Abstract
Background
Pulse amplitude index (PAx), a descriptor of cerebrovascular reactivity, correlates the changes of the pulse amplitude of the intracranial pressure (ICP) waveform (AMP) with changes in mean arterial pressure (MAP). AMP relies on cerebrovascular compliance, which is modulated by the state of the cerebrovascular reactivity. PAx can aid in prognostication after acute brain injuries as a tool for the assessment of cerebral autoregulation and could potentially tailor individual management; however, invasive measurements are required for its calculation. Our aim was to evaluate the relationship between noninvasive PAx (nPAx) derived from a novel noninvasive device for ICP monitoring and PAx derived from gold standard invasive methods.
Methods
We retrospectively analyzed invasive ICP (external ventricular drain) and non-invasive ICP (nICP), via mechanical extensometer (Brain4Care Corp.). Invasive and non-invasive ICP waveform morphology data was collected in adult patients with brain injury with arterial blood pressure monitoring. The time series from all signals were first treated to remove movement artifacts. PAx and nPAx were calculated as the moving correlation coefficients of 10-s averages of AMP or non-invasive AMP (nAMP) and MAP. AMP/nAMP was determined by calculating the fundamental frequency amplitude of the ICP/nICP signal over a 10-s window, updated every 10-s. We then evaluated the relationship between invasive PAx and noninvasive nPAx using the methods of repeated-measures analysis to generate an estimate of the correlation coefficient and its 95% confidence interval (CI). The agreement between the two methods was assessed using the Bland–Altman test.
Results
Twenty-four patients were identified. The median age was 53.5 years (interquartile range 40–70), and intracranial hemorrhage (84%) was the most common etiology. Twenty-one (87.5%) patients underwent mechanical ventilation, and 60% were sedated with a median Glasgow Coma Scale score of 8 (7–15). Mean PAx was 0.0296 ± 0.331, and nPAx was 0.0171 ± 0.332. The correlation between PAx and nPAx was strong (R = 0.70, p < 0.0005, 95% CI 0.687–0.717). Bland–Altman analysis showed excellent agreement, with a bias of − 0.018 (95% CI − 0.026 to − 0.01) and a localized regression trend line that did not deviate from 0.
Conclusions
PAx can be calculated by conventional and noninvasive ICP monitoring in a statistically significant evaluation with strong agreement. Further study of the applications of this clinical tool is warranted, with the goal of early therapeutic intervention to improve neurologic outcomes following acute brain injuries.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cerebral autoregulation (CA) refers to the central nervous system’s ability to maintain cerebral blood flow relatively constant within a certain range of slow changes in mean arterial pressure (MAP) [1, 2]. Impaired CA has been correlated with worse outcomes in patients with acute brain injuries [3,4,5,6,7]. Ongoing trials of CA-guided blood pressure management hold the promise of individualized patient management strategies with improved patient outcomes [8].
Today’s bedside monitoring technology used to assess CA and cerebrovascular reactivity in the clinical setting includes transcranial Doppler, which measures cerebral blood flow velocity; near-infrared spectroscopy, which measures regional cerebral oxygen saturation; and invasive brain oxygen monitors, which measure tissue oxygen partial pressure [9]. Although these methodologies for the assessment of CA have been described, the best validated methods require the placement of invasive intracranial pressure (ICP) probes, which limits their widespread use [10,11,12]. Furthermore, current noninvasive techniques of CA assessment have well-known limitations, including surface insonation windows and extracerebral vasculature contamination, and provide only moderate agreement with invasive standards [9, 13,14,15]. The validation of a CA index based on the ICP waveform obtained noninvasively would represent a novel development that could have implications in the monitoring and management of patients with acute brain injuries.
The use of a novel noninvasive mechanical extensometer has been recently validated [16,17,18]. This technology is designed to detect small skull expansions originating from pulsatile beat-to-beat ICP variations, which subsequently generates a surrogate ICP waveform (Fig. 1). As extensively reported in the literature, an ICP waveform analysis carries significant clinical and pathophysiologic information, including associated parameters relating to intracranial dynamics and compliance [19,20,21,22].
