Abstract
Background
Although the placement of an intraventricular catheter remains the gold standard technique for measuring intracranial pressure (ICP), the method has several limitations. Therefore, noninvasive alternatives to ICP (ICPni) measurement are of great interest. The main objective of this study was to compare the correlation and agreement of wave morphology between ICP (standard intraventricular ICP monitoring) and a new ICPni monitor in patients admitted with stroke. The second objective was to estimate the discrimination of the noninvasive method to detect intracranial hypertension.
Methods
We prospectively collected data of adults admitted to an intensive care unit with subarachnoid hemorrhage, intracerebral hemorrhage, or ischemic stroke in whom an invasive ICP monitor was placed. Measurements were simultaneously collected from two parameters [time-to-peak (TTP) and the ratio regarding the second and first peak of the ICP wave (P2/P1 ratio)] of ICP and ICPni wave morphology monitors (Brain4care). Intracranial hypertension was defined as an invasively measured sustained ICP > 20 mm Hg for at least 5 min.
Results
We studied 18 patients (subarachnoid hemorrhage = 14; intracerebral hemorrhage = 3; ischemic stroke = 1) on 60 occasions with a median age of 52 ± 14.3 years. A total of 197,400 waves (2495 min) from both ICP (standard ICP monitoring) and the ICPni monitor were sliced into 1-min-long segments, and we determined TTP and the P2/P1 ratio from the mean pulse. The median invasively measured ICP was 13 (9.8–16.2) mm Hg, and intracranial hypertension was present on 18 occasions (30%). The correlation and agreement between invasive and noninvasive methods for wave morphology were strong for the P2/P1 ratio and moderate for TTP using categoric (κ agreement 88.1% and 71.3%, respectively) and continuous (intraclass correlation coefficient 0.831 and 0.584, respectively) measures. There was a moderate but significant correlation with the mean ICP value (P2/P1 ratio r = 0.427; TTP r = 0.353; p < 0.001 for all) between noninvasive and invasive techniques. The areas under the curve to estimate intracranial hypertension were 0.786 [95% confidence interval (CI) 0.72–0.93] for the P2/P1 ratio and 0.694 (95% CI 0.60–0.74) for TTP.
Conclusions
The new ICPni wave morphology monitor showed a good agreement with the standard invasive method and an acceptable discriminatory power to detect intracranial hypertension.
Clinical trial registration Trial registration: NCT05121155.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Invasive intraventricular intracranial pressure (ICPi) monitoring coupled with external ventricular drainage (EVD) is considered the gold standard measurement of intracranial pressure (ICP) for its accuracy and for allowing cerebrospinal fluid (CSF) drainage for therapeutic purposes [1, 2]. However, ICPi monitoring can be particularly difficult in patients with small ventricles, has a 5–7% risk of hemorrhage during insertion, and has a progressive cumulative risk of intracranial infection (up to 25% after 5 days) [3]. Additionally, a basic assumption is that the ICP reading at one point in the intracranial system adequately reflects the average pressure of the entire skull because CSF pressure, under normal conditions, should be in balance throughout the CSF space. However, pressure gradients may develop under pathological conditions, leading to unequal or asymmetrical ICP levels between the hemispheres or compartments [4]. Moreover, even when the ICP is homogeneous throughout the system, it may lose accuracy depending on the method used or time of use [5].
Because of these limitations of the standard method, the search for a noninvasive ICP monitoring technique capable of real-time monitoring has become an essential topic in neurological intensive care. Several noninvasive technologies have been described, but no method has achieved the required accuracy, availability, and ability of continuous analysis [6,7,8]. A new opportunity emerged when the Monro–Kellie doctrine was questioned in its fundamental principle that after the fontanelles closure, the cranial volume remains the same [9].
The first attempt to correlate a change in the cranial diameter to ICP was published in 1985 using brains from human and dog cadavers to demonstrate physiological and pathological variations in brain volume [10]. Another study published in 2009 related deformity in rat skulls with variations in ICP and confirmed the previous findings with great accuracy [11].
