Abstract
This study proposes new molecularly imprinted solid-phase microextraction (MIP-SPME) coatings based on a sandwich method for the extraction of 2,4-dichlorophenoxyacetic acid (2,4-D) from milk samples. MIP-SPME coatings were prepared by sandwich surface polymerization, using 2,4-dichlorophenoxyacetic acid (2,4-D) as the template, 4-vinylpyridine as the functional monomer and PTFE membrane as supporting material. MIP-SPME coatings were characterized by Fourier transform infrared spectroscopy, scanning electron microscopy, Brunner Emmet Teller measurements and adsorption experiments. The adsorption capacities of nonimprinted polymer solid-phase microextraction and MIP-SPME coatings were 41.8 mg/g and 31.8 mg/g, respectively. Regeneration adsorption was achieved in 137 runs (relative standard deviation, RSD ≤ 6.35%) with an indication of high stability and reusability. The parameters, such as loading solvent, time and washing and elution solvents were optimized and found to be methanol–water (1/9, v/v), 1 h, water and acetonitrile–methanol (1/9, v/v), respectively. Under the optimized conditions, the developed SPME method was successfully applied for the selective extraction and determination of 2,4-D in milk samples coupled with HPLC, with a recovery of 88.8–96.6% (RSD ≤ 5.1%). The limits of determination and the limits of quantitation were found to be 0.03 and 0.1 mg L−1, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
2,4-Dichlorophenoxyacetic acid (2,4-D) is one of the most commonly used herbicides (Wang et al. 2016; Heydari and Feyzianpour 2021). The chemical structure of 2,4-D resembles indoleacetic acid, a naturally occurring hormone produced by plants to regulate their own growth (Yang et al. 2013). Studies have shown that the 2,4-D can cause serious harmful effects on humans and animals such as nervous system dysfunction, metabolic endocrine disorders, immune deficiency syndromes and cancers (Sheng et al. 2017). Therefore, it is necessary to establish a simple, sensitive and environmentally friendly method for the extraction and separation of 2,4-D in real samples.
Analytical methods reported for 2,4-D determination include high-performance liquid chromatography (HPLC) (Zheng et al. 2018; Wu et al. 2020b) capillary electrophoresis (CE) (Espina-Benitez et al. 2017), enzyme-linked immunosorbent assay (ELISA) (Vdovenko et al. 2013), surface-enhanced Raman spectroscopy (SERS) (Hua et al. 2018; Xu et al. 2020; Hassan et al. 2021) and liquid chromatography coupled with tandem mass spectrometry (LC–MS/MS) (Peng et al. 2018). However, nearly all these methods rely on sample pretreatment for example, solid-phase microextraction (SPME). SPME was first introduced by Pawliszyn and his coworkers in 1990 (Arthur and Pawliszyn 1990) as a solvent-free sample preparation technique that integrates sampling, extraction, preconcentration and sample introduction into a single step.
The SPME coating material and its immobilization on a solid support are the focus of this technique. Porous structural materials including carbon nanotubes, graphene or graphene oxides (GOs), metal organic frameworks (MOFs) and covalent organic frameworks (COFs) are highly favoured in coatings due to their high surface area (Peng et al. 2022). However, these materials can adsorb interfering substances together with the analytes because of their low selectivity. To address this issue, molecularly imprinted polymers (MIPs) which possess specific binding comparable with that of natural receptors (He et al. 2021; Yang et al. 2020) were introduced as coating materials. MIPs are prepared in the presence of the template by polymerization and can rebind selectively with the template and its analogous (Pandey et al. 2020; Singh et al. 2020a). MIPs have been widely applied in SPME (He et al. 2021; Turiel and Martín-Esteban 2019; Yigaimu et al. 2019).
In addition, conventional SPME suffers from limitations such as low selectivity of the sorbent material, and poor durability and repeatability of the coating especially when used in the liquid phase. Thin SPME coatings have a low enrichment capacity for target analytes, while thick coatings need a long adsorption time. To have both high adsorption capacity and a fast adsorption rate, research has focused on increasing the specific surface area of coating materials without decreasing mass transfer rates.
