Abstract
Purpose
To compare the effectiveness of liquid L-T4 (L-thyroxine) and tablet L-T4 in patients on L-T4 replacement or suppressive therapy.
Methods
The Cochrane Library, PubMed, Embase, and Web of Science databases were searched to identify relevant articles. All prospective or randomized controlled studies (RCTs) comparing liquid L-T4 and tablet L-T4 in patients on L-T4 replacement or suppressive therapy were included in the analysis.
Results
Overall, the initial search of the four databases identified 1278 published studies; of these, eight studies were ultimately included in the meta-analysis. TSH (thyroid stimulating hormone) levels were significantly suppressed in patients on liquid L-T4 compared with those on tablet L-T4, in patients on L-T4 suppressive therapy with L-T4 malabsorption (Mean Difference (MD) = −2.26, 95% Confidence Interval (CI): −3.59, −0.93; P = 0.0009)). However, liquid L-T4 and tablet L-T4 did not show a statistically significant difference in patients on L-T4 suppressive therapy without malabsorption (MD = 0.08, 95% CI: −0.31, 0.47; P = 0.69). TSH levels were significantly normalized in patients on liquid L-T4 compared with those on tablet L-T4, in Patients on L-T4 replacement therapy with L-T4 malabsorption (MD = −3.20, 95% CI: −5.08, −1.32; P = 0.0009). However, liquid L-T4 and tablet L-T4 did not show a statistically significant difference in patients on L-T4 replacement therapy without malabsorption (MD = 0.91, 95% CI: −0.03, 1.86; P = 0.06).
Conclusion
Liquid L-T4 is more efficient than tablet L-T4 in patients on L-T4 replacement or suppressive therapy with malabsorption. No significant differences were observed in patients without malabsorption. Further studies should be conducted to verify these findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Levothyroxine (L-thyroxine or L-T4) is used worldwide for replacement purposes in patients with primary or central hypothyroidism [1, 2]. It is also used at high doses for thyroid-stimulating hormone (TSH)-suppressive purposes in high- risk patients who underwent thyroidectomy for thyroid cancer, to decrease the risk of recurrence [3].
Acquired primary hypothyroidism is the most common type of thyroid disease dysfunction, noticing that Hashimoto’s thyroiditis is the leading cause. An emerging cause of acquired primary hypothyroidism is represented by drugs, for instance tyrosine kinase inhibitors [4, 5]. Recent studies have shown that hypothyroidism has some correlations with cardiovascular diseases and metabolic syndrome such as hypertension, diabetes and dyslipidemia [6,7,8]. Therefore, an adequate L-T4 replacement therapy can reverse or delay the events of those co-morbidities.
Differentiated thyroid cancer (DTC), which includes both papillary thyroid cancer (PTC) and follicular thyroid cancer (FTC), is the most common form of thyroid cancer. Approximately 90% of thyroid cancers are differentiated thyroid cancers [9]. While most patients diagnosed with DTC have a good prognosis, a significant proportion has persistent or recurrent disease. TSH-suppressive therapy has been shown to be effective not only in those with persistent metastatic disease but also in high- risk “disease –free” patients to reduce the risk of recurrence. In recently updated guidelines from the American Thyroid Association (ATA), the serum TSH suppression should be maintained <0.1 mU/L for high–risk thyroid cancer patients, 0.1–0.5 mU/L for intermediate–risk thyroid cancer patients and 0.5–2.5 mU/L for low–risk thyroid cancer patients [3].
In some cases, L-T4 is used at TSH-suppressive doses also to treat patients with nodular goiter even though this purpose is still debated [10, 11].
L-thyroxine in tablet is widely used in clinic. To obtain a good L-T4 tablet dissolution, an acid intragastric pH is necessary, and about 70% of the orally ingested L-T4 is absorbed mostly in the upper intestine (duodenum, jejunum and ileum) [12].