An illustration of the mechanical extensometer. As arterial blood pressure drives blood into the intracranial space, producing pulsatile expansions of the cerebral arteries walls, the skull then experiences small deformations owing to these volume and pressure variations (as depicted in an amplified manner) (a). By adjusting the sensor’s headband (red line) (b) to a certain tension extent, the pin of the sensor touches the head and keeps the device in place on the frontotemporal region of the head above the ears. The skull deformations can then be detected by the sensor as displacements of the pin that bends the sensor’s cantilever beam c, The displacement detected by the sensor, in which the resulting displacement (d3) is the difference between the initial (d1) and final (d2) positions (d3 = d1 − d2), having the support bar of the sensor as a reference. Extracted and modified from Andrade et al. [23]
The pulse amplitude index (PAx), a descriptor of cerebrovascular reactivity, is a validated index of cerebrovascular reactivity that correlates the changes of pulse amplitude of ICP (AMP) with changes in MAP [22]. The pulsatile component of ICP reflects the beat-to-beat changes in cerebral blood volume [20,21,22, 24]. Cardiac pulsations in arterial blood pressure produce pulsatile expansions of the cerebral arteries walls and consequently pulsations in cerebral blood volume, which in turn are transmitted to the surrounding brain parenchyma and cerebrospinal fluid. This transmission depends on the compliance of the cerebrovascular bed, which is a function of the vasomotor tone; therefore, AMP is modulated by the state of the cerebrovascular reactivity. AMP is calculated as the frequency amplitude of the first (fundamental) spectral harmonic component of the ICP waveform [4, 24, 25]. In previous studies, AMP monitored over time appeared to present better stability than mean ICP values because AMP is not subject to drifts and shifts of the absolute values of ICP because it is obtained in the frequency domain [26, 27].
Although PAx can aid in prognostication and potentially management after acute brain injuries as a tool for the assessment of CA, invasive ICP methods are required for its calculation [5]. A non-invasive PAx (nPAx) surrogate could provide cerebrovascular reactivity monitoring in patients without an ICP monitor who remain at high risk for neurologic deterioration. Our aim was to evaluate the agreement between nPAx, derived from a novel device for ICP waveform monitoring (nICP), and PAx obtained from gold standard invasive methods.
Methods
Study Design
This is a single-center retrospective study of adult patients admitted to Cleveland Clinic’s neurointensive care unit between January 2021 and December 2021 with acute brain injury who underwent nICP monitoring with a US Food and Drug Administration–approved novel mechanical extensometer device (Brain4Care Corp.). To be included in the present study, patients also must have undergone ventriculostomy insertion and arterial catheter placement as part of standard medical care as determined by the neurosurgical and neurointensive care teams.
All methods were performed in accordance with the relevant guidelines and regulations. An investigational review board exempt protocol was obtained for the creation of a multimodal monitoring database in which relevant clinical data are stored in a Health Insurance Portability and Accountability Act–compliant, password-protected server in place at our institution since 2019. To ascertain high-quality reporting of this observational study, the Strengthening the Reporting of Observational Studies in Epidemiology guidelines were followed [28].
Monitoring Protocol
Monitoring data from patients with acute brain injuries, who, at the discretion of the managing team and as part of standard clinical care, undergo invasive ICP monitoring, invasive cerebral oximetry, transcranial Doppler ultrasonography, nICP monitoring, and/or near-infrared cerebral oximetry, are stored in the multimodal monitoring database for subsequent offline review. In the present investigation, we selected patients who had nICP monitoring as well as concomitant continuous arterial blood pressure and invasive ICP monitoring in place.
The nICP sensor consists of a support for a sensor bar for the detection of local skull bone deformations, adapted with mechanical extensometers (Brain4care Corp.) (Fig. 1). Detection of these deformations is obtained by a cantilever bar modeled by finite elements calculations. To this bar, strain gauges are attached for strain detection. Noninvasive contact with the skull is obtained by adequate pressure directly on the scalp by a pin. Changes in ICP cause deformations in the skull bone detected by the sensor bar. Variations in ICP lead to deformations in the bar, which are captured by the strain sensors. The equipment filters, amplifies, and digitalizes the signal from the sensor and sends the data to the bedside patient monitor.
