Abstract
Levothyroxine (T4) is a critical-dose drug, because little variations in the blood concentration may cause treatment failure as well as iatrogenic thyrotoxicosis. Despite the dose response of this drug being more carefully titrated nowadays, several papers still report that a significant fraction of patients treated with levothyroxine demonstrate a TSH which is not on target. Moreover, some widespread gastrointestinal disorders as well as interfering drugs and foods may cause the “refractoriness” of a significant number of patients to an expected dose of thyroxine. The increasing awareness of the mechanisms interfering with the oral thyroid hormone bioavailability and the body of evidence regarding the complexity of treatment in certain classes of patients prompted pharmaceutical research to identify new hormonal formulations to optimize the performance of this drug. In this brief review, the progression of the scientific knowledge of novel T4 formulations use has been analyzed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Synthesized in 1927 by Harrington [1] and obtained as sodium salt in 1950 [2], levothyroxine (T4) has subsequently obtained the role of gold standard in the treatment of hypothyroid patients [3]. Prescribed worldwide, it ranks within the top ten most used drugs in western countries [4]. Levothyroxine is a critical-dose drug, since slight variations in blood concentration may result in treatment failure as well as iatrogenic thyrotoxicosis [5]. Therefore, both over- and undertreatment have been increasingly recognized in clinical practice and in scientific literature [6, 7], suggesting the need for an individualization of oral T4 treatment. Unfortunately, a daily dose of levothyroxine fitting all patients has been sought without success [3] and different levothyroxine doses are used to treat patients of different ages and with specific clinical conditions [8].
The daily requirement of levothyroxine depends on patients’ lean body mass [9], the leading cause of hypothyroidism and the therapeutic goal [e.g. replacement or TSH suppressive treatment]. Over the last few years, the knowledge of pharmacokinetic features of orally administered levothyroxine has led to a better individualization of treatment with a progressive decrease in administered doses [3]. Despite the fact that the dose response of this drug is currently more carefully titrated, several papers still report that a significant fraction of levothyroxine treated patients (including elderly and pregnant subjects) shows a TSH not on target [10]. While for a long time, nonadherence to levothyroxine treatment has been claimed as the major cause of increased T4 need, this condition may be detected in only 13–17% of patients, as demonstrated by specific questionnaires [11, 12]. These percentages also include the uncommon intentional nonadherence to treatment, frequently associated with psychiatric disorders (pseudomalabsorption) [13]. However, the need to fast for the correct absorption of the active ingredient represents the most relevant issue leading to patients’ nonadherence to a thyroxine schedule. In fact, Hays [14] clearly showed that orally administered thyroxine is incompletely absorbed in the small intestine, and more recent evidence has been provided to demonstrate that tablets must be previously dissolved in the gastric fluid [15]. In healthy humans, the fasted state warrants an intact gastric acid secretion that ensures a normal gastric juice pH, usually ranging from 1.5 to 2.4, increasing up to 5 after a meal, thus returning to normal within 3–4 h [15]. The crucial role of an acidic gastric pH has been ascertained both in vitro and in vivo. Indeed, a pH-dependent dissolution of oral thyroxine has been demonstrated in vitro [16] while, in clinical practice, an increased need for thyroxine has been observed in patients chronically treated with proton pump inhibitors or bearing H pylori infection or gastric atrophy [17, 18]. Calcium supplementation compounds and aluminum hydroxide also appear to interfere with thyroxine absorption through this mechanism [19]. In clinical practice, the interval between food and/or drug and thyroxine ingestion seems to be a limiting step for the subsequent intestinal absorption [20, 21]. Aside from the aforementioned causes of an increased need for thyroxine, some conditions and diseases may enhance daily T4 requirement by affecting the actual site of thyroxine absorption: drugs and foods adsorbing thyroxine in the intestinal lumen or interfering with TH intestinal transporters activity [19], celiac disease [22], lactose intolerance [23], and infections [24] are the most frequent conditions. Specific strains of gut microbiota have been involved in the processes leading to thyroid hormones recycling, thus potentially affecting pharmacologic thyroxine homeostasis [25, 26]. The increasing awareness of the mechanisms interfering with oral thyroid hormone bioavailability and the body of evidence on the complexity of treatment in certain classes of patients has led to the definition of “difficult” patient [27]. Figure 1 describes the long route of sodium levothyroxine to reach its final effect.
The refractoriness of a significant number of patients to a “normal dose” of thyroxine [28] has prompted pharmaceutical research to identify new hormonal formulations in an attempt to obtain a better performance for this widely prescribed drug.