There are many conditions that can interfere with L-T4 absorption. In fact, studies showed that dietary fiber and coffee interfere with L-T4 absorption [13, 14]. Malabsorption is also reported in disorders such as Helicobacter pylori infection, atrophic gastritis, inflammatory bowel disease, celiac disease, and lactose intolerance [14, 15]. Many commonly used drugs such as cholestyramine, colesevelam, lanthanum, calcium carbonate, calcium citrate, calcium acetate, iron sulfate, ciprofloxacin, aluminum hydroxide, sevelamer, or proton pump inhibitors (PPIs) have also been shown to interfere with L-T4 absorption [14]. In addition, some conditions such as enteral feeding tube can also interfere with drug absorption [16].
The usual formulation of thyroxine is in tablet, but new formulations in soft gel capsule or liquid form exist in clinical application as well [17]. When the target serum thyroid–stimulating hormone(TSH) levels are not reached with the L-T4 tablet treatment, physicians can increase the daily dose [12]. It was reported that nearly 40% of patients undergoing treatment with levothyroxine are either overtreated or undertreated [18]. Therefore, any new formulation with a better outcome would be welcomed.
Many studies have been recently conducted. They compared the efficacy of liquid L-T4 and tablet L-T4 in patients on L-T4 therapy with or without malabsorption [19,20,21]. Grussendorf et al. reported the bioequivalence of tablet L-T4 and liquid L-T4 in the treatment of hypothyroid patients [22]. However, other studies showed that the two formulations are not statistically bioequivalent, especially in patients with malabsorption [12, 20, 23].
To further evaluate the efficacy of the new liquid L-T4 formulation, we performed a meta-analysis to compare liquid L-T4 with tablet L-T4 in patients on L-T4 replacement or suppressive therapy, since there has never been any meta-analysis on this topic.
Materials and methods
Study selection and data extraction
The PubMed, EMBASE, Web of Science, and Cochrane library databases were searched for relevant papers. The last search was performed on October 15/2017. To identify all the relevant studies, the search terms were “liquid levothyroxine” or “liquid L-thyroxine” or “liquid L-T4” and “tablet levothyroxine” or “tablet L-thyroxine” or “tablet L-T4” and “hypothyroidism” or “surgery” or “thyroid cancer” or “absorption”.
The eligibility criteria were as follows:1) the study should be prospective or randomized controlled trial; 2) the study comparing liquid L-thyroxine with tablet L-thyroxine in patients on L-thyroxine replacement or suppressive therapy; 3) the study should be written in english; 4) the study should be published as full text; 5) the study with complete outcome. The studies which did not fulfill the eligibility criteria were excluded.
To specifically assess the efficacy of the liquid formulation, the included studies were divided into four subgroups: a) patients on L-thyroxine replacement therapy with malabsorption; b) patients on L-thyroxine replacement therapy without malabsorption; c) patients on L-thyroxine suppressive therapy with malabsorption; d) patients on L-thyroxine suppressive therapy without malabsorption.
Any condition that can interfere with L-thyroxine absorption was considered as malabsorption, including a) patients after bariatric surgery, b) patients on calcium or iron supplementation, c) patients with enteral tube feeding, d) patients on PPIs, e) patients on concomitant intake of multiple drugs, f) patients with helicobacter pylori infection.
Study quality and risk of bias assessment
The authors worked independently to search for and assess studies for their methodological quality. The Cochrane collaboration’s tool for assessing the risk of bias was used. It includes seven entries: the random sequence generation, the allocation concealment, blinding of participants, blinding of personnel, blinding of outcome assessor, incomplete outcome data, selective reporting. If one study has more than two “high risk” entries, it was considered to be of low quality; otherwise it was considered to be of high quality [24]. Any disagreement in the study was resolved by consensus and, if necessary, a senior staff member was consulted.
Statistical analysis
Mean differences (MDs) with 95% confidence intervals (CIs) were calculated to assess the effect of continuous data using Review Manager Version 5.3 software. I2 and P-values were calculated to assess the heterogeneity among studies (I2 > 50% and /or P < 0.05 were considered statistically significant). We used the method of Xiang Wan et al. [25], in case that data were not presented as mean and standard deviation. Therefore, the mean and standard deviation were then estimated.The MDs were pooled using only a random effects model to calculate a more conservative result. MDs <0 indicated a better outcome for using liquid L-T4 while MDs >0 indicated a worse outcome for using liquid L-T4. Subgroup analyses were performed in patients on L-thyroxine replacement or suppressive therapy, and with or without malabsorption. Publication bias was not assessed due to the small number of studies in our meta-analysis. The Cochrane meta-analysis guidelines suggest the use of Egger’s test for publication bias for analyses with more than 10 studies.