The sensor pin is placed about two inches above the level of the external auditory meatus, far away from any palpable arterial pulses, and held in place by a headband. Because of the known artifactual mechanical drift that is present soon after placement of the nICP sensor, data recording does not typically start until 20 min after placement. Although the duration of the monitoring session varies, it usually ranges from 20 to 40 min to obtain sufficient data for agreement analysis.
Data Retrieval and Processing
Once patients who met all inclusion criteria were identified, monitoring data for ICP, nICP, and MAP were retrieved with the ICM + software platform (Cambridge Enterprise, Cambridge, UK) with a sampling frequency of 300 Hz. MAP was monitored invasively through the radial artery using a standard pressure monitoring kit. ICP was monitored using an external ventricular drain, which was closed during the monitoring time frame. The time series from all signals were first treated to remove movement artifacts identified by visual inspection.
PAx and nPAx were calculated as moving Pearson correlation coefficients between changes in 30 consecutive 10-s averages of the MAP signal and corresponding pulse amplitudes of ICP and nICP (with 50% overlap of data), as previously described. The AMPs of ICP and nICP were obtained using a fast Fourier transform to convert the time-series signals into the frequency domain and to extract the fundamental first harmonic amplitude using tools implemented on ICM + software.
Statistical Analysis
Patient descriptive characteristics and categorical data were presented as absolute frequencies and percentages. Quantitative data were presented as median values with their interquartile range (IQR) or mean ± standard deviation based on Shapiro–Wilk’s normality tests. Repeated-measures correlation was used to evaluate the relationship between the invasive PAx and nPAx to ensure that the relationship was not curvilinear. Bland–Altman analysis was used to test the agreement between invasive PAx and nPAx. All statistical analyses were performed using RStudio software (version 4.0.3) and SAS v 9.4. A p value < 0.05 was considered statistically significant.
Results
The monitored cohort included 24 patients with acute brain injury. There were 9 female patients, and the median age was 53.5 years (IQR 40–70). The admission neurologic evaluation depicted a median Glasgow Coma Scale (GCS) score of 8 (IQR 7–15). The majority of patients were admitted with an intracerebral hemorrhage (46%) or aneurysmal subarachnoid hemorrhage (37.5%) diagnosis. Summary statistics for baseline demographics and comorbidities are shown in Table 1.
Intensive Care Management on Day of Monitoring
On the day of monitoring, 21 patients (87.5%) required mechanical ventilation (Table 1). During the monitoring session, the majority (67%) received sedative agents by either continuous administration, bolus therapy, or a combination of both. Fentanyl (54%) was the most common sedative agent used, followed by propofol (21%). A Richmond Agitation Sedation Scale score of less than − 2 was common. Four patients (17%) were monitored while on vasopressor therapy. No patients underwent renal replacement therapy on the day of monitoring.
Monitoring and Outcome Data
A single monitoring session was completed for each patient, and the median time for the total monitored period was 40 min (IQR 36–42.5). Continuous physiologic data yielded a median MAP of 88 mm Hg (IQR 77–94 mm Hg) and invasive ICP of 9.3 mm Hg (IQR 5.4–12.9 mm Hg). Ten patients underwent active cerebral edema management, with the majority requiring hypertonic saline (29%) decided by the clinical team. For the cohort, 83% (20 of 24 patients) survived, with a median discharge GCS score of 13 (IQR 9.75–15) and a median modified Rankin scale score of 4 (IQR 3–5). Table 2 summarizes discharge location for the survivors.
Correlation Between PAx and nPAx
The mean PAx calculated by invasive ICP measurements was 0.0296 ± 0.331. The mean nPAx calculated by noninvasive ICP measurements was 0.0171 ± 0.332. Figure 2 illustrates the linear relationship between the two measures PAx and nPAx (R = 0.70, p < 0.0005, 95% confidence interval [CI] 0.687–0.717).
