treatment of infectious diseases and as an additive to animal feeds because of their broad-spectrum antibacterial activity and cost effectiveness. However, these pharmaceuticals are very difficult to be metabolized completely, and the residues in the environment can induce the development of antibiotic-resistant pathogens and pose adverse health effects to humans (Ji et al. 2011). The US Food and Drug Administration (FDA) has set the maximum residue limits for the sum of tetracycline, chlortetracycline, and oxytetracycline residues as 2 mg/kg in muscle and 0.4 mg/kg in milk (U.S. Code of Federal Regulations 2003). In order to protect humans to be exposed to TCs in the edibles of animal origin foods. Traditional methods for determination of TC include microbiological inhibition tests (Pellinen et al. 2002), immunoassays (Pastor-Navarro et al. 2007), and chemical-physical methods, e.g., gas/liquid chromatographic (GC/LC) analysis (Schneider et al. 2007) and Liquid chromatography–tandem mass spectrometry (LC–MS/MS) (Kaufmann et al. 2008).
Molecularly imprinted polymers (MIPs) possessing tailor-made recognition sites exhibit the ability of specifically rebinding to a target molecule in preference to analogous compounds. During the preparation of MIPs, three-dimension structure cavities were generated after polymerization and template extraction (Gao et al. 2007; Pérez-Moral and Mayes 2007). MIPs possess excellent recognition ability toward template molecules, and these specific binding affinities between the multifunctional MIPs and the target molecule have been proven. MIPs displayed outstanding advantages, such as predetermined recognition ability, stability, and effortless and less expensive preparation (Alexander et al. 2006). MIPs have been widely used as artificial receptors in separations (López et al. 2012), sensors (Pardieu et al. 2009), catalysis (Volkmann and Brüggemann 2006), and drug development and screening (Shi et al. 2007). To achieve the necessary levels of sensitivity, an enrichment step is needed before chromatographic analysis (Bagheri et al. 2004). MIPs provide high selectivity to SPE, allowing analytes to be eluted from cartridges almost free of co-extracted compounds. Nowadays, solid-phase extraction (SPE) is a well-established technique and has been used for the preconcentration and cleaning up of numerous different classes of compounds in a variety of matrices (Masqué et al. 2000; Figueiredo et al. 2007). Coupling of MIPs and SPE is possible to combine the two advantages of molecular recognition and traditional separation methods. Thus, molecularly imprinted solid-phase extraction (MISPE) would be an ideal separation material presenting high specificity and sensitivity (Zhang et al. 2014; Wang et al. 2015).
Molecular imprinting for the recognition of TCs has been already reported. Caro et al. (2005), Cai and Gupta (2004), and Suedee et al. (2004) prepared the TC MIPs using bulk polymerization to obtain bulky MIPs, which were traditional and exhibited some disadvantages including incomplete template removal, small binding capacity, slow mass transfer, and irregular materials shape. To overcome these disadvantages, surface polymerization has been proposed to prepare the MIP film on a solid support substrate, which can improve mass transfer and reduce permanent entrapment of the template (Bonini et al. 2007; Gao et al. 2008). Up to date, the molecular imprinting at the surface of various substrates remains a challenge, and there are few reports with respect to the synthesis of the tetracycline MIP material on silica surface by the surface molecular printing technique and its application to real sample analysis.
The aim of this work was to develop a new programmed surface molecular printing approach to imprint tetracycline antibiotics at the surface of silica particles. The surface structure and the physicochemical properties of the polymers were characterized; the adsorption capacity and the selectivity toward the template were determined. The performance of the developed MIPs for the separation and preconcentration of TCs from milk samples was also evaluated.
