Summary
Intracranial pressure (ICP) is an important invasive monitoring parameter in management of patients with acute brain injury and compromised compliance. This study aimed to compare waveforms obtained from standard ICP monitoring and noninvasive ICP monitoring (nICP) methods.
We analyzed continuous arterial blood pressure (ABP) waves, ICP (with standard monitoring), and nICP recorded simultaneously. All signal recordings were sliced into data chunks, each 1 min in duration, and from the mean pulse, we determined the time to peak (Tp) and the ratio between tidal and percussion waves (P2/P1). We also calculated the Isomap projection of the pulses into a bidimensional space—K1 and K2. The defined nICP and ICP parameters were compared using a unilateral Wilcoxon–Mann–Whitney test. The Pearson correlation coefficient and normalized mutual information were used to verify the association between parameters.
In total, 1504 min of monitoring from ten patients were studied. Nine of the patients were male. The mean age of the patients was 58.4 ± 10.4 years, and they had an initial Glasgow Coma Scale of 9 ± 4, a mean Simplified Acute Physiology Score (SAPS II) of 45.6, and an intensive care unit stay of 44 ± 45 days. With the exception of Tp, all parameters showed a weak linear association but presented a strong nonlinear association.
Mutual information analysis and a bigger sample size would be helpful to build more refined models and to improve understanding of the waveform relationships.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Intracranial pressure (ICP), which is usually measured in millimeters of mercury (mmHg), is an important invasive monitoring parameter in management of patients with acute brain injury and compromised brain compliance in an intensive care unit (ICU) setting. Likewise, there are a large number of minor neurological conditions that can benefit from continuous or occasional ICP monitoring as a diagnostic aid, as in cases of hydrocephalus, improper operation of ventriculoperitoneal valves, or intracranial hypertension caused by central nervous system infections [1].
The Monro–Kellie hypothesis states that the cranial compartment (comprising three main elements: the brain, the cerebrospinal fluid, and the blood) is an inelastic structure that maintains the same constant volume, even throughout pathological changes. Therefore, an increase in the volume of one of those elements will inevitably lead to a decrease in one or both of the others [1].
In an ICU context, ICP is monitored by an invasive sensor (with a pressure transducer) inserted into the intraventricular or intraparenchymal spaces. Although this is an extremely accurate method, it is complex and expensive, as it carries higher risks of infection and bleeding, and it can be performed only in a restricted number of hospitals that have a trained and specialized neurosurgical team available [1].
Taking into consideration the disadvantages mentioned above, the need to monitor ICP in patients whose medical condition does not justify a high-risk, continuous, and invasive procedure such as neurosurgical insertion of an internal sensor has prompted efforts to develop a noninvasive method of ICP monitoring.
In this study, we compared the waveforms of standard invasive ICP and noninvasive ICP (nICP) monitoring methods. The noninvasive values were obtained by a strain gauge mechanism (a mechanical extensometer) applied over the scalp in the temporal window (the parietal region lateral to the sagittal suture).
Our goal was to corroborate the similarities between the two ICP waveforms (invasive and noninvasive), as well as the radial arterial blood pressure (ABP), in order to validate the noninvasive method as an alternative to invasive measurements in situations where the waveform can give enough clinical information.
We also compared nICP with arterial ABP waveforms to verify the possible influence of the peripheral circulation on the nICP signal, which is one of the possible limitations of the present method.
Methods
Subjects
Fifteen patients were screened for the study. After application of the patient selection criteria, ten of them were included in the study: three with a traumatic brain injury, three with a subarachnoid hemorrhage, three with an intracranial hemorrhage, and one who had suffered an ischemic stroke.
Nine of the ten patients were male. The mean age was 58.4 ± 10.4 years, the initial Glasgow Coma Scale (GCS) score was 9 ± 4, the mean Simplified Acute Physiology Score (SAPS II) was 45.6, and the mean length of stay (LOS) in the ICU was 44 ± 45 days.
Experimental Protocol
A strain gauge mechanism was first applied over the left or right side of the skull, in the parietal region, avoiding the temporal arteries. The system then recorded up to 30 min of waveforms (nICP, ICP, and ABP), using ICM+ software. After a minimum recording time of 30 min on the left side, the mechanism was switched over to the right side of the skull and the recording process was repeated. The times at which the recording started and ended on the right or left side were noted, as was other relevant information such as times when the patient was moved for cleaning or physiotherapy, or was turned by the nursing or medical team, which produced artifacts in the recording.
Inclusion Criteria
The study included only patients who were at least 18 years old, with an acute brain injury. Patients who had undergone decompressive craniectomy or who had an intraventricular drain were excluded.
