Abstract
A rapid, specific, and sensitive method has been developed using molecularly imprinted polymers (MIPs) as solid-phase extraction sorbents for extraction of trace tetracycline antibiotics (TCs) in foodstuffs. MIPs were prepared by precipitation polymerization using tetracycline as the template. Under the optimal condition, the imprinting factors for MIPs were 4.1 (oxytetracycline), 7.0 (tetracycline), 7.4 (chlortetracycline), 7.7 (doxycycline), respectively. Furthermore, the performance of MIPs as solid-phase extraction sorbents was evaluated and high extraction efficiency of molecularly imprinted solid-phase extraction (MISPE) procedure was demonstrated. Compared with commercial sorbents, MISPE gave a better cleanup efficiency than C18 cartridge and a higher recovery than Oasis HLB cartridge. Finally, the method of liquid chromatography–tandem mass spectrometry coupled with molecular-imprinted solid-phase extraction was validated in real samples including lobster, duck, honey, and egg. The spiked recoveries of TCs ranged from 94.51% to 103.0%. The limits of detection were in the range of 0.1–0.3 μg kg−1.

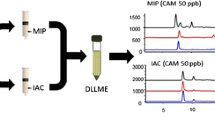
Chromatograms obtained by direct injection of the spiked egg extracts (5 × 10-3 mmol L−1) and purification with MISPE
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tetracycline antibiotics (TCs) have been commonly used in veterinary medicine for both therapeutic and prophylactic purposes in food-producing animals. Tetracycline (TC), oxytetracycline (OTC), chlortetracycline (CTC), and doxycycline (DOX) are four members of this antibiotic group and their chemical structures are given in Fig. 1. In recent years, the abundant and in some cases improper use of TCs has resulted in the presence of residues in edible animal tissues, which is toxic and dangerous for human health [1]. In Belgium, the action limit for the group of tetracycline was preliminarily set at 50 μg kg−1. Since July 1, 2002, this value has been fixed at 20 μg kg−1. France applies a nonconformity limit for tetracycline in honey of 15 μg kg−1; the reporting limit in Great Britain is 50 μg kg−1, while the tolerance levels in Switzerland are 20 μg kg−1 [2]. Therefore, a suitable monitor for the fate of TCs in foodstuff is of great importance.
Foodstuff samples cannot be analyzed without any preliminary sample preparations because analytes are present in trace amount and the matrix is rather complex [3]. Sample preparation, such as extraction, concentration, and isolation of analytes, greatly influences the reliability and accuracy of the whole assay. Solid-phase extraction (SPE) has been proposed as a well-established method for sample cleanup and preconcentration at trace level, owing to its simplicity and economy in terms of time and solvent consumption [4–6]. However, the main problem associated with solid-phase extraction cartridge packed with common stationary phases (such as reversed-phase materials or ion exchange) is the low selectivity for analyte [7, 8].
Recently, molecularly imprinted polymers (MIPs) have been recognized as useful materials for solid-phase extraction [7, 9–11]. Because of the selective absorption of MIPs for a particular analyte or group of analytes, SPE using MIPs as sorbent materials allows preconcentration of these analytes and removal of the interfering compounds from the sample matrix. In addition, other advantages of MIPs as sorbents are the low cost of synthesis, high stability to harsh chemical conditions, and being reusable [12]. Nowadays, the most common methodology for molecular imprinting is the bulk polymerization. This process is tedious and time-consuming and the obtained particles show a random shape and size, so its applicability is limited [13]. Precipitation polymerization has recently emerged as a desirable and scalable approach to adopt for the production of high-quality, uniform, and spherical imprinted particles. Although a large amount of template molecules are needed for the preparation process, the feasibility of preparing highly selective molecularly imprinting polymers using precipitation polymerization has been demonstrated [14–17].
Liquid chromatography–tandem mass spectrometry (LC–MS/MS) provides specific, selective, and sensitive quantitative results and allows unequivocal identification of trace contaminants in complex foodstuff matrices. It has become one of the major tools in the foodstuff analysis and Public Health Agencies in many countries rely on MS detection for unambiguous confirmation of antibiotics in foodstuffs. Thus, several analytical procedures based on LC–MS/MS have been developed for TCs residue determination in foodstuffs [18–23].