P < 0.05 was considered to have a statistically significant difference in the outcomes between liquid L-thyroxine and tablet L-thyroxine.
Results
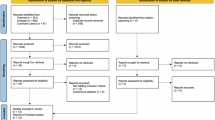
A total of 1278 papers were retrieved from the four databases, among which 971 papers were duplicates.16 potential studies were included for full text view after reviewing the titles and abstracts. With further screening, a total of eight studies met the inclusion criteria [26,27,28,29,30,31,32,33]. Flowchart of study selection is shown in Fig. 1. The main characteristics of eligible studies are summarized in Table 1. The publication dates of all included studies vary between June/2014 and June/2017.
Study characteristics
In four studies, there was a comparison between liquid L-T4 and tablet L-T4 in patients on L-T4 replacement therapy [28, 29, 31, 33]. In two studies, there was a comparison of liquid L-T4 and tablet L-T4 in patients on L-T4 replacement or suppressive therapy [26, 27]. In the remaining 2 studies, liquid L-T4 was compared with tablet L-T4 in patients on L-T4 suppressive therapy [30, 32]. In each study included, the daily dose of L-T4 was similar for both tablet and liquid formulations.
In five studies, all participants have at least one factor of malabsorption [26, 27, 29, 31, 33]. In one study, the efficacy of tablet and oral liquid L-T4 formulation was compared in patients with or without Helicobacter pylori infection [28]. In the remaining studies, there was not any condition influencing absorption being mentioned [30, 32]. In all the studies, the liquid L-T4 used, was Tirosint soluzione orale (IBSA Italia s.r.l).
Patients characteristics
The baseline characteristics such as sample size, sex, age, body mass index (BMI), TSH, Free thyroxine (f T4), free triiodothyronine (fT3), were comparable between patients on liquid L-thyroxine and patients on tablet L-thyroxine. There were no statistically significant differences between the patients in the two groups (Group on liquid L-T4 and group on tablet L-T4).
Publication bias
All included studies were considered to be of high quality.
Outcome
Patients on L-T4 suppressive therapy with drug malabsorption
TSH levels were remarkably suppressed in patients on liquid L-T4 in comparison with those on tablet L-T4 (Fig. 2), and with a statistically significant difference [(MD = −2.26, 95% Confidence interval (CI): −3.59, −0.93; P = 0.0009)]. The heterogeneity between studies was not significant (I2 = 0%, P = 0.62).
Patients on L-T4 suppressive therapy without drug malabsorption
TSH suppression seemed to be more obvious in patients on tablet L-T4 than those on liquid L-T4 (Fig. 2). However, there was not a statistically significant difference (MD = 0.08, 95% CI: −0.31, 0.47; P = 0.69). The heterogeneity between studies was significant (I2 = 59%, P = 0.12).
Patients on L-T4 replacement therapy with drug malabsorption
TSH levels were remarkably normalized in patients on liquid L-T4 compared to those on tablet L-T4 (Fig. 3), and with a statistically significant difference (MD = −3.20, 95% CI: −5.08, −1.32; P = 0.0009). The heterogeneity between studies was significant (I2 = 81%, P < 0.0001).
Patients on L-T4 replacement therapy without drug malabsorption
TSH normalization seemed to be more obvious in patients on tablet L-T4 than those on liquid L-T4 (Fig. 3). However, there was not a statistically significant difference (MD = 0.91, 95%CI: −0.03, 1.86; P = 0.06). The heterogeneity between studies was not significant (I2 = 0%, P = 0.47).
Discussion
The results of this meta-analysis show that liquid L-T4 is better than tablet L-T4 in patients on L-T4 replacement or suppressive therapy with malabsoption (Figs. 2 and 3). However there is not a statistically significant difference when liquid L-T4 is compared with tablet L-T4 in patients without malabsorption (Figs. 2 and 3).
