Abstract
Oral levothyroxine (L-T4) is the mainstay of hypothyroidism treatment. Many factors may influence its absorption, including the timing of administration. Objective of the study is to demonstrate the therapeutic equivalence of administering liquid L-T4 with breakfast or 10 min before breakfast. This was a pilot study conducted with a crossover design AB/BA where A stays for L-T4 with breakfast and B for L-T4 10 min before breakfast. A post hoc analysis was conducted to compare L-T4 administered at breakfast or 10 min before breakfast with L-T4 administered 30 min before breakfast. Sixty-one hypothyroid patients were enrolled and assigned to one of the two treatment sequences. All patients were evaluated for TSH levels at the end of each period. Fifty-nine patients completed the study. The mean thyrotropin concentration was 1.52 ± 0.73 µU/ml when L-T4 was administered with breakfast and 1.46 ± 0.81 µU/ml when it was taken 10 min before breakfast, without clinically and statistically significant differences (P = 0.59), regardless of treatment sequence and period. The mean thyrotropin concentration was 1.54 ± 0.9 µU/ml when L-T4 was administered at 0–10 min intervals before breakfast and 1.25 ± 0.7 µU/ml when it was taken 30 min before breakfast (ratio = 1.23, within our definition of equivalence set at 0.8–1.25). There is therapeutic equivalence between liquid L-T4 administration at breakfast or 10 min before breakfast. We can also hypothesize that there are no clinically relevant differences between liquid L-T4 administration 30 min before breakfast or at shorter intervals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypothyroidism is a common endocrine disorder resulting from deficiency of thyroid hormone and oral levothyroxine (L-T4) is the mainstay of its treatment. Oral L-T4 treatment is often used lifelong and the search for the optimal daily dose may be a challenge for the physician [1].
Many different L-T4 products are available (tablets, drops, and the recently introduced oral solution) that are thought to have different absorption rates because of different excipients and different formulations [2].
Approximately 62–82 % of L-T4 is absorbed after oral administration of the tablet formulation [3–5]. This absorption occurs within the first 3 h after ingestion and is localized mainly in the jejunum and ileum [6]. Many factors can affect the L-T4 dose required to normalize a particular patient’s TSH and the absorbed dose of L-T4 may be considered the resultant of physiological, paraphysiological, pharmacological, or pathological conditions [7]. Some of these factors are drugs that can affect L-T4 metabolism, absorption, or transport [8, 9]. Other factors are patient-related and include body weight and lean body mass [10], age [11–13], gender [10, 14], pregnancy [15], adherence to therapy [16], etiology of hypothyroidism [17, 18], TSH goal [18], deiodinase polymorphism [19, 20]. Pathological conditions that may result in less than optimal absorption of orally administered L-T4 include some gastrointestinal disorders [8], such as gastritis related either to Helicobacter pylori [21, 22] or autoimmunity (atrophic gastritis) [22, 23], celiac disease [24], lactose intolerance [25], or inflammatory bowel diseases. The timing of L-T4 administration also has a significant impact on L-T4 absorption. The absorption of L-T4 is maximal when the stomach is empty, reflecting the importance of timing of food intake and of gastric acidity in this process [5, 26]. The acidic gastric environment is fundamental either for the dissolution of the tablets or for the solubilization of the hormone with release of native L-T4 from the sodium salt contained in the tablets [22]. Studies show optimal intestinal absorption of L-T4 under fasting conditions and a 40–80 % reduction in assimilation with concurrent food ingestion [26]. Therefore, the standard of care for patients requiring L-T4 is to prescribe its administration when the stomach is empty, preferably 1 h before breakfast [26]. However, in order to enhance patient compliance, as convention the drug is usually given half an hour before breakfast, but many patients with hypothyroidism find that this practice is inconvenient and interferes with their lifestyle [27].
Due to its different pharmacokinetics from the conventional tablets, the liquid L-T4 preparation, that lacks the dissolution phase, being ready for a faster uptake by the small intestine mucosa, may allow to overcome this issue.
In a small retrospective study, Cappelli et al. did not observe significant differences in thyroid hormone concentrations when patients consumed oral liquid L-T4 at breakfast or 30 min before breakfast, demonstrating that oral liquid formulations could reduce the problem of L-T4 malabsorption caused by coffee observed using traditional tablet formulations [28].