Liquid preparations
Beside the liquid formulation in drops, a monodose vial preparation has recently become available in some parts of the world. This formulation, in which sodium levothyroxine is dissolved in ethanol and glycerol, has proven to be bioequivalent to the traditional preparations, but the mean time to reach maximal concentrations appears to be shorter than that of softgel or tablet formulations (1.96 vs 2.38 vs 2.25 h) [29]. The use of this formulation has been studied in newborns with congenital hypothyroidism in which the rapid attainment of thyroid homeostasis is a key to avoid neuropsychological sequelae. Cassio et al. [30] observed a faster TSH recovery in severe hypothyroid babies treated with liquid formulation; however, in the same group, there was a higher percentage of newborns showing suppressed TSH values. As a result, the authors suggested a careful individualized approach to avoid overtreatment [30]. The consistent efficacy of liquid formulation has been also described in 78 congenital hypothyroid newborns in the first two weeks, when a significantly lower TSH was observed in liquid T4-treated newborns [31]. The data available after the 8-month checkup are even more interesting: the better performance of liquid persisted despite the baby’s weaning, supporting the lower sensitivity of liquid formulation to food interference [31]. These data have been confirmed in 20 enterally fed patients [32] in whom there was no need to stop nutrition to attain the therapeutic target when treated with the liquid solution, as is the case for those treated with crushed T4 tablets.
Cappelli et al. [33] in a double-blind placebo-controlled trial specifically examined the interference of breakfast with the efficacy of liquid T4 in 77 patients; they concluded that liquid thyroxine might be ingested at breakfast time; this result has been confirmed in a similar [34] and in a larger cohort of patients [35]. Contrasting results were instead obtained in the quality of life [QoL] of patients treated with liquid T4 preparation. In fact, when QoL, either in mental or physical terms, was evaluated using Short-Form 12 questionnaires, regardless of the timing of liquid T4 intake, no difference emerged [34]. On the contrary, a study based on the validated ThyTSQ questionnaire submitted to 102 patients, who were unsatisfied with their tablet thyroxine treatment, revealed that some 2/3 of patients recognized an improved QOL and an easier compliance with treatment, following the switch to liquid thyroxine [36]. A lower rate of subjective symptoms was also reported in 54 patients, thyroidectomized for differentiated thyroid cancer, who had been switched to liquid formulation [37].
Different gastrointestinal disorders are known to reduce the efficacy of tablet thyroxine (22–24) and, therefore, the efficacy of liquid thyroxine has also been studied in these patients. This formulation has proven, essentially in case series, useful in 28 patients with active H pylori infection [38], in five patients bearing atrophic gastritis [39], in five subjects bearing lactose intolerance [40], in two patients with liver cirrhosis [41] and in one patient with Giardia intestinalis infestation [22]. The superior performance of this formulation over the T4 tablet was also claimed in 22 patients who have undergone various types of bariatric surgery: these included sleeve gastrectomy (restrictive surgery) [42], Roux en-Y gastric bypass (a restrictive/malabsorptive technique) [43, 44] and biliary pancreatic diversions (a purely malabsorptive surgery) [44].
The concomitant ingestion with other drugs of levothyroxine treatment represents a growing clinical problem: polypharmacy is in fact a widespread condition not only in the elderly patients [45]. Drug interference may act on thyroxine’s route of absorption at multiple levels i.e. (gastric acid neutralization, intestinal binding of T4, trapping, entero-hepatic recycling, competition for intestinal transporter etc.) [See [19] for rev]. On clinical ground, the liquid T4 formulation has been proven to overcome the co-ingestion effect of proton pump inhibitors [46, 47], calcium and iron supplements [48, 49], aluminum/magnesium hydroxide, sodium alginate, and sevelamer [50]. Notably, a population-based study of 55,000 thyroxine users demonstrated a significant reduction in the number of thyroid-stimulating hormone measurements after switching from tablet to liquid preparation, particularly in patients using drugs that potentially interfere with levothyroxine absorption [51]. A better control of serum TSH has been reported in elderly [52], pregnant [53], and unselected patients without evident malabsorption [54, 55] even if bearing subclinical hypothyroidism [56] or thyroidectomized for cancer [57], but with no substantial reduction of dose between liquid and tablet T4.
A case series on three thyroidectomized patients with refractory hypothyroidism identified the possibility of treating patients using a liquid preparation with a sublingual route of administration [58]. The highly permeable and vascularized sublingual mucosa allows direct access to systemic circulation, bypassing the steps preceding thyroxine absorption (Fig. 1). A study comparing the efficacy of liquid thyroxine dissolution in a glass of water and squeezed directly into the mouth revealed significant variable buccal absorption of the active ingredient [59]. This promising alternative deserves pharmacokinetic studies to assess a more extensive use of this route.
Liquid formulation performance, as compared with the traditional one, has been assessed in two meta-analyses: the first indicated that patients on tablet T4 showing suboptimal TSH values may reach the desired TSH when switching to liquid T4 formulation at the same daily dose [60]. The second meta-analysis demonstrated that liquid T4 is more efficient than tablet T4 in patients with malabsorption on thyroxine treatment either in replacement or in suppressive schedule [61]. In contrast, this finding is no more evident in patients with no malabsorption.