In the previous reports, 2,4-D MIPs were prepared by the conventional bulk polymerization (Wu et al. 2020a, b). The use of the bulk polymerization method for MIP synthesis suffers from intrinsic limitations because the polymer particles have irregular shapes and sizes, which significantly limits their use. Coatings prepared by surface molecular imprinting technology (Mirnaghi and Pawliszyn 2012; Dong et al. 2021; Li et al. 2022) have high separation efficiency, and high accessibility to the binding sites, and can reduce the “embedding” phenomenon. Delnia et al. (Bahari et al. 2020) successfully prepared surface-imprinted polymers of polydopamine (PDA) with significant advantages, such as high template removal efficiency, fast mass transfer and a controllable and regular shape. An et al. (2018) prepared a porous water-compatible surface-imprinted coating for the extraction of phenolic compounds from environmental water samples; however, there are drawbacks, such as the uncontrollable thickness of the prepared coating. Wu et al. (2008) combined molecular imprinting technology with fluorescence to design and prepare a composite imprinted polymer film with a fluorescence response to phenylalanine by the sandwich method. The polymer coating thickness is controllable; however, the polymer coating is brittle and easily peels off. Therefore, it is necessary to improve the stability of the coating. The stability of the coating may be improved by adding a carrier film and chemical immobilization.
The aim of this study was to develop a simple, versatile, rapid and low-cost sandwich method to prepare imprinted polymer coatings on the surface of glass substrates via covalent bonding, as shown in Fig. 1. A porous membrane was sandwiched between two kinds of glass slides, a bare slide and a slide immobilized with 3-(methacryloyloxy) propyltrimethoxysilane. Monomers in the membrane were copolymerized on the slide. MIP-SPME coatings with controlled thickness and high stability were successfully prepared. In addition, the selectivity and reusability of the SPME coatings were evaluated. A method for the determination of 2,4-D in milk using the imprinted polymer microextraction coatings combined with high-performance was developed.
Experimental
Materials
Chemicals
Ethylene glycol dimethacrylate (EGDMA), 3-(methacryloyloxy) propyltrimethoxysilane (MPS) and 2,4-dichlorophenoxyacetic acid (2,4-D) were obtained from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). 2,4-Dichlorophenylacetic acid (DPAc), 4-chlorophenoxyacetic acid (CPOAc), 4-vinylpyridine (4-VP), 4-chlorophenylacetic acid (CPAc) and 2,2-azobisisobutyronitrile (AIBN) were supplied by J&K Chemical (Beijing, China). Methanol, acetonitrile and acetic acid were purchased from Zhiyuan Chemical Reagent (Tianjin, China). Methanol (HPLC grade) was obtained from Sigma Aldrich (Tianjin, China). All other chemicals were of analytical reagent grade. Polytetrafluoroethylene (PTFE) microporous membranes were obtained from Shanghai XingYa Purifying Material Factory (Shanghai, China). PTFE membranes for medical use were kindly provided by Zhejiang Kertice Hi-Tech Fluro Material Co, Ltd, (Zhejiang, China). Quartz chips were obtained from Zhejiang Yixing Glass Instrument Factory (Zhejiang, China). Ultrapure water was produced by a UPT-I-10T ULUPURE apparatus (Chengdu, China).
Instrumentation
UV–Vis spectroscopy was conducted using a UV-1800 spectrophotometer (Shimadzu, Japan). FTIR spectra were obtained using KBr pellets on an EQUINOX 55 (Bruker Optics, Germany). Scanning electron microscopy (SEM) and selected area mapping were prepared using a Hitachi SU 8010 scanning electron microscope (Honshu, Japan). Water contact angle measurements were conducted using a DCAT21 instrument from Dataphysics (Stuttgart, Germany). The specific surface area was measured using nitrogen adsorption and desorption with the aid of an Autosorb-IQ2-MP (Quantachrome Instruments Co. Ltd., America). pH was measured using a pH meter (FE28, Shanghai).
Chromatographic analysis was conducted using an HPLC-20AD system (Shimadzu, Japan) equipped with a pump and SPD-20A detector. A Waters XBridge C18 analytical column (4.6 × 250 mm, 5 μm) was used for separation of analytes. The analysis was performed with a mobile phase consisting of methanol and pH = 3 KH2PO4-H3PO4 (60/40, v/v) at a flow rate of 1.0 mL min−1, with injection of 20 μL of sample and detection at 283 nm at room temperature.
Preparation of stock and working standard solutions
Stock solutions of 2,4-D and its analogues (DPAc, CPAc and CPOAc) 1000 mg L−1 were dissolved in methanol and stored in a refrigerator at 4 °C. The stock solutions were diluted with methanol–water (1/9, v/v) to obtain a series of 2,4-D calibration solutions in the range of 0.1–10 mg L−1 for the construction of the calibration curve.
Pretreatment of milk samples
The sample preparation was carried out according to a previously described method with a slight modification (Ji et al. 2019; Singh et al. 2020b). In each 5.0-mL centrifuge tube, 1.0 g of the raw milk (local market) was added and then spiked with 2,4-D stock solutions. After adding 2.5 mL of methanol, the sample was vortexed for 2 min, and kept in a thermostat at 45 °C for 10 min so that the proteins were precipitated, followed by centrifugation at 10,000 rpm for 5 min. The supernatant was separated and collected in another tube. The extraction was carried out in triplicate. The sample pH was adjusted to 3.00 before extraction (Yasen et al. 2021).
