Abstract
In this study, a molecularly imprinted polymer that can selectively recognize dimethoate, isocarbophos, and methyl parathion was synthesized. Using this compound as sorbent, a new method for simultaneous determination of the three organophosphorus pesticides by molecularly imprinted solid-phase extraction coupled with high-performance liquid chromatography was established. The factors affecting the preconcentration and sensitivity were studied in detail. Under optimal conditions, the limits of detection of this method for dimethoate, isocarbophos, and methyl parathion were 19.78, 8.73, and 17.41 μg/kg, respectively. The relative standard deviation for five replicates of 0.01 mg/L-mixed solutions was in the range of 1.8–4.2 %. Cucumber sample spiked with the three organophosphorus pesticides at levels of 0.125 and 0.250 μg/g were examined using this method, good recoveries were obtained ranging from 82.5 to 95.6 %. Moreover, this method was also successfully used for the detection of the three organophosphorus pesticides in cauliflower sample.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Organophosphorus pesticides play an important role in the improvement of agricultural output by controlling diseases, and they have become some of the most commonly used pesticides in industrialized countries (Pérez-Ruiz et al. 2005). However, their universal application in agriculture also gives rise to high-level residues of organophosphorus pesticides in food and the environment, and this is harmful to human health because of their high toxicities (Taira et al. 2006; Casida and Quistad 2004). Therefore, monitoring and controlling the levels of these compounds in food and the environment are crucial and necessary.
In the past decades, many studies have been reported on the determination of organophosphorus pesticides using methods such as gas chromatography (GC) with flame photometric detection (FPD) (Liu et al. 2014) or nitrogen–phosphorus detection (NPD) (Tian et al. 2014), high-performance liquid chromatography (HPLC) with diode array detection (Pirsaheb et al. 2013), gas chromatography–mass spectrometry (GC-MS) (Chen et al. 2009) or liquid chromatography-mass spectrometry (Ingelse et al. 2001), capillary electrophoresis (CE) (Zhao et al. 2014; Chen and Fung 2010), immunoassays (Garcés-García et al. 2006), and chemiluminescence methods (Meng et al. 2011). Among these methods, GC and HPLC are the classical methods, but their high limits of detection are insufficient for the analysis of trace pesticide residues in samples (Xin et al. 2012; Wang et al. 2013). So, the development of effective preconcentration and separation procedures prior to analysis is of great significant.
In recent years, many pretreatment techniques have been used such as dispersive liquid–liquid microextraction (DLLME) (You et al. 2013), solid-phase extraction (SPE) (Xie et al. 2013), supercritical fluid extraction (SFE) (Cuong et al. 2012), solid-phase microextraction (SPME) (Saraji et al. 2013), matrix solid-phase dispersion extraction (MSPDE) (Guan et al. 2011), and stir bar sorptive extraction (SBSE) (Yu and Hu 2009). Among them, SPE is the most widely used and commercialized technique because it is simple, stable, and easily automated (Zhao et al. 2014). Currently, C18 and C8-bonded silica are commonly used as solid-phase sorbents. However, the interaction between the adsorbent and target in SPE is non-specific, which leads to poor purification efficiency. Sometimes, it is also difficult to remove matrix interference, which is unfavorable for the determination of target analytes.
The molecular imprinting technique (MIT) is one of the most ideal and promising methods to prepare tailor-made materials for the development of specific sorbents. The resulting molecularly imprinted polymers (MIPs), which are stable, simple to prepare, reusable, and highly selective, have been applied as SPE sorbents in many fields, including biology (Ričanyová et al. 2010), environmental studies (Núñez et al. 2010), pharmaceuticals (Zhang et al. 2009), and food analysis (Theodoridis et al. 2006). However, traditional MIPs can only specifically recognize the template molecule and have low adsorption for other substances. Their applications in multi-pesticide residue analysis have therefore been limited.
4-(Dimethoxyphosphorothioylamino)butanoic acid has common functional groups with, and a similar structure to, organophosphorus pesticides and has been used as a hapten to immunize animals to obtain antibodies that can selectively recognize multi-pesticides (Zhang et al. 2007). In this study, a new MIP that can selectively recognize dimethoate, isocarbophos, and methyl parathion was prepared using 4-(dimethoxyphosphorothioylamino)butanoic acid as the template. Using this functionalized material as sorbent, a new method of molecularly imprinted solid-phase extraction (MISPE) coupled with high-performance liquid chromatography (MISPE-HPLC) for the separation and determination of three organophosphorus pesticide residues in vegetables was established. The factors affecting preconcentration were optimized. The accuracy and applicability of the method were also evaluated.