Braincare Inc. (Brazil) developed a novel noninvasive monitoring technology [12], which can detect small changes in the skull dimension created by the changes in ICP (Fig. 1). The monitor works by connecting a pin to a voltage sensor that touches the skin surface in the temporoparietal region lateral to the sagittal suture [13] delivering continuous real-time information on the ICP waveform [14, 15].
The ICP pulse wave is divided into three peaks: P1 is associated with the systolic arterial pressure transferred by the choroid plexus to the cerebrospinal fluid, P2 is associated with the reflection (rebound) of the blood pressure wave in the cerebral parenchyma, and P3 is related to the aortic valve closure. Intracranial hypertension (IH) affects the characteristics of the waveform. For example, an increased amplitude of the three peaks indicates a mean increase in ICP. The reduced amplitude of P1 suggests a loss of cerebral perfusion pressure, and a relative increase of P2 suggests a loss of cerebral compliance (CC). Additionally, the presence of Lundberg A waves sustained increases in the mean ICP lasting 5–20 min may also signify decreased compliance [16]. Therefore, noninvasive analysis of the ICP wave morphology seems a promising technique for monitoring neurocritical patients.
The main objective of this study was to measure the correlation and agreement of the wave morphology between the noninvasive measurements of ICP (Braincare Corp., US 2013/0085400 A1) and the standard invasive method (ICPi monitoring with EVD) in patients hospitalized for stroke. A secondary aim was to estimate the discriminatory power of the noninvasive method to detect IH.
Methods
Adult inpatients from a dedicated neurological intensive care unit with ischemic stroke (IS) or hemorrhagic stroke who needed ICPi monitoring were prospectively evaluated from March 2019 to March 2020 (before the COVID-19 pandemic). We excluded patients with chronic neurological diseases (demyelinating diseases, chronic hydrocephalus, pseudotumor brain), patients with suspected brain death, and patients monitored with nonventricular sensors (e.g., subdural or epidural).
There was no direct public or private funding in this study, except for availability of the device and the technical support given by Brain4care (B4c) Inc. This clinical trial study protocol was approved by the local ethics committee on February 1, 2019 (register: 03843118.0.0000.5505), and the patients’ legal representative signed a free informed consent form. Registered under number NCT05121155 at ClinicalTrials.gov.
Patient Monitoring
Data were obtained from both the invasive and the noninvasive methods simultaneously 5 min after the closure of the EVD drainage. ICP assessment was performed using a catheter inserted into the ventricle and connected to a pressure transducer and a drainage system (Codman, Johnson and Johnson Medical Ltd., Raynham, MA). The Braincare sensor was positioned on the patient’s scalp without the need for trichotomy, surgical incision, or trepanation, and the morphology of the ICP waves was acquired through a strain sensor that could detect and monitor skull bone deformations caused by changes in ICP. All patients were monitored using this noninvasive system [Braincare Monitor 2000 and Braincare Intracranial Pressure Noninvasive sensor (BcSsNI2000), Braincare Corp] for at least 30 min per session. All recordings were digitized, filtered, and amplified by the device and then transferred to a computer for analysis. The waves of the B4c monitor and the gold standard technique were accessed and interpreted blindly, including the absolute values of the ICPi method, which were not included in the data sent. P1 and P2 were automatically determined (B4c system analytical software) and confirmed by visual inspection of the waveforms. Amplitudes were obtained by detecting the highest point of these peaks and subtracting the baseline value of the ICP waveform. The P2/P1 ratio was calculated by dividing the amplitude at these two time points. Time-to-peak (TTP) was defined as the time between the wave onset and its global peak. P2/P1 ratio and TTP parameters of the ICP waveform were calculated using the average ICPi and B4c morphology after excluding artifacts. The average pulse wave was used to calculate the amplitudes of P1 and P2 peaks, and when in doubt, the peak of the arterial pressure wave was used to define P1 [15].