In patients withL-T4 malabsorption, liquid L-T4 seems to be a better alternative therapy when the target serum thyroid –stimulating hormone (TSH) levels are not reached with the L-T4 tablet. Our results support previous findings. In fact, recent studies have proved that liquid L-T4 has a better therapeutic effect than tablet in patients undergoing bariatric surgery [31, 34], under PPIs or concomitant intake of multiple drugs [26, 35], with Helicobacter pylori infection [28] or when administered with breakfast [36, 37]. Liquid L-T4 pharmacokinetic properties explain the mechanism whereby liquid L-T4 can escape from multiple interferes. First, L-T4 in the liquid formulation does not need a dissolution phase in the stomach, which is necessary for the tablet and occurs at acidic pH [38]. Therefore, any interferer that increases or tampons the gastric pH, as proton pump inhibitors, calcium supplementation or sodium alginate, does not reduce the amount of L-T4 that is conveyed to the small intestine. Second, liquid L-T4 appears to be refractory also to the binding by drugs that sequester it and form insoluble complexes in the intestinal lumen, resulting in reduced absorption [27]. At last, the ethanol contained in the liquid formulation may enhance L-T4 absorption [39].
The liquid formulation could be helpful in saving a large amount of money. In fact, the repeated biochemical monitoring in L-T4 treated hypothyroid patients under simultaneous supplementation with iron (men and women) or calcium carbonate (women) in the European Community could be as high as one–eighth of billion Euros [33]. Negro et al. [40], interestingly, also found that a less proportion of patients on the liquid L-T4 had TSH values outside the normal range at follow up visit. The same authors suggested that the reduction of TSH variability by the use of liquid formulation, may give rise to reduced consultations, fewer laboratory tests, fewer dose adjustment and better outcomes for patients. Furthermore, Helicobacter pylori are very common in less developed countries [41, 42]. Thus, the new formulation should be chosen in those patients suspected of helicobacter pylori infection.
As both L-T4 and PPI are top prescribed drugs worldwide, it is common to meet patients who take them concurrently [43]. The physicians usually increase tablet L-T4 dose, which is a real risk of iatrogenic hyperthyroidism. In addition, overtreatment of L-T4 is particularly dangerous in old patients, in whom the risk of arrhythmia or fracture is higher [26]. Therefore, the use of new formulation may be safer and welcomed.
The obesity is worldwide a growing problem [44]. In the last decades, demand for bariatric surgery has globally increased [45, 46]. This procedure can lead to drug malabsorption [47]. After bariatric surgery, liquid L-T4 has been shown to be more efficient than tablet L-T4.It has resolved the problem of tablet L-T4 malabsorption observed in patients on L-T4 therapy after bariatric surgery [31].
Pirola et al. [29] showed that liquid L-T4 can be administered immediately through nasoenteric tube without the need for an empty stomach. The same authors also found that this formulation was more easily managed by nurses than with the tablet formulation in patients under enteral nutrition through a feeding tube. Peroni et al. [48] concluded liquid L-T4 might be more useful in congenital hypothyroid infants, especially around 6 months of treatment, at the time of weaning, when the intake of new types of food could interfere with drug absorption and deleteriously affect treatment efficacy. Thus, the use of liquid L-T4 in patients with malabsorption is of great importance.
This meta-analysis showed a better efficacy of liquid L-T4 in patients with malabsorption. However, its use remains controversial in elderly or secondary hypothyroid patients [37]. In fact, elderly patients are more susceptible to adverse effect of thyroid hormone excess. Therefore, careful titration of L-T4 dose, particularly when a formulation that ensures a better absorption is used, is needed to avoid iatrogenic thyrotoxicosis. In addition, overtreatment or under-treatment may often occur in secondary hypothyroid patients on L-T4 and a careful monitoring of circulating thyroxine should be assessed when using liquid L-T4.
Some recent studies have shown that liquid L-T4 is even more efficient than tablet L-T4 in patients without malabsorption [12, 20]. However, our meta-analysis has not found a statistically significant difference between the two formulations in patients without malabsorption (Figs. 2 and 3). Nevertheless, our findings correlate with previous findings where no significant difference was found between the two formulations in patients without malabsorption [28].