Although there are a few reports using TCs as templates to prepare MIPs, to the best of our knowledge, the accepted method for preparation of MIPs was bulk polymerization [24–27]. One aim of this work is to synthesize uniform and spherical MIPs by precipitation polymerization. Then, the specificity of molecularly imprinted solid-phase extraction (MISPE) and the sensitivity of LC–MS/MS together demonstrated the determination of TCs in different foodstuffs. The results indicated this method was suitable for analysis of tetracycline antibiotics residue in different foodstuffs.
Experimental
Reagents and chemicals
TC, OTC, CTC, DOX, methacrylic acid (MAA), 4-vinylpyridine (4-VP), 2,2-azobisisobutyronitrile (AIBN), ethylene glycol dimethacrylate (EGDMA), and trimethylolpropane trimethylacrylate (TRIM) were purchased from Sigma Company (St. Louis, MO, USA). Methanol, acetonitrile, and acetone were obtained from KeMiOu Chemical Reagent Company (Tianjin, China). MAA, 4-VP, EGDMA, and TRIM were purified prior to use via general distillation method in vacuum under argon protection to remove the polymerization inhibitor. AIBN was recrystallized from methanol and dried at room temperature in a vacuum. Ultrapure water was obtained from a Milli-R04 purification system (Millipore, Germany). McIlvaine buffer was prepared by dissolving 11.8 g of citric acid monohydrate, 13.72 g of dibasic sodium phosphate, and 33.62 g of ethylenediaminetetraacetic acid disodium salt in 1 L of water. A 1-mmol L−1 stock standard solution of tetracycline antibiotics was prepared in methanol and diluted to the final concentration with acetonitrile when used. All solutions were stored at 4 °C in the dark and made up every 2 weeks.
Samples
Lobster, duck, honey, and egg samples used for this study were collected from local market. Before use, all samples were determined to be free of the antibiotics considered.
Apparatus and analytical conditions
Extraction equipment
SPE was performed with a Supelco (Bellefonte, PA, USA) 12-position SPE manifold equipped with vacuum control valve and poly(tetrafluoroethylene) cartridge adapters. Empty 3-mL Isolute SPE cartridges and polyethylene frits for MISPE were purchased from Biotage Company (Uppsala, Sweden). The ultrasonic water bath (150 W, 35 kHz) was purchased from Raypa (Barcelona, Spain). The Commercial C18 cartridges (100 mg, Supelclean LC-C18, Supelclean C18-ODS Supelco) and HLB cartridge (100 mg, Oasis HLB, Waters (Milford, MA, USA)) were used for comparison.
LC–MS/MS analysis
All measurements were performed using an Agilent 1200 series high-performance liquid chromatograph (Palo Alto, CA, USA) equipped with a quaternary pump, a standard autosampler, a thermostatted cartridge compartment, and a UV detector and was interfaced to an ABI Sciex API 3000 triple–quadrupole mass spectrometer (Applied Biosystems, Toronto, Canada). The chromatographic separation was carried out with a Restek C18 (150 × 2.1 mm id, particle size 5 μm) column (Bellefonte, PA, USA) using methanol/acetonitrile/100 mmol L−1 oxalic acid solution (1/2/7, v/v/v) as mobile phase. The flow rate was kept at 0.25 mL min−1 and the injection volume was 10 μL. The multireaction monitoring mode was used for quantitative analysis. High-purity nitrogen was used as drying gas, curtain gas, and collision gas. The nebulizer gas was set as 7 psi; curtain gas was set as 9 psi at 7 mL min−1; source voltage gas was set as 5.5 kV and the electrospray source was set as 500 °C. The precursor ion, product ions, collision energy, declustering energy, and dwell time were optimized and reported in Table 1.