Data Analysis
Qualitative variables were summarized in absolute (n) and relative (percentage) frequencies, and quantitative variables were expressed as mean ± standard deviation and medians and minimum and maximum values. Sample sizes were determined by convenience due to great numbers of waves in each patient.
We used Pearson’s linear correlation to assess the association between B4c and ICP wave morphology (P2/P1 ratio and TTP). The intraclass correlation coefficient (ICC) and the Bland–Altman plot were used to analyze the agreement when analyzed continuously; Cohen’s κ, when analyzed binary categorically (P2/P1 ratio > 1 and TTP > 0.2 s) on the basis of previous studies [17, 18].
The relationship of the noninvasive method with the absolute value of ICP was evaluated using Pearson’s correlation. To define the cutoff points of the noninvasive method, receiver operating characteristic (ROC) curves for repeated measures were constructed for each variable of the method; the presence or absence of IH, defined as a mean ICP above 20 mm Hg for at least 5 min, was considered as outcome. The area under the curve (AUC) was used to describe discriminatory power of the methods, and the Youden index was used to define the cutoff for each method.
We performed a sensitivity analysis in three different ways. First, we combined the wave morphology parameters (TTP and P2/P1 ratio) to evaluate their impact on accuracy; secondly, we evaluated the parameters to estimate cerebral perfusion pressure under 60 mm Hg; and thirdly, we evaluated their accuracy to predict ICP above 25 and 30 mm Hg. There were no missing data. Statistical analysis was performed using the JAMOVI software (version 1.8.1.).
Results
Description of the Population
From a total of 30 patients with ICPi monitoring evaluated in our hospital between March 2019 and March 2020 (pre-COVID-19), 18 patients were included. A total of 60 monitorizations were performed (Fig. 2). The clinical characteristics of patients are detailed in Table 1.
We included 14 patients with subarachnoid hemorrhage (SAH), one with IS, and three with intracerebral hemorrhage (ICH). All cases of SAH were aneurysmatic, all patients with ICH were hypertensive, and the only IS case was a cardioembolic malignant middle cerebral artery stroke. Patients with SAH had a median Hunt–Hess scale of 3.0 points [mean 2.95, interquartile range (IQR) 2.0], ranging from 0 to 5. The median ICH score in patients with intraparenchymal hemorrhage was 3 (mean 3.14, IQR 0.14). The median National Institutes of Health Stroke Scale score was 17.0 (95% confidence interval [CI] 15.9–18.8) in patients with SAH, 12 (95% CI 10.2–15.3) in patients with ICH, and 6 in the only patient with IS. Seven patients died (37.3%). The distribution of the modified Rankin scale score was as follows: 2 in 5% of the patients, 3 in 21.7%, 4 in 18.3%, and 5 in 18.3%. There were no side effects from the use of the noninvasive method.
Combined Invasive and B4c Monitoring
Median ICP values for each stroke subtype were as follows: 9 for SAH (95% CI 10.0.3–15.1, IQR 12.0), 19.5 for ICH (95% CI 8.64–21.2, IQR 15.3), and 3.5 for the only patient with IS. The mean ICP was greater than 20 mm Hg in 30% of the measurements. The invasive and noninvasive monitoring time for each patient averaged 45 min (ranging from 35 to 60 min), with 3,290 waves on average (number of waves = heart rate × minutes monitored) per monitoring (ranging from 2080 to 4310), totaling 197,400 waves for both B4c and ICPi monitoring in 2495 min.
Wave Morphology of the P2/P1 Ratio and TTP of the B4c and ICPi Methods and Correlation and Agreement Between the B4c and ICPi Methods
The description of the wave morphology of the B4c and ICPi methods is shown in Table 2. There was a positive correlation between all wave characteristics between the methods and between TTP and the P2/P1 ratio within each method (Table 3). The highest correlation was between P2/P1 ratios (Pearson 0.754, p < 0.0001), and the lowest correlation was between TTPs (Pearson 0.395, p < 0.0001) between the methods.