Experimental
Materials
Cucumber sample was purchased from the Tai’an Yaxiya Food Co., Ltd. (Shandong, China), and the cauliflower sample was purchased randomly from a market in Taian (Shandong, China) in May 2014.
Chemicals
Analytical standard dimethoate (>98.3 %), isocarbophos (>99.0 %), and methyl parathion (>99.0 %) were obtained from the Institute for the Control of Agrochemicals of the Ministry of Agriculture (Beijing, China). 4-Aminobutyric acid was purchased from the TCI Development Corp. (Shanghai, China). O,O-Dimethyl phosphorochloridothioate and ethylene glycol dimethacrylate (EGDMA) were supplied by the Sigma-Aldrich Co., Ltd. (St Louis, MO, USA). Acrylamide (AM) was purchased from the Meryer Chemical Technology Co., Ltd. (Shanghai, China). 2,2-Azobis(isobutyronitrile) (AIBN) was obtained from Tianjin Chemical Reagent Factory (Tianjin, China), and it was purified by recrystallization before use. HPLC-grade methanol and acetonitrile (ACN) were supplied by the Shandong YuWang Industrial Co., Ltd. (Dezhou, China). Doubly distilled water (DDW) obtained from an Aike ultrapure water instrument (Tangshi Kangning Technology, Chengdu, China) was used in all of the experiments. Other reagents were of the highest available purity and at least of analytical grade.
Apparatus
Analysis was performed using an HPLC equipped with two Shimadzu LC-20AT pumps, a Shimadzu SPD-M20A ultraviolet (UV) detector, and an automatic injector (Shimadzu, Kyoto, Japan). All separations were performed using an analytical reversed-phase Thermo C18 column (4.6 mm × 250 mm; Agela Technologies, Newark, DE, USA), at a mobile phase flow rate of 0.8 mL/min. ChemStation software was used to acquire and process spectroscopic and chromatographic data. The mobile phase was methanol/DDW (60:40, v/v). The injection volume was 10 μL, and the detection was operated at 200 nm.
The C18 SPE columns (Strata Scx, 200 mg/3 mL) were purchased from Phenomenex (Torrance, CA, USA). Fourier-transform infrared (FT-IR) spectra (4000–400 cm−1) in KBr were recorded using a Vector 22 spectrometer (Bruker, German).
Methods
Synthesis of 4-(dimethoxyphosphorothioylamino)butanoic Acid
In this study, 4-(dimethoxyphosphorothioylamino)butanoic acid was prepared using the method reported by Wang et al. (Wang et al. 2013). Briefly, 1.032 g of 4-aminobutyric acid (10 mmol) was dissolved in 10 mL of 2.5 mol/L NaOH (4 °C). After stirring for 30 min (4 °C), 1.215 mL of O,O-dimethyl phosphorochloridothioate was gradually added to the mixture. The solution pH was then adjusted to 10 with 2.5 mol/L NaOH, and the solution was stirred for another 6 h while the temperature gradually increased to room temperature. Impurities were removed by washing with diethyl easter, and then the pH of mixture was adjusted to 2.0 by addition of 1.0 mol/L HCl. Finally, the mixture was extracted with diethyl easter (3 × 25 mL). The organic layer was dried with Na2SO4 overnight, and the product was obtained by rotary evaporation.
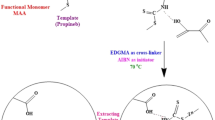
Preparation of MIP
The MIP was prepared according to the method of our previous study as follows: 0.227 g of 4-(dimethoxyphosphorothioylamino)butanoic acid (1 mmol) and 0.213 g of AM (3 mmol) were dissolved in 5.0 mL of ACN. After stirring for 30 min, 1.585 g of EDGMA (8 mmol) and 40 mg of AIBN were added. The mixture was stirred for another 15 min to form a homogenous solution. After ultrasonication for 15 min and purging with nitrogen for 15 min, the mixture was incubated in a water bath at 60 °C for 8 h. When polymerization was ended, the obtained rigid polymer was ground and sieved with a 200-mesh sieve. The MIP was extracted with 200 mL of methanol/acetic acid (4:1, v/v) for 8 h, and then with 200 mL of methanol for 2 h to remove the template molecules. Finally, the product was dried under vacuum at 60 °C for 12 h.