The possibility to reduce the time interval with breakfast implies less impact on lifestyle and could improve patient compliance and quality of life, with a significant therapeutic value. In this respect, our is a pilot randomized crossover study primarily aimed at demonstrating that the administration of oral liquid L-T4 with breakfast or 10 min before breakfast are equivalent in terms of obtained TSH concentration and of patient’s quality of life (QoL). Clarification of this point could be important for the design of further studies. We also conducted a post hoc explorative analysis to verify if taking liquid L-T4 at breakfast or 10 min before breakfast is as effective as taking it 30 min before breakfast.
Subjects and methods
Patients and study drug
Enrolled patients included subjects affected by primary hypothyroidism of any nature (Hashimoto’s thyroiditis, total thyroidectomy, radioactive iodine treatment) in whom substitutive treatment with L-T4 was indicated. All subjects were older than 18 and were able to express and sign an informed consent. Exclusion criteria included assumption of any drug at breakfast time, positive history for gastrointestinal diseases, positive anti-gastric parietal cell and anti-transglutaminase antibody titers, pregnancy and, for ethical reasons, thyroid cancer history. Before extending the study to more fragile patients (i.e., thyroid cancer patients, older people or patients taking interfering drugs such as proton pump inhibitors), we preferred to demonstrate the therapeutic efficacy of the two modalities of liquid L-T4 administration. All patients signed an informed consent.
The study drug was a liquid L-T4 formulation (Tirosint® Oral Solution, IBSA Group) available in prefilled 25, 50, 75, and 100 µg vials. Patients were invited to use the appropriate vial or combinations of vials in order to take the established dose.
Study design
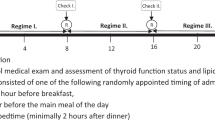
This was a pilot study conducted with a crossover design AB/BA (Fig. 1). In the first instance, enrolled patients were submitted to a L-T4 oral solution dose titration, taking the drug 30 min before breakfast, aimed at obtaining a TSH in the range 0.5–2 µUI/ml. Once the treatment goal was reached, they were enrolled and entered a 6-weeks run-in phase taking the drug 30 min before breakfast. At the end of this phase, the patients were randomized to the AB or BA treatment sequences, where A stays for a 6-week period in which the interval between the assumption of the drug and breakfast was 0 min and B for a 6-weeks period in which the interval was 10 min. A 6-week washout phase separated periods 1 and 2 of each treatment sequence in order to minimize a possible carry-over effect. TSH was measured at the end of each phase using the HYPERsensitive hTSH assay (Beckman Coulter Inc.) according to manufacturer’s recommendations. The study was approved by the local ethical committee and by the Italian Medicine Agency (AIFA). Moreover, it was recorded in the European EudraCT database (EudraCT code: 2012-005709-35).
Design of the study. After L-T4 oral solution dose titration, enrolled patients entered a 6-week run-in phase taking the drug 30 min before breakfast. At the end of this phase, the patients were randomized to the AB or BA treatment sequences, where A stays for a 6-week period in which the interval between the assumption of the drug and breakfast was 0 min and B for a 6-week period in which the interval was 10 min. A 6-week washout phase, in which the interval between the assumption of the drug and breakfast was 30 min, separated periods one and two of each treatment sequence in order to minimize a possible carry-over effect
QoL evaluation
Possible variations in the QoL associated with the different intervals between assumption of the drug and breakfast were evaluated using a Short-Form 12 questionnaire, which represents a synthetic version of the more common Short-Form 36 questionnaire [29]. In detail, SF-12 allows to describe the mental and physical health status using two questions for each of the following SF-36 scales: physical activity, physical health and role, emotional status and role, and mental health. Moreover, one question is formulated for the remaining four scales, which include physical pain, general health, vitality, and social activities.
Statistical analysis
Sample size
The study was sized hypothesizing the non-equivalence of the two treatment modalities in a predefined 20 % range and assuming a 35 % variance coefficient of serum TSH concentration. Assuming a power of 80 % and a statistical significance of 5 %, the minimal sample size was fixed at 52 patients. Considering the possible drop-outs, the sample size was corrected (+20 %) in order to maintain the same power and significance.