Wave morphology of the ICPi method showed a P2 greater than P1 (P2 > P1) in 54.3% of the minutes (1356 of the total 2495 min), whereas wave morphology of the B4c method showed a P2 greater than P1 64.5% of the time (1598 of 2495 min). The agreement between the methods, when we considered the ability to demonstrate a P2/P1 ratio > 1, was strong, with a κ of 0.76 (p < 0.001). Much of the disagreement occurred because of more P2 > P1 findings in the B4c method than in the ICPi method. The sensitivity of the B4c method for detection of a P2/P1 ratio > 1 morphology of ICPi monitoring was 98.3%, and the specificity was 76.8% [positive predictive value (PPV) of 83.4% and negative predictive value (NPV) of 97.5%], with an accuracy of 88.4%.
The TTP of ICPi and B4c monitoring was greater than 0.2 s in 998 min (39.1%) and in 1307 min (52.3%), respectively. The agreement between the methods, when we considered the ability to demonstrate a TTP > 0.2 s, was moderate, with a κ of 0.43 (p < 0.001). Most of the disagreement occurred because of a higher TTP > 0.2 s in the B4c method than in the ICPi method. The sensitivity and specificity of the B4c method for detection of a TTP > 0.2 s morphology of ICPi monitoring were 80.3% and 65.8% (PPV 70.1%, NPV 83.7%), respectively, with an accuracy of 71.3%.
Continuous Agreement Using ICC
The ICC of the mean P2/P1 ratio between the ICPi and B4c methods was 0.83 (95% CI 0.72–0.90). The ICC of the mean TTP between the methods was 0.58 (95% CI 0.32–0.76).
Continuous Agreement Using the Bland–Altman Plot
The agreement between the ICPi and B4c methods regarding the wave morphology parameters (P2/P1 ratio and TTP) was analyzed using the Bland–Altman plot (Fig. 3). The mean bias for the P2/P1 ratio between the methods was − 0.065 (95% CI − 0.077 to − 0.053), and the 95% limits of agreement were − 0.64 (95% CI − 0.664 to − 0.626) and 0.513 (95% CI 0.492–0.534). The interval of the agreement up to 1.96 standard deviations between the methods was 0.97. For the TTP, the mean bias was − 0.0426 (95% CI − 0.0476 to − 0.0377), and the 95% limits of agreement were − 0.280 (95% CI − 0.0288 to − 0.271) and 0.194 (95% CI 0.186–0.2030). The interval region of the agreement up to 1.96 standard deviations between the methods for TTP was 0.98.
Correlation Between B4c and ICPi Waves with the ICP Value
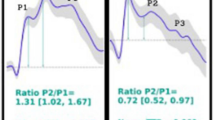
The mean correlation values of the P2/P1 ratio from the ICPi and B4c methods with the absolute mean value of ICP were 0.41 for ICPi and 0.43 for B4c (both with a p value < 0.001). The correlation value between TTP and ICP was 0.39 for ICPi and 0.35 for B4c (both with a p value < 0.01). Figure 4 shows the distribution of the P2/P1 ratio and mean TTP values pertaining to the presence or absence of IH. All patients with IH had a mean P2/P1 ratio above 1 and a TTP above 0.2 s.
ROC Curve with the P2/P1 Ratio and the TTP of the B4c Method to Predict IH
The mean P2/P1 ratio of the B4c method showed a sensitivity of 100%, specificity of 45.4%, PPV of 36.8%, and NPV of 100% to predict IH with a cutoff value of 1.06. The TTP of the B4c method showed a sensitivity of 85.7%, specificity of 50.0%, PPV of 35.3%, and NPV of 91.7% to predict IH with a mean ICP more significant than 20 mm Hg with a cutoff value of 0.2. Concerning the AUC, the mean P2/P1 ratio had an AUC of 0.79, whereas mean TTP had an AUC of 0.69 to predict IH (Fig. 5).