For comparison, a non-imprinted polymer (NIP) was prepared using the same procedure as for the MIP except for the addition of a molecular template.
Procedures of MISPE-HPLC
To evaluate the applicability of the prepared MIP as a sorbent for the extraction and determination of trace organophosphorus pesticides in vegetables, 100 mg of MIP or NIP was packed into an empty SPE cartridge. The cartridge was conditioned with 6 mL of methanol and DDW, and then 100 mL of mixed standard aqueous solution containing three organophosphorus pesticides was loaded at a rate of 1.5 mL/min. As a result, the analytes (dimethoate, isocarbophos, and methyl parathion) were selectively adsorbed onto the cartridge. When the sample loading was finished, the MIP cartridge was eluted with 2.0 mL of methanol/acetic acid (95:5, v/v). The eluent was collected in a test tube and condensed to dryness under a gentle flow of nitrogen at room temperature. The analytes were then accurately redissolved with 0.5 mL methanol. After filtration with a 0.22 μm filter membrane, 10 μL of the filtrate were injected into the HPLC for analysis (Fig. 1). Finally, the MIP cartridge was rinsed with 6.0 mL of methanol/acetic acid (9:1, v/v) for the next procedure.
Sample Preparation
To test the accuracy of the developed MISPE-HPLC method, the fortified cucumber sample, which had been determined to be free of the three organophosphorus pesticides before spiking, were prepared. Briefly, 2.0 g of cucumber was cut into slices and weighed into a 100-mL breaker. The samples were then spiked with 1.0 mL of a mixed standard solution (0.25 or 0.50 mg/L) containing 0.25 or 0.50 μg of the three organophosphorus pesticides. After incubation for 4 h, the spiked samples were sonicated and extracted with 3 × 30 mL of DDW for 20 min. The resulting extractions were collected and then constant their volume 100 mL. Finally, the supernatants were used for the MISPE-HPLC procedure.
To evaluate the applicability of this method, cauliflower sample randomly purchased from a market in Tai’an were detected. Cauliflower sample (2.0 g) was prepared according to the above process, except for spiking with the standard solutions, and the levels of dimethoate, isocarbophos, and methyl parathion were determined.
Results and Discussions
Characterization of MIP by FT-IR
The FT-IR spectra of the MIP before and after extraction of the template molecules, and the NIP are shown in Fig. 2. The peak at 1730 cm−1 was assigned to C = O stretch and the peak near 2980 cm−1 was the C–H stretch. The peaks at 3440 cm−1 and 3450 cm−1 were –OH vibrations, the slight shift in the position of this stretch for the MIP might result from interactions between the –COOH group of the template molecule and the –NH2 or C = O groups of AM. These results indicated that the template had reacted with AM, and the imprinted polymer had been synthesized.
The spectra of the MIP after extraction (Fig. 2b) and the NIP (Fig. 2c) showed major bonds with similar locations and appearances, and this demonstrated that the template molecules had been completely removed after extraction (Xin et al. 2012).
Optimization of MISPE Conditions
To achieve good precision and sensitivity, the MISPE process conditions were optimized, including the eluent composition and volume, and the sample loading flow rate.
The eluent has an important effect on elution and the recovery of target molecules in the MISPE procedure. In this study, eluent with different polarities, namely methanol, acetonitrile, acetone, and ethyl acetate were used to desorb the three organophosphorus pesticides from the MIP cartridge. The results (Fig. 3) showed that good recoveries were achieved for all three organophosphorus pesticides when methanol was used.
It is well known that the recognition principle of most MIPs is based on the hydrogen bonding between the analytes and the polymer functional groups (Zhu et al. 2002). Acetic acid can increase the eluting strength, weaken the strong interactions between the MIP and the analyte, and release the template from the imprinted cavity more quickly (Stafiej et al. 2007). In this study, various volumes of acetic acid (1–10 %) were added to the elution solvent to test the effect of acetic acid on the recoveries. The results indicated that the addition of 5 % acetic acid gave good recoveries for all three organophosphorus pesticides. As a result, a mixture of methanol/acetic acid (95:5, v/v) was selected as the eluent for the subsequent experiments.