Test of Method Agreement
Agreement between PAx and nPAx methods was analyzed using the Bland–Altman test (Fig. 3). The bias between the two measurement systems was − 0.018 (95% CI − 0.026 to − 0.01). The pattern of the locally weighted scatterplot smoothing line remained close to the mean of the differences, there were no excursions outside the 95% CI, the differences were normally distributed, and there were no patterns in the data. The plot and the fit confirm the two methods are in agreement.
Discussion
We aimed to compare the agreement of an index of CA (PAx) obtained with conventional and noninvasive methods for ICP waveform monitoring. The mechanical extensometer demonstrated comparable performance to the invasive standard to generate a surrogate ICP waveform, which can then be analyzed to monitor cerebrovascular reactivity (Fig. 4). Individual Bland–Altman plots do indicate patient-to-patient changes in the bias of the measurements. Given the difference in patients, as well as differing locations of placement of the noninvasive device, these differences are not unexpected. Overall, our results demonstrate that PAx can be calculated by a noninvasive ICP monitoring method in patients with acute neurologic injury. To our knowledge, no previous studies have sought to evaluate noninvasive surrogates of ICP waveform for a CA index.
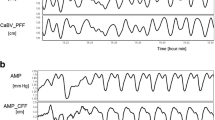
Real-time waveform data over 5-s from a selected representative patient. The top panel represents arterial blood pressure (ART), the middle panel represents invasive intracranial pressure (ICP) from external ventricular drain, and the lower panel represents noninvasive ICP from mechanical extensometer (P4) waveforms
The strong agreement between the conventional and noninvasive ICP monitoring of PAx supports other recent studies that have validated the use of mechanical extensometer [16,17,18,19]. A small prospective study reported that the noninvasive ICP waveform monitor depicted agreement with the standard invasive method with an acceptable discriminatory power to detect intracranial hypertension via waveform morphology parameters (P2/P1 ratio) [19]. Although our study did not evaluate the detection of intracranial hypertension and rather focused on cerebrovascular reactivity, the findings presented here support the use of the mechanical extensometer to obtain secondary CA indices from surrogate noninvasive ICP waveform analysis.
Total intracranial compliance is a complex, nonlinear relationship among the different compartmental compliances of the intracranial system (i.e., cerebrospinal fluid, arterial and venous circulations). The pulse amplitude of the ICP waveform (AMP) can approximate the compliance of the cerebral arterial bed (relating to the arterial circulation compliance), which is modulated by cerebrovascular reactivity [20,21,22, 24]. The clinical utility of this novel tool to obtain a noninvasive CA index will rely on future prospective trials with targeted, CA-directed therapy showing significant clinical benefits and improved patient outcomes. Such clinical trials of autoregulation-guided hemodynamic management are ongoing and have shown early promise in patients with traumatic brain injuries and patients with acute ischemic stroke undergoing large vessel recanalization [29, 30]. Whether nPAx could be used to guide therapeutic interventions in these or other patient populations remains to be further defined.
Invasive devices remain the gold standard for ICP monitoring; however, they can be associated with significant complications [9]. Given the evidence from our data that the ICP waveform morphology can be recorded noninvasively to obtain a cerebrovascular reactivity index, this has the potential to increase our pathophysiologic understanding of intracranial complications of several diseases. Furthermore, our findings suggest that nPAx can be estimated in a vast majority of patients who traditionally are not considered good candidates for invasive ICP probe placement, which potentially may help expand its clinical use.
This study has several limitations. This is a small retrospective single-center study. Different monitoring periods as well as different treatment protocols (such as presence/mode of ventilation, sedative medications, and cerebral edema optimization) were used during monitoring sessions. Therefore, we cannot rule out the impact of treatment on cerebral dynamics. Another limitation is the presence of a heterogeneous population with brain injury. It is unknown if the presence of intracranial hematoma, cerebral edema, or subarachnoid blood affects the ability to obtain a noninvasive ICP waveform. The heterogeneous group of patients and monitoring conditions support the noninvasive device’s adaptability and performance in various settings. The mechanical extensometer’s sensor pin cannot allow for prolonged monitoring periods without consequence of skin breakdown. Unlike its invasive counterpart, this sensor has a limited role in continuous monitoring; however, serial daily monitoring can still detect changes in cerebral dynamics, and an improved design of the sensor may eventually allow for safe continuous monitoring.