Preparation of MIP microsphere
The polymers were prepared by precipitation polymerization, based on the practical experience in our laboratory [28–30]. Tetracycline (0.75 mmol) as a template, MAA (6 mmol) as a functional monomer, TRIM (3 mL) as a cross-linker, and AIBN (30 mg) as a free radical initiator were dissolved in 65 mL of a mixture of methanol and acetonitrile (30/35, v/v) in a 100-mL round-bottomed flask. The solution was deoxygenated with oxygen-free nitrogen for 5 min. Then, the flask was attached to a rotoevaporator and, after sealing, polymerization took place at 55 °C for 24 h with continuous shaking at about 50 rpm. After polymerization, the templates were removed by Soxhlet apparatus with acetic acid/methanol (1/9, v/v) for 24 h. Then, MIPs were washed with methanol for 12 h to remove the acetic acid and dried at 60 °C to reach a constant weight. Nonimprinted polymers (NIPs) were prepared under identical conditions except that there was no template present during polymerization.
Morphological observations
The surface morphology of MIPs was observed using an FEI (Hillsboro, OR, USA) Sirion 200 ultrahigh-resolution Schottky field emission scanning electron microscope (FESEM). All samples were sputter-coated with gold before FESEM analysis.
Frontal chromatography and selectivity evaluation
To estimate the breakthrough volume and the absorptive capacity, frontal chromatography was performed using an HPLC column (40 × 4.6 mm) packed with MIPs. The column was coupled to a HPLC system and washed with methanol, until a stable baseline was obtained. Tetracycline solutions, spiked at different concentrations (5, 10, 20, 40, 60, 80, 100, 200, ×10−3 mmol L−1), were tested as mobile phase. After a certain time, the MIP binding sites were saturated and tetracycline was not retained any further. Then, the dissociation constant (K d) and the total amount of the immobilized ligand (B t) were calculated from the Eq. 1. The A 0 was the spiked concentration of tetracycline solution. The breakthrough volume (V) was calculated as the time in which TC reached the detector and the flow rate. The breakthrough volume for noninteracting molecule (V 0) was measured by eluting the column with acetone.
To evaluate the absorptive property of the MIPs, 10 μL of standard solution (0.1 mmol L−1) and the void marker (acetone) were injected onto the column. Methanol was used as mobile phase; the flow rate was 0.5 mL min−1 and the wavelength of detection was 350 nm. The capacity factors (k mip and k nip) were defined as k mip(nip) = (t − t 0 ) / t 0 , where t is the retention time of tetracycline and t 0 is the retention time of acetone. The imprinting factor (I) was defined as I = k mip / k nip.
Preparation and optimization of MISPE cartridges
A quantity of 100 mg of MIPs or NIPs was suspended in methanol and slurry-packed into SPE cartridges with polyethylene frits. After conditioning the cartridge with 5 mL of acetic acid/methanol (1/9, v/v), 3 mL of methanol and 5 mL of acetonitrile sequentially, 3 mL of standard solutions (0.01 mmol L−1 each, respectively) were passed through the cartridge. Different SPE protocols were applied by utilizing different solvents when loading, washing, and eluting the MISPE cartridge. All of the applied fractions were collected separately and the amount of the recovered compound was quantified by LC–MS/MS.
Foodstuff sample preparation and solid-phase extraction
Spiked foodstuff samples (lobster, duck, and honey) were prepared by adding 500-mL different concentrations of TCs aqueous solution (10, 20, 30 μg/mL) into 1 kg of homogenized sample and shaken in dark conditions overnight at room temperature. The preliminary extraction of spiked sample (lobster, duck, and honey) was optimized, based on the practical experience in our laboratory. A 5-g lobster subsample was placed into a 50-mL centrifuge tube and 20 mL of McIlvaine buffer (adjust to pH 4.0 with 5% HCl solution) was added. The mixture was then placed in ultrasonic bath for 10 min and centrifuged at 3,500 rpm for 5 min. Subsequently, the supernatant was transferred to a clean tube and evaporated to dryness at 40 °C under a stream of N2. Three milliliters of acetonitrile were then added to redissolve the residue for the MISPE process.
Five-gram honey and duck subsamples were preliminary extracted by the above procedure. But the extraction solutions were 5% HCl and acetic acid/methanol (1/9, v/v), respectively.