ROC curves of the morphological variables of B4c referring to mean ICP greater than 20 mm Hg. a Curve referring to P2/P1 ratio B4c (AUC: 0.786). b Curve referring to TTP B4c (AUC:0.694). AUC area under the curve, B4c Brain4care monitor, ICP intracranial pressure, ROC receiver operating characteristic, TTP time-to-peak
The sensitive analysis aggregating the wave morphology parameters did not bring better accuracy. Waveform analysis variables were less accurate to predict Cerebral Perfusion Pressure (CPP) less than 60 mm Hg than to predict IH.
Discussion
Our study demonstrated a high correlation and agreement between the wave morphology (P2/P1 ratio and TTP) between the invasive (ICPi) and noninvasive (B4c) methods of ICP monitoring in patients with stroke. With a sensitivity of nearly 100%, this noninvasive method could be used as a screening tool to identify patients who need invasive intracranial monitoring. Only two previous studies have sought to evaluate the characteristics of the ICP waveform between an invasive and a noninvasive method. The first study that compared the invasive method with the B4c method [19] included seven patients from Portugal with simultaneous measurements of B4c and intraparenchymal ICPi methods totaling 337 h of monitoring. The analysis, however, was not of the wave morphology but of the nature of the waves (power spectral density). This previous case series also differs from ours in the diagnosis of the patients included (traumatic brain injury) and in the location of the monitor (intraparenchymal). Recently, another study [17] compared the wave morphology of an intraparenchymal monitor and of the B4c method in a single measurement in ten patients (three patients with traumatic brain injury, three with SAH, three with ICH, and one with IS), totaling 1504 min of monitorization. Like in our series, all signals were analyzed in blocks of 1 min, and from the mean pulse obtained, the TTP and the P2/P1 ratio were determined. In contrast with our study, there was a low correlation between the B4c and ICPi methods [TTP 0.30 (95% CI 0.26–0.36), P2/P1 ratio 0.40 (95% CI 0.35–0.46)]. Some reasons for this inconsistency of results might include the smaller number of measurements (ten), the inclusion of heterogeneous diseases, the use of an intraparenchymal monitor [20,21,22], and the low prevalence of IH (7%) in their study.
The B4c method showed adequate performance when we considered the correlation of the B4c waves and the mean value of the ICP, similar to the waves of ICPi itself, corroborating its agreement. The ability to detect IH was better with the P2/P1 ratio than with TTP. The prediction of CPP < 60 mm Hg with the noninvasive P2/P1 ratio was less accurate than the prediction of IH. These results are in line with previous research showing that the intracranial compliance, measured by the ICP wave morphology and CPP, was not significantly related [23].
Even more interesting is the analysis of how the morphology curve behaves pertaining to the presence or absence of IH (Fig. 3). All patients with IH had the expected morphological findings with a P2/P1 ratio greater than 1 and a TTP greater than 0.2. However, for patients with normal ICP, we observed both normal and abnormal waves. Our findings fit perfectly with the expectation of morphological changes of the brain volume–pressure curve that precede IH [24,25,26,27]. A recent study interpreting wave morphology as a measure of CC [28] evaluated three noninvasive methods and their relationship with CC. It evaluated records of 36 patients with ICP monitoring who underwent intracranial volume infusion testing with continuous and parallel monitoring of ICP and flow velocity by transcranial Doppler (TCD). Three methods were used to evaluate CC: a mathematical fluid model, evaluation of changes in the cerebral arterial blood volume, and ratio of the amplitudes of the P2/P1 peaks of the pulse wave. All methods showed a strong and positive correlation with increased ICP, and the P2/P1 ratio showed the best correlation with CC with an r of 0.94. When we consider the hypothesis that the wave morphology changes before the rise of ICP, the P2/P1 ratio and TTP must be altered in IH but may be normal or altered in normal ICP values, depending on the brain compliance (CC). Therefore, the accuracy of the P2/P1 ratio and TTP was only moderate for different thresholds as depicted in our ROC curve.