Obtaining the noninvasive ICP waveform using the mechanical extensometer device was easily prone to artifactual noise (such as a strong respiratory pattern) that could eliminate the waveform, as well as the need currently for manual artifact removal, which can be time consuming. Although artifact removal is currently performed manually, it is feasible that computer algorithms could be developed to aid in this task. The presence of these factors and other sources of special cause variation undoubtedly contributed to large confidence intervals in the Bland–Altman analysis. An examination and elimination of their effect will reduce the observed differences between the methods that are clinically acceptable. Despite these limitations, the main aim of this investigation was to test agreement between invasive and noninvasive methods for PAx calculation, which was found to be comparable.
Conclusions
PAx can be calculated by conventional and noninvasive ICP monitoring in a statistically significant evaluation with strong agreement. Further study of the applications of this clinical tool is warranted, with the goal of intervening early to improve neurologic outcomes following acute brain injuries.
References
Brassard P, Labrecque L, Smirl JD, et al. Losing the dogmatic view of cerebral autoregulation. Physiol Rep. 2021;9(15): e14982.
Donnelly J, Aries MJ, Czosnyka M. Further understanding of cerebral autoregulation at the bedside: possible implications for future therapy. Expert Rev Neurother. 2015;15(2):169–85.
Castro P, Azevedo E, Sorond F. Cerebral autoregulation in Stroke. Curr Atheroscler Rep. 2018;20(8):37.
Aries MJ, Czosnyka M, Budohoski KP, et al. Continuous monitoring of cerebrovascular reactivity using pulse waveform of intracranial pressure. Neurocrit Care. 2012;17(1):67–76.
Radolovich DK, Aries MJ, Castellani G, et al. Pulsatile intracranial pressure and cerebral autoregulation after traumatic brain injury. Neurocrit Care. 2011;15(3):379–86.
Reinhard M, Neunhoeffer F, Gerds TA, et al. Secondary decline of cerebral autoregulation is associated with worse outcome after intracerebral hemorrhage. Intensive Care Med. 2010;36:264–71.
Ma H, Guo ZN, Liu J, et al. Temporal course of dynamic cerebral autoregulation in patients with intracerebral hemorrhage. Stroke. 2016;47:674–81.
Calviello LA, Donnelly J, Zeiler FA, et al. Cerebral autoregulation monitoring in acute traumatic brain injury: what’s the evidence? Minerva Anestesiol. 2017;83(8):844–57. https://doi.org/10.23736/S0375-9393.17.12043-2.
Rivera-Lara L, Zorrilla-Vaca A, Geocadin RG, et al. Cerebral autoregulation-oriented therapy at the bedside: a comprehensive review. Anesthesiology. 2017;126(6):1187–99.
Liu X, Czosnyka M, Donnelly J, Cardim D, et al. Wavelet pressure reactivity index: a validation study. J Physiol. 2018;596(14):2797–809. https://doi.org/10.1113/JP274708.
Sorrentino E, Diedler J, Kasprowicz M, et al. Critical thresholds for cerebrovascular reactivity after traumatic brain injury. Neurocrit Care. 2012;16(2):258–66.
Steiner LA, Czosnyka M, Piechnik SK, et al. Continuous monitoring of cerebrovascular pressure reactivity allows determination of optimal cerebral perfusion pressure in patients with traumatic brain injury. Crit Care Med. 2002;30(4):733–8.
Czosnyka M, Miller C. Participants in the international multidisciplinary consensus conference on multimodality monitoring. Monitoring of cerebral autoregulation. Neurocrit Care. 2014;21(Suppl 2):S95-102.
Selb J, Wu KC, Sutin J, et al. Prolonged monitoring of cerebral blood flow and autoregulation with diffuse correlation spectroscopy in neurocritical care patients. Neurophotonics. 2018;5(4): 045005.
Panerai RB. Transcranial doppler for evaluation of cerebral autoregulation. Clin Auton Res. 2009;19(4):197–211.