Experimental
Materials
Tetracycline, oxytetracycline, chlortetracycline, trimethylolpropane trimethacrylate (TRIM, technical grade), and methacrylic acid (MAA) were obtained from Aladdin Reagent (Shanghai, China). Ammonium persulfate and 3-methacryloylpropyl trimethoxysilane (MPS) were obtained from Shanghai Chemical Reagent Plant (Shanghai, China). Ethylene glycol diglycidyl ether (EGDE) was purchased from Wuxi Wanrong Material Ltd. (Jiangsu, China); HPLC grade methanol and acetonitrile were obtained from Sigma (St. Louis, MO, USA); and acetic acid (HAC), hydrogen chloride, methanol, and acetonitrile were purchased from the Tianjin chemical reagent company (Tianjin, China). Silica gel (200–300 mesh) was purchased from Qingdao Marine chemical factory. Milk samples were purchased from a local supermarket. MAA was purified prior to use via the general distillation method in vacuum under nitrogen protection to remove the polymerization inhibitor. Double distilled water was used throughout the experimental procedures. All other reagents were of analytical grade. All solutions used for HPLC were filtered through a 0.45-μm nylon filter before use.
Instruments
All chromatographic tests were performed using an LC-20AT chromatographic system (Shimadzu, Japan), including two LC-20AT pumps and a SPD-20A UV–VIS detector. Samples were injected through a Rheodyne 7725 valve. Thermogravimetric analyses (TGA) were carried out simultaneously using a ZRY-2P instrument. JSM-7500F electron scanning microscope (JEOL Co., Japan) and VarioEL III elemental Analyzer (Elementar Co., Germany) were used. Fourier transform infrared spectrometer (Shimadzu, Japan). TU-1810-type ultraviolet spectrophotometer (Beijing General Instrument Co., Ltd., China) and TG16-WS high-speed centrifuge (Centrifuge Factory, China) were used in this experiment.
Preparation of Tetracycline Molecularly Imprinted Polymer
Bonding of 3-methacryloxypropyl Trimethoxysilane on the Surface of Silica Gel
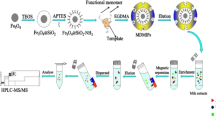
Two grams of silica gel particles which were activated with hydrochloric acid and 2.5 mL of MPS were added into a 100 mL mixed solvent of ethanol–water (1:1, v/v). The modification reaction was carried out at 50 °C for 24 h. Then, the products were extracted into anhydrous ethanol in a Soxhlet extractor for 24 h to remove the polymer absorbed on the surface of the silica gel. The surface-modified particles MPS/SiO2 was obtained.
Grafting Poly-methylacrylic Acid on MPS/SiO2
Two grams of particles MPS/SiO2 and 7 mL of MAA were added into 150 mL of water, and the graft polymerization was performed by initiating (NH4)2S2O8 under N2 atmosphere at 70 °C for 7 h. The resultant particles were extracted with ethanol in a Soxhlet extractor to remove the polymers physically attaching to the particles. After drying under vacuum, the grafted particles MAA/SiO2 were obtained.
Preparation of Tetracycline MIPs
Two grams MAA/SiO2 was added into 50 mL of 10 mmol/L tetracycline mixed solution of methanol–acetonitrile (6:7, v/v). The mixture was shaken at 25 °C for 6 h until the grafted PMAA on MPS/SiO2 particles was fully swelled and the adsorption of MAA/SiO2 toward tetracycline reached the equilibrium. After filtrating, the particles MAA/SiO2, which had adsorbed tetracycline in saturation, were dried under vacuum. Two grams of the MAA/SiO2 particles with adsorbed tetracycline was placed in 50 mL of 4 mmol/L tetracycline in 50 % aqueous methanol–acetonitrile (6:7, v/v) solution. The pH value of the solution was adjusted to pH 8.0, and 2.5 mL of crosslinker EGDE was added. The reaction was performed at 50 °C for 8 h. Then, the MIP was extracted into acetic acid–methanol (1:9, v/v) solution in a Soxhlet extractor for 24 h to remove the unreacted crosslinker EGDE and the template thoroughly. After that, the MIP was washed with ultrapure water until neutral. The non-imprinted polymer (NIP) was synthesized using the same procedure mentioned above (Gao et al. 2008), except without adding a template molecule.
Kinetic Adsorption
In the kinetic experiments, 50 mg of MIP or NIP was added to 10 mL of 8 mmol/L TC mixed solution of methanol–acetonitrile (6:7, v/v). The mixture was mechanically shaken with different adsorption times of 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 12, and 14 h at room temperature and then separated centrifugally. The unbound TC in the supernatant was measured by UV spectrometry at 355 nm.