Statistical methods
The main statistical analysis was the comparison of the absolute and relative differences between the intra-person means of serum TSH concentrations after 6 weeks of L-T4 oral solution treatment assuming the drug 10 min before breakfast or together with breakfast. All the analyses were performed in accordance with an intention-to-treat principle. In order to calculate the statistical significance of the treatment differences, a t test for paired data with correction for period and crossover sequence was applied. Moreover, the effect of baseline and washout TSH on the treatment differences of the respective periods was evaluated applying a linear model for covariance analysis (ANCOVA), which included TSH concentration as continuous variable. Finally, although the study design included a long washout period, specifically introduced to minimize a potential carry-over effect, an analysis to test formally the occurrence of this effect was performed including a specific term in the ANCOVA model [30]. In order to conduct an explorative analysis on the comparison of serum TSH concentrations during the study periods and the corresponding baselines, descriptive statistics and ANOVA models for repeated measures were used, applying the Bonferroni correction when appropriate.
Results
Clinical characteristics of the patients’ cohort
We identified 105 patients with primary hypothyroidism (88 females and 17 males) aged 20-78 years; among them, 61 responded to the considered inclusion criteria and were enrolled (52 females and 9 males) (Table 1). Thirty-two patients were assigned to AB treatment sequence (group 1) and 29 to BA treatment sequence (group 2).
There were no differences between group 1 and 2 regarding gender distribution, age, body weight, hypothyroidism etiology, breakfast habits, and drug therapies (Table 1).
Fifty-nine patients completed the study: 31 in group 1 and 28 in group 2.
Comparison of TSH between the two modalities of L-T4 assumption
In the first instance, we verified the equivalence between liquid L-T4 intake with breakfast or 10 min before breakfast. The mean TSH value in group 1 (AB treatment sequence) was 1.58 ± 0.69 µUI/ml during period 1 (when L-T4 was assumed with breakfast) and 1.63 ± 0.87 µUI/ml during period 2 (when L-T4 was assumed 10 min before breakfast) (difference of the means −0.05; P = 0.80). For patients in group 2 (BA treatment sequence), the mean TSH value was 1.26 ± 0.70 µUI/ml during period 1 (when L-T4 was assumed 10 min before breakfast) and 1.45 ± 0.79 µUI/ml during period 2 (when L-T4 was assumed with breakfast) (difference of the means 0.19; P = 0.31) (Table 2). Similarly, comparison of the differences of the means of the TSH levels between the two groups did not turn out statistically different (P = 0.56) (Table 2).
In accordance with these data, the dispersion of TSH levels in each phase of treatment was similar in both treatment sequences and in both periods (Fig. 2a). Similarly, mean TSH concentration trend was superimposable in patients that followed AB or BA treatment sequences (Fig. 2b). Moreover, the mean thyrotropin concentration of group 1 and group 2 was globally 1.52 ± 0.73 µU/ml when levothyroxine was administered with breakfast and 1.46 ± 0.81 µU/ml when it was taken 10 min before breakfast, in the absence of any statistically significant difference (difference of the means 0.07, P = 0.59) (Table 2; Fig. 2c). Similarly, the mean thyrotropin concentration of group 1 and group 2 was globally 1.43 ± 0.71 µU/ml in period 1 and 1.55 ± 0.83 µU/ml in period 2, in the absence of any statistical significant difference (difference of the means −0.12, P = 0.33) (Table 2; Fig. 2d). Finally, no differences could be detected between the two different treatment sequences (AB or BA) comparing mean intra-patient TSH concentration trend in all the series (Data not shown).
Comparison of TSH between the two modalities of L-T4 assumption. a Box plot of TSH dispersion in the different treatment phases and periods. L-T4 assumed 0 min before breakfast during period 1 or during period 2 is indicated as A; L-T4 assumed 10 min before breakfast during period 1 or during period 2 is indicated as B. b TSH concentration trend during AB and BA treatment sequences. Light continuous line: TSH trend in each patient following the AB sequence. Light interrupted line: TSH trend in each patient following the BA sequence. Marked continuous line: Mean TSH trend in patients following the AB sequence. Marked interrupted line: Mean TSH trend in patients following the BA sequence. c TSH means for each treatment phase. d TSH means for each treatment period
Altogether, these data indicate that assuming liquid L-T4 with breakfast or 10 min before breakfast does not produce statistically significant differences on TSH concentration, regardless of treatment sequence or considered study period.
We also conducted an analysis to evaluate a potential carry-over effect after the washout period. Using a linear model which included among other factors a term “patient-in-sequence,” we did not find a statistically significant carry-over effect (P = 0.1288) (Data not shown).