The current study presents some limitations. In fact, we did not find enough and large studies comparing liquid L-T4 and tablet L-T4, especially in patients without malabsorption. All the included studies originate from Italy and therefore one cannot exclude the possibility that other populations might behave differently. In addition, as all selected studies are from Italian research groups, there is a possible overlapping of study methodology and redundant findings that may influence the main results of the present study. Thus, further large-sized studies or RCTs should be conducted.
Conclusion
This is the first meta-analysis comparing liquid L-T4 and tablet L-T4 in patients on L-T4 replacement or suppressive therapy. Our findings proved again that liquid L-T4 is significantly more efficient than tablet L-T4 in patients on L-T4 replacement or suppressive therapy with malabsorption. No significant differences were found in patients without malabsorption. Further large–sized studies or RCTs should be conducted to verify these findings.
Data availability
The datasets during and/ or analyzed during the current study are available from the corresponding author on reasonable request.
References
J.R. Garber, R.H. Cobin, H. Gharib, J.V. Hennessey, I. Klein, J.I. Mechanick, R. Pessah-Pollack, P.A. Singer, K.A. Woeber, Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr. Pract. 18(6), 988–1028 (2012). https://doi.org/10.4158/ep12280.gl
B. Biondi, L. Wartofsky, Treatment with thyroid hormone. Endocr. Rev. 35(3), 433–512 (2014). https://doi.org/10.1210/er.2013-1083
B.R. Haugen, E.K. Alexander, K.C. Bible, G.M. Doherty, S.J. Mandel, Y.E. Nikiforov, F. Pacini, G.W. Randolph, A.M. Sawka, M. Schlumberger, K.G. Schuff, S.I. Sherman, J.A. Sosa, D.L. Steward, R.M. Tuttle, L. Wartofsky, 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 26(1), 1–133 (2016). https://doi.org/10.1089/thy.2015.0020
J. Przytulska, K. Tupikowski, G. Bednarek-Tupikowska, [Thyroid dysfunction in patients treated with tyrosine kinase inhibitors]. Pol. Merkur. Lek. 36(211), 42–44 (2014)
L. Bianchi, L. Rossi, F. Tomao, A. Papa, F. Zoratto, S. Tomao, Thyroid dysfunction and tyrosine kinase inhibitors in renal cell carcinoma. Endocr. Relat. Cancer 20(5), R233–R245 (2013). https://doi.org/10.1530/erc-13-0201
P. Wolf, Y. Winhofer, M. Krssak, M. Krebs, Heart, lipids and hormones. Endocr. Connect. 6(4), R59–r69 (2017). https://doi.org/10.1530/ec-17-0031
M. Gutch, S. Rungta, S. Kumar, A. Agarwal, A. Bhattacharya, S.M. Razi, Thyroid functions and serum lipid profile in metabolic syndrome. Biomed. J. 40(3), 147–153 (2017). https://doi.org/10.1016/j.bj.2016.12.006
A.P. Delitala, G. Fanciulli, G.M. Pes, M. Maioli, G. Delitala, Thyroid Hormones, Metabolic Syndrome and Its Components. Endocr. Metab. Immune Disord. Drug Targets 17(1), 56–62 (2017). https://doi.org/10.2174/1871530317666170320105221
R.B. Lv, Q.G. Wang, C. Liu, F. Liu, Q. Zhao, J.G. Han, D.L. Ren, B. Liu, C.L. Li, Low versus high radioiodine activity for ablation of the thyroid remnant after thyroidectomy in Han Chinese with low-risk differentiated thyroid cancer. OncoTargets Ther. 10, 4051–4057 (2017). https://doi.org/10.2147/ott.s135145
E. Fiore, P. Vitti, Serum TSH and risk of papillary thyroid cancer in nodular thyroid disease. J. Clin. Endocrinol. Metab. 97(4), 1134–1145 (2012). https://doi.org/10.1210/jc.2011-2735