Softgel capsule
In liquid gel cap form, sodium levothyroxine is dissolved in water and glycerin and then placed into a gelatin matrix, protecting the active ingredient from degradation. It is entirely free of gluten, lactose, alcohol, dyes, or sugar [62]. Pabla et al. [16] compared in vitro the dissolution processes of two tablet [one branded and one generic] and one softgel thyroxine preparations. They clearly demonstrated that the softgel formulation dissolves better than the tablet formulations at different medium pH; this formulation, in fact, showed a lower pH sensitivity compared with traditional formulations [16]. The dissolution process of one softgel capsule was also directly observed during an endoscopy performed on a 35-year-old healthy woman [63]. Ten minutes after ingestion, the capsule volume was reduced by 50%, having completely disappeared 11 min later [63].
Softgel formulation has proven to be bioequivalent to tablet thyroxine in healthy subjects and in fasting conditions, [64]. Interestingly, a pharmacokinetic study by Seng Yue et al. [65] showed that in patients in which esomeprazole administration increased the gastric pH, the thyroxine peak exposure with the tablet was 16% lower than that obtained using softgel capsules. These data were previously predicted in a case report [66]. On clinical ground, Santaguida et al. [67] showed the efficacy of this new formulation in patients with definite gastric disorders. In this study, 31 patients showing a stable increased need for tablet thyroxine were switched to a lower dose of softgel preparation. Two-thirds of patients benefited from this variation, maintaining TSH on target despite the reduced dose of thyroxine. In some of those that did not show improvement, an occult intestinal disorder was eventually diagnosed, which, in a multivariate analysis, was the unique factor associated with the lack of response to this new formulation. The efficacy of softgel T4 has also been studied in further clinical conditions. A study by Di Donna et al. [68] examined the daily requirement of thyroxine in 103 thyroidectomized patients by comparing softgel and tablet preparations. The daily thyroxine requirement was similar using both preparations, but in patients treated with softgel formulation, serum TSH values were 30% lower compared with patients treated with tablet T4. The authors highlighted a possible role of softgel T4, chiefly in patients in whom a narrower therapeutic TSH goal is required, such as patients monitored for high risk differentiated thyroid cancer and hypothyroid pregnant women. Also, in patients with central hypothyroidism, the success of this formulation in reaching more normal circulating levels of FT4 compared with the traditional one has been demonstrated [69]. In two case reports [70, 71], describing patients bearing type 1 diabetes also featuring a gastroparesis, softgel T4 helped to overcome a tablet T4 refractory hypothyroidism due to a delayed gastric emptying. Few studies have been performed on nonselected patients to test the potential benefit of softgel formulation. A retrospective analysis was carried out on 99 randomly selected hypothyroid patients (24 with gastrointestinal comorbidity) who switched from tablet to softgel T4 treatment. In this paper, Ernst et al. [72] observed that, despite the fact that most of the patients did not experience changes in TSH levels, almost two-thirds of patients reported an improved control of hypothyroid symptoms. Furthermore, they observed a decreased number of dose changes following the switch, anticipating cuts to health costs in the long-term.
Softgel T4 effectiveness appears to be less sensitive to nutritional interference due to concomitant breakfast and/or coffee ingestion. Since the more efficient absorption of tablet T4 appears to require fasting for 60 min after drug ingestion [21, 73, 74], food interference with T4 gained interest. One study evaluated the efficacy of softgel and liquid T4 preparations taken with breakfast in 30 thyroidectomized patients [75]. The authors concluded that, similarly to liquid T4, softgel T4 may be taken at the time of breakfast. However, while the liquid T4 efficacy remained stable over time, that of softgel appeared to be slightly reduced, thus warranting confirmatory studies. A more recent paper examined the usefulness of gel capsules in 18 hypothyroid patients with no increased need for thyroxine [76]. The effect of the switch from tablet to softgel in these patients was evaluated after 3 months. When using tablet T4, serum TSH values were in the normal range in only 11 out of 18 patients, whereas, following the switch, the target TSH was reached in 16/18 subjects and the median TSH value was lower than that reached with the traditional preparation. In this study, however, the ingestion of thyroxine 30 min before breakfast for both preparations may have been optimal for softgel but not for tablet absorption [19, 21, 73, 74]. The effectiveness of softgel preparation was observed in a crossover study on eight patients taking thyroxine shortly prior to drinking coffee, demonstrating a serum TSH not on target [77]. The desired TSH was obtained by switching tablet T4 to the same dose of softgel T4. However, due to the small sample size, further studies would be useful. An acute oral load test enforced these data: in fact, eight volunteers ingested an Americano coffee concomitantly with 600 mcg of tablet T4 and a further eight ingested the same dose of softgel T4 preparation: over a 12-h postingestion time course, AUC and Cmax were greater in those treated with softgel T4 [74]. The safety of softgel preparation was only questioned in one study [78].