Static Adsorption Test
To measure the adsorption capacity of the polymer, 50 mg of MIP or NIP sorbent was mixed with a series of concentrations (0.5, 1, 2, 3, 4, 5, 6, 7, 8, 9 mmol/L) of TC mixed solution of methanol–acetonitrile (6:7, v/v) and was shaken for 8 h. The resulting supernatant was measured for unbound TC by UV spectrometry at 355 nm.
where Q (mg/g) was the mass of TC adsorbed per gram of polymers, C i (mg/L) and C f (mg/L) are the initial and final concentrations of TC in solution, respectively, V (L) is the total volume of the solution, and m (g) is the mass of polymers.
The saturation binding data were further processed to generate a Scatchard equation to estimate the binding properties of MIP and NIP. The Scatchard equation (Hiratsuka et al. 2013) was as follows:
where Q was the amount of TC bound to polymers at equilibrium, Q max was the apparent maximum adsorption capacity, C free was the free TC concentration at equilibrium, and K d was the dissociation constant. The values of K d and Q max could be calculated from the slope and intercept of the linear curve plotted at Q/C free versus Q.
Selective Binding Experiments
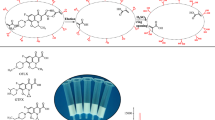
To investigate the rebinding selectivity of the prepared MIP towards TC molecule, oxytetracycline (OTC) and chlortetracycline (CTC) (Fig. 1) were selected as the structural analogue. The MIP (50 mg) was added to a flask containing 10 mL of 8 mmol/L TC, OTC, and CTC, respectively, shaken at room temperature for set times and then separated centrifugally.
The distribution coefficients (K D), selectivity coefficients (k), and relative selectivity coefficients (k ′) of OTC and CTC with respect to TC can be obtained according to the following equations:
where Q e (mg/g) and C e (mg/L) are the equilibrium binding amount and the equilibrium concentration of the TC and competitive antibiotics, respectively. K Dj represents the distribution coefficients of competition species. k MIP and k NIP are the selectivity coefficients of MIP and NIP, correspondingly.
Solid-Phase Extraction Procedure on MIP
Five hundred milligrams of the polymer was poured into 10 mL volume SPE cartridges. The cartridge was preconditioned with 5 mL of acetic acid–methanol (1:9, v/v). The sorbent was conditioned with 5 mL of methanol and 5 mL of acetonitrile. One milliliter of sample extracts spiked with TC, OTC, and CTC was loaded on the MIP-SPE cartridge. After a washing step with 5 % methanol (2 mL), it was eluted with 5 mL of 100 mmol/L potassium hydroxide solution–methanol (3:2, v/v) (Jing et al. 2009). The eluting fraction was collected and evaporated to dryness under a stream of N2 at 50 °C. The residue was redissolved with 1 mL of mobile phase and analyzed by HPLC. All results were confirmed by duplicated experiments.
Milk Sample Treatment
A portion of 5 mL milk was mixed with TCs mixed standard solution in methanol–acetonitrile (6:7, v/v) mixture and was homogenized. A step of protein precipitation was carried out before MIP-SPE. Thus, equal volumes (5 mL) of milk and l mL of l mol/L hydrochloric acid and 15 mL of acetonitrile were added. The spiked samples were extracted by ultrasonic-assisted extraction at room temperature for 10 min; the mixed sample was transferred to two 10-mL centrifuge tubes. The supernatant extract was isolated after centrifugation for 10 min at a speed of 4000 rpm. A portion of 2 mL of the clear filtrate was evaporated until dryness under a gentle stream of nitrogen at ambient temperature. The solid residue was then dissolved in 1 mL of acetonitrile and loaded on MIP-SPE cartridge. The spiking concentrations for each TCs were set with three levels of 300.0, 500.0, and 700.0 μg/L, respectively.
HPLC Analysis
The analyses of sample were performed on Shimadzu LC-20A HPLC system under the gradient condition: methanol–acetonitrile (1:4, v/v) as solvent A and 0.03 mol/L oxalic acid solution as solvent B. The conditions of gradient elution are shown in Table 1. All separations were achieved on an analytical reversed-phase column (250 × 4.6 mm) packed with ODS C18 stationary phase. The flow rate was 1.0 mL/min, and the detection wavelength was set at 355 nm (the maximal absorbance wavelength of TCs).