Comparison of QoL between the two modalities of L-T4 assumption
In the second instance, we evaluated the QoL associated with the changes in the interval between assumption of the drug and breakfast. In detail, we calculated for each patient, in each study phase, mental and physical health status scores, using the Short-Form 12 questionnaire. We did not observe significant differences either in the physical component or in the mental component score regardless of timing of L-T4 intake (30 min before breakfast, with breakfast or 10 min before breakfast) and of the treatment sequence or period (Tables 3, 4; Fig. 3). Also the dispersion grade of the scores and the score trend resulted similar in patients that followed AB or BA treatment sequences (Data not shown).
Comparison of QoL between the three modalities of L-T4 assumption: 30 min before breakfast, at breakfast, or 10 min before breakfast. a PCS (Physical Component Score) when L-T4 was assumed 30 min before breakfast or with breakfast and when LT-4 was assumed 30 min before breakfast or 10 min before breakfast. b MCS (Mental Component Score) when L-T4 was assumed 30 min before breakfast or with breakfast and when LT-4 was assumed 30 min before breakfast or 10 min before breakfast
Altogether, these data indicate that assuming liquid L-T4 30 min before breakfast, with breakfast, or 10 min before breakfast does not produce statistically significant differences on QoL features, regardless of treatment sequence or the considered study period.
Post hoc explorative analysis
Finally, we conducted a post hoc explorative analysis to verify if taking liquid L-T4 at breakfast or 10 min before breakfast was as effective as taking it 30 min before breakfast. The mean thyrotropin concentration was 1.54 ± 0.9 µU/ml when L-T4 was administered with breakfast or 10 min before and 1.25 ± 0.7 µU/ml when it was taken 30 min before breakfast (ratio = 1.23, within our definition of equivalence set at 0.8–1.25).
Similarly, the equivalence hold splitting the TSH values in accordance with the treatment modality (L-T4 assumption at breakfast or 10 min before breakfast). In detail, in the first setting, the mean thyrotropin concentration was 1.56 ± 0.8 µU/ml when L-T4 was administered with breakfast and 1.24 ± 0.7 µU/ml when it was taken 30 min before breakfast (ratio = 1.25). Moreover, in the second setting, the mean thyrotropin concentration was 1.52 ± 0.9 µU/ml when L-T4 was administered 10 min before breakfast and 1.26 ± 0.7 µU/ml when it was taken 30 min before breakfast (ratio = 1.20).
Altogether, these data indicate that assuming liquid L-T4 with breakfast or 10 min before breakfast might be clinically equivalent to assuming it 30 min before breakfast.
Discussion
Hypothyroidism is the most frequent endocrine disease and the most common thyroid dysfunction in humans. In western countries, about 4–10 % of the population is affected by this condition [31–34] and almost an equivalent number of subjects are treated with L-T4. Even if some patient subgroups may benefit from a combination of L-T4 and L-triiodothyronine, L-T4 monotherapy remains the most commonly employed treatment modality for hypothyroidism [35]. Identification of the efficacious L-T4 dose for each individual needs a fine titration of the drug taking into consideration timing of the drug assumption and many factors either physiologic or pathologic that might interfere with L-T4 absorption [8]. Moreover, the therapeutic window of the drug is small, and easily patients can experience under- or over-treatments [36]. This latter notion prompts a frequent monitoring of thyroid function tests and the need to standardize the treatment as much as possible [37]. In detail, timing of the drug assumption is very critical [26]. Because food can interfere with L-T4 solubilization and intestinal uptake, L-T4 assumption is recommended in the morning when the subject is in the fasting state, at least 30 min before breakfast, in order to minimize absorption lack or day-by-day absorption variability. In clinical practice, most of the patients adapt to this recommendation, although it is generally felt that this habit interferes with the accomplishment of the first daily activities.
These considerations have pushed the search for novel L-T4 preparations featuring a greater solubility and a faster intestinal absorption. In these regards, oral L-T4 solution has appeared very convenient because it lacks the gastric dissolution phase and appears to be characterized by a faster and greater absorption from the gastrointestinal tract. At equal interval with breakfast, compared to tablets, when food arrives to small intestine, liquid solution is predictably almost all absorbed. Furthermore, the absorption of the liquid formulation appears to be poorly affected by the altered pH of the gastric environment associated with food consumption, gastritis, and use of proton pump inhibitors in comparison with other L-T4 products [38]. Walter-Sack et al. demonstrated that the rate of liquid solution absorption is 30 % faster than that observed with conventional tablets [39] and this likely allows to reduce the interval between L-T4 assumption and breakfast. In this respect, recent studies have shown that also soft gel L-T4 formulation (in which T4 is dissolved in glycerin and which has a protecting shell made of soft gel gelatin) should be preferred both in patients with gastric-related [40] or coffee-related [41] L-T4 malabsorption. Indeed, the physico-chemical characteristics of liquid formulation appear to allow a reduction in the interval between drug assumption and breakfast, without significant changes in drug efficacy, but with improvement in quality of life of the patients and increase in therapeutic compliance.