Data Acquisition and Data Processing
The data were continuously recorded as raw data with ICM+ software. The signal preprocessing pipeline was composed of several steps: the continuously recorded physiological signals (ABP, ICP, and nICP) were initially parsed and sliced into data chunks, each 1 min in duration, and stored on a MongoDB database (MongoDB Inc., New York, NY, USA) for further analysis; the segmented signals were then decomposed to extract their pulsatile components, using spline interpolation [2]; a low-pass finite impulse response (FIR) filter with a cutoff frequency of 15 Hz was then applied to the resulting signal to eliminate high-frequency noise; pulse identification was done via the phase of the Hilbert transform [3, 4]; in sequence, the pulses obtained for each signal were aligned with the maximum slope of the pulse, and the mean pulses per minute were calculated using a nonparametric bootstrap method with a 95% confidence interval [5].
From the mean pulses obtained from the data preprocessing pipeline, we calculated the following parameters of the ICP and nICP pulses: the time to peak, the ratio between the “tidal wave” (P2) and the “percussion wave” (P1), and the Isomap [6] projections K1 and K2. The time to peak was defined as the difference between the maximal slope of the pulse and the time in which the pulse reached its maximum. The ratio between P2 and P1 waves was identified using the corresponding ABP pulse as an auxiliary signal. The Isomap dimensionality reduction method was used to summarize the pulse waveform into two dimensions (K1 and K2), preserving the similarity between the pulses in the original space. The amplitude and length of the pulses were normalized before application of the Isomap (Fig. 1).
Intracranial pressure and noninvasive intracranial pressure monitoring signal-processing pipeline. (a) Raw data and the respective spline interpolation curve. (b) Detrended signal. (c) Pulse identification based on the Hilbert transform. The red dots show the starts of the pulses. (d) Pulse alignment using, as a reference, the maximal slope before the pulse maximum. (e) Average pulse with bootstrap confidence interval (CI) (a = 0.05, N = 1000). (f) Parameter estimation. In the case of the tidal wave/percussion wave (P2/P1) ratio, we use the respective arterial blood pressure (ABP) waveform (not shown) to identify the peaks. Time to peak (TTP)
All signal processing and analysis were performed using custom programs written in Python, using the libraries numpy [7], scipy [8], scikit-learn [9], and matplotlib [10].
Statistical Analysis
Comparisons between ICP and nICP parameters were evaluated using linear (Pearson) and nonlinear (normalized mutual information) correlations [11]. The mutual information was calculated on the basis of the respective parameters’ joint histograms, using histogram bin size extrapolation as the bias correction method [12].
Results
After application of criteria for signal preprocessing to detect good-quality waveforms (signals recorded in ICM+ software that had continuous and simultaneous ABP, ICP, and nICP pulses) and slicing of those pulses into 1-min data chunks, a total of 1504 min of monitoring were studied.
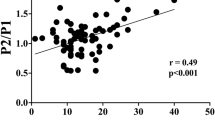
Primarily, a linear correlation analysis was done to evaluate the correlation of the obtained data with ICP versus nICP signals. The calculated Pearson’s coefficient showed a weak linear association in all of the ICP/nICP parameters (Table 1).
In light of these preliminary weak linear results, and to further investigate whether there was a statistical dependence between the ICP/nICP parameters, a nonlinear correlation method—normalized mutual information—was used. Normalized mutual information ranges between 0 (no statistical dependence) and 1 (maximal statistical dependence between variables). We also used a nonlinear dimensionality reduction technique—Isomap [6] projection of the pulses into a bidimensional space (K1 and K2)—to compare the entire waveform shape of the invasive and noninvasive ICP signals (Fig. 2). This last statistical analysis showed a strong nonlinear association in the K1, K2, and P2/P1 ratio variables. An exception was found in the time-to-peak variable, where the nonlinear association remained weak (Table 1, Fig. 2).
Joint histograms of intracranial pressure (ICP) and noninvasive ICP monitoring (nICP) parameters. Top left: Time to peak (Tp). Top right: Tidal wave/percussion wave (P2/P1) ratio. Bottom left: Isomap K1. Bottom right: Isomap K2. Each section of the figure contains three panels: the main panel in which the joint density function approximation is shown and two adjacent panels in which the marginal histograms for the parameters can be observed. In each main panel, dark lines represent a higher concentration of points, as the color bar indicates. The values shown in square brackets are the lower and upper confidence limits. The densities were approximated by using a kernel density estimation method with optimized bandwidth selection
Discussion
Although the two methods we used for comparison have very different measurement approaches (one being invasive and the other over the skull), both waveforms are generated by blood inflow into the brain and therefore should share some resemblance, at least in the dominant parameters that define and summarize the main waveform characteristics [13, 14].