To optimize the eluent volume, different levels of methanol/acetic acid (95:5, v/v) solution from 0.5 to 3 mL were investigated in this study. It was found that the recoveries of the three organophosphorus pesticides increased quickly with eluent volume increasing from 0.5 to 1.5 mL, and then increased slightly when the eluent volume was in the range of 1.5–3.0 mL. Finally, an eluent consisting of 2.0 mL of methanol/acetic acid (95:5, v/v) was selected for the MISPE procedure to ensure complete elution of the three absorbed organophosphorus pesticides.
The loading flow rate is also an important parameter that affects the binding of target analytes. In this study, 100 mL of 0.01 mg/L-mixed standard solution was used for adsorption, with a flow rate ranging from 1.0 to 5.0 mL/min. When the loading time was increased from 20 min to 60 min, the recoveries of the three organophosphorus pesticides were gradually increased. When the time was varied from 60 to 100 min, the recoveries had no obvious increasing. To ensure the complete desorption of the three organophosphorus pesticides, 1.5 mL/min was therefore selected as the loading flow rate.
Selectivity of MISPE-HPLC Method
To test the selectivity of the prepared MIP, 100 mL of mixed standard solutions (0.01 mg/L) containing the three organophosphorus pesticides was passed through a MIP or NIP cartridge, respectively. Compared to the NIP chromatogram (Fig. 4b), the signals of the three organophosphorus pesticides were more obvious in the MIP chromatogram (Fig. 4a). These results indicated that the three organophosphorus pesticides were selectively adsorbed onto the MIP cartridge, and the MIP sorbent had higher selectivity toward the dimethoate, isocarbophos, and methyl parathion than the NIP sorbent.
Analytical Parameters of the MISPE-HPLC Method
The analytical parameters of the developed MISPE-HPLC method for simultaneous separation and determination of the three organophosphorus pesticides were evaluated under the optimal conditions. The limit of detections (LODs) (S/N = 3) of the three organophosphorus pesticides were 19.78 μg/kg for dimethoate, 8.73 μg/kg for isocarbophos, and 17.41 μg/kg for methyl parathion, respectively, which were lower than the maximum residue limits (MRLs) in both China and the European Union (EU) (Table 1). The linear ranges of the calibration graph were all between 0.001 and 1.0 mg/L. The relative standard deviation (RSD) for five replicate extractions of 0.01 mg/L of mixed solution was in the range of 1.8–4.2 %.
Accuracy and Applicability of the MISPE-HPLC Method
To evaluate the accuracy of the developed MISPE-HPLC method, cucumber sample spiked with the three organophosphorus pesticides at level of 0.125 or 0.25 μg/g was extracted and analyzed using the method. Three measurements were performed for each concentration. Good recoveries ranging from 82.5 to 95.6 % were achieved (Table 2). The peak area precision (RSD) for three replicate extractions at each concentration was 2.5–5.9 %. These results showed that the developed MISPE-HPLC method was accurate and applicable.
To evaluate the practical application of the developed MISPE-HPLC method, cauliflower sample purchased randomly from a market in Tai’an was analyzed. Methyl parathion was not found in the cauliflower sample, possibly because its use on fruit trees in China was prohibited. Dimethoate and isocarbophos were quantitatively detected at levels of 0.11 and 0.33 mg/kg, respectively, which are higher than the MRLs in the EU. Therefore, more work should be done on monitoring organophosphorus pesticides in vegetables.
Merits of the Developed Method
A lot of methods including GC (Liu et al. 2014; Tian et al. 2014), HPLC (Pirsaheb et al. 2013), GC-MS (Chen et al. 2009), LC-MS-MS (Ingelse et al. 2001), CE (Chen and Fung 2010). and enzyme-linked immunosorbent assay (ELISA) (Garcés-García et al. 2006) have been reported for the detection of organophosphorus pesticide residues, and they are summarized in Table 3.
In comparison, each method has its advantages and disadvantages in terms of sensitivity and interference of matrix compounds. GC and GC-MS are classic methods for the determination of organophosphorus pesticides, but the accuracy of the result is relatively low because organophosphorus pesticides are unstable and degrade easily at high temperature. ELISA is sensitive, accurate, and specific, but it is time consuming, expensive, and has difficulties associated with antibody production. These drawbacks have restricted the use of ELISA in the determination of pesticide residues. HPLC and CE have been reported for the analysis of organophosphorus pesticides, but they are limited because of their low sensitivity to UV detectors.