E. Garcia-Garcia, M. Lopez-Gonzalez, R. Cabello-Laureano, E. Navarro-Gonzalez, Multinodular goiter in children: treatment controversies. J. Pediatr. Endocrinol. Metab. 30(8), 847–850 (2017). https://doi.org/10.1515/jpem-2016-0368
P. Fallahi, S.M. Ferrari, A. Antonelli, In patients with subclinical hypothyroidism while in therapy with tablet L-T4, the liquid L-T4 formulation is more effective in restoring euthyroidism. Endocr. Pract. 23(2), 170–174 (2017). https://doi.org/10.4158/ep161545.or
R. Vita, G. Saraceno, F. Trimarchi, S. Benvenga, A novel formulation of L-thyroxine (L-T4) reduces the problem of L-T4 malabsorption by coffee observed with traditional tablet formulations. Endocrine 43(1), 154–160 (2013). https://doi.org/10.1007/s12020-012-9772-2
M. Skelin, T. Lucijanic, D. Amidzic Klaric, A. Resic, M. Bakula, A.M. Liberati-Cizmek, H. Gharib, D. Rahelic, Factors affecting gastrointestinal absorption of levothyroxine: a review. Clin. Ther. 39(2), 378–403 (2017). https://doi.org/10.1016/j.clinthera.2017.01.005
M. Ruchala, E. Szczepanek-Parulska, A. Zybek, The influence of lactose intolerance and other gastro-intestinal tract disorders on L-thyroxine absorption. Endokrynol. Pol. 63(4), 318–323 (2012)
N.T. Williams, Medication administration through enteral feeding tubes. Am. J. Health-Syst. Pharm. 65(24), 2347–2357 (2008). https://doi.org/10.2146/ajhp080155
R. Vita, P. Fallahi, A. Antonelli, S. Benvenga, The administration of L-thyroxine as soft gel capsule or liquid solution. Expert Opin. Drug Deliv. 11(7), 1103–1111 (2014). https://doi.org/10.1517/17425247.2014.918101
G.J. Canaris, N.R. Manowitz, G. Mayor, E.C. Ridgway, The Colorado thyroid disease prevalence study. Arch. Intern. Med. 160(4), 526–534 (2000)
D. Brancato, A. Scorsone, G. Saura, L. Ferrara, A. Di Noto, V. Aiello, M. Fleres, V. Provenzano, Comparison of TSH levels with liquid formulation versus tablet formulations of levothyroxine in the treatment of adult hypothyroidism. Endocr. Pract. 20(7), 657–662 (2014). https://doi.org/10.4158/ep13418.or
P. Fallahi, S.M. Ferrari, A. Antonelli, Oral L-thyroxine liquid versus tablet in patients with hypothyroidism without malabsorption: a prospective study. Endocrine 52(3), 597–601 (2016). https://doi.org/10.1007/s12020-015-0836-y
P. Fallahi, S.M. Ferrari, I. Ruffilli, A. Antonelli, Reversible normalisation of serum TSH levels in patients with autoimmune atrophic gastritis who received L-T4 in tablet form after switching to an oral liquid formulation: a case series. BMC Gastroenterol. 16, 22 (2016). https://doi.org/10.1186/s12876-016-0439-y
M. Grussendorf, R. Vaupel, K. Wegscheider, [Bioequivalence of L-thyroxine tablets and a liquid L-thyroxine solution in the treatment of hypothyroid patients]. Med. Klin. 99(11), 639–644 (2004). https://doi.org/10.1007/s00063-004-1096-4
G. Ianiro, F. Mangiola, T.A. Di Rienzo, S. Bibbo, F. Franceschi, A.V. Greco, A. Gasbarrini, Levothyroxine absorption in health and disease, and new therapeutic perspectives. Eur. Rev. Med. Pharmacol. Sci. 18(4), 451–456 (2014)
X.S. Qi, Y.X. Bao, M. Bai, W.D. Xu, J.N. Dai, X.Z. Guo, Nonselective beta-blockers in cirrhotic patients with no or small varices: A meta-analysis. World J. Gastroenterol. 21(10), 3100–3108 (2015). https://doi.org/10.3748/wjg.v21.i10.3100
X. Wan, W. Wang, J. Liu, T. Tong, Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 14, 135 (2014). https://doi.org/10.1186/1471-2288-14-135