Polymer coating preparation
A polymer solution was prepared according to the work reported by K Haupt (Haupt et al. 1998). Briefly, 2,4-D (1 mmol), the monomer 4-VP (4 mmol), EGDMA (20 mmol), and free-radical initiator AIBN (0.31 mmol) were dissolved in 5.0 mL of methanol and water (4/1, v/v). After sonication for 10 min, the mixture was purged with N2 for 2 min to remove oxygen and sealed. Finally, PTFE membranes were cut into 26 mm × 13 mm, pieces and cleaned using ultrapure water and methanol in an ultrasonic bath for 5 min each, respectively. The membrane was then placed between an MPS immobilized glass slide as the substrate (Chen et al. 2017) and a clean glass slide as the cover to form a sandwich structure (Wu et al. 2008). The assembled structure was immersed into a screw-capped glass vial containing the polymerization solution. After that, the system was vacuumed under sonification. The sandwich structure was polymerized at 45 °C for 4 h, followed by 2 h at 60 °C. Finally, the cover glass slide was carefully removed to obtain the poly (4-VP-co-EGDMA) coated slide imprinted against 2.4-D. The NIP coatings were prepared using the same procedures but without the template (2,4-D).
The coatings were washed in methanol/acetic acid (7:3) (2 ×), acetonitrile/acetic acid (9:1) (2 ×), acetonitrile (1 ×) and methanol (2 ×) for 2 h each time using a shaking oscillation. After confirming the complete removal of the template with a UV detector, the obtained coatings were dried under vacuum.
SPME procedure
The MIP and NIP coatings were placed in a 20 mL screw-capped glass vial, respectively, and the coatings were cleaned with methanol for 30 min. Then, the polymer coatings were activated with 4.0 mL of methanol–water (1/9, v/v) in a mechanical shaker. The polymer coatings were placed in 5.0 mL of 2,4-D solution in methanol–water (1/9, v/v) and incubated for 1 h at room temperature. The polymer coating was washed with 4.0 mL of water. The analyte was then eluted with 5.0 mL of methanol on a shaker for 1 h at room temperature, the eluent was collected and evaporated to dryness by gentle nitrogen blowing, and the residue was then redissolved in the mobile phase (0.5 mL), followed by filtration through a 0.22-μm microporous nylon membrane. Finally, an aliquot of 20 µL of filtrate was injected into the HPLC.
Statistical analysis
The data were analysed by one-way analysis of variance (ANOVA) in IBM SPSS software 24. Mean differences were compared by Duncan's multiple range test at the 95% confidence level. The error level for statistics was a P value of less than 0.05 for statistically significant differences.
Results and discussion
Characterization
FTIR
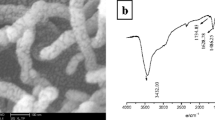
Figure 2A shows the FTIR spectra of the 2,4-D MIP and NIP coatings. The peaks at 2953.47 cm−1, 1215.16 cm−1 and 1156.36 cm−1 correspond to the stretching vibrations of C–H and asymmetric and symmetric stretching vibrations of C–O–C, respectively. The absorption peaks at 1598.28 cm−1 and 1636.82 cm−1 are characteristic of 4-VP skeleton vibrations (Liu et al. 2014) and are the evidence that MIPs and NIPs were polymerized successfully. The peak at 1727.10 cm−1 is attributed to the stretching vibrations of C=O on the polymer coatings. The peak at 637 cm−1 is assigned to C–Cl stretching on the template (Arivazhagan and Meenakshi 2011). Compared to 2,4-D MIPs with the template, NIPs have weaker absorbance at 1215 and 1156 cm−1 corresponding to C–O–C antisymmetric and symmetric vibrations due to the contribution of the template molecule, indicating the successful preparation of MIP and NIP coatings.