Given the evidence from our data that the morphology of the ICP waveform can be evaluated by a noninvasive, continuous method that is simple to install and has no side effects, we have opened a considerable avenue to increase pathophysiological studies of intracranial complications of several diseases and scenarios. To reinforce the benefit of this monitoring, it would be necessary to evaluate whether guiding therapy by wave morphology can bring better outcomes for patients than the absolute value of ICP alone. The only randomized trial that relied on this premise using wave amplitude did show a significant clinical benefit [29]. Patients with aneurysmal SAH were evaluated using the ICP wave amplitude or the ICP value to guide therapy, with the modified Rankin scale as the clinical outcome in 12 months. The group treated on the basis of the wave amplitude had a better prognosis than the group treated using the ICP.
It is worth comparing these findings with other noninvasive monitoring techniques, such as TCD, that have been tested in patients with acute brain injury, with the goal to identify patients with IH. In a multicenter pilot study that included 36 patients, TCD had an AUC of 0.96, with 100% sensitivity and 91.2% specificity, for excluding IH [30]. Unlike our cohort, their sample was mostly composed of patients with traumatic brain injury and ICH. However, other studies did not show similar performances, which emphasizes the need of further studies in different populations to evaluate the real prediction accuracy of these methods [31,32,33] or to investigate the best combination of complementary noninvasive methods [34].
Our study has some limitations. It was a single-center study evaluating only 18 consecutive patients with stroke; however, the number of measurements was reasonable (n = 60), and the number of waves was satisfactory (n = 197,400 waves in 2495 min). The time between admission and evaluation varied among patients, as well as the interval between measurements within the same patient. Another limitation was the fixed cutoff for IH (ICP > 20 mm Hg) and not individualized measurements, such as the RAP [correlation coefficient (R) between AMP amplitude (A) and mean ICP pressure (P)] index, that would have added information on the status of cerebral autoregulation [35, 36]. Finally continuous CO2 measurement was not available during our monitorization.
Conclusions
Our study showed a high correlation and agreement in the analysis of wave morphology between the new B4c method when compared with ICPi monitoring, especially the P2/P1 ratio, opening the possibility for the routine use of this noninvasive technology as a screening or even follow-up method in patients who have contraindications to ICPi monitorization methods. It becomes an option that will need further studies to clarify its role in daily neurocritical practice.
References
Fried HI, Nathan BR, Rowe AS, Zabramski JM, Andaluz N, Bhimraj A, et al. The insertion and management of external ventricular drains: an evidence-based consensus statement: a statement for healthcare professionals from the neurocritical care society. Neurocrit Care. 2016;24(1):61–81.
Srinivasan VM, O’Neill BR, Jho D, Whiting DM, Oh MY. The history of external ventricular drainage: historical vignette. J Neurosurg. 2014;120(1):228–36.
Helbok R, Olson DW, Le Roux P, Vespa P, Menon DK, Vespa P, et al. Intracranial pressure and cerebral perfusion pressure monitoring in non-TBI patients: special considerations. Neurocrit Care. 2014;21(2):85–94.
Cardim D, Robba C, Bohdanowicz M, Donnelly J, Cabella B, Liu X, et al. Non-invasive monitoring of intracranial pressure using transcranial Doppler ultrasonography: Is it possible? Neurocrit Care. 2016;25(3):473–91.
Zacchetti L, Magnoni S, Di Corte F, Zanier ER, Stocchetti N. Accuracy of intracranial pressure monitoring: systematic review and meta-analysis. Crit Care. 2015;19(1):1–8. https://doi.org/10.1186/s13054-015-1137-9.
de Moraes FM, Silva GS. Noninvasive intracranial pressure monitoring methods: a critical review. Arq Neuropsiquiatr. 2021;79(5):437–46.