Concluding remarks
The availability of novel preparations of a drug widely diffused worldwide is always welcomed, as it widens paraphernalia for the treatment of patients with differentiated therapeutic needs. What we have learned from these studies shows, as usual, lights and shadows, which are indicated in Table 1.
These concepts clearly envisage more extensive and carefully controlled studies, also using shared therapeutic schedules. Keeping these observations in mind, however, the promising results of the aforementioned studies may fit well with the perspective of a real patient-tailored thyroxine treatment plan, according to the rules of precision medicine.
References
C.R. Harington, G. Barger, Chemistry of thyroxine: constitution and synthesis of thyroxine. Biochem. J. 21, 169–183 (1927)
J.V. Hennessey, The emergence of levothyroxine as a treatment for hypothyroidism. Endocrine 55, 6–18 (2017)
J. Jonklaas, A.C. Bianco, A.J. Bauer, K.D. Burman, A.R. Cappola, F.S. Celi, D.S. Cooper, B.W. Kim, R.P. Peeters, M.S. Rosenthal, A.M. Sawka, American Thyroid Association Task Force on Thyroid Hormone Replacement, Guidelines for the treatment of hypothyroidism: prepared by the american thyroid association task force on thyroid hormone replacement. Thyroid 24, 1670–1751 (2014)
https://www.statista.com/statistics/780284/levothyroxine-prescriptions-number-in-the-us/ Accessed 6 May 2019
R.B. Shah, J.S. Collier, V.A. Sayeed, A. Bryant, M.J. Habib, M.A. Khan, Tablet splitting of a narrow therapeutic index drug: a case with levothyroxine sodium. AAPS PharmSciTech 11, 1359–1367 (2010)
B. Biondi, D.S. Cooper, The clinical significance of subclinical thyroid dysfunction. Endocr. Rev. 29, 76–131 (2008). https://doi.org/10.1210/er.2006-0043
B. Biondi, D.S. Cooper, Subclinical hyperthyroidism. N. Engl. J. Med. 378, 2411–2419 (2018). https://doi.org/10.1056/NEJMcp1709318
B. Biondi, L. Wartofsky, Treatment with thyroid hormone. Endocr. Rev. 35, 433–512 (2014). https://doi.org/10.1210/er.2013-1083
F. Santini, A. Pinchera, A. Marsili, G. Ceccarini, M.G. Castagna, R. Valeriano, M. Giannetti, D. Taddei, R. Centoni, G. Scartabelli, T. Rago, C. Mammoli, R. Elisei, P. Vitti, Lean body mass is a major determinant of levothyroxine dosage in the treatment of thyroid diseases. J. Clin. Endocrinol. Metab. 90, 124–127 (2005)
V. Eligar, P.N. Taylor, O.E. Okosieme, G.P. Leese, C.M. Dayan, Thyroxine replacement: a clinical endocrinologist’s viewpoint. Ann. Clin. Biochem. 53, 421–433 (2016). https://doi.org/10.1177/0004563216642255
C. Cappelli, R. Castello, F. Marini, A. Paoletta, M. Marchetti, M. Saullo, A. Cristiano, I. Pirola, E. Gandossi, A. Ferlin, M. Castellano, Adherence to levothyroxine treatment among patients with hypothyroidism: a Northeastern Italian Survey. Front Endocrinol. 9, 699 (2018). https://doi.org/10.3389/fendo.2018.00699
H.M. Robertson, A.K. Narayanaswamy, O. Pereira, S.A. Copland, R. Herriot, A.W. McKinlay, J.S. Bevan, P. Abraham, Factors contributing to high levothyroxine doses in primary hypothyroidism: an interventional audit of a large community database. Thyroid 24, 1765–1771 (2014). https://doi.org/10.1089/thy.2013.0661
N. Van Wilder, B. Bravenboer, S. Herremans, N. Vanderbruggen, B. Velkeniers, Pseudomalabsorption of levothyroxine: a challenge for the endocrinologist in the treatment of hypothyroidism. Eur. Thyroid J. 6, 52–56 (2017). https://doi.org/10.1159/000452489
M.T. Hays, Localization of human thyroxine absorption. Thyroid 1, 241–248 (1991)
S.S. Jambhekar, P.J. Breen, Drug dissolution: significance of physicochemical properties and physiological conditions. Drug Discov. Today 18, 1173–1184 (2013)
D. Pabla, F. Akhlaghi, H. Zia, A comparative pH-dissolution profile study of selected commercial levothyroxine products using inductively coupled plasma mass spectrometry. Eur. J. Pharm. Biopharm. 72, 105–110 (2009)