A The FTIR spectra of MIP and NIP coatings. B The SEM images of different PTFE membranes with different magnifications of 30,000 (B1), 500 (B2) and 2000 (B3); different polymer coatings with different magnifications of 30,000 (B4), 500 (B5) and 2000 (B6); the elemental mapping images of (a) MIP coatings with 45 μm thickness and (b) MIP coatings with 140 μm thickness
SEM and elemental mapping
The morphology of different PTFE membranes and composite polymer coatings was investigated by the scanning electron microscopy (SEM). The SEM images of the membranes and polymer coatings are shown in Figure B1–B2 and B3–B6. Clearly, thicknesses of the 45-μm and 140-μm PTFE membranes before coating formation presented various network structures with layers and networks. This structure facilitated the formation of a firm polymer coating. Figures B2 and B4, and B6 show that the polymer coatings have some tiny holes, uneven surfaces, and layered and crosslinked pore structures, which are completely different from those of the bare support membrane. This indicates that the polymer solution penetrated the PTFE membrane and polymerized to form a relatively uniform and dense surface. The evenly distributed microstructure on the surface provides a favourable environment for the adsorption of the analyte. It can be proposed that the polymer coatings prepared by the sandwich method have a smoother surface and more uniform thickness compared to other methods. Elemental mapping analysis (Fig. 2a, b) further revealed that F, and other constituent elements (C, O and N) were uniformly distributed in the composite materials. This clearly demonstrated that the coating was successfully formed on the PTFE membranes successfully. In addition, the C and O elements demonstrate that the dispersion of O essentially overlapped with that of C, indicating a uniform doping of O on the carbon body.
Contact angle measurement
Measurement of the contact angle of the polymer coatings was performed by placing a drop of water on the surface of the material. Figure 3 shows the contact angles of the polymer coatings and bare PTFE membranes with different thicknesses (45 and 140 μm). The bare PTFE membranes showed high hydrophobicity with a contact angle of approximately 121° (Fig. 3A, B). After polymerization, the coating surface presented a contact angle close to 62°, which was much lower than that of the bare membrane, showing a water wettable surface (Fig. 3a, b). The results indicated that a hydrophilic composite polymer coating was successfully prepared for the selective extraction of 2,4-D.
Surface area measurements
The pore size distribution and Brunauer‒Emmett‒Teller (BET) surface area were calculated from the nitrogen adsorption–desorption isotherm that was obtained by using the Brunauer‒Emmett‒Teller method. The nitrogen adsorption and desorption isotherms of MIP and NIP coatings with 45 μm and 140 μm are shown in Fig. S1. The nitrogen adsorption–desorption isotherm of 45-μm and 140-μm MIP and NIP coatings show an adsorption at low pressures, an increase in adsorption with increasing pressure, and hysteresis upon desorption. The structural parameters of the 45-μm and 140-μm-thick polymer coatings are listed in Table 1. The surface area and pore volume increase with the increasing of membrane thickness. By comparison, the MIP coatings were observed to have a higher surface area and pore volume, which was helpful to improve the binding capacity. This is also the main contributing factor of the imprinting effect.
Optimization of the polymer coating SPME method
Several parameters associated with SPME efficiency, such as the thickness of the coating, adsorption selectivity and loading and elution solvents were optimized.
Effect of the thickness of the supported membrane
Coating thickness is a decisive factor of SPME extraction performance. Herein, polymer coatings were prepared with the supported membranes with thicknesses of 45 and 140 μm. The coatings have a uniform and highly controlled thickness. Due to the low adsorption capacity of thin coatings, the extraction capacity of analytes is not ideal. The application of a thick coating is an effective way to improve extraction efficiency and analytical sensitivity. Figure 4a shows that the adsorption capacity of the thin coating was lower than that of the thick coating. The supported membrane with the greater thickness and porous structure can not only guaranteed fast adsorption/desorption of the target compounds but also provided high extraction capacity.
a Effect of the thickness on the adsorption capacity of polymer coatings (n = 3) (4.0 mL of 10 mg L−1 2,4-D solution in methanol–water (1/9, v/v) and one piece of coating used for the adsorption); b Reusability of MIP coatings (n = 3). (Stirring speed of 200 rpm, 14 mL of 20 mg L−1 2,4-D solution in methanol–water (1/9, v/v) and four pieces of coatings used for the adsorption)
Regeneration of the SPME coating
To be an effective SPME device, the regeneration and reuse of SPME coatings are essential. Under the same conditions, the regeneration of the coatings (n = 4) was verified by interbatch experiments. The RSD value was found to be 2.89% (n = 3). In this study, the MIP coatings were regenerated by eluting with 10% acetic acid–methanol and methanol, respectively. As shown in Fig. 4b, the adsorption capacity of MIPs did not change significantly after 137 runs (RSD less than 6.35%), indicating that MIP coatings have outstanding stability and repeatability. Therefore, they have good reusability and can be used as a potential SPME material for the separation and enrichment of trace 2,4-D in real samples.