Vinciguerra L, Bösel J. Noninvasive neuromonitoring: current utility in subarachnoid hemorrhage, traumatic brain injury, and stroke. Neurocrit Care. 2017;27(1):122–40.
Harary M, Dolmans RGF, Gormley WB. Intracranial pressure monitoring—review and avenues for development. Sensors (Switzerland). 2018;18(2):3–7.
Sonig A, Jumah F, Raju B, Patel NV, Gupta G, Nanda A. The historical evolution of intracranial pressure monitoring. World Neurosurg. 2020;138:491–7.
Pitlyk PJ, Piantanida TPPD. Noninvasive intracranial pressure monitoring. Neurosurgery. 1985;17:581–4.
Xy LW. Deformation of skull bone as intracranial pressure changing. Afr J Biotechnol. 2009;8:745–50.
Mascarenhas S, Vilela GHF, Carlotti C, Damiano LEG, Seluque W, Colli B, et al. The new ICP minimally invasive method shows that the monro-kellie doctrine is not valid. Acta Neurochir Suppl. 2012;114:117–20.
Mascarenhas SVG. Noninvasive intracranian pressure system. United States; Patent No. WO/2013/041973., 2013.
Fan JY, Kirkness C, Vicini P, Burr RMP. Intracranial pressure waveform morphology and intracranial adaptive capacity. Am J Crit Care. 2008;6:545–56.
Cabella B, Vilela GHF, Mascarenhas S, Czosnyka M, Smielewski P, Dias C, et al. Validation of a new noninvasive intracranial pressure monitoring method by direct comparison with an invasive technique. Acta Neurochir Suppl. 2016;122:93–6.
Kasprowicz M, Lalou DA, Czosnyka M, Garnett M, Czosnyka Z. Intracranial pressure, its components and cerebrospinal fluid pressure–volume compensation. Acta Neurol Scand. 2016;134(3):168–80.
Gomes I, Shibaki J, Padua B, Silva F, Gonçalves T, Spavieri-Junior DL, Frigieri G, Mascarenhas SDC. Comparison of waveforms between noninvasive and invasive monitoring of intracranial pressure. Acta Neurochir Suppl. 2021;131:135–40. https://doi.org/10.1007/978-3-030-59436-7_28.
Brasil S, Taccone FS, Wayhs Y, Tomazini BM, Annoni F, Fonseca S, et al. Cerebral Hemodynamics and intracranial compliance impairment in critically Ill COVID-19 patients: a pilot study. Brain Sci. 2021;11:874.
Frigieri G, Andrade RAP, Wang CC, Spavieri D, Lopes L, Brunelli R, et al. Analysis of a minimally invasive intracranial pressure signals during infusion at the subarachnoid spinal space of pigs. Acta Neurochir Suppl. 2018;126:75–7.
Eide PK. Comparison of simultaneous continuous intracranial pressure (ICP) signals from ICP sensors placed within the brain parenchyma and the epidural space. Med Eng Phys. 2008;30(1):34–40.
Nucci CG, De Bonis P, Mangiola A, Santini P, Sciandrone M, Risi A, et al. Intracranial pressure wave morphological classification: automated analysis and clinical validation. Acta Neurochir (Wien). 2016;158(3):581–8.
Teichmann D, Lynch JC, Heldt T. Distortion of the intracranial pressure waveform by extraventricular drainage system. IEEE Trans Biomed Eng. 2021;68(5):1646–57.
Portella G, Cormio M, Citerio G, Contant C, Kiening K, Enblad P, et al. Continuous cerebral compliance monitoring in severe head injury: Its relationship with intracranial pressure and cerebral perfusion pressure. Acta Neurochir (Wien). 2005;147(7):707–13.
Carrera E, Kim DJ, Castellani G, Zweifel C, Czosnyka Z, Kasparowicz M, et al. What shapes pulse amplitude of intracranial pressure? J Neurotrauma. 2010;27(2):317–24.