R. Vita, G. Saraceno, F. Trimarchi, S. Benvenga, Switching levothyroxine from the tablet to the oral solution formulation corrects the impaired absorption of levothyroxine induced by proton-pump inhibitors. J. Clin. Endocrinol. Metab. 99(12), 4481–4486 (2014). https://doi.org/10.1210/jc.2014-2684
R. Vita, F. Di Bari, S. Benvenga, Oral liquid levothyroxine solves the problem of tablet levothyroxine malabsorption due to concomitant intake of multiple drugs. Expert Opin. Drug Deliv. 14(4), 467–472 (2017). https://doi.org/10.1080/17425247.2017.1290604
D. Ribichini, G. Fiorini, A. Repaci, V. Castelli, L. Gatta, D. Vaira, R. Pasquali, Tablet and oral liquid L-thyroxine formulation in the treatment of naïve hypothyroid patients with Helicobacter pylori infection. Endocrine 57(3), 394–401 (2017). https://doi.org/10.1007/s12020-016-1167-3
I. Pirola, L. Daffini, E. Gandossi, D. Lombardi, A. Formenti, M. Castellano, C. Cappelli, Comparison between liquid and tablet levothyroxine formulations in patients treated through enteral feeding tube. J. Endocrinol. Invest 37(6), 583–587 (2014). https://doi.org/10.1007/s40618-014-0082-9
M. Giusti, L. Mortara, N. Machello, E. Monti, G. Pera, M. Marenzana, Utility of a liquid formulation of levo-thyroxine in differentiated thyroid cancer patients. Drug Res. 65(6), 332–336 (2015). https://doi.org/10.1055/s-0034-1384535
P. Fallahi, S.M. Ferrari, S. Camastra, U. Politti, I. Ruffilli, R. Vita, G. Navarra, S. Benvenga, A. Antonelli, TSH normalization in bariatric surgery patients after the switch from L-thyroxine in tablet to an oral liquid formulation. Obes. Surg. 27(1), 78–82 (2017). https://doi.org/10.1007/s11695-016-2247-4
C. Cappelli, I. Pirola, E. Gandossi, C. Casella, D. Lombardi, B. Agosti, F. Marini, A. Delbarba, M. Castellano, TSH variability of patients affected by differentiated thyroid cancer treated with levothyroxine liquid solution or tablet form. Int J. Endocrinol. 2017, 7053959 (2017). https://doi.org/10.1155/2017/7053959
S. Benvenga, F. Di Bari, R. Vita, Undertreated hypothyroidism due to calcium or iron supplementation corrected by oral liquid levothyroxine. Endocrine 56(1), 138–145 (2017). https://doi.org/10.1007/s12020-017-1244-2
I. Pirola, A.M. Formenti, E. Gandossi, F. Mittempergher, C. Casella, B. Agosti, C. Cappelli, Oral liquid L-thyroxine (L-t4) may be better absorbed compared to L-T4 tablets following bariatric surgery. Obes. Surg. 23(9), 1493–1496 (2013). https://doi.org/10.1007/s11695-013-1015-y
C. Cappelli, I. Pirola, L. Daffini, A. Formenti, C. Iacobello, A. Cristiano, E. Gandossi, E. Agabiti Rosei, M. Castellano, A double-blind placebo-controlled trial of liquid thyroxine ingested at breakfast: results of the TICO study. Thyroid 26(2), 197–202 (2016). https://doi.org/10.1089/thy.2015.0422
S. Morelli, G. Reboldi, S. Moretti, E. Menicali, N. Avenia, E. Puxeddu, Timing of breakfast does not influence therapeutic efficacy of liquid levothyroxine formulation. Endocrine 52(3), 571–578 (2016). https://doi.org/10.1007/s12020-015-0788-2
A.M. Formenti, G. Mazziotti, R. Giubbini, A. Giustina, Treatment of hypothyroidism: all that glitters is gold? Endocrine 52(3), 411–413 (2016). https://doi.org/10.1007/s12020-016-0882-0
M. Centanni, L. Gargano, G. Canettieri, N. Viceconti, A. Franchi, G. Delle Fave, B. Annibale, Thyroxine in goiter, Helicobacter pylori infection, and chronic gastritis. New Engl. J. Med. 354(17), 1787–1795 (2006). https://doi.org/10.1056/NEJMoa043903
C. Cappelli, I. Pirola, E. Gandossi, A. Formenti, M. Castellano, Oral liquid levothyroxine treatment at breakfast: a mistake? Eur. J. Endocrinol. 170(1), 95–99 (2014). https://doi.org/10.1530/eje-13-0693