M. Centanni, L. Gargano, G. Canettieri, N. Viceconti, A. Franchi, G. Delle Fave, B. Annibale, Thyroxine in goiter, Helicobacter pylori infection, and chronic gastritis. N. Engl. J. Med. 354, 1787–1795 (2006)
M. Cellini, M.G. Santaguida, C. Virili, S. Capriello, N. Brusca, L. Gargano, M. Centanni, Hashimoto’s thyroiditis and autoimmune gastritis. Front. Endocrinol. 8, 92 (2017)
C. Virili, A. Antonelli, M.G. Santaguida, S. Benvenga, M. Centanni, Gastrointestinal malabsorption of thyroxine. Endocr. Rev. 40, 118–136 (2019). https://doi.org/10.1210/er.2018-00168
K.W. Wenzel, H.E. Kirschsieper, Aspects of the absorption of oral L-thyroxine in normal man. Metabolism 26, 1–8 (1977)
T.G. Bach-Huynh, B. Nayak, J. Loh, S. Soldin, J. Jonklaas, Timing of levothyroxine administration affects serum thyrotropin concentration. J. Clin. Endocrinol. Metab. 94, 3905–3912 (2009)
A. Tortora, D. La Sala, M. Vitale, Switch from tablet levothyroxine to oral solution resolved reduced absorption by intestinal parasitosis. Endocrinol. Diabetes Metab. Case Rep. https://doi.org/10.1530/EDM-19-0026
C. Virili, G. Bassotti, M.G. Santaguida, R. Iuorio, S.C. Del Duca, V. Mercuri, A. Picarelli, P. Gargiulo, L. Gargano, M. Centanni, Atypical celiac disease as cause of increased need for thyroxine: a systematic study. J. Clin. Endocrinol. Metab. 97, E419–E422 (2012). https://doi.org/10.1210/jc.2011-1851
M. Cellini, M.G. Santaguida, I. Gatto, C. Virili, S.C. Del Duca, N. Brusca, S. Capriello, L. Gargano, M. Centanni, Systematic appraisal of lactose intolerance as cause of increased need for oral thyroxine. J. Clin. Endocrinol. Metab. 99, E1454–E1458 (2014). https://doi.org/10.1210/jc.2014-1217
C. Virili, M. Centanni, Does microbiota composition affect thyroid homeostasis? Endocrine 49, 583–587 (2015)
C. Virili, M. Centanni, “With a little help from my friends”—the role of microbiota in thyroid hormone metabolism and enterohepatic recycling. Mol. Cell Endocrinol. 458, 39–43 (2017)
L.S. Ward, The difficult patient: drug interaction and the influence of concomitant diseases on the treatment of hypothyroidism. Arq. Bras. Endocrinol. Metabol. 54, 435–442 (2010)
M. Centanni, S. Benvenga, I. Sachmechi, Diagnosis and management of treatment-refractory hypothyroidism: an expert consensus report. J. Endocrinol. Investig. 40, 1289–1301 (2017). https://doi.org/10.1007/s40618-017-0706-y
C.S. Yue, C. Scarsi, M.P. Ducharme, Pharmacokinetics and potential advantages of a new oral solution of levothyroxine vs. other available dosage forms. Arzneimittelforschung 62, 631–636 (2012). https://doi.org/10.1055/s-0032-1329951
A. Cassio, S. Monti, A. Rizzello, I. Bettocchi, F. Baronio, G. D’Addabbo, M.O. Bal, A. Balsamo, Comparison between liquid and tablet formulations of levothyroxine in the initial treatment of congenital hypothyroidism. J. Pediatr. 162, 1264–1269 (2013). https://doi.org/10.1016/j.jpeds.2012.11.070
E. Peroni, M.C. Vigone, S. Mora, L.A. Bassi, C. Pozzi, A. Passoni, G. Weber, Congenital hypothyroidism treatment in infants: a comparative study between liquid and tablet formulations of levothyroxine. Horm. Res. Paediatr. 81, 50–54 (2014). https://doi.org/10.1159/000356047
I. Pirola, L. Daffini, E. Gandossi, D. Lombardi, A. Formenti, M. Castellano, C. Cappelli, Comparison between liquid and tablet levothyroxine formulations in patients treated through enteral feeding tube. J. Endocrinol. Investig. 37, 583–587 (2014). https://doi.org/10.1007/s40618-014-0082-9
C. Cappelli, I. Pirola, L. Daffini, A. Formenti, C. Iacobello, A. Cristiano, E. Gandossi, E. Agabiti Rosei, M. Castellano, A double-blind placebo-controlled trial of liquid thyroxine ingested at breakfast: results of the TICO study. Thyroid 26, 197–202 (2016). https://doi.org/10.1089/thy.2015.0422
I. Pirola, E. Gandossi, D. Brancato, F. Marini, A. Cristiano, A. Delbarba, B. Agosti, M. Castellano, C. Cappelli, TSH evaluation in hypothyroid patients assuming liquid levothyroxine at breakfast or 30 min before breakfast. J. Endocrinol. Investig. 41, 1301–1306 (2018). https://doi.org/10.1007/s40618-018-0867-3