Eide PK. The correlation between pulsatile intracranial pressure and indices of intracranial pressure–volume reserve capacity: results from ventricular infusion testing. J Neurosurg. 2016;125(6):1493–503.
Avezaat CJJ, Van Eijndhoven JHM, Wyper DJ. Cerebrospinal fluid pulse pressure and intracranial volume–pressure relationships. J Neurol Neurosurg Psychiatry. 1979;42(8):687–700.
Cardoso ER, Rowan JO, Galbraith S. Analysis of the cerebrospinal fluid pulse wave in intracranial pressure. J Neurosurg. 1983;59(5):817–21.
Kazimierska A, Kasprowicz M, Czosnyka M, Placek MM, Baledent O, Smielewski P, et al. Compliance of the cerebrospinal space: comparison of three methods. Acta Neurochir (Wien). 2021;163:1979–89.
Eide PK, Bentsen G, Sorteberg AG, Marthinsen PB, Stubhaug A, Sorteberg W. A randomized and blinded single-center trial comparing the effect of intracranial pressure and intracranial pressure wave amplitude-guided intensive care management on early clinical state and 12-month outcome in patients with aneurysmal subarachnoid hemo. Neurosurgery. 2011;69(5):1105–15.
Bellner J, Romner B, Reinstrup P, Kristiansson K-A, Ryding E, Brandt L. Transcranial Doppler sonography pulsatility index (PI) reflects intracranial pressure (ICP). Surg Neurol. 2004;62:45–51.
De Riva N, Budohoski KP, Smielewski P, Kasprowicz M, Zweifel C, Steiner LA, et al. Transcranial Doppler pulsatility index: What it is and what it isn’t. Neurocrit Care. 2012;17(1):58–66.
Behrens A, Lenfeldt N, Ambarki K, Malm J, Eklund A, Koskinen LO. Transcranial Doppler pulsatility index: Not an accurate method to assess intracranial pressure. Neurosurgery. 2010;66(6):1050–7.
Zweifel C, Czosnyka M, Carrera E, Deriva N, Pickard JD, Smielewski P. Reliability of the blood flow velocity pulsatility index for assessment of intracranial and cerebral perfusion pressures in head-injured patients. Neurosurgery. 2012;71(4):853–61.
Robba C, Robba C, Pozzebon S, Moro B, Vincent JL, Creteur J, et al. Multimodal non-invasive assessment of intracranial hypertension: an observational study. Crit Care. 2020;24(1):1–10.
Kim DJ, Czosnyka Z, Kasprowicz M, Smieleweski P, Baledent O, Guerguerian AM, et al. Continuous monitoring of the Monro–Kellie doctrine: Is it possible? J Neurotrauma. 2012;29(7):1354–63.
Hall A, O’Kane R. The best marker for guiding the clinical management of patients with raised intracranial pressure—the RAP index or the mean pulse amplitude? Acta Neurochir (Wien). 2016;158(10):1997–2009. https://doi.org/10.1007/s00701-016-2932-z.
Funding
None.
Author information
Authors and Affiliations
Contributions
FMdM: conceptualization, investigation, writing (original draft). ER: investigation. FCDB: investigation. MM: investigation. RAV: investigation. JBCdA: investigation. FGRF: conceptualization, supervision. FEACN: conceptualization, supervision. GSS: conceptualization, supervision, writing (review and editing). All authors contributed equally to this work. The final manuscript was approved by all authors.
Corresponding author
Ethics declarations
Conflicts of interest
None.
Ethical Approval/Informed Consent
We confirm adherence to ethical guidelines and indicate ethical approvals (institutional review board) and use of informed consent, as appropriate.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Moraes, F.M., Rocha, E., Barros, F.C.D. et al. Waveform Morphology as a Surrogate for ICP Monitoring: A Comparison Between an Invasive and a Noninvasive Method. Neurocrit Care 37, 219–227 (2022). https://doi.org/10.1007/s12028-022-01477-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-022-01477-4