In this study, the sensitivity of the developed MISPE-HPLC method had been improved because of the good adsorption ability and selectivity of the MIP. The LODs of the three organophosphorus pesticides were much lower than the MRLs of China and EU (Table 1). Therefore, this present method has enough sensitivity for the determination of trace multi-pesticide residues in samples for export. Furthermore, the prepared MISPE column is stable and can be reused for more than 15 times, so the cost per analysis of the MISPE-HPLC method is low.
Conclusions
In this study, a MISPE-HPLC method for the simultaneous determination of three trace organophosphorus pesticides was developed. The results showed that the method was simple, sensitive, and reliable. This study established a preparation methodology for MIP that can selectively recognize of many structural analogs. Furthermore, we will provide a promise tool for determination of trace multi-pesticide residues in food samples.
References
Casida JE, Quistad GB (2004) Organophosphate toxicology: safety aspects of nonacetylcholinesterase secondary targets. Chem Res Toxicol 17:983–998
Chen QD, Fung YS (2010) Capillary electrophoresis with immobilized quantum dot fluorescence detection for rapid determination of organophosphorus pesticides in vegetables. Electrophoresis 31:3107–3114
Chen H, Chen RW, Feng R, Li SQ (2009) Simultaneous analysis of carbamate and organophosphorus pesticides in water by single-drop microextraction coupled with GC–MS. Chromatographia 70:165–172
Cuong LP, Evgen’ev MI, Gumerov FM (2012) Determination of pesticides in the hair of Vietnamese by means of supercritical CO2 extraction and GC–MS analysis. J Supercrit Fluid 61:86–91
Garcés-García M, Brun EM, Puchades R, Maquieira Á (2006) Immunochemical determination of four organophosphorus insecticide residues in olive oil using a rapid extraction process. Anal Chim Acta 556:347–354
Guan SX, Yu ZG, Yu HN, Song CH, Song ZQ, Qin Z (2011) Multi-walled carbon nanotubes as matrix solid-phase dispersion extraction adsorbent for simultaneous analysis of residues of nine organophosphorus pesticides in fruit and vegetables by rapid resolution LC–MS–MS. Chromatographia 73:33–41
Ingelse BA, Dam RCJV, Vreeken RJ, Mol HGJ, Steijger OM (2001) Determination of polar organophosphorus pesticides in aqueous samples by direct injection using liquid chromatography–tandem mass spectrometry. J Chromatogr A 918:67–78
Liu HM, Kong WJ, Qi Y, Gong B, Miao Q, Wei JH, Yang MH (2014) Streamlined pretreatment and GC–FPD analysis of multi-pesticide residues in perennial Morinda roots: a tropical or subtropical plant. Chemosphere 95:33–40
Meng L, Qiao XG, Song JM, Xu ZX, Xin JH, Zhang Y (2011) Study of an online molecularly imprinted solid phase extraction coupled to chemiluminescence sensor for the determination of trichlorfon in vegetables. J Agric Food Chem 59:12745–12751
Núñez L, Turiel E, Martin-Esteban A, Tadeo JL (2010) Molecularly imprinted polymer for the extraction of parabens from environmental solid samples prior to their determination by high performance liquid chromatography-ultraviolet detection. Talanta 80:1782–1788
Pérez-Ruiz T, Martínez-Lozano C, Sanz A, Bravo E (2005) Determination of organophosphamidon pesticides in water, vegetables and grain by automated SPE and MEKC. Chromatographia 61:493–498
Pirsaheb M, Fattahi N, Shamsipur M (2013) Determination of organophosphorous pesticides in summer crops using ultrasound-assisted solvent extraction followed by dispersive liquid–liquid microextraction based on the solidification of floating organic drop. Food Control 34:378–385
Ričanyová J, Gadzała-Kopciuch R, Reiffova K, Bazel Y, Buszewski B (2010) Molecularly imprinted adsorbents for preconcentration and isolation of progesterone and testosterone by solid phase extraction combined with HPLC. Absorption 16:473–483
Saraji M, Rezaei B, Boroujeni MK, Bidgoli AAH (2013) Polypyrrole/sol–gel composite as a solid-phase microextraction fiber coating for the determination of organophosphorus pesticides in water and vegetable samples. J Chromatogr A 1279:20–26