S. Morelli, G. Reboldi, S. Moretti, E. Menicali., N. Avenia., E. Puxeddu, Timing of breakfast does not influence therapeutic efficacy of liquid levothyroxine formulation. Endocrine 52, 571–578 (2015). https://doi.org/10.1007/s12020-015-0788-2
R. Guglielmi, F. Grimaldi, R. Negro, A. Frasoldati, I. Misischi, F. Graziano, C. Cipri, E. Guastamacchia, V. Triggiani, E. Papini, Shift from levothyroxine tablets to liquid formulation at breakfast improves quality of life of hypothyroid patients. Endocr. Metab. Immune Disord. Drug Targets 18, 235–240 (2018)
M. Giusti, L. Mortara, N. Machello, E. Monti, G. Pera, M. Marenzana, Utility of a liquid formulation of levo-thyroxine in differentiated thyroid cancer patients. Drug Res. 65, 332–336 (2014)
D. Ribichini, G. Fiorini, A. Repaci, V. Castelli, L. Gatta, D. Vaira, R. Pasquali, Tablet and oral liquid L-thyroxine formulation in the treatment of naïve hypothyroid patients with Helicobacter pylori infection. Endocrine 57, 394–401 (2017). https://doi.org/10.1007/s12020-016-1167-3
P. Fallahi, S.M. Ferrari, I. Ruffilli, A. Antonelli, Reversible normalisation of serum TSH levels in patients with autoimmune atrophic gastritis who received L-T4 in tablet form after switching to an oral liquid formulation: a case series. BMC Gastroenterol. 24, 16–22 (2016). https://doi.org/10.1186/s12876-016-0439-y
P. Fallahi, S.M. Ferrari, S. Marchi, N. De Bortoli, I. Ruffilli, A. Antonelli, Patients with lactose intolerance absorb liquid levothyroxine better than tablet levothyroxine. Endocrine 57, 175–178 (2017). https://doi.org/10.1007/s12020-016-1090-7
S. Benvenga, G. Capodicasa, S. Perelli, S.M. Ferrari, P. Fallahi, A. Antonelli, Increased requirement of replacement doses of levothyroxine caused by liver cirrhosis. Front. Endocrinol. 9, 150 (2018). https://doi.org/10.3389/fendo.2018.00150
C. Hommel, E. Delgrange, Resistance to levothyroxine in a bariatric surgery patient: an indication for liquid formulation? Acta Clin. Belg. 72, 72–75 (2017). https://doi.org/10.1080/17843286.2016.1196861
I. Pirola, A.M. Formenti, E. Gandossi, F. Mittempergher, C. Casella, B. Agosti, C. Cappelli, Oral liquid L-thyroxine (L-T4) may be better absorbed compared to L-T4 tablets following bariatric surgery. Obes. Surg. 23, 1493–1496 (2013). https://doi.org/10.1007/s11695-013-1015-y
P. Fallahi, S.M. Ferrari, S. Camastra, U. Politti, I. Ruffilli, R. Vita, G. Navarra, S. Benvenga, A. Antonelli, TSH normalization in bariatric surgery patients after the switch from L-thyroxine in tablet to an oral liquid formulation. Obes. Surg. 27, 78–82 (2017). https://doi.org/10.1007/s11695-016-2247-4
R.A. Payne, The epidemiology of polypharmacy. Clin. Med. 16, 465–469 (2016)
R. Vita, G. Saraceno, F. Trimarchi, S. Benvenga, Switching levothyroxine from the tablet to the oral solution formulation corrects the impaired absorption of levothyroxine induced by proton-pump inhibitors. J. Clin. Endocrinol. Metab. 99, 4481–4486 (2014). https://doi.org/10.1210/jc.2014-2684
D. Brancato, A. Scorsone, G. Saura, L. Ferrara, A. Di Noto, V. Aiello, M. Fleres, V. Provenzano, Comparison of TSH levels with liquid formulation versus tablet formulations of levothyroxine in the treatment of adult hypothyroidism. Endocr. Pract. 20, 657–662 (2014). https://doi.org/10.4158/EP13418.OR
S. Benvenga, F. Di Bari, R. Vita, Undertreated hypothyroidism due to calcium or iron supplementation corrected by oral liquid levothyroxine. Endocrine 56, 138–145 (2017). https://doi.org/10.1007/s12020-017-1244-2
E. Morini, A. Catalano, A. Lasco, N. Morabito, S. Benvenga, In thyroxine-replaced hypothyroid postmenopausal women under simultaneous calcium supplementation, switch to oral liquid or softgel capsule L-thyroxine ensures lower serum TSH levels and favorable effects on blood pressure, total cholesterolemia and glycemia. Endocrine. https://doi.org/10.1007/s12020-019-01908-x.