Because the quantitation of TCs in egg extracts was interfered by the matrix obtained by the above-mentioned pretreatment method, matrix solid-phase dispersion procedure was proposed for cleanup of the extracts. A 5-g egg sample was placed in a glass mortar containing 10 g of silica gel. Different amounts of TCs (0.05, 0.1, 0.15 μg) were added and the mixture was grinded gently to yield a homogeneous material. The blended sample was then transferred to a 25-mL glass syringe packed with filter paper at the bottom and TCs were eluted with 20 mL acetic acid/methanol (1/9, v/v). Subsequently, the eluting solution was evaporated to dryness at 40 °C under a stream of N2. Three milliliters of acetonitrile were added to redissolve the residue for the MISPE process.
For the MISPE process, the cartridge was preconditioned with 5 mL of acetic acid/methanol (1/9, v/v), 3 mL of methanol and 5 mL acetonitrile. After loading 3 mL of sample extracts, the MISPE cartridge was washed with 3 mL of acetonitrile/water (7/3, v/v) and eluted with 5 mL of methanol/100 mmol L−1 potassium hydroxide solution (4/1, v/v). The eluting fraction was collected and evaporated to dryness under a stream of N2. The residue was redissolved with 1 mL of methanol and analyzed by LC–MS/MS.
SPE on commercial C18 and Oasis HLB cartridges
According to the literature [31–34], the C18 cartridges were optimized as follows: conditioning sequentially with 4 mL methanol, 4 mL water, and 4 mL McIlvaine buffer, loading with 3 mL of spiked McIlvaine buffer, washing with 4 mL of water, and eluting with 3 mL of methanol; the Oasis HLB cartridges were optimized as follows: conditioning with 4 mL methanol, 4 mL water, and 2 mL 40 mmol L−1 citric acid buffer (pH 4.7), loading with 3 mL of spiked water, washing with 2 mL of 100 mmol L−1 potassium acetate solution, and eluting with 3 mL of methanol.
Results and discussion
Preparation and evaluation of MIPs and NIPs
In the precipitation polymerization, the polymer was synthesized in the presence of a larger volume of porogen solvent than that used in the bulk polymerization method. The growing polymer chains do not overlap or coalesce but continue to grow individually by capturing newly formed oligomers and monomers in this diluted reaction system and then separate from the solution with microspherical morphologies [11]. In this procedure, although some parameters had been discussed in our literature [35], porogenic solvents and cross-linking agent were further optimized in this study, which still greatly influenced the imprinting effect of polymers.
It is known that the nature and level of porogenic solvents determine the strength of noncovalent interactions and influence polymer morphology, which directly affects the performance of MIPs [36]. Because of the high polarity of TCs, methanol as a necessary solvent was mixed with other less polar solvents to favor the formation of hydrogen bonds between TCs and the monomer. The results indicated that with the ratio of acetonitrile to methanol increasing, the immobilized ligand (B t) gradually increased (Table 2). When the volume ratio of porogen was higher than 35:30, the pressure of packed cartridge was too high to exceed the range of the HPLC system, which might be because the pore size was extremely small. Thus, the optimal volume ratio of acetonitrile/methanol was chosen as 35:30 and the resulting polymers were porous enough and showed high selectivity.
In addition, the selectivity of MIPs is greatly influenced by the kind and amount of cross-linking agent [37]. TRIM, with three allyl groups, can much more favorably form the porous structure of polymers than EGDMA. And it was shown that the imprinted factor (I) of TC for MIP7 was 7.0 and the immobilized ligand (B t) was 2.3 mmol g−1, which demonstrated that MIPs prepared with 3 mL TRIM had the highest load capacity (Table 3). Finally, several studies proved that MIPs polymerized at lower temperatures had greater selectivity versus those made at elevated temperatures, and better selectivity was obtained at 55 °C.
Under the optimal polymerization condition, the immobilized ligand (B t) was 2.3 mmol g−1 and the imprinting factors (I) were 4.1 (OTC), 7.0 (TC), 7.4 (CTC), and 7.7 (DOX), respectively. It was shown that a large number of selective sites and three-dimensional cavities, which were complementary in both shape and chemical functionality arrangement to the template, were generated in imprinting process.