Effect of loading solvent
When the loading conditions were fixed, the maximum capacity of the imprinted material was a constant value. Suitable organic or water–organic solvents are necessary to extract analytes using polymer coatings (Karlsson et al. 2001). Therefore, to study the effect of the loading solvent on the adsorption of polymer coatings, different proportions of methanol in water were tested as loading solvents. As shown in Fig. 5a, the adsorption capacity of 2,4-D increased with decreasing methanol, and the highest adsorption capacity of 2,4-D by the coating was achieved when methanol–water (1/9, v/v) was used as the loading solvent. Therefore, methanol–water (1/9, v/v) was used as the optimal solvent for further experiments.
a Effect of the ratios of methanol–water ratio in the loading solvent on the adsorption capacity of the polymer coatings; b adsorption kinetic curves of 2,4-D on the MIP and NIP coatings; c effect of washing solvents on the elution rate; and d effect of the elution solvent on the elution rate; (n = 3) (4.0 mL of 10 mg L−1 2,4-D solution in methanol–water (1/9, v/v) and one piece of coating used for the adsorption; *MeOH (methanol), ACN (acetonitrile), HAc (acetic acid))
Effect of extraction time
The adsorption kinetic curves of 2,4-D MIPs and NIPs are shown in Fig. 5b, which were obtained by the in-situ adsorption method (Beltran et al. 2010). As the adsorption time was prolonged, the adsorption amount slowly increased. Both MIPs and NIPs reached approximately 75% of the equilibrium adsorption within 60 min in methanol–water (1/9, v/v) which indicating that the polymers have a fast adsorption for 2,4-D. Subsequently, the change in the adsorption capacity gradually decreased, and the adsorption equilibrium was basically reached in 3 h. Furthermore, clearly, it can be clearly seen that the adsorption capacities of MIPs for 2,4-D were higher than those of NIPs under the same conditions, due to the existence of recognition sites in MIPs having complementary shapes and sizes with 2,4-D. Here, one hour was selected as the extraction time in the following tests.
Effect of washing solvent
The washing step is essential to remove interfering components and reduce nonspecific interactions in the sample matrix. During the washing process, the type of solvent will directly affect the elution efficiency. In this work, water and 10% and 15% methanol in water were selected as washing solvents. In Fig. 5c, the highest elution efficiency was obtained with water, so it was chosen as the washing solution.
Effect of elution solvent
The imprinted polymers are bound to the target molecule by hydrogen bonding, electrostatic interactions, etc., such as 2,4-D molecularly imprinted polymers (Aya et al. 2019). The elution solvent determines the efficiency of elution of the imprinted material. Many organic solvents can disrupt the hydrogen bond between the template and the cavity. The selection of an appropriate eluent is very important for the elution process. In addition, considering the consistency with the mobile phase used in liquid chromatography, the eluent is limited to solvents such as methanol, acetonitrile and purified water. However, 2,4-D is insoluble in water. Therefore, a certain ratio of methanol with acetonitrile as the eluent was tested. Figure 5d shows that three different solvents (acetic acid–methanol (1/9, v/v), acetonitrile–methanol (1/9, v/v) and methanol) were used to evaluate the elution efficiency. When acetonitrile–methanol (1/9, v/v) was selected, the elution rate reached 96.55%. The differences between acetonitrile–methanol (1/9, v/v) and other elution solvents were significant (p < 0.05). Therefore, acetonitrile–methanol (1/9, v/v) was selected as the elution solvent.
Adsorption selectivity
The selectivity of MIPs is based on the configuration of the binding sites and the orientation of the functional groups in the sites. To determine the selectivity of MIPs and NIPs towards 2,4-D, the structural analogues (DPAc, CPOAc and CPAc) were tested (Fig. S2). It can be seen that 2,4-D has the largest adsorption capacity, followed by DPAc, CPAc and CPOAc. Although they have a very similar structure resembling 2,4-D, MIPs presented higher adsorption capacity towards the template (2,4-D) than towards the three analogues, indicating that 2,4-D MIPs could recognize the template due to their specific imprinting sites and cavities (Yasen et al. 2021). 2,4-D MIPs have an imprinting factor of 2.02.
Analytical performance
The method performance was evaluated by the determination of the linearity and sensitivity. The linearity of the calibration curve (y = 1.0694 × 104x + 0.9307 × 103) was obtained by determination of the peak areas from the analysis of 0.1–10 mg L−1 2,4-D without enrichment, and the R2 value was 0.9973. According to three and ten times the signal-to-noise ratio, the LODs and LOQs were calculated. The LOD and LOQ were found to be 0.03 and 0.1 mg L−1, respectively. The blank samples of milk were selected and spiked with standard solutions at 0.1 and 1.1 mg L−1 concentration levels in the range of 0.1–10 mg L−1. The determination followed the optimized above-mentioned protocol. The recoveries were 91.20–101.67%, and the RSDs were between 4.06 and 5.14%. Briefly, the developed method can provide trace analysis of 2,4-D.