R. Vita, F. Di Bari, S. Benvenga, Oral liquid levothyroxine solves the problem of tablet levothyroxine malabsorption due to concomitant intake of multiple drugs. Expert Opin. Drug Deliv. 14, 467–472 (2017). https://doi.org/10.1080/17425247.2017.1290604
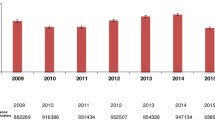
R. Ferrara, V. Ientile, V. Arcoraci, C. Ferrajolo, C. Piccinni, A. Fontana, S. Benvenga, G. Trifirò, Treatment pattern and frequency of serum TSH measurement in users of different levothyroxine formulations: a population-based study during the years 2009–2015. Endocrine 58, 143–152 (2017). https://doi.org/10.1007/s12020-017-1242-4
C. Cappelli, I. Pirola, L. Daffini, E. Gandossi, B. Agosti, M. Castellano, Thyroid hormonal profile in elderly patients treated with two different levothyroxine formulations: a single institute survey. Eur. Geriatr. Med. 5, 382–385 (2014)
C. Cappelli, R. Negro, I. Pirola, E. Gandossi, B. Agosti, M. Castellano, Levothyroxine liquid solution versus tablet form for replacement treatment in pregnant women. Gynecol. Endocrinol. 20, 1–3 (2015)
P. Fallahi, S.M. Ferrari, A. Antonelli, Oral L-thyroxine liquid versus tablet in patients with hypothyroidism without malabsorption: a prospective study. Endocrine 52, 597–601 (2016). https://doi.org/10.1007/s12020-015-0836-y
R. Negro, R. Valcavi, D. Agrimi, K.A. Toulis, Levothyroxine liquid solution versus tablet for replacement treatment in hypothyroid patients. Endocr. Pract. 20, 901–906 (2014). https://doi.org/10.4158/EP13378.OR
P. Fallahi, S.M. Ferrari, A. Antonelli, In patients with subclinical hypothyroidism while in therapy with tablet L-T4, the liquid L-T4 formulation is more effective in restoring euthyroidism. Endocr. Pract. 23, 170–174 (2017). https://doi.org/10.4158/EP161545.OR
P. Fallahi, S.M. Ferrari, G. Materazzi, F. Ragusa, I. Ruffilli, A. Patrizio, P. Miccoli, A. Antonelli, Oral L-thyroxine liquid versus tablet in patients submitted to total thyroidectomy for thyroid cancer (without malabsorption): a prospective study. Laryngoscope Investig. Otolaryngol. 3, 405–408 (2018). https://doi.org/10.1002/lio2.186
C. Peirce, S. Ippolito, A. Lanas, M. Pesce, G. Pontieri, D. Arpaia, G. Sarnelli, B. Biondi, Treatment of refractory and severe hypothyroidism with sublingual levothyroxine in liquid formulation. Endocrine 60, 193–196 (2018). https://doi.org/10.1007/s12020-017-1367-5
S. Benvenga, F.Di Bari, Intestinal absorption and buccal absorption of liquid levothyroxine. Endocrine 58, 591–594 (2017). https://doi.org/10.1007/s12020-017-1250-4
C. Virili, L. Giovanella, P. Fallahi, A. Antonelli, M.G. Santaguida, M. Centanni, P. Trimboli, Levothyroxine therapy: changes of TSH levels by switching patients from tablet to liquid formulation. a systematic review and meta-analysis. Front Endocrinol. 9, 10 (2018). https://doi.org/10.3389/fendo.2018.00010
I. Laurent, S. Tang, M. Astère, K.R. Wang, S. Deng, L. Xiao, Q.F. Li, Liquid L-thyroxine versus tablet L-thyroxine in patients on L- thyroxine replacement or suppressive therapy: a meta-analysis. Endocrine 61, 28–35 (2018). https://doi.org/10.1007/s12020-018-1574-8
R. Vita, P. Fallahi, A. Antonelli, S. Benvenga, The administration of L-thyroxine as softgel capsule or liquid solution. Expert Opin. Drug Deliv. 11, 1103–1111 (2014)
G. Fiorini, D. Ribichini, R. Pasquali, D. Vaira, In vivo dissolution of levothyroxine softgel capsules. Intern. Emerg. Med. 11, 1151–1152 (2016)
P. Colucci, P. D’Angelo, G. Mautone, C. Scarsi, M.P. Ducharme, Pharmacokinetic equivalence of a levothyroxine sodium soft capsule manufactured using the new food and drug administration potency guidelines in healthy volunteers under fasting conditions. Ther. Drug Monit. 33, 355–361 (2011). https://doi.org/10.1097/FTD.0b013e318217b69f
C. Seng Yue, S. Benvenga, C. Scarsi, L. Loprete, M.P. Ducharme, When bioequivalence in healthy volunteers may not translate to bioequivalence in patients: differential effects of increased gastric pH on the pharmacokinetics of levothyroxine capsules and tablets. J. Pharm. Pharm. Sci. 18, 844–855 (2015)