Therefore, we compared the ICP and nICP waveforms with the radial artery blood pressure waveform in an attempt to quantify and eliminate the interference of peripheral circulation in the nICP waveform parameters (one possible limitation of this approach). Such a limitation could be minimized if the noninvasive sensor positioning was optimized, expressly away from major vessels in the parietal region (approximating its pattern to the direct ICP waveform). Another limitation of our work was that as yet, the method developed by our team does not yield pressure values calibrated in millimeters of mercury (mmHg). Because of this, it would be interesting to evaluate and compare the waveform morphology between groups of patients with different diagnoses (when we have a larger number of patients). In that way, we could assess whether there is a waveform pattern specific to one condition or another that could provide relevant information about the state and pathophysiology of the patient without using the direct mmHg value [1].
Conclusion
Although the compared waveform parameters of ICP and nICP showed strong nonlinear coupling in all but one parameter, the results presented in this work are preliminary. Therefore, mutual information analysis of a larger patient sample with a longer monitoring time would be helpful to build refined models and to improve the understanding of the waveform relationships [1].
Thus, we intend to proceed to a demographic analysis of the P2/P1 ratio and the normalized time to peak. Given the importance of ICP waveform analysis in management of the clinical state of patients with an acute brain injury, and the similarities given by the strong nonlinear correlation between the invasive and noninvasive parameters, we believe that the noninvasive method could be used for monitoring relative changes in ICP, despite the absence of absolute values in mmHg.
References
Kawoos U, McCarron RM, Auker CR, Chavko M (2015) Advances in intracranial pressure monitoring and its significance in managing traumatic brain injury. Int J Mol Sci 16(12):28979–28997. https://doi.org/10.3390/ijms161226146
Hastie T, Tibshirani R, Friedman J (2013) The elements of statistical learning: data mining, inference, and prediction. Springer, New York
Benitez D, Gaydecki PA, Zaidi A, Fitzpatrick AP (2001) The use of the Hilbert transform in ECG signal analysis. Comput Biol Med 31:399–406
Mitrou N, Laurin A, Dick T, Inskip J (2017) A peak detection method for identifying phase in physiological signals. Biomed Signal Process Control 31:452–462. https://doi.org/10.1016/j.bspc.2016.07.001
Zoubir AM, Boashash B (1998) The bootstrap and its application in signal processing. IEEE Signal Process Mag 15:56–76. https://doi.org/10.1109/79.647043
Tenenbaum JB (2000) A global geometric framework for nonlinear dimensionality reduction. Science 290:2319–2323. https://doi.org/10.1126/science.290.5500.2319
Oliphant TE (2015) Guide to NumPy, 2nd edn. Continuum, Austin
Oliphant TE (2007) Python for scientific computing. Comput Sci Eng 9:10–20. https://doi.org/10.1109/mcse.2007.58
Garreta R, Moncecchi G (2013) Learning scikit-learn: machine learning in Python. Packt, Birmingham
Hunter JD (2007) Matplotlib: a 2D graphics environment. Comput Sci Eng 9(3):90–95. https://doi.org/10.1109/mcse.2007.55
Vu T, Mishra A, Konapala G (2018) Information entropy suggests stronger nonlinear associations between hydro-meteorological variables and ENSO. Entropy 20(1):38
Strong SP, Koberle R, Van Steveninck RRDR, Bialek W (1998) Entropy and information in neural spike trains. Phys Rev Lett 80(1):197
Frigieri G, Andrade RAP, Dias C, Spavieri DL Jr, Brunelli R, Cardim DA, Wang CC, Verzola RMM, Mascarenhas S (2018) Analysis of a non-invasive intracranial pressure monitoring method in patients with traumatic brain injury. Acta Neurochir Suppl 126:107–110
Vilela GH, Cabella B, Mascarenhas S et al (2016) Validation of a new minimally invasive intracranial pressure monitoring method by direct comparison with an invasive technique. Acta Neurochir Suppl 122:97–100
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
Juliana Shibaki, Bruno Padua, Felipe Silva, Thauan Gonçalves, Deusdedit Spavieri-Junior, Gustavo Frigieri, and Sérgio Mascarenhas have received financial support from Braincare Health Technologies. Inês Gomes and Celeste Dias have no commercial relationship with the company.
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Gomes, I. et al. (2021). Comparison of Waveforms Between Noninvasive and Invasive Monitoring of Intracranial Pressure. In: Depreitere, B., Meyfroidt, G., Güiza, F. (eds) Intracranial Pressure and Neuromonitoring XVII. Acta Neurochirurgica Supplement, vol 131. Springer, Cham. https://doi.org/10.1007/978-3-030-59436-7_28
Download citation
DOI: https://doi.org/10.1007/978-3-030-59436-7_28
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-59435-0
Online ISBN: 978-3-030-59436-7
eBook Packages: MedicineMedicine (R0)