In this study, we compared the liquid L-T4 absorption in two conditions: taking it at breakfast or 10 min before breakfast.
Comparison of the two treatment modalities did not show neither clinically nor statistically significant TSH differences and indicated their equivalence regardless of treatment sequence (AB or BA) or treatment period (period 1 or period 2). Coherently, analysis of QoL during the different phases of the two treatment sequences did not show any significant difference neither in the physical component score (PCS) nor in the mental component score (MCS). Because mean PCS or MCS scores of 50 ± 10 are considered normal, the values between 40 and 60, detected in our patients, indicated always a good QoL and an equivalence of the impact on QoL of the two treatment modalities regardless of treatment sequence (AB or BA) or treatment period (period 1 or period 2). Mean PCS or MCS scores were not different even comparing LT-4 assumption at breakfast or 10 min before breakfast with the assumption 30 min before breakfast.
Although the study was designed only to compare the efficacy of taking liquid L-T4 10 min before breakfast or at breakfast, it was enriched by a large set of TSH values obtained in the same patients during the assumption of liquid L-T4 30 min before breakfast at the end of the run-in phase or of the washout phase. This design allowed the conduction of a post hoc explorative analysis to verify if taking liquid L-T4 at breakfast or 10 min before breakfast was as effective as taking it 30 min before breakfast. Interestingly, all the comparisons showed TSH value ratios within our definition of equivalence set at 0.8–1.25. Thus, we can speculate the absence of clinically significant differences between the three treatment modalities. Of course, the formal proof of that hypothesis needs the design of a therapeutic equivalence study aimed at demonstrating that liquid L-T4 assumption 10 min before breakfast or at breakfast is clinically not inferior with respect to the 30 min interval.
In summary, this study allowed to demonstrate that there is clinical equivalence between liquid L-T4 administration at breakfast or 10 min before breakfast, without changes even in the quality of life.
References
M. Centanni, A. Franchi, M.G. Santaguida, C. Virili, S. Nardo, L. Gargano, Oral thyroxine treatment: towards an individually tailored dose. Recenti Prog. Med. 98, 445–451 (2007)
R. Vita, P. Fallahi, A. Antonelli, S. Benvenga, The administration of L-thyroxine as soft gel capsule or liquid solution. Expert Opin. Drug Deliv. 11, 1103–1111 (2014)
L.H. Fish, H.L. Schwartz, J. Cavanaugh, M.W. Steffes, J.P. Bantle, J.H. Oppenheimer, Replacement dose, metabolism, and bioavailability of levothyroxine in the treatment of hypothyroidism. Role of triiodothyronine in pituitary feedback in humans. N. Engl. J. Med. 316, 764–770 (1987)
M.T. Hays, Absorption of oral thyroxine in man. J. Clin. Endocrinol. Metab. 28, 749–756 (1968)
K.W. Wenzel, H.E. Kirschsieper, Aspects of the absorption of oral L-thyroxine in normal man. Metabolism. 26, 1–8 (1977)
M.T. Hays, Localization of human thyroxine absorption. Thyroid. 1, 241–248 (1991)
M. Centanni, Thyroxine treatment: absorption, malabsorption, and novel therapeutic approaches. Endocrine 43, 8–9 (2013)
L. Liwanpo, J.M. Hershman, Conditions and drugs interfering with thyroxine absorption. Best Pract. Res. Clin. Endocrinol. Metab. 23, 781–792 (2009)
M.I. Surks, R. Sievert, Drugs and thyroid function. N. Engl. J. Med. 333, 1688–1694 (1995)