R. Vita, S. Benvenga, Tablet levothyroxine (L-T4) malabsorption induced by proton pump inhibitor; a problem that was solved by switching to L-T4 in softgel capsule. Endocr. Pract. 20, e38–e41 (2014)
M.G. Santaguida, C. Virili, S.C. Duca, M. Cellini, I. Gatto, N. Brusca, C. De Vito, L. Gargano, M. Centanni, Thyroxine softgel capsule in patients with gastric-related T4 malabsorption. Endocrine 49, 51–57 (2015). https://doi.org/10.1007/s12020-014-0476-7
V. Di Donna, R.M. Paragliola, C. de Waure, G. Papi, A. Pontecorvi, S.M. Corsello, Is levothyroxine requirement the same for tablet and softgel formulations? Endocrine 59, 458–460 (2018). https://doi.org/10.1007/s12020-017-1311-8
S. Benvenga, G. Capodicasa, S. Perelli, l-thyroxine in an oral liquid or softgel formulation ensures more normal serum levels of free T4 in patients with central hypothyroidism. Front. Endocrinol. 8, 321 (2017). https://doi.org/10.3389/fendo.2017.00321
P.J. Kim, I. Sachmechi, Levothyroxine malabsorption induced by diabetic gastroparesis exacerbated during pregnancies: effect of intramuscular levothyroxine injections and levothyroxine softgel capsules. AACE Clin. Case Rep. 1, e73–e78 (2015)
D.P. Reardon, P.S. Yoo, Levothyroxine tablet malabsorption associated with gastroparesis corrected with gelatin capsule formulation. Case Rep. Endocrinol. 2016, 1316724 (2016). https://doi.org/10.1155/2016/1316724
F.R. Ernst, W. Sandulli, R. Elmor, J. Welstead, A.B. Sterman, M. Lavan, Retrospective study of patients switched from tablet formulations to a gel cap formulation of levothyroxine: results of the CONTROL Switch Study. Drugs R. D. 17, 103–115 (2017). https://doi.org/10.1007/s40268-016-0150-z
S. Benvenga, L. Bartolone, S. Squadrito, F. Lo Giudice, F. Trimarchi, Delayed intestinal absorption of levothyroxine. Thyroid 5, 249–253 (1995)
M. Centanni, Thyroxine treatment: absorption, malabsorption, and novel therapeutic approaches. Endocrine 43, 8–9 (2013). https://doi.org/10.1007/s12020-012-9814-9
C. Cappelli, I. Pirola, E. Gandossi, A. Cristiano, L. Daffini, B. Agosti, C. Casella, M. Castellano, Thyroid hormone profile in patients ingesting softgel capsule or liquid levothyroxine formulations with breakfast. Int J. Endocrinol. 2016, 9043450 (2016). https://doi.org/10.1155/2016/9043450
P. Trimboli, C. Virili, M. Centanni, L. Giovanella, Thyroxine treatment with softgel capsule formulation: usefulness in hypothyroid patients without malabsorption. Front Endocrinol. 9, 118 (2018). https://doi.org/10.3389/fendo.2018.00118
R. Vita, G. Saraceno, F. Trimarchi, S. Benvenga, A novel formulation of L-thyroxine (L-T4) reduces the problem of L-T4 malabsorption by coffee observed with traditional tablet formulations. Endocrine 43, 154–160 (2013). https://doi.org/10.1007/s12020012-9772-2
E. Messina, F. Ferraù, S. Cannavò, Oral mucositis induced by treatment with softgel formulation of levothyroxine. Endocrine 59, 226–227 (2018). https://doi.org/10.1007/s12020-017-1312-7
Acknowledgements
The support of Mrs Jaclyn McLoughlin in revising the paper is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
C.V. received reimbursement from Institut Biochimique SA (IBSA) for travel expenses to attend the annual ATA and ETA Meeting. P.T. has nothing to disclose. M.C. received reimbursements from Institut Biochimique SA (IBSA), Pambio Noranco, CH, for travel expenses to attend some international meetings. M.C. received some reimbursement for his participation in advisory boards from Akrimax Pharmaceuticals, Cranford, NY, USA.
Ethical approval
As a review article, this paper does not contain any original study with human participants or animals performed by any of the authors.
Informed consent
As a review article, no informed consent is available.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Virili, C., Trimboli, P. & Centanni, M. Novel thyroxine formulations: a further step toward precision medicine. Endocrine 66, 87–94 (2019). https://doi.org/10.1007/s12020-019-02049-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-019-02049-x