Results and Discussion
Preparation of MIP
In the current study, the surface of the silica gel was first silylated with 3-(methacryloxy) propyltrimethoxysilane to obtain vinyl end groups, then the methylacrylic acid (MAA) was grafted to the surface of modified silica, and the grafted particles MAA/SiO2 were obtained. The MAA was used as the monomer, which could form hydrogen bond between MAA and the 3-OH and 4-N(CH3)2 of TC to enhance selectivity and affinity of MIP. As the adsorption of MAA/SiO2 toward TC reached a saturated state by hydrogen bonding and electrostatic interaction, crosslinker EGDE was added. As a result, TC molecule was enveloped in the polymer. As the template molecules were washed away, a great deal of TC caves would remain in the thin polymer layer on the surface of silica gel particles, and the TC imprinted material MIP was obtained. The schematic diagram of MIP preparation is shown in Fig. 2.
Characterization of MIPs
Element Analysis
The polymers of MAA/SiO2 and MIP were characterized by using elemental analysis. Elemental analysis data are listed in Table 2. The content of C and H elements increased obviously compared MIP with MAA/SiO2. This evidences indicated that crosslinker EGDE had grafted to the surface of MAA/SiO2 successfully.
Thermogravimetric Analysis
Thermogravimetric analysis plays an important role in assessing the surface coverage of the polymer-modified silica. The weight loss between 200 and 600 °C was found to be related to the loss of the organic groups attached to the surface (Lumley et al. 2004). Table 3 shows the thermogravimetric date of MPS/SiO2, MAA/SiO2, and MIP, respectively. For MPS/SiO2, the weight loss from 200 to 600 °C was attributed to the loss of the initiator-bonded silica gel. For MAA/SiO2, the weight loss was due to the MAA moieties. The gravity of MIP was caused by the bonding on its surface of functional monomers and crosslinker losses. The immobilization percentages of MPS/SiO2, MAA/SiO2, and MIP in thermogravimetric analysis were about 13.1, 14.0, and 15.8 %, respectively. These results indicated that the molecularly imprinted materials were successfully prepared.
FT-IR Characterization of MAA/SiO2 and MIP
FT-IR spectrums of MAA/SiO2 and MIP are shown in Fig. 3. There is an obvious broad and strong adsorption peak at about 1092 cm−1 which represents the Si–O antisymmetric stretching vibration. The adsorption peaks at 806 and 471 cm−1 imply the Si–O symmetric stretching vibration and bending vibration, respectively. The C=O stretching vibration absorption peak appeared at about 1721 cm−1 in Fig. 3a. The strong bands appeared at 3408∼3563 cm−1 corresponding to the –OH stretching vibration of carboxyl groups. These results indicated that the MAA was successfully immobilized on the surface of silica. In MIP, 1093 cm−1 peak strength enhancement is due to the introduction of crosslinker C–O–C stretching vibration. In the peak of 3563 cm−1 strength enhancement, because one end of crosslinker EGDE could react with hydroxyl group exposed on the MAA/SiO2 through open-ring bonded reaction, the number of free hydroxyl increased, suggesting that crosslinker EGDE successful polymerization on the surface of MAA/SiO2.
Scanning Electron Analysis
Figure 4 shows the SEM image of the surface structure of the MIP (right), as well as that of the silica (left) which was used for comparison. It was obvious that silica possess a very smooth surface, spherical shape before imprinted. However, the MIP had rough surface which was morphological features after a step-by-step coating procedure. The resulting MIP had a large number of mesopores on their surface, which facilitated template molecules closed to the “reservation” cavity and interacted with the recognition sites of imprinted polymer through these tunnels.
Binding Properties of the MIP and NIP
Figure 5 presents the adsorption kinetics of TC solution on the MIP. Adsorption equilibrium of MIP nanoparticles in TC solution was determined at different adsorption times. The initial concentration of TC was kept constantly at 8 mmol/L. In 0.5∼4 h, the binding amount of TC increased rapidly and reached an equilibrium after 8 h; the adsorption capacity rapidly increase at the initial stage and then slow down to reach the adsorption equilibrium.