Cabella B, Vilela GH, Mascarenhas S, et al. Validation of a new noninvasive intracranial pressure monitoring method by direct comparison with an invasive technique. Acta Neurochir Suppl. 2016;122:93–6.
Frigieri G, Andrade RAP, Dias C, et al. Analysis of a non-invasive intracranial pressure monitoring method in patients with traumatic brain injury. Acta Neurochir Suppl. 2018;126:107–10.
Mascarenhas S, Vilela GHF, Carlotti C, et al. The new ICP minimally invasive method shows that the monrokellie doctrine is not valid. Acta Neurochir Suppl. 2012;114:117–20.
de Moraes FM, Rocha E, Barros FCD, et al. Waveform morphology as a surrogate for ICP monitoring: a comparison between an invasive and a noninvasive method. Neurocrit Care. 2022;24:1–9.
Holm S, Eide PK. The frequency domain versus time domain methods for processing of intracranial pressure (ICP) signals. Med Eng Phys. 2008;30(2):164–70.
Bentsen G, Stubhaug A, Eide PK. Differential effects of osmotherapy on static and pulsatile intracranial pressure. Crit Care Med. 2008;36(8):2414–9.
Eide PK, Sorteberg W. Intracranial pressure levels and single wave amplitudes, Glasgow Coma Score and Glasgow Outcome Score after subarachnoid haemorrhage. Acta Neurochir (Wien). 2006;148(12):1267–75.
Andrade RDAP, et al. A nanometer resolution wearable wireless medical device for non invasive intracranial pressure monitoring. IEEE Sens J. 2015;21(20):22270–84.
Carrera E, Kim DJ, Castellani G, et al. What shapes pulse amplitude of intracranial pressure? J Neurotrauma. 2010;27(2):317–24.
Radolovich DK, Aries MJ, Castellani G, et al. Pulsatile intracranial pressure and cerebral autoregulation after traumatic brain injury. Neurocrit Care. 2011;15:379–86.
Patel HC, Menon DK, Tebbs S, Hawker R, Hutchinson PJ, Kirkpatrick PJ. Specialist neurocritical care and outcome from head injury. Intensive Care Med. 2002;28(5):547–53.
Marmarou A, Lu J, Butcher I, et al. Prognostic value of the Glasgow Coma Scale and pupil reactivity in traumatic brain injury assessed pre-hospital and on enrollment: an IMPACT analysis. J Neurotrauma. 2007;24(2):270–80.
Cuschieri S. The STROBE guidelines. Saudi J Anaesth. 2019;13(Suppl 1):S31–4.
Tas J, Beqiri E, van Kaam CR, et al. An update on the COGiTATE phase II study: feasibility and safety of targeting an optimal cerebral perfusion pressure as a patient-tailored therapy in severe traumatic brain injury. Acta Neurochir Suppl. 2021;131:143–7.
Petersen NH, Silverman A, Strander SM, Kodali S, Wang A, Sansing LH, Schindler JL, Falcone GJ, Gilmore EJ, Jasne AS, Cord B, Hebert RM, Johnson M, Matouk CC, Sheth KN. Fixed compared with autoregulation-oriented blood pressure thresholds after mechanical thrombectomy for ischemic stroke. Stroke. 2020;51(3):914–21.
Funding
There is no funding to disclose for this study.
Author information
Authors and Affiliations
Contributions
CEH: conceptualization, data curation, investigation, methodology, writing of the original draft, and review and editing. SPU: data curation. RB: formal analysis. NZM: review and editing. DC: conceptualization, data curation, formal analysis, and review and editing. JAG: conceptualization, data curation, investigation, methodology, writing of the original draft, and review and editing.
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflicts of interest to disclose.
Ethical approval/informed consent
The article adheres to ethical guidelines and was approved by Cleveland Clinic’s Institutional Research Board for retrospective study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hassett, C.E., Uysal, S.P., Butler, R. et al. Assessment of Cerebral Autoregulation Using Invasive and Noninvasive Methods of Intracranial Pressure Monitoring. Neurocrit Care 38, 591–599 (2023). https://doi.org/10.1007/s12028-022-01585-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-022-01585-1