F. Santini, A. Pinchera, A. Marsili, G. Ceccarini, M.G. Castagna, R. Valeriano, M. Giannetti, D. Taddei, R. Centoni, G. Scartabelli, T. Rago, C. Mammoli, R. Elisei, P. Vitti, Lean body mass is a major determinant of levothyroxine dosage in the treatment of thyroid diseases. J. Clin. Endocrinol. Metab. 90, 124–127 (2005)
J. Jonklaas, Sex and age differences in levothyroxine dosage requirement. Endocr. Pract. 16, 71–79 (2010)
R.L. Rosenbaum, U.S. Barzel, Levothyroxine replacement dose for primary hypothyroidism decreases with age. Ann. Intern. Med. 96, 53–55 (1982)
C.T. Sawin, T. Herman, M.E. Molitch, M.H. London, S.M. Kramer, Aging and the thyroid. Decreased requirement for thyroid hormone in older hypothyroid patients. Am. J. Med. 75, 206–209 (1983)
M. Devdhar, R. Drooger, M. Pehlivanova, G. Singh, J. Jonklaas, Levothyroxine replacement doses are affected by gender and weight, but not age. Thyroid. 21, 821–827 (2011)
J.A. Loh, L. Wartofsky, J. Jonklaas, K.D. Burman, The magnitude of increased levothyroxine requirements in hypothyroid pregnant women depends upon the etiology of the hypothyroidism. Thyroid. 19, 269–275 (2009)
D.J. Lips, M.T. van Reisen, V. Voigt, W. Venekamp, Diagnosis and treatment of levothyroxine pseudomalabsorption. Neth. J. Med. 62, 114–118 (2004)
M.B. Gordon, M.S. Gordon, Variations in adequate levothyroxine replacement therapy in patients with different causes of hypothyroidism. Endocr. Pract. 5, 233–238 (2004)
L.A. Burmeister, M.O. Goumaz, C.N. Mariash, J.H. Oppenheimer, Levothyroxine dose requirements for thyrotropin suppression in the treatment of differentiated thyroid cancer. J. Clin. Endocrinol. Metab. 75, 344–350 (1992)
M. Torlontano, C. Durante, I. Torrente, U. Crocetti, G. Augello, G. Ronga, T. Montesano, L. Travascio, A. Verrienti, R. Bruno, S. Santini, P. D’Arcangelo, B. Dallapiccola, S. Filetti, V. Trischitta, Type 2 deiodinase polymorphism (threonine 92 alanine) predicts L-thyroxine dose to achieve target thyrotropin levels in thyroidectomized patients. J. Clin. Endocrinol. Metab. 93, 910–913 (2008)
K.A. Heemstra, H.C. Hoftijzer, W.M. van der Deure, R.P. Peeters, E. Fliers, B.C. Appelhof, W.M. Wiersinga, E.P. Corssmit, T.J. Visser, J.W. Smit, Thr92Ala polymorphism in the type 2 deiodinase is not associated with T4 dose in athyroid patients or patients with Hashimoto thyroiditis. Clin. Endocrinol. 71, 279–283 (2009)
E. Lahner, C. Virili, M.G. Santaguida, B. Annibale, M. Centanni, Helicobacter pylori infection and drugs malabsorption. World J. Gastroenterol. 20, 10331–10337 (2014)
M. Centanni, L. Gargano, G. Canettieri, N. Viceconti, A. Franchi, G. Delle Fave, B. Annibale, Thyroxine in goiter, Helicobacter pylori infection, and chronic gastritis. N. Engl. J. Med. 354, 1787–1795 (2006)
S. Checchi, A. Montanaro, L. Pasqui, C. Ciuoli, V. De Palo, M.C. Chiappetta, F. Pacini, L-thyroxine requirement in patients with autoimmune hypothyroidism and parietal cell antibodies. J. Clin. Endocrinol. Metab. 93, 465–469 (2008)
C. Virili, G. Bassotti, M.G. Santaguida, R. Iuorio, S.C. Del Duca, V. Mercuri, A. Picarelli, P. Gargiulo, L. Gargano, M. Centanni, Atypical celiac disease as cause of increased need for thyroxine: a systematic study. J. Clin. Endocrinol. Metab. 97, 419–422 (2012)
M. Cellini, M.G. Santaguida, I. Gatto, C. Virili, S.C. Del Duca, N. Brusca, S. Capriello, L. Gargano, M. Centanni, Systematic appraisal of lactose intolerance as cause of increased need for oral thyroxine. J. Clin. Endocrinol. Metab. 99, 1454–1458 (2014)
T.G. Bach-Huynh, B. Nayak, J. Loh, S. Soldin, J. Jonklaas, Timing of levothyroxine administration affects serum thyrotropin concentration. J. Clin. Endocrinol. Metab. 94, 3905–3912 (2009)