Optimization of the MISPE procedure
The SPE procedure was optimized and the performance of the imprinted polymers for extraction of TCs was compared with the nonimprinted polymers. First of all, the loading step was optimized. After preconditioning, 3 mL of different loading solvents including methanol, water, methanol/acetonitrile (1/1, v/v), and acetonitrile, spiked at 0.01 mmol L−1 of TCs, were passed through the cartridge packed with 100 mg of MIPs. It was shown that when acetonitrile was used as loading solvent, TCs were fully retained on the MISPE whereas nonspecific binding to the NISPE ranged from 86.6% to 90.2%, indicating that acetonitrile was a suitable loading solvent. The next step was to optimize the concentration of loading solution and 3 mL acetonitrile, spiked at different levels of TCs, was passed through the cartridge. The results demonstrated that, when the loading concentration was more than 0.02 mmol L−1, the breakthrough in MISPE happened.
Secondly, after loading the cartridge with 3 mL of acetonitrile (spiked at 0.01 mmol L−1), several solvents such as acetonitrile, acetonitrile/water solution, and acetonitrile/methanol solution were investigated as washing solvents. It was well known that the template could be retained on MIPs by selective and nonspecific interactions but NIPs by nonspecific interactions. Thus, a washing solution with moderate elution strength was used to damage the nonspecific interactions (measured using the NIPs) and to let the target analyte be retained by specific interactions [38]. The results indicated that when the ratio of acetonitrile to water was 7:3, 12.7% of TC was washed from MISPE but 39.5% of that from NISPE. Moreover, the amount of TCs washed from MISPE was greatly increased with increasing ratio of water because the specific interaction was damaged. Thus, acetonitrile/water (7/3, v/v) was the most effective in disrupting nonspecific interactions, while keeping the specific binding between TCs and the polymers. Then, different volumes of this washing solution were tested (Fig. 2). The specific interactions between MISPE and TCs were maximized using 3-mL washing solution. Thus, it was shown that an optimized cleanup procedure was received.
Finally, it was known that the interaction of template and monomer, based on noncovalent interactions, was damaged by eluting solution [39]. When methanol/water (4/1, v/v) was used as eluting solution, 32.5% of TC was still retained on the MIPs. But when the mixture of methanol/water was replaced by methanol/100 mmol L−1 potassium hydroxide solutions, the recoveries increased significantly. This was probably because the specific interactions between TCs and MIPs were damaged at this pH. Then, the volume of the elution solvent was studied. It was shown that any volume less than 5 mL was not sufficient to elute the TCs completely from the polymers.
Therefore, the optimal MISPE protocol was loading with 3 mL acetonitrile, washing with 3 mL acetonitrile/water (7/3, v/v), and eluting with 5 mL methanol/100 mmol L−1 potassium hydroxide (4/1, v/v). Under the optimal protocol, different standard solutions (spiked at 2.5, 5, 7.5, 10, 20, ×10−3 mmol L−1, respectively) were loaded onto the MISPE cartridges. It was shown that the recoveries of TCs on MISPE ranged from 79.5% to 89.1%. The interday relative standard deviation (RSD) was lower than 8.8% and the intraday RSD was lower than 3.1% (Table 4).
Specificity of MISPE
In order to investigate the potential of MIPs for selective entrapment of a target analyte, experiments were performed on LC-C18 cartridges, C18-ODS cartridges, Oasis HLB cartridges, and MISPE. Under the optimized condition, 3 mL of standard solutions (5 × 10−3 mmol L−1) were diluted to different volumes (10, 30, 50, 100 mL, respectively) and loaded onto LC-C18 cartridges, C18-ODS cartridges, Oasis HLB cartridges, and MISPE. It was shown that the recovery of TC on MISPE was 75.1%, even though the loading volume was 100 mL. Compared with MIPs, the recoveries of TCs on commercial sorbents were greatly decreased with the increase of loading volumes because of the absence of selective interaction (Table 5). This feature may have important implications for trace analysis when large volumes of biological and environmental samples need to be processed.