Static adsorption tests were performed on the MIP and NIP, with different initial concentrations of TC (Fig. 6). The MIP and NIP particles were put in the concentrations of TC solutions ranging from 0.5 to 9 mmol/L, when the concentration of TC reached 6 mmol/L and more, the bound amounts achieved a saturated plateau for MIP and NIP. The static adsorption capacities of the MIP and NIP for TC were 42.30 and 22.32 mg/g, respectively, which displayed better steric matching with the imprinted molecule TC, indicating that the MIP had a higher affinity than the NIP for the template molecule. The calculated maximum monolayer adsorption capacities of the MIP exhibited better than the literature value which is 12.10 mg/g (Dai et al. 2012). In general, MIP possesses both specific and nonspecific binding sites, while NIP only has nonspecific binding sites, which enable MIP to take up more TC than NIP. The difference in adsorption capacity between MIP and NIP is equivalent to the contribution from the specific binding sites on MIP.
As can be seen from Fig. 7, the Scatchard plot for MIP was not a single linear curve but rather consisted of two linear parts with different slopes, which suggested that there were two different types of binding sites for MIP toward TC molecular. The K d and Q max values can be calculated according to the slopes and intercepts of the two linear portions of the Scatchard plot. The results are shown in Table 4.
Adsorption Selectivity
To evaluate the selectivity of the TC imprinted MIP, two TC structural analogs OTC and CTC were selected as the controls to determine their binding capacities on MIP and NIP, respectively. The initial concentrations of TC, OTC, and CTC used were 8 mmol/L. It can be seen in Fig. 8 that the bound amount of TC for MIP was much higher than that of the other two competitive analogs. MIP exhibited much higher selectivity to TC than to NIP. The k values for OTC and CTC were 20.11 and 9.07, respectively. The k′ values for OTC and CTC were 2.98 and 0.60, respectively. These results showed that the preparation of molecularly imprinted materials had good selective adsorption ability for TC.
Molecularly Imprinted Solid-Phase Extraction of TC from Spiked Samples
The prepared MIP and NIP were packed into a solid-phase extraction cartridge to evaluate their characteristics. In order to optimize the chromatographic extraction conditions, different loading, washing, and eluting solutions were tested. The highest extraction efficiency was achieved by using acetonitrile as loading solution, 2 mL 5 % methanol as washing solution, and 5 mL of 100 mmol/L potassium hydroxide solution/methanol (3/2, v/v) as loading, washing, and eluting solution, respectively.
In order to validate the linearity of the MIP-SPE/HPLC method, standard calibration curves were constructed by using the three TCs from the working standard solution. Linear regression analysis was performed by measuring the peak areas against their respective analyte concentrations (Table 5). The calibration curves were established with R above 0.9972. Limit of detection (LOD) was calculated as the concentration corresponding to a signal three times the standard deviation of the baseline noise, respectively. The LODs of the proposed method were 25 μg/L for TC, 15 μg/L for OTC, and 40 μg/L for CTC, respectively. The MRLs for tetracyclines in milk samples from animals were 100 μg/kg in European Union. The LODs were relatively lower than the maximum residue limits of tetracyclines in milk provided by European Union.
Solid-phase extraction prior to chromatographic quantitative determination was used in order to achieve high sample cleanup and pre-concentration. Figure 9 shows the chromatograms obtained for TCs standard solution (Fig. 9a), blank milk sample (Fig. 9b) and spiked milk with a cleanup of MISPE (Fig. 9c), and NISPE (Fig. 9d), respectively. As can be seen in Table 6, the mean recoveries of TC, OTC, and CTC in milk were evaluated by three spiking samples with different concentrations (300, 500, and 700 μg/L) were 79.3∼83.3, 51.5∼55.0, and 46.2∼59.1 %, respectively, with relative standard deviations (RSDs) of 2.5∼10.0 % (Table 6), indicating that the MIP can be used for the selective enrichment of TC in milk. In contrast with the MIP-SPE, using NIP as sorbent was also investigated. Since some analytes were eluted out the cartridge during the washing step of NIP-SPE, the relative recoveries of TC using NIP were lower (50.7–52.1 %) and some interferences originating from the biological matrix were eluted. In MIP-SPE, due to their special selectivity for conducting target analyte isolation, MIPs provided better selection, yielding higher recovery values and a clearer extract.