R. Rajput, S. Chatterjee, M. Rajput, Can levothyroxine be taken as evening dose? Comparative evaluation of morning versus evening dose of levothyroxine in treatment of hypothyroidism. J. Thyroid Res. 505239 (2011). doi:10.4061/2011/505239
C. Cappelli, I. Pirola, E. Gandossi, A. Formenti, M. Castellano, Oral liquid levothyroxine treatment at breakfast: a mistake? Eur. J. Endocrinol. 170, 95–99 (2013)
J. Ware Jr, M. Kosinski, S.D. Keller, A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med. Care 34, 220–233 (1996)
S.S. Senn, Cross-over Trials in Clinical Research (John Wiley, Chichester, 2002)
M.P. Vanderpump, W.M. Tunbridge, J.M. French, D. Appleton, D. Bates, F. Clark, J. Grimley Evans, D.M. Hasan, H. Rodgers, F. Tunbridge, E.T. Young, The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham Survey. Clin. Endocrinol. 43, 55–68 (1995)
C.T. Sawin, W.P. Castelli, J.M. Hershman, P. McNamara, P. Bacharach, The aging thyroid. Thyroid deficiency in the Framingham Study. Arch. Intern. Med. 145, 1386–1388 (1985)
J.G. Hollowell, N.W. Staehling, W.D. Flanders, W.H. Hannon, E.W. Gunter, C.A. Spencer, L.E. Braverman, Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J. Clin. Endocrinol. Metab. 87, 489–499 (2002)
G.J. Canaris, N.R. Manowitz, G. Mayor, E.C. Ridgway, The Colorado thyroid disease prevalence study. Arch. Intern. Med. 160, 526–534 (2000)
J.R. Garber, R.H. Cobin, H. Gharib, J.V. Hennessey, I. Klein, J.I. Mechanick, R. Pessah-Pollack, P.A. Singer, K.A. Woeber, American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults, Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr. Pract. 18, 988–1028 (2012)
M.I. Surks, E. Ortiz, G.H. Daniels, C.T. Sawin, N.F. Col, R.H. Cobin, J.A. Franklyn, J.M. Hershman, K.D. Burman, M.A. Denke, C. Gorman, R.S. Cooper, N.J. Weissman, Subclinical thyroid disease. Scientific review and guidelines for diagnosis and management. JAMA 291, 228–238 (2004)
W. Wiersinga, Thyroid hormone replacement therapy. Horm. Res. 56, 74–81 (2001)
R. Vita, G. Saraceno, F. Trimarchi, S. Benvenga, Switching levothyroxine from the tablet to the oral solution formulation corrects the impaired absorption of levothyroxine induced by proton-pump inhibitors. J. Clin. Endocrinol. Metab. 99, 4481–4486 (2014)
I. Walter-Sack, C. Clanget, R. Ding, C. Goeggelmann, V. Hinke, M. Lang, J. Pfeilschifter, Y. Tayrouz, K. Weqscheider, Assessment of levothyroxine sodium bioavailability: recommendations for an improved methodology based on the pooled analysis of eight identically designed trials with 396 drug exposures. Clin. Pharmacokinet. 43, 1037–1053 (2004)
M.G. Santaguida, C. Virili, S.C. Del Duca, M. Cellini, I. Gatto, N. Brusca, C. De Vito, L. Gargano, M. Centanni, Thyroxine softgel capsule in patients with gastric-related T4 malabsorption. Endocrine 49, 51–57 (2015)
R. Vita, G. Saraceno, F. Trimarchi, S. Benvenga, A novel formulation of L-thyroxine (L-T4) reduces the problem of L-T4 malabsorption by coffee observed with traditional tablet formulations. Endocrine 43, 154–160 (2013)
Acknowledgments
The authors would like to thank the nurse team (Nadia Biccari, Alberto Pambianco, and Lorena Urbani) of the Endocrine Clinic at the Department of Medicine, University of Perugia, for their excellent assistance. This research did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Morelli, S., Reboldi, G., Moretti, S. et al. Timing of breakfast does not influence therapeutic efficacy of liquid levothyroxine formulation. Endocrine 52, 571–578 (2016). https://doi.org/10.1007/s12020-015-0788-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-015-0788-2