Many investigations into analytical troubleshooting encountered with LC–MS/MS detection have focused on matrix effects. The matrix interference results in reduction of signal intensity and consequently inferior performance of the analytical method with regard to sensitivity, precision, and accuracy [39]. To establish if matrix effects were present in the extracts, comparison tests between the MIPs and commercial sorbents were performed with spiked egg extracts. Chromatograms confirmed that an improved extraction efficiency was achieved with MISPE, obtained by direct injection of the spiked egg extracts (5 × 10−3 mmol L−1, Fig. 3a), purification of the spiked egg extracts with LC-C18 cartridges (Fig. 3b), C18-ODS cartridges (Fig. 3c), Oasis HLB cartridges (Fig. 3d), MISPE (Fig. 3e), NISPE (Fig. 3f), and the blank sample with MISPE (Fig. 3g). After being extracted by LC-C18 cartridges and C18-ODS cartridges, the complex matrix affected the quantification of TCs. Compared with MISPE, similar matrix interference appeared, but the recovery of TC on Oasis HLB cartridges (35.6%) was much less than that of MISPE (89.1%). MISPE gave a better cleanup efficiency than C18 cartridges and a higher recovery than Oasis HLB cartridge. This confirmed that satisfactory sample cleanup was achieved with MISPE.
Analysis of foodstuff samples
The reliability of liquid chromatography–tandem mass spectrometry coupled with molecular-imprinted solid-phase extraction (MISPE-LC–MS/MS) was evaluated with different spiked samples. Under the optimized condition, TCs of spiked samples were extracted and determined with the MISPE-LC–MS/MS procedure. Results were listed in Table 6. The spiked recoveries of TCs from MISPE-LC–MS/MS ranged from 94.5% to 103.0%, and the RSD was lower than 4.6%. The limits of detection were in the range of 0.1–0.3 μg kg−1, which was defined as three times of the noise of LC–MS/MS profile, and the limits of quantification of this method were in the range of 0.2–1.1 μg kg−1, which was defined as ten times of the noise of LC–MS/MS profile. The result indicated that the MISPE-LC–MS/MS method had good accuracy and precision for determination of TCs in different foodstuffs.
Stability and carryover
Generally, a single MIP cartridge can be employed for 20 consecutive cycles without more treatment being required between cycles. The cartridge only needed to be preconditioned with 5 mL of acetic acid/methanol (1/9, v/v), 3 mL of methanol and 5 mL acetonitrile between extractions. TCs were not detected in blank foodstuff extracts from reused cartridges, indicating that there were no carryover effects.
Conclusion
A fast, accurate, and selective analytical method using MIPs as solid-phase extraction sorbents for extraction of trace TCs in foodstuffs had been developed. MIPs were prepared by precipitation polymerization using tetracycline as template. After polymerization, MIPs were used as SPE sorbents and the results indicated that MIPs could not only concentrate but also selectively separate the target analytes from various sample matrices. Under the optimal protocol, MISPE gave a better cleanup efficiency than C18 cartridges and a higher recovery than Oasis HLB cartridge. In addition, the enriching capacity of MISPE was tested and acceptable TCs recoveries were obtained even though the loading volume was 100 mL. Finally, the specificity of MISPE and the sensitivity of LC–MS/MS together demonstrated the determination of TCs in different foodstuffs. Therefore, developing MIPs for the highly selective retention and cleanup of tetracyclines and combining this with the LC–MS/MS method would be useful for regulators concerned about foodstuffs and for researchers characterizing the fate and extent of antibiotic pollution.