Conclusions
We used surface imprinted technique to prepare TC on silica gel. Kinetic, isotherm, selectivity and regeneration analyses were used to estimate the adsorption performance. The adsorption equilibrium time, capacity, and selectivity of MIP/SiO2 are significantly better than that of NIP/SiO2. Thus, a method was successfully developed by using the MIP/SiO2 polymer as the MISPE coupled with HPLC for enrichment and analysis of trace TC in milk. The high recoveries and satisfied precision for TC proved that the method was valid for the analysis of trace TC in milk samples.
References
Alexander C, Andersson HS, Andersson LI, Ansell RJ, Kirsch N, Nicholls IA, O’Mahony J, Whitcombe MJ (2006) Molecular imprinting science and technology: a survey of the literature for the years up to and including 2003. J Mol Recognit 19:106–180
Bagheri H, Mohammadi A, Salemi A (2004) On-line trace enrichment of phenolic compounds from water using a pyrrole-based polymer as the solid-phase extraction sorbent coupled with high-performance liquid chromatography. Anal Chim Acta 513:445–449
Bonini F, Piletsky S, Turner APF, Speghini A, Bossi A (2007) Surface imprinted beads for the recognition of human serum albumin. Biosens Bioelectron 22:2322–2328
Cai W, Gupta RB (2004) Molecularly-imprinted polymers selective for tetracycline binding. Sep Purif Technol 35:215–221
Caro E, Marce RM, Cormack PAG, Sherrington DC, Borrull F (2005) Synthesis and application of an oxytetracycline imprinted polymer for the solid-phase extraction of tetracycline antibiotics. Anal Chim Acta 552:81–86
Dai JD, Pan JM, Xu LC, Li XX, Zhou ZP, Zhang RX, Yan YS (2012) Preparation of molecularly imprinted nanoparticles with superparamagnetic susceptibility through atom transfer radical emulsion polymerization for the selective recognition of tetracycline from aqueous medium. J Hazard Mater 205:179–188
Figueiredo EC, Tarley CRT, Kubota LT, Rath S, Arruda MAZ (2007) On-line molecularly imprinted solid phase extraction for the selective spectrophotometric determination of catechol. Microchem J 85:290–296
Gao DM, Zhang ZP, Wu MH, Xie CG, Guan GJ, Wang DP (2007) A surface functional monomer-directing strategy for highly dense imprinting of TNT at surface of silica nanoparticles. J Am Chem Soc 129:7859–7866
Gao BJ, Wang J, An FQ, Liu Q (2008) Molecular imprinted material prepared by novel surface imprinting technique for selective adsorption of pirimicarb. Polymer 49:1230–1238
Hiratsuka Y, Funaya N, Matsunaga H, Haginaka J (2013) Preparation of magnetic molecularly imprinted polymers for bisphenol A and its analogues and their application to the assay of bisphenol A in river water. J Pharm Biomed 75:180–185
Ji LL, Wan YQ, Zheng SR, Zhu DQ (2011) Adsorption of tetracycline and sulfamethoxazole on crop residue-derived ashes: implication for the relative importance of black carbon to soil sorption. Environ Sci Technol 45:5580–5586
Jing T, Gao XD, Wang P, Wang Y, Lin YF, Hu XZ, Hao QL, Zhou YK, Mei SR (2009) Determination of trace tetracycline antibiotics in foodstuffs by liquid chromatography–tandem mass spectrometry coupled with selective molecular-imprinted solid-phase extraction. Anal Bioanal Chem 393:2009–2018
Kaufmann A, Butcher P, Maden K, Widmer M (2008) Quantitative multiresidue method for about 100 veterinary drugs in different meat matrices by sub 2-μm particulate high-performance liquid chromatography coupled to time of flight mass spectrometry. J Chromatogr A 1194:66–79
López MMC, Pérez MCC, García MSD, Vilarino JML, Rodriguez MVG, Losada LFB (2012) Preparation, evaluation and characterization of quercetin-molecularly imprinted polymer for preconcentration and clean-up of catechins. Anal Chim Acta 721:68–78