R. Negro, R. Valcavi, D. Agrimi, K.A. Toulis, Levothyroxine liquid solution versus tablet for replacement treatment in hypothyroid patients. Endocr. Pract. 20(9), 901–906 (2014). https://doi.org/10.4158/ep13378.or
I. Muller, P. Yap, P. Steinmann, B.P. Damons, C. Schindler, H. Seelig, N.S. Htun, N. Probst-Hensch, M. Gerber, R. du Randt, U. Puhse, C. Walter, J. Utzinger, Intestinal parasites, growth and physical fitness of schoolchildren in poor neighbourhoods of Port Elizabeth, South Africa: a cross-sectional survey. Parasites Vectors 9(1), 488 (2016). https://doi.org/10.1186/s13071-016-1761-5
K. Thevakumar, J.R. Chandren, G.I. Perez-Perez, E.G. Chua, L.K. Teh, M.Z. Salleh, J.A. Tan, A.H. Leow, K.L. Goh, A.C. Tay, B.J. Marshall, J. Vadivelu, M.F. Loke, L.P. Wong, Assessment of risk and Sero-Prevalence of Helicobacter pylori colonization among remote Orang Asli Tribes in Peninsula Malaysia. PloS One 11(7), e0159830 (2016). https://doi.org/10.1371/journal.pone.0159830
I. Sachmechi, D.M. Reich, M. Aninyei, F. Wibowo, G. Gupta, P.J. Kim, Effect of proton pump inhibitors on serum thyroid-stimulating hormone level in euthyroid patients treated with levothyroxine for hypothyroidism. Endocr. Pract. 13(4), 345–349 (2007). https://doi.org/10.4158/ep.13.4.345
N. Bahreini Esfahani, N. Ganjali Dashti, M. Ganjali Dashti, M.I. Noorv, P.B. Koon, R.A. Talib, S.H. Lubis, Dietary predictors of overweight and obesity in Iranian adolescents. Iran. Red. Crescent Med. J. 18(9), e25569 (2016). https://doi.org/10.5812/ircmj.25569
G.A. Bray, G. Fruhbeck, D.H. Ryan, J.P. Wilding, Management of obesity. Lancet 387(10031), 1947–1956 (2016). https://doi.org/10.1016/s0140-6736(16)00271-3
G. Fruhbeck, Bariatric and metabolic surgery: a shift in eligibility and success criteria. Nat. Rev. Endocrinol. 11(8), 465–477 (2015). https://doi.org/10.1038/nrendo.2015.84
A.D. Miller, K.M. Smith, Medication and nutrient administration considerations after bariatric surgery. Am. J. Health-Syst. Pharm. 63(19), 1852–1857 (2006). https://doi.org/10.2146/ajhp060033
E. Peroni, M.C. Vigone, S. Mora, L.A. Bassi, C. Pozzi, A. Passoni, G. Weber, Congenital hypothyroidism treatment in infants: a comparative study between liquid and tablet formulations of levothyroxine. Horm. Res Paediatr. 81(1), 50–54 (2014). https://doi.org/10.1159/000356047
Acknowledgements
The study was supported by National Natural Science Foundation of China Grants (81370954, 81670785); Fundamental Science and Advanced Technology Research of Chongqing Major Project (cstc2015jcyjBX0096); Chongqing Science and Technology Committee Innovation Project, Technology Development and Application of Precision Medicine (cstc2016shms-ztzx1003).The authors thank Fengfan Zheng, Ting Luo, and John Belly for their advices. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for integrity of the work as whole, and have given approval for the version to be published. All authors approved the final version.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
These authors contributed equally: Irakoze Laurent, Siying Tang.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Laurent, I., Tang, S., Astère, M. et al. Liquid L-thyroxine versus tablet L-thyroxine in patients on L- thyroxine replacement or suppressive therapy: a meta-analysis. Endocrine 61, 28–35 (2018). https://doi.org/10.1007/s12020-018-1574-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-018-1574-8