References
Samanidou VF, Nikolaidou KI, Papadoyannis IN (2005) J Sep Sci 28:2247–2285
Reybroeck W, Ooghe S, Brabander HD, Daeseleire E (2007) J Agric Food Chem 55:8359–8366
Möller K, Nilsson U, Crescenzi C (2004) J Chromatogr B 811:171–176
Farrington K, Magner E, Regan F (2006) Anal Chim Acta 566:60–68
Tarley CRT, Kubota LT (2005) Anal Chim Acta 548:11–19
Baggiani C, Baravalle P, Giraudi G, Tozzi C (2007) J Chromatogr A 1141:158–164
Ou JJ, Kong L, Pan CS, Su XY, Lei XY, Zou HF (2006) J Chromatogr A 1117:163–169
Boyd B, Bjork H, Billing J, Shimelis O, Axelsson S, Leonora M, Yilmaz E (2007) J Chromatogr A 1174:63–71
Ou JJ, Hu LH, Hu LG, Li X, Zou HF (2006) Talanta 69:1001–1006
Song S, Wu A, Shi X, Li R, Lin Z, Zhang D (2008) Anal Bioanal Chem 390:2141–2150
Qiao FX, Sun HW, Yan HY, Row KH (2006) Chromatographia 64:625–634
Michailof C, Manesiotis P, Panayiotou C (2008) J Chromatogr A 1182:25–33
Bravo JC, Garcinuño RM, Fernández P, Durand JS (2007) Anal Bioanal Chem 388:1039–1045
Kan XW, Zhao Q, Zhang Z, Wang ZL, Zhu JJ (2008) Talanta 75:22–26
Pichon V (2007) J Chromatogr A 1152:41–53
Haginaka J, Futagami A (2008) J Chromatogr A 1185:258–262
Wei S, Mizaikoff B (2007) J Sep Sci 30:1794–1805
Debayle D, Dessalces G, Grenier-Loustalot MF (2008) Anal Bioanal Chem 391:1011–1020
Bogialli S, Curini R, Corcia AD, Lagana A, Rizzuti G (2006) J Agric Food Chem 54:1564–1570
Aga DS, O’Connor S, Ensley S, Payero JO, Snow D, Tarkalson D (2005) J Agric Food Chem 53:7165–7171
Lopez MI, Pettis JS, Smith IB, Chu PS (2008) J Agric Food Chem 56:1553–1559
Cherlet M, Schelkens M, Croubels S, De Backer P (2003) Anal Chim Acta 492:199–213
Kaufmann A, Butcher P, Maden K, Widmer M (2008) J Chromatogr A 1194:66–79
Suedee R, Srichana T, Chuchome T, Kongmark U (2004) J Chromatogr B 811:191–200
Cai WS, Gupta RB (2004) Sep Purif Technol 35:215–221
Caro E, Marce RM, Cormack PAG, Sherrington DC, Borrull F (2005) Anal Chim Acta 552:81–86
Hu X, Pan J, Hu Y, Huo Y, Li G (2008) J Chromatogr A 1188:97–107
Hu M, Jiang M, Wang P, Mei S, Lin Y, Hu X, Shi Y, Lu B, Dai K (2007) Anal Bioanal Chem 387:1007
Zhang J, Jiang M, Zou L, Shi D, Mei S, Zhu Y, Shi Y, Dai K, Lu B (2006) Anal Bioanal Chem 385:780–786
Jiang M, Zhang J, Mei S, Shi Y, Zou L, Zhu Y, Dai K, Lu B (2006) J Chromatogr A 1110:27–34
Arikan OA, Sikora LJ, Mulbry W, Khan SU, Foster GD (2007) Bioresour Technol 98:169–176
Li J, Chen L, Wang X, Jin H, Ding L, Zhang K, Zhang H (2008) Talanta 75:1245–1252
De Ruyck H, De Ridder H (2007) Rapid Commun Mass Spectrom 21:1511–1520
Pena A, Lino C, Alonso R, Barceloa D (2007) J Agric Food Chem 55:4973–4979
Jing T, Gao XD, Wang P, Wang Y, Lin YF, Zong XC, Zhou YK, Mei SR (2007) Chin Chem Lett 18:1535–1538
Sanderson H, Ingerslev F, Brain RA, Halling-Soensen BJ, Bestari C, Wilson J, Johnson DJ (2005) Chemosphere 60:619–629
Anderson CR, Rupp HS, Wu WH (2005) J Chromatogr A 1075:23–32
Qiao F, Sun H, Yan H, Row KH (2006) Chromatographia 64:625–634
Boyd B, Bjork H, Billing J, Shimelis O, Axelsson S, Leonora M, Yilmaz E (2007) J Chromatogr A 1174:63–71
Acknowledgement
This work was supported by the National Nature Science Foundation of China (grant no. 20407009, 30771807) and the Entry–Exit Inspection and Quarantine Bureau of PRC (grant no. 2006IK152).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jing, T., Gao, XD., Wang, P. et al. Determination of trace tetracycline antibiotics in foodstuffs by liquid chromatography–tandem mass spectrometry coupled with selective molecular-imprinted solid-phase extraction. Anal Bioanal Chem 393, 2009–2018 (2009). https://doi.org/10.1007/s00216-009-2641-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-009-2641-z