Lumley B, Khong TM, Perrett D (2004) The characterisation of chemically bonded chromatographic stationary phases by thermogravimetry. Chromatographia 60:59–62
Masqué N, Marcé RM, Borrull F, Cormack PAG, Sherrington DC (2000) Synthesis and evaluation of a molecularly imprinted polymer for selective on-line solid-phase extraction of 4-nitrophenol from environmental water. Anal Chem 72:4122–4126
Pardieu E, Cheap H, Vedrine C, Lazerges M, Lattach Y, Garnier F, Remita S, Pernelle C (2009) Molecularly imprinted conducting polymer based electrochemical sensor for detection of atrazine. Anal Chim Acta 649:236–245
Pastor-Navarro N, Morais S, Maquieira A, Puchades R (2007) Synthesis of haptens and development of a sensitive immunoassay for tetracycline residues: application to honey samples. Anal Chim Acta 594:211–218
Pellinen T, Bylund G, Virta M, Niemi A, Karp M (2002) Detection of traces of tetracyclines from fish with a bioluminescent sensor strain incorporating bacterial luciferase reporter genes. J Agric Food Chem 50:4812–4815
Pérez-Moral N, Mayes AG (2007) Molecularly imprinted multi-layer core-shell nanoparticles—a surface grafting approach. Macromol Rapid Commun 28:2170–2175
Schneider MJ, Darwish AM, Freeman DW (2007) Simultaneous multiresidue determination of tetracyclines and fluoroquinolones in catfish muscle using high performance liquid chromatography with fluorescence detection. Anal Chim Acta 586:269–274
Shi XZ, Wu AB, Qu GR, Li RX, Zhang DB (2007) Development and characterisation of molecularly imprinted polymers based on methacrylic acid for selective recognition of drugs. Biomaterials 28:3741–3749
Suedee R, Srichana T, Chuchome T, Kongmark U (2004) Use of molecularly imprinted polymers from a mixture of tetracycline and its degradation products to produce affinity membranes for the removal of tetracycline from water. J Chromatogr B 811:191–200
U.S. Code of Federal Regulations (2003) Title 21, Part 556, Sections 150, 500, and 720, US Government Printing Office, Washington DC, (Chapter 1)
Volkmann A, Brüggemann O (2006) Catalysis of an ester hydrolysis applying molecularly imprinted polymer shells based on an immobilised chiral template. React Funct Polym 66:1725–1733
Wang QQ, Zhang X, Xu ZX, Gao HJ (2015) Simultaneous determination of three trace organophosphorus pesticide residues in vegetables using molecularly imprinted solid-phase extraction coupled with high-performance liquid chromatography. Food Anal Methods 8:2044–2051
Zhang J, Wang LL, Ma JQ, Wang YL (2014) Preparation of ofloxacin poly (glycidyl methacrylate-co-ethylenedimethacrylate)(PGMA/EDMA) molecularly imprinted microspheres and their application to the analysis of quinolones in milk. Food Anal Methods 7:721–729
Acknowledgments
This research was supported by National Natural Science Foundation of China (No. 21565001 and 31271868) and the National Innovative Experiment (No.14HGG03).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Bolin Gong declares that he has no conflict of interest. Yuling Niu declares that she has no conflict of interest. Cong’an Liu declares that he has no conflict of interest. Jin Yang declares that she has no conflict of interest. Meihua Ma declares that she has no conflict of interest. Yanru Gong declares that she has no conflict of interest. Yue Wang declares that she has no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Not applicable.
Rights and permissions
About this article
Cite this article
Niu, Y., Liu, C., Yang, J. et al. Preparation of Tetracycline Surface Molecularly Imprinted Material for the Selective Recognition of Tetracycline in Milk. Food Anal. Methods 9, 2342–2351 (2016). https://doi.org/10.1007/s12161-016-0422-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-016-0422-9