Real sample analysis
The developed method was applied to the determination of 2,4-D in milk samples. The sample was passed through a 0.22-μm microporous nylon membrane to eliminate particulate matter prior to SPME. The chromatograms of spiked milk samples before and after treatment by polymer coating SPME are shown in Fig. 6. The milk sample was not found to contain 2,4-D and spiked with the analyte at a known concentration of 0.1 mg L−1, the recoveries of MIPs and NIPs were 91.20% and 62.20%, respectively, and the RSDs of MIPs and NIPs were 5.14% and 6.96%. Compared to NIP coatings, some interfering substances were eliminated by MIP-SPME coatings after extraction, and its peak area was much larger, which demonstrated the higher selectivity and affinity of the MIP coatings (Fig. 6a). This result indicated that the MIP coatings can be used for the preconcentration and selective extraction of 2,4-D from milk samples.
HPLC chromatograms of milk samples (a) 2,4-D spiked milk sample after extraction with NIP coatings and (b) MIP coatings. (c) Without extraction. (Column: Waters XBridge C18 column (4.6 × 250 mm, 5 μm), mobile phase: methanol: KH2PO4-H3PO4 (60/40, v/v, pH = 3.00), flow rate: 1.0 mL min−1, λ = 283 nm. concentration: 0.1 mg L−1)
Method comparison
Previously reported methods and this newly developed method for the determination of 2,4-D in real samples are listed in Table 2. As shown in Table 2, 200 mL of diethyl ether was consumed in the determination of 2,4-D in milk, as reported by Marquardt et al. (1951), and 25 mL of acetonitrile containing 1% formic acid was consumed in the determination of 2,4-D in milk, as reported by Hua et al. (2018). However, the MIP-SPME-HPLC used in this study consumes less organic solvents in the determination of 2,4-D in milk, so the MIP-SPME-HPLC used in this study is green and pollution-free. Yang et al. (2013) determined 2,4-D in river water, which consumed less organic solvent but was time-consuming. Liu et al. (2014) determined 2,4-D in tap water, and although the consumption of organic solvents was relatively low and the time required was short, the synthesis of MIPs using the traditional bulk polymerization method resulted in high product loss due to tedious grinding and screening processes, and mechanical crushing promoted the destruction of the binding cavities, resulting in small adsorption capacities. Comparable RSDs and better recoveries were obtained for the 2,4-D compared to other methods. The SPME coating can be recycled 137 times, indicating that it has good reusability. Thus, the developed method has the advantages of being applied for the determination of 2,4-D in complex samples.
Conclusions
The MIP-SPME coatings for the selective recognition of 2,4-D were prepared by the sandwich method. The sandwich-type polymer coatings also provided several advantages over traditional methods such as ease of use, relatively low-cost, and robustness. Compared with NIP-SPME coatings, MIP-SPME coatings exhibited large adsorption capacity, fast binding ability and excellent selectivity towards the template 2,4-D, suggesting the successful imprinting. The results demonstrated that the surface-imprinted coatings could significantly improve the adsorption capacity, adsorption kinetics and mass transfer due to the recognition sites situated at the surface of the MIP-SPME coatings. It is believed that these simple, low-cost and long-life of the materials can be promising as a kind of SPME coating for the enrichment and detection of other kinds of phytohormones in food samples.
Data availability
The raw data required to reproduce these findings are available to download from the website of European Polymer Journal in the form of supplementary information.
References
An Q, Huang T, Shi F (2018) Covalent layer-by-layer films: chemistry, design, and multidisciplinary applications. Chem Soc Rev 47:5061–5098. https://doi.org/10.1039/c7cs00406k
Arivazhagan M, Meenakshi R (2011) Quantum chemical studies on structure of 1–3-dibromo-5-chlorobenzene. Spectrochim Acta: Part A Mol Biomol Spectrosc 82:316–326. https://doi.org/10.1016/j.saa.2011.07.055
Arthur CL, Pawliszyn J (1990) Solid phase microextraction with thermal desorption using fused silica optical fibers. Anal Chem 62:2145–2148. https://doi.org/10.1021/ac00218a019