Stafiej A, Pyrzynska K, Regan F (2007) Determination of anti-inflammatory drugs and estrogens in water by HPLC with UV detection. J Sep Sci 30:985–991
Taira K, Aoyama Y, Kawamata M (2006) Long QT and ST-T change associated with organophosphate exposure by aerial spray. Environ Toxicol Pharmacol 22:40–45
Theodoridis G, Lasáková M, Škeříková V, Tegou A, Giantsiou N, Jandera P (2006) Molecular imprinting of natural flavonoid antioxidants: application in solid-phase extraction for the sample pretreatment of natural products prior to HPLC analysis. J Sep Sci 29:2310–2321
Tian F, Liu WJ, Fang HS, An M, Duan SS (2014) Determination of six organophosphorus pesticides in water by single-drop microextraction coupled with GC-NPD. Chromatographia 77:487–492
Wang XL, Qiao XG, Ma Y, Zhao T, Xu ZX (2013) Simultaneous determination of trace nine organophosphorous pesticide residues in fruit samples using molecularly imprinted matrix solid-phase dispersion followed by gas chromatography. J Agric Food Chem 61:3821–3827
Xie J, Liu TS, Song GX, Hu YM, Deng CH (2013) Simultaneous analysis of organophosphorus pesticides in water by magnetic solid-phase extraction coupled with GC–MS. Chromatographia 76:535–540
Xin JH, Qiao XG, Ma Y, Xu ZX (2012) Simultaneous separation and determination of 8 organophosphorous pesticide residues in vegetables through molecularly imprinted solid-phase extraction coupled to gas chromatography. J Sep Sci 35:3501–3508
You XW, Xing ZK, Liu FM, Jiang NW (2013) Air-assisted liquid–liquid microextraction used for the rapid determination of organophosphorus pesticides in juice samples. J Chromatogr A 1311:41–47
Yu CH, Hu B (2009) Sol–gel polydimethylsiloxane/poly(vinylalcohol)-coated stir bar sorptive extraction of organophosphorus pesticides in honey and their determination by large volume injection GC. J Sep Sci 32:147–153
Zhang Q, Wang LB, Ahn KC, Sun Q, Hu BS, Wang J, Liu FQ (2007) Hapten heterology for a specific and sensitive indirect enzyme-linked immunosorbent assay for organophosphorus insecticide fenthion. Anal Chim Acta 596:303–311
Zhang ZH, Liu L, Li H, Yao SZ (2009) Synthesis, characterization and evaluation of uniformly sized core–shell imprinted microspheres for the separation trans-resveratrol from giant knotweed. Appl Surf Sci 255:9327–9332
Zhao T, Gao HJ, Wang XL, Zhang LM, Qiao XG, Xu ZX (2014) Study on a molecularly imprinted solid-phase extraction coupled to capillary electrophoresis method for the determination of trace trichlorfon in vegetables. Food Anal Method 7:1159–1165
Zhu QZ, Degelmann P, Niessner R, Knopp D (2002) Selective trace analysis of sulfonylurea herbicides in water and soil samples based on solid-phase extraction using a molecularly imprinted polymer. Environ Sci Technol 36:5411–5420
Acknowledgments
The authors are grateful for the financial support from the National Natural Science Foundation of China (Project No. 31171699 and 31471649) and the Agricultural Science and Technology Achievements Transformation Project of China (Project No. 2013GB2C600276).
Compliance with Ethics Requirements
We have no financial relationship with the organization that sponsored the research. Dr. Zhixiang Xu has received research grants from the National Natural Science Foundation of China and the Agricultural Science and Technology Achievements Transformation Project of China.
Conflict of Interest
Qingqing Wang, Xin Zhang, Zhixiang Xu and Huiju Gao declare that they have no conflict of interest. This article does not contain any studies with human or animal subjects.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors Xin Zhang and Qingqing Wang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wang, Q., Zhang, X., Xu, Z. et al. Simultaneous Determination of Three Trace Organophosphorus Pesticide Residues in Vegetables Using Molecularly Imprinted Solid-Phase Extraction Coupled with High-Performance Liquid Chromatography. Food Anal. Methods 8, 2044–2051 (2015). https://doi.org/10.1007/s12161-014-0086-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-014-0086-2