Aya GA, Yang JC, Hong SW, Park JY (2019) Replicated pattern formation and recognition properties of 2,4-dichlorophenoxyacetic acid-imprinted polymers using colloidal silica array molds. Polymers (basel) 11:1332. https://doi.org/10.3390/polym11081332
Bahari D, Babamiri B, Salimi A (2020) An eco-friendly MIP-solid surface fluorescence immunosensor for detection of CA 19–9 tumor marker using Ni nanocluster as an emitter labels. J Iran Chem Soc 17:2283–2291. https://doi.org/10.1007/s13738-020-01924-z
Beltran A, Marcé RM, Cormack PAG, Borrull F (2010) Synthetic approaches to parabens molecularly imprinted polymers and their applications to the solid-phase extraction of river water samples. Anal Chim Acta 677:72–78. https://doi.org/10.1016/j.aca.2010.07.021
Chen L, Muhammad T, Yakup B, Piletsky SA (2017) New immobilisation protocol for the template used in solid-phase synthesis of MIP nanoparticles. Appl Surf Sci 406:115–121. https://doi.org/10.1016/j.apsusc.2017.02.105
Dong C, Shi H, Han Y et al (2021) Molecularly imprinted polymers by the surface imprinting technique. Eur Polym J 145:110231. https://doi.org/10.1016/j.eurpolymj.2020.110231
Espina-Benitez M, Araujo L, Prieto A et al (2017) Development of a new microextraction fiber combined to on-line sample stacking capillary electrophoresis UV detection for acidic drugs determination in realwater samples. Int J Environ Res Public Health 14:1–16. https://doi.org/10.3390/ijerph14070739
Hassan MM, Zareef M, Jiao T et al (2021) Signal optimized rough silver nanoparticle for rapid SERS sensing of pesticide residues in tea. Food Chem. https://doi.org/10.1016/j.foodchem.2020.127796
Haupt K, Dzgoev A, Mosbach K (1998) Assay system for the herbicide 2,4-dichlorophenoxyacetic acid using a molecularly imprinted polymer as an artificial recognition element. Anal Chem 70:628–631. https://doi.org/10.1021/ac9711549
He S, Zhang L, Bai S et al (2021) Advances of molecularly imprinted polymers (MIP) and the application in drug delivery. Eur Polym J 143:110179. https://doi.org/10.1016/j.eurpolymj.2020.110179
Heydari R, Feyzianpour R (2021) Determination of 2,4-dichlorophenoxyacetic acid in water and edible seeds samples using salt-assisted liquid-liquid extraction coupled with high-performance liquid chromatography. Food Anal Methods 14:561–567. https://doi.org/10.1007/s12161-020-01903-3
Hua MZ, Feng S, Wang S, Lu X (2018) Rapid detection and quantification of 2,4-dichlorophenoxyacetic acid in milk using molecularly imprinted polymers–surface-enhanced Raman spectroscopy. Food Chem 258:254–259. https://doi.org/10.1016/j.foodchem.2018.03.075
Ji Z, Yu Y, Jin Q et al (2019) Determination of naturally occurring thyreostats in bovine milk by high performance liquid chromatography combined with fluorescence detection. Microchem J 145:892–898. https://doi.org/10.1016/j.microc.2018.11.052
Karlsson JG, Andersson LI, Nicholls IA (2001) Probing the molecular basis for ligand-selective recognition in molecularly imprinted polymers selective for the local anaesthetic bupivacaine. Anal Chim Acta 435:57–64. https://doi.org/10.1016/S0003-2670(00)01182-X
Li X, Lin M, Zhang H et al (2022) N-terminal epitope surface imprinted particles for high selective cytochrome c recognition prepared by reversible addition- fragmentation chain transfer strategy. Chem Pap. https://doi.org/10.1007/s11696-022-02134-y
Liu Y, He Y, Jin Y et al (2014) Preparation of monodispersed macroporous core-shell molecularly imprinted particles and their application in the determination of 2,4-dichlorophenoxyacetic acid. J Chromatogr A 1323:11–17. https://doi.org/10.1016/j.chroma.2013.11.002
Marquardt R, Luce E (1951) Determination of small amounts of 2, 4: dichloro- phenoxyacetic acid in milk. Anal Chem 23:1484–1486. https://doi.org/10.1021/ac60058a033
Mirnaghi FS, Pawliszyn J (2012) Development of coatings for automated 96-blade solid phase microextraction-liquid chromatography-tandem mass spectrometry system, capable of extracting a wide polarity range of analytes from biological fluids. J Chromatogr A 1261:91–98. https://doi.org/10.1016/j.chroma.2012.07.012
Pandey H, Khare P, Singh S, Singh SP (2020) Carbon nanomaterials integrated molecularly imprinted polymers for biological sample analysis: a critical review. Mater Chem Phys 239:121966. https://doi.org/10.1016/j.matchemphys.2019.121966
Peng MM, Han YQ, Xia H et al (2018) Rapid and sensitive detection of the phenoxy acid herbicides in environmental water samples by magnetic solid-phase extraction combined with liquid chromatography–tandem mass spectrometry. J Sep Sci 41:2221–2228. https://doi.org/10.1002/jssc.201701325
Peng S, Huang X, Huang Y et al (2022) Novel solid-phase microextraction fiber coatings: a review. J Sep Sci 45:282–304. https://doi.org/10.1002/jssc.202100634
Sheng L, Jin Y, He Y et al (2017) Well-defined magnetic surface imprinted nanoparticles for selective enrichment of 2,4-dichlorophenoxyacetic acid in real samples. Talanta 174:725–732. https://doi.org/10.1016/j.talanta.2017.07.002
Singh M, Singh S, Singh SP, Patel SS (2020a) Recent advancement of carbon nanomaterials engrained molecular imprinted polymer for environmental matrix. Trends Environ Anal Chem 27:e00092. https://doi.org/10.1016/j.teac.2020.e00092
Singh M, Srivastava A, Sharma YK et al (2020b) CVD grown carbon nanofibers: an efficient DSPE sorbent for cleanup of multi-class pesticide residue in high fat and low water commodities by QuEChERS using GC-ECD. Microchim Acta 187:490. https://doi.org/10.1007/s00604-020-04464-8
Turiel E, Martín-Esteban A (2019) Molecularly imprinted polymers-based microextraction techniques. TrAC - Trends Anal Chem 118:574–586. https://doi.org/10.1016/j.trac.2019.06.016
Vdovenko MM, Stepanova AS, Eremin SA et al (2013) Quantification of 2,4-dichlorophenoxyacetic acid in oranges and mandarins by chemiluminescent ELISA. Food Chem 141:865–868. https://doi.org/10.1016/j.foodchem.2013.04.060
Wang X, Yu J, Wu X et al (2016) A molecular imprinting-based turn-on Ratiometric fluorescence sensor for highly selective and sensitive detection of 2,4-dichlorophenoxyacetic acid (2,4-D). Biosens Bioelectron 81:438–444. https://doi.org/10.1016/j.bios.2016.03.031
Wu Z, Tao CA, Lin C et al (2008) Label-free colorimetric detection of trace atrazine in aqueous solution by using molecularly imprinted photonic polymers. Chem: A Eur J 14:11358–11368. https://doi.org/10.1002/chem.200801250
Wu B, Muhammad T, Aihebaier S et al (2020a) A molecularly imprinted polymer based monolith pipette tip for solid-phase extraction of 2,4-dichlorophenoxyacetic acid in an aqueous sample. Anal Methods 12:4913–4921. https://doi.org/10.1039/d0ay01587c
Wu X, Jiao T, Xu C et al (2020b) Preparation of molecularly imprinted polymers for sensing of 2,4-dichlorophenoxyacetic acid residues in environmental water and mixed juice. J Mater Sci 55:6848–6860. https://doi.org/10.1007/s10853-020-04516-7
Xu Y, Hassan MM, Ali S et al (2020) SERS-based rapid detection of 2,4-dichlorophenoxyacetic acid in food matrices using molecularly imprinted magnetic polymers. Microchim Acta 187:454. https://doi.org/10.1007/s00604-020-04408-2
Yang W, Jiao F, Zhou L et al (2013) Molecularly imprinted polymers coated on multi-walled carbon nanotubes through a simple indirect method for the determination of 2,4- dichlorophenoxyacetic acid in environmental water. Appl Surf Sci 284:692–699. https://doi.org/10.1016/j.apsusc.2013.07.157
Yang X, Muhammad T, Yang J et al (2020) In-situ kinetic and thermodynamic study of 2,4-dichlorophenoxyacetic acid adsorption on molecularly imprinted polymer based solid-phase microextraction coatings. Sensors Actuators, A Phys 313:112190. https://doi.org/10.1016/j.sna.2020.112190
Yasen A, Muhammad T, Yang W et al (2021) A novel sandwich method to prepare robust SPME polymer coating on glass slide with controllable thickness for direct analysis through fluorescence and MS imaging. Prog Org Coat 151:106076. https://doi.org/10.1016/j.porgcoat.2020.106076
Yigaimu A, Muhammad T, Yang W et al (2019) Magnetic molecularly imprinted polymer particles based micro-solid phase extraction for the determination of 4-nitrophenol in lake water. Macromol Res 27:1089–1094. https://doi.org/10.1007/s13233-019-7151-z
Zheng S, He M, Chen B, Hu B (2018) Melamine-based porous organic polymers inline solid phase extraction coupled with high performance liquid chromatography for the analysis of phytohormones in juice samples. J Chromatogr A 1567:64–72. https://doi.org/10.1016/j.chroma.2018.07.003
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (22064015 and 21565025).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jian, P., Yasen, A., Muhammad, T. et al. Preparation of molecularly imprinted polymer coatings based on via a sandwich method for solid-phase microextraction of 2,4-dichlorophenoxyacetic acid from milk. Chem. Pap. 77, 219–228 (2023). https://doi.org/10.1007/s11696-022-02471